Abstract
Recent studies suggest that epigenetic programming may mediate the relationship between early life environment, including parental socioeconomic position, and adult cardiometabolic health. However, interpreting associations between early environment and adult DNA methylation may be difficult because of time-dependent confounding by life-course exposures. Among 613 adult women (mean age = 32 years) of the Jerusalem Perinatal Study Family Follow-up (2007–2009), we investigated associations between early life socioeconomic position (paternal occupation and parental education) and mean adult DNA methylation at 5 frequently studied cardiometabolic and stress-response genes ( ABCA1 , INS-IGF2 , LEP , HSD11B2 , and NR3C1 ). We used multivariable linear regression and marginal structural models to estimate associations under 2 causal structures for life-course exposures and timing of methylation measurement. We also examined whether methylation was associated with adult cardiometabolic phenotype. Higher maternal education was consistently associated with higher HSD11B2 methylation (e.g., 0.5%-point higher in 9–12 years vs. ≤8 years, 95% confidence interval: 0.1, 0.8). Higher HSD11B2 methylation was also associated with lower adult weight and total and low-density lipoprotein cholesterol. We found that associations with early life socioeconomic position measures were insensitive to different causal assumption; however, exploratory analysis did not find evidence for a mediating role of methylation in socioeconomic position-cardiometabolic risk associations.
Keywords: epigenetic biomarker, life course, marginal structural models, methylation, time-dependent confounding
Early life exposure to stressful environments such as poverty, famine, and war may increase adult susceptibility to mortality ( 1 ), cardiovascular disease ( 2 , 3 ), and poor pregnancy outcomes ( 4–6 ). Programming of the fetal epigenome through changes in DNA methylation ( 7–10 ) is a putative mechanism. The response of methylation in gene promoter regions to intrauterine environment ( 11–13 ) and persistence into adulthood ( 6–10 ) may mediate the association of early life exposures with adult health. Importantly, DNA methylation of growth, metabolism, and stress response genes ( 8 , 11–14 ) may be programmed by prenatal stressors, including those related to socioeconomic position ( 7 , 15 , 16 ).
Several studies have found associations between severe maternal stressors and candidate gene DNA methylation in cardiometabolic and stress response genes in neonates ( 17 ), adolescents ( 18 ), and adults ( 12 , 19 ). Early life socioeconomic position as measured by parental education and other factors has also been associated with systemic, differential offspring methylation ( 20–23 ) and, recently, with functionally relevant candidate genes ( 16 ). However, studies investigating associations between parental socioeconomic position and offspring methylation in specific cardiometabolic and stress response genes, such as the nuclear receptor subfamily 3 group C member 1 gene ( NR3C1 ) or the insulin–insulin-like growth factor 2 readthrough gene region ( INS-IGF2 ) ( 24 , 25 ), may provide evidence for a mechanistic role of DNA methylation in early life socioeconomic position-adult health relationships ( 26–29 ). Moreover, investigating associations among women may be of particular interest, because their own early life environment may affect the intrauterine environment for their offspring ( 6 , 26 , 27 ).
However, a major identified gap in the empirical literature is identifying whether observed associations between early life factors and adult DNA methylation may be due to life-course experiences ( 16 , 30 ), rather than strictly early life programming. For example, adolescent adiposity or obesity may both mediate the early life-adult methylation relationship through developmental programming ( 30 ), as well as confound relationships between adult socioeconomic position (due to health selection ( 31 )) or smoking (due to desire for weight loss ( 32 )) and adult methylation. To avoid overcorrecting for such mediators in estimating the direct effects of early life environment, we may find that indirect adjustment for these variables though marginal structural models may be necessary ( 3 ).
We used several alternative life-course models and modeling techniques to investigate associations between measures of early life socioeconomic position and DNA methylation in commonly studied cardiometabolic ( 33 ) and stress-related ( 7 , 34 , 35 ) genes among young adult women (mean age = 32 years). Properly accounting for complex relationships among life-course variables by using marginal structural models may provide stronger evidence that developmental programming is responsible for early life socioeconomic position-adult methylation associations. Additionally, we investigated adult DNA methylation-adult health associations and explored potential mediation of early life socioeconomic position-adult health associations by DNA methylation.
METHODS
Study setting and population
Our investigation was conducted in the Jerusalem Perinatal Study that included all 17,003 births to Jerusalem residents between 1974 and 1976 ( 36 , 37 ). Around the time of birth, maternal medical history, pregnancy course, and offspring birth weight were abstracted from birth certificates or maternity ward logs. Additionally, mothers were interviewed 1 or 2 days postpartum. In the subsequent Jerusalem Perinatal Study Family Follow-up Study, a sample of 1,400 mother-offspring dyads was drawn from the Jerusalem Perinatal Study, oversampled by maternal prepregnancy body mass index (BMI, weight (kg)/height (m) 2 ) ≥27 and offspring birth weight ≤2,500 g or ≥4,000 g, and restricted to singletons born ≥36 weeks without overt congenital malformations. Between 2007 and 2009, offspring (mean age = 32 years) were interviewed by telephone about sociodemographic, behavioral, and health history. In later physical examinations, peripheral blood samples were collected, assayed, and stored ( 36–38 ). All female Jerusalem Perinatal Study Family Follow-up Study offspring with stored blood ( n = 613) were included in the current study. Jerusalem Perinatal Study Family Follow-up Study protocols were approved by the University of Washington (Seattle, Washington) and Hadassah-Hebrew University Medical Center (Jerusalem, Israel) institutional review boards.
Data collection
Early life socioeconomic position
We utilized 4 measures for early life socioeconomic position: 1) 6-category paternal occupational class (i.e., 6 = lowest/manual and 1 = highest/professional) as reported during the maternal postpartum interview; 2) binary paternal occupational class (high = 1–3 and low = 4–6); and total years of 3) maternal and 4) paternal education ( 36–39 ). The class measure was derived from ranking 108 occupations by mean years of education ( 37 ). Representative occupations include teachers (class 1), rabbis (class 2), accountants (class 3), electricians (class 4), skilled agricultural workers (class 5), and unskilled laborers (class 6) ( Supplementary Data available at http://aje.oxfordjournals.org/ ). This measure has been shown to be associated with a number of outcomes in the Jerusalem Perinatal Study population ( 37 ). Maternal occupation was not included, as a majority of women in the study (59%) were not employed outside the home ( 37 ). We also categorized parental education as ≤8, 9–12, and ≥13 years.
Candidate gene selection and methylation profiling
Five genes were selected on the basis of prior literature and putative cardiometabolic (adenosine triphosphate binding cassette subfamily A member 1 gene ( ABCA1 ), INS-IGF2 , and leptin gene ( LEP )) and stress response (hydroxysteroid (11-β) dehydrogenase 2 gene ( HSD11B2 ) and NR3C1 ) function. These genes are related to cholesterol homeostasis ( ABCA1 ) ( 24 , 33 ), insulin production and growth ( INS-IGF2 ) ( 24 , 25 , 33 ), energy balance ( LEP ) ( 33 ), cortisol inactivation ( HSD11B2 ) ( 40–42 ), and cortisol signaling ( NR3C1 ) ( 17 , 18 , 43 ), respectively. Using stored peripheral blood samples obtained at the physical examination, we performed quantitative assessment of methylation by use of the MassARRAY procedure (Sequenom, Inc., San Diego, California) ( 44 , 45 ) at Roswell Park Cancer Institute's Genomics Shared Resource (Buffalo, New York). Briefly, 1 µg of genomic DNA was bisulfite converted by using an EZ DNA Methylation Kit (Zymo Research, Orange, California). Converted DNA was then amplified by polymerase chain reaction under standard conditions ( 44 ; Supplementary Data ) using primers designed in MethPrimer ( 45 ) to coincide with prior literature ( 17 , 18 , 24 , 25 , 33 , 39–4 ; Supplementary Data , Supplementary Data ). Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry was conducted to analyze cleavage products, with methylation calls performed by EpiTyper v1.0 (Sequenom) and written to an Oracle 8i database. Each cleavage product corresponded to 1 CpG (cytosine-phosphate-guanine site of methylation) unit, with the output value being proportion methylated among several proximal CpG sites ( Supplementary Data ). Region-specific methylation was calculated as the arithmetic mean of all CpG units in the region. Control runs of 0%, 50%, and 100% methylation were included for quality control. CpG units with >25% failed methylation calls were excluded (3 for ABCA1 , 2 for LEP , and 4 for NR3C1 ). No subjects were excluded on the basis of these criteria.
Adult cardiometabolic and pregnancy outcomes
At the subject's physical examination, height, weight, waist and hip circumference, and systolic and diastolic blood pressures were measured by study staff ( 36 ). Additionally, a concurrently drawn fasting (≥8 hours since last meal) peripheral blood sample was assayed for total cholesterol and lipids on the VITROS 5,1 FS Chemistry System (Ortho Clinical Diagnostics, Raritan, New Jersey), and high- and low-density lipoprotein cholesterol concentrations were calculated ( 36 ). We constructed 3 binary outcomes: obesity (BMI ≥30); metabolic syndrome defined on the basis of International Diabetes Federation criteria ( 46 ); and, among women reporting any children ( n = 451), whether they had any term, low birth weight offspring (<2,500 g; yes/no). We were interested in the latter because our previous work suggested that grandmaternal education is positively associated with grandchild birth weight ( 47 ).
Early life confounders
Data on maternal prepregnancy overweight status (prepregnancy BMI ≥27 based on oversampling criteria), age at birth, any prenatal smoking, and parity from postpartum interview, as well as subject's birth weight from medical records ( 36–38 ), were included. Previous studies have shown maternal prepregnancy BMI, age, ethnicity ( 39 ), and prenatal smoking and offspring birth weight ( 25 ) to be confounders of early life socioeconomic position-methylation associations. Although all mothers in this study reported Jewish ethnicity, we included whether a mother reported immigrating from the West as a confounder ( 38 ). Because birth order ( 48 ) and age ( 9 ) may be related to methylation, we also adjusted for maternal parity and subject's age at blood draw.
Life-course measures
Although methylation patterns are established early ( 8 , 9 , 21 ), they may also be influenced by a subject's life course. We included the following variables from the interview at age 32 years: self-reported adolescent overweight status (yes/no) in grades 4–6 (∼10 to 12 years), total years of education, religiosity (secular, traditional, religious, or ultra-Orthodox), marital status, parity, and alcohol or tobacco use. In particular, overweight status may be a time-dependent confounder (i.e., both a consequence of early programming and a confounder of later mediator-outcome relationships) ( 47 ). Because recall may be biased, we included an objectively measured BMI at age 17 years available for some participants ( n = 374) ( 37 , 38 ) in sensitivity analyses.
We constructed a traditional family role factor score and dummy for any drinking/smoking (yes/no). The traditional role factor score was estimated by principal components factor analysis of: religiosity, marital status, parity, and years of education. Higher scores corresponded to greater religiosity and parity, being married, and fewer years of education. These characteristics have been shown to be related to each other as well as drinking, smoking, and adiposity among Israeli ( 49 , 50 ) and non-Israeli women ( 51 ). In turn, drinking, smoking, and adiposity may affect adult methylation ( 23 , 30 ). We dichotomized this score at the median.
Hypothesized causal models
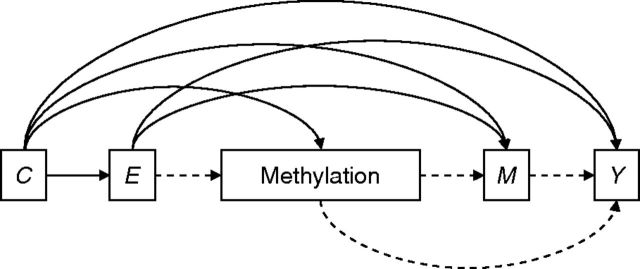
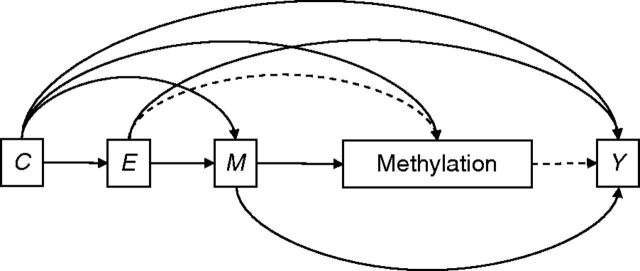
Because variation of DNA methylation over the life course is understudied ( 30 , 52 , 53 ), we must make certain causal structure assumptions to assess associations between early life socioeconomic position and DNA methylation. We used 2 alternate models: Under an “early programming” model, we assumed that methylation is set in early life and does not change in adulthood (Figure 1 ). Under a “cumulative effects” model, we assumed that some variation in methylation is due to life course (Figure 2 ).
Figure 1.
“Early programming” model, Jerusalem Perinatal Family Follow-up Study, 1974–2009. Methylation is established in early life. Under this hypothesized causal structure, a woman's methylation status is established early in life by birth socioeconomic position ( E ) and perinatal characteristics ( C ) and is independent of subsequent life-course mediators ( M ) such as childhood overweight, attained education, marital status, religiosity, childbearing, or substance use. The mediated effect of E on adult phenotype ( Y ) through methylation status (i.e., the indirect effect) is given by the dashed lines, and M need not be controlled for in examining exposure-methylation associations.
Figure 2.
“Cumulative effects” model, Jerusalem Perinatal Family Follow-up Study, 1974–2009. Methylation is affected by life-course mediators. Under this hypothesized causal structure, an adult woman's methylation status is determined by birth socioeconomic position ( E ), perinatal characteristics ( C ), and life-course mediators ( M ). One possible mediated effect of E on adult phenotype ( Y ) is given by the dashed lines. We are interested in the dashed paths, so M should be adjusted for in analyses. Note that adolescent overweight (a component of M ) may be an important biological pathway for early life development, so we adjust for it indirectly through weighting.
Statistical analysis
First, we described the study population by using means or proportions and methylation by means and quartiles. To estimate early life socioeconomic position-adult methylation associations, we fit multivariable linear regression models predicting average methylation at each gene region ( ABCA1 , HSD11B2 , INS-IGF2 , LEP , NR3C1 ) by each of the 4 socioeconomic position measures, adjusted for the following: Under “Cumulative Effects,” adolescent overweight status is an important biological mediator for early life socioeconomic position, but it may also confound relationships between adult risk factors (such as smoking) and methylation. Consequently, we used categorical socioeconomic position measures and binary life course factors to also fit marginal structural models, indirectly adjusting for adolescent overweight by inverse probability weighting ( 3 , 54 ), using the outcome model:
Subject's age at blood draw and stratification variables (maternal prepregnancy BMI ≥27 and subject's birth weight ≤2,500, 2,501–3,999, or ≥4,000 g) (“ Crude” Model );
Also all early life confounders (“ Early Programming ” Model );
Also all life-course measures, except adolescent overweight (“ Cumulative Effects ” Model ).
where Yi is mean methylation, Xi is early life socioeconomic position category, Ti is high traditional role score, and Si is any drinking/smoking. For each predictor of Y i , stabilized weights were estimated by fitting multinomial logistic regression models to predict the probability of a given level of exposure by hypothesized causal parents. Each individual was then assigned an overall weight by the following set of equations:
Here, C is a vector of confounders: maternal Western immigrant status, any prenatal smoking, parity, prepregnancy BMI, and subject's age at blood draw; B is subject's birth weight; and O is adolescent overweight. By weighting, the functional causal order is C , X , B , O , T , S . We also fit linear regression models using these predictors (i.e., X , T , and S ), to investigate whether any differences in marginal structural models estimates may be due to different parameterizations. Multiple imputation was used to estimate the influence of missingness ( n = 19 with any missing values).
Secondary investigation of methylation-adult health associations
We were also interested in whether observed DNA methylation was related to adult health. However, methylation was measured from the same samples used to assay other biomarkers. Nonetheless, we estimated cross-sectional associations between methylation and adult phenotype by fitting multivariable regression models, adjusting for all confounders, and life-course variables. Continuous biomarkers (e.g., total cholesterol) were normally distributed and left untransformed ( 36 ). We also conducted exploratory analyses to see if DNA methylation mediated any indirect effects of early life socioeconomic position on adult phenotypes.
We conducted all data analyses using Stata MP 13.1 software (StataCorp LP, College Station, Texas). Given our a priori socioeconomic position measures and specific gene regions, we performed no multiple testing corrections.
RESULTS
Overall, our subjects ( n = 613) did not differ substantially from the total female population of the Jerusalem Perinatal Study Family Follow-up Study ( n = 715) ( 36 ). High paternal class was associated with more years of parental education, Western-origin mothers, and less maternal smoking (Table 1 ). Overall, peripheral blood methylation was tightly controlled; interquartile ranges were generally less than 10% (Table 2 ). Compared with subjects who had low occupational class fathers, those with high class fathers had slightly higher mean methylation at ABCA1 (20.2% vs. 19.6%), INS-IGF2 (77.4% vs. 77.2%), LEP (22.7% vs. 21.5%), and lower methylation at NR3C1 (6.4% vs. 6.8%) (Table 2 ).
Table 1.
Study Population Characteristics by Father's Occupational Class, Jerusalem Perinatal Family Follow-up Study, 1974–2009
|
Overall (
n
= 613)
|
Father's Occupational Class
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Low (Class 4–6) (
n
= 256)
|
High (Class 1–3) (
n
= 357)
|
||||||||
| % | No. | Mean (SD) | % | No. | Mean (SD) | % | No. | Mean (SD) | |
| Socioeconomic position at birth | |||||||||
| Low paternal occupational class a | 41.8 | 256 | |||||||
| Maternal education, years | 11.8 (3.3) | 10.0 (3.0) | 13.0 (3.0) | ||||||
| Paternal education, years | 12.2 (4.0) | 9.6 (2.9) | 14.0 (3.6) | ||||||
| Maternal perinatal characteristics | |||||||||
| Age at delivery, years | 28.3 (5.8) | 27.8 (6.0) | 28.7 (5.6) | ||||||
| Prepregnancy BMI b | 24.3 (3.9) | 24.5 (4.0) | 24.2 (3.8) | ||||||
| Parity c | 2.0 (2.0) | 2.0 (1.8) | 2.0 (2.2) | ||||||
| Any smoking in pregnancy | 12.6 | 77 | 18.4 | 47 | 8.4 | 30 | |||
| Immigrant from the West | 16.8 | 103 | 8.6 | 22 | 22.7 | 81 | |||
| Daughter's perinatal characteristics | |||||||||
| Birth weight, g | 3,298 (599) | 3,302 (597) | 3,296 (602) | ||||||
| Birth weight <2,500 g | 13.1 | 80 | 11.3 | 29 | 14.3 | 51 | |||
| Daughter's life-course mediators | |||||||||
| Adolescent overweight d | 20.9 | 128 | 18.8 | 48 | 22.4 | 80 | |||
| Married | 78.7 | 474 | 76.8 | 192 | 80.1 | 282 | |||
| Ultraorthodox | 18.7 | 112 | 6.5 | 16 | 27.4 | 96 | |||
| Years of education | 14.9 (2.6) | 14.4 (2.5) | 15.3 (2.6) | ||||||
| Number of children | 2.4 (2.1) | 1.9 (1.6) | 2.7 (2.4) | ||||||
| Daughter's adult phenotype | |||||||||
| Height, cm | 162.0 (6.1) | 161.7 (6.1) | 162.2 (6.2) | ||||||
| Weight, kg | 68.0 (14.7) | 68.0 (15.1) | 68.0 (14.5) | ||||||
| BMI b | 25.9 (5.4) | 26.0 (5.4) | 25.9 (5.5) | ||||||
| Waist-to-hip ratio (× 100) | 78.7 (6.2) | 78.6 (5.8) | 78.7 (6.5) | ||||||
| Systolic blood pressure, mm Hg | 99.6 (10.5) | 99.3 (11.4) | 99.9 (9.8) | ||||||
| Diastolic blood pressure, mm Hg | 69.6 (8.7) | 69.5 (9.8) | 69.6 (7.9) | ||||||
| Serum total cholesterol, mg/dL | 183.7 (33.8) | 186.0 (35.7) | 182.1 (32.3) | ||||||
| Serum HDL-C, mg/dL | 57.0 (15.1) | 56.4 (14.7) | 57.5 (15.3) | ||||||
| Serum LDL-C, mg/dL | 108.1 (28.5) | 110.2 (29.8) | 106.6 (27.4) | ||||||
| Obese, BMI ≥30 | 20.0 | 122 | 20.4 | 52 | 19.6 | 70 | |||
| Metabolic syndrome e | 5.1 | 31 | 6.3 | 16 | 4.2 | 15 | |||
| Any term, low birth weight children | 13.5 | 61 | 13.5 | 25 | 13.4 | 36 | |||
Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SD, standard deviation.
a Defined by father's occupational class being in the bottom half (i.e., classes 4–6 ).
b Weight (kg)/height (m) 2 .
c Number of self-reported previous livebirths, including those who have since died.
d Self-reported as “slightly overweight” or “significantly overweight” in grades 4–6 (around age 10–12 years).
e Based on International Diabetes Federation year 2006 criteria, a subject has metabolic syndrome if she has central obesity defined as a waist circumference ≥80 cm or BMI ≥30 and at least 2 of the following: triglycerides ≥150 mg/dL, HDL <50 mg/dL, systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥85 mm Hg, and/or fasting glucose ≥100 mg/dL.
Table 2.
Detailed Methylation Distribution of Candidate Gene Promoter Regions by Paternal Occupational Class, Jerusalem Perinatal Family Follow-up Study, 1974–2009
| Gene | Chromosome | Location a | No. of CpGs |
Overall (
n
= 613)
|
Mean % (SD)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean % (SD) | Minimum | 25th Percentile | Median | 75th Percentile | Maximum | Low Class ( n = 256) | High Class ( n = 357) | ||||
| ABCA1 | 9 | 107,690,502–107,690,821 | 27 | 20.0 (7.3) | 6.6 | 14.5 | 18.6 | 24 | 58.5 | 19.6 (7.2) | 20.2 (7.4) |
| HSD11B2 | 16 | 67,464,230–67,464,442 | 6 | 5.7 (2.1) | 0.5 | 4.5 | 5.3 | 6.3 | 22.8 | 5.7 (2.2) | 5.7 (2.1) |
| INS-IGF2 | 11 | 2,182,336–2,182,640 | 4 | 77.3 (5.4) | 44.5 | 75 | 77.8 | 80.5 | 97.5 | 77.2 (5.4) | 77.4 (5.3) |
| LEP | 7 | 127,881,051–127,881,408 | 32 | 22.2 (11.7) | 2.5 | 13.4 | 20.3 | 29.7 | 62.6 | 21.5 (11.2) | 22.7 (11.9) |
| NR3C1 | 5 | 142,783,506–142,783,905 | 47 | 6.5 (2.7) | 2.8 | 5.4 | 6.2 | 7 | 48.8 | 6.8 (3.7) | 6.4 (1.7) |
Abbrevations: ABCA1 , adenosine triphosphate binding cassette subfamily A member 1 gene; CpG, cytosine-phosphate-guanine site of methylation; HSD11B2 , hydroxysteroid (11-β) dehydrogenase 2 gene; INS-IGF2 , insulin–insulin-like growth factor 2 readthrough gene region; LEP , leptin gene; NR3C1 , nuclear receptor subfamily 3 group C member 1 (“glucocorticoid receptor”) gene; SD, standard deviation.
a Locations refer to Genome Reference Consortium human genome build 37 (GRCh37).
In the crude “early programming” (controlling for parental confounders; Figure 1 ) and “cumulative effects” (also controlling for offspring life course; Figure 2 ) models estimated by standard linear regression, each higher category of paternal occupational class was associated with 0.5%-points higher ABCA1 methylation (Tables 3 and 4 ). However, when marginal structural models were used to account for time-dependent confounding, this association was weaker (Table 6). Notably, this was not due to different covariate parameterizations, as demonstrated by the linear regression using the same predictors (Table 4 ). Greater maternal education was associated with higher HSD11B2 methylation in all models (e.g., β = 0.5 per category, 95% confidence interval: 0.1, 0.8) (Table 4 ). A sensitivity analysis using the 374 individuals with BMI measured at age 17 ( 37 ) found even stronger evidence for association ( Supplementary Data ). No evidence of associations was found between socioeconomic position and LEP , INS-IG2 , or NR3C1 . Missingness had a minimal influence on any estimates ( Supplementary Data ).
Table 3.
Associations Between Early Life Socioeconomic Position and Percent Candidate Gene Methylation, Jerusalem Perinatal Family Follow-up Study, 1974–2009
| Exposure a |
ABCA1
|
HSD11B2
|
INS-IGF2
|
LEP
|
NR3C1
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β Coefficient | 95% CI | P Value | β Coefficient | 95% CI | P Value | β Coefficient | 95% CI | P Value | β Coefficient | 95% CI | P Value | β Coefficient | 95% CI | P Value | |
| Crude Model b | |||||||||||||||
| High paternal occupational class c | 0.5 | −0.9, 1.9 | 0.5 | −0.04 | −0.4, 0.3 | 0.8 | 0.2 | −0.7, 1.1 | 0.7 | 1.2 | −1.0, 3.4 | 0.3 | −0.4 | −0.9, 0.2 | 0.2 |
| Increasing paternal occupational class d | 0.5 | 0.04, 0.9 | 0.03 | −0.02 | −0.1, 0.1 | 0.8 | 0.1 | −0.2, 0.4 | 0.5 | 0.4 | −0.4, 1.1 | 0.3 | −0.1 | −0.3, 0.1 | 0.3 |
| Mother's years of education | 0.2 | −0.02, 0.4 | 0.09 | 0.07 | 0.01, 0.1 | 0.01 | −0.04 | −0.2, 0.1 | 0.6 | 0.3 | −0.04, 0.6 | 0.09 | 0.02 | −0.07, 0.1 | 0.7 |
| Father's years of education | 0.1 | −0.05, 0.3 | 0.2 | 0.03 | −0.01, 0.08 | 0.2 | −0.09 | −0.2, 0.03 | 0.1 | 0.2 | −0.1, 0.4 | 0.3 | −0.02 | −0.08, 0.04 | 0.6 |
| “Early Programming” Model e | |||||||||||||||
| High paternal occupational class c | 0.4 | −1.1, 1.8 | 0.6 | 0.0002 | −0.4, 0.4 | 1.0 | 0.3 | −0.7, 1.2 | 0.6 | 0.8 | −1.4, 3.0 | 0.5 | −0.4 | −1.1, 0.2 | 0.2 |
| Increasing paternal occupational class d | 0.5 | 0.004, 0.9 | 0.05 | −0.008 | −0.1, 0.1 | 0.9 | 0.1 | −0.2, 0.4 | 0.4 | 0.2 | −0.5, 1.0 | 0.5 | −0.1 | −0.3, 0.1 | 0.3 |
| Mother's years of education | 0.2 | −0.003, 0.5 | 0.05 | 0.08 | 0.02, 0.1 | 0.01 | −0.1 | −0.2, 0.1 | 0.5 | 0.2 | −0.1, 0.6 | 0.2 | 0.02 | −0.1, 0.1 | 0.7 |
| Father's years of education | 0.1 | −0.1, 0.3 | 0.2 | 0.04 | −0.003, 0.1 | 0.07 | −0.1 | −0.2, 0.04 | 0.2 | 0.1 | −0.2, 0.4 | 0.5 | −0.02 | −0.1, 0.04 | 0.5 |
Abbreviations: ABCA1 , adenosine triphosphate binding cassette subfamily A member 1 gene; CI, confidence interval; HSD11B2, hydroxysteroid (11-β) dehydrogenase 2 gene; INS-IGF2 , insulin–insulin-like growth factor 2 readthrough gene region; LEP , leptin gene; NR3C1 , nuclear receptor subfamily 3 group C member 1 (“glucocorticoid receptor”) gene.
a β coefficients represent percentage-point difference in methylation per unit exposure. Two-tailed P values are given (H 0 : β = 0).
b Adjusted for maternal prepregnancy overweight (≥27 kg), daughter's birth weight category (≤2,500 g, 2,501–3,999 g, ≥4,000 g), and daughter's age at blood draw.
c High early life socioeconomic position (father's occupational classes 1–3) versus low early life socioeconomic position (classes 4–6).
d Per unit father's occupational class increase (e.g., class 5 vs. class 6 or class 1 vs. class 2).
e In addition to variables from footnote b, also adjusted for maternal age at birth, any maternal smoking during pregnancy, maternal Western origin, and mother's parity.
Table 4.
Associations Between Early Life Socioeconomic Position and Candidate Gene Methylation by “Cumulative Effects” Model, Jerusalem Perinatal Family Follow-up Study, 1974–2009
| Model |
ABCA1
|
HSD11B2
|
INS-IGF2
|
LEP
|
NR3C1
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β Coefficient | 95% CI | P Value | β Coefficient | 95% CI | P Value | β Coefficient | 95% CI | P Value | β Coefficient | 95% CI | P Value | β Coefficient | 95% CI | P Value | |
| High (Classes 1–3) Versus Low (Classes 4–6) Paternal Occupational Class a | |||||||||||||||
| Multivariable, observables b | 0.4 | −1.1, 1.9 | 0.6 | 0.004 | −0.4, 0.4 | 1.0 | 0.3 | −0.7, 1.3 | 0.5 | 0.6 | −1.8, 3.0 | 0.6 | −0.4 | −0.9, 0.08 | 0.1 |
| Multivariable, constructed scores c | 0.4 | −1.0, 1.9 | 0.6 | −0.02 | −0.4, 0.4 | 0.9 | 0.2 | −0.8, 1.1 | 0.7 | 0.8 | −1.5, 3.0 | 0.5 | −0.5 | −1.0, 0.02 | 0.06 |
| Inverse probability weighted, constructed scores d | 1.0 | −0.5, 2.5 | 0.2 | −0.2 | −0.6, 0.2 | 0.4 | 0.2 | −0.8, 1.2 | 0.7 | 1.0 | −1.6, 3.7 | 0.4 | −0.6 | −1.3, 0.08 | 0.08 |
| Increasing Paternal Occupational Class (Lowest (Class 6) to Highest (Class 1)) a | |||||||||||||||
| Multivariable, observables b | 0.5 | 0.002, 1.0 | 0.05 | −0.001 | −0.1, 0.1 | 1.0 | 0.2 | −0.2, 0.5 | 0.3 | 0.2 | −0.6, 1.0 | 0.6 | −0.1 | −0.3, 0.06 | 0.2 |
| Multivariable, constructed scores c | 0.5 | 0.02, 1.0 | 0.04 | −0.01 | −0.1, 0.1 | 0.8 | 0.1 | −0.2, 0.4 | 0.5 | 0.3 | −0.5, 1.0 | 0.5 | −0.1 | −0.3, 0.05 | 0.2 |
| Inverse probability weighted, constructed scores d | 0.4 | −0.2, 0.9 | 0.2 | −0.04 | −0.2, 0.1 | 0.7 | −0.03 | −0.4, 0.4 | 0.9 | 0.8 | −0.3, 2.0) | 0.1 | −0.1 | −0.3, 0.01 | 0.08 |
| Increasing Maternal Education (≤8 Years, 9–12 Years, ≥13 Years) a | |||||||||||||||
| Multivariable, observables b | 0.9 | −0.2, 2.0 | 0.1 | 0.3 | 0.03, 0.6 | 0.03 | −0.2 | −1.0, 0.5 | 0.5 | 1.4 | −0.4, 3.2 | 0.1 | 0.07 | −0.3, 0.4 | 0.7 |
| Multivariable, constructed scores c | 0.6 | −0.5, 1.7 | 0.3 | 0.3 | 0.003, 0.5 | 0.05 | −0.4 | −1.1, 0.3 | 0.3 | 1.5 | −0.2, 3.1 | 0.08 | 0.1 | (−0.3, 0.5) | 0.6 |
| Inverse probability weighted, constructed scores d | 1.4 | −0.3, 3.1 | 0.1 | 0.5 | 0.1, 0.8 | 0.008 | −0.5 | −1.5, 0.5 | 0.3 | −0.04 | −2.9, 2.8 | 1.0 | 0.3 | −0.4, 0.9 | 0.4 |
| Increasing Paternal Education (≤8 Years, 9–12 Years, ≥13 Years) a | |||||||||||||||
| Multivariable, observables b | 0.8 | −0.3, 1.8 | 0.2 | 0.1 | −0.1, 0.4 | 0.3 | −0.4 | −1.1, 0.3 | 0.3 | 1.0 | −0.7, 2.7 | 0.3 | −0.3 | −0.6, 0.1 | 0.2 |
| Multivariable, constructed scores c | 0.5 | −0.5, 1.5 | 0.3 | 0.1 | −0.1, 0.3 | 0.4 | −0.5 | −1.1, 0.2 | 0.2 | 1.0 | −0.6, 2.6 | 0.2 | −0.1 | −0.5, 0.2 | 0.4 |
| Inverse probability weighted, constructed scores d | 1.7 | −0.2, 3.6 | 0.07 | 0.2 | −0.2, 0.7 | 0.3 | −0.7 | −1.7, 0.4 | 0.2 | 1.6 | −1.8, 4.9 | 0.4 | 0.3 | −0.4, 1.0 | 0.4 |
Abbreviations: ABCA1 , adenosine triphosphate binding cassette subfamily A member 1 gene; CI, confidence interval; HSD11B2, hydroxysteroid (11-β) dehydrogenase 2 gene; INS-IGF2 , insulin–insulin-like growth factor 2 readthrough gene region; LEP , leptin gene; NR3C1 , nuclear receptor subfamily 3 group C member 1 (“glucocorticoid receptor”) gene.
a Adjusted for maternal prenatal characteristics (age at delivery, Western origin, any maternal smoking during pregnancy, parity, and prepregnancy overweight), daughter's perinatal characteristics (birth weight category), and daughter's life-course mediators (age at blood draw, years of completed education, marital status, religiosity, number of children, and frequency of alcohol and cigarette use) as continuous or binary variables.
b β coefficients represent percentage-point difference in methylation per unit of exposure. Two-tailed P values are given (H 0 : β = 0).
c Adjusted for prenatal maternal characteristics (age at delivery, Western origin, any maternal smoking during pregnancy, parity, and prepregnancy overweight), daughter's birth weight category, and daughter's age at blood draw as continuous or binary variables. To parallel the marginal structural model, the daughter's life-course mediators were adjusted for as dichotomized variables: adolescent overweight (self-report), “high” traditional family role (higher than median factor score base on fewer years of completed education, married status, greater religiosity, and greater number of children), and any alcohol or cigarette use (yes to either).
d Marginal structural model estimate for the controlled direct effect of higher early life socioeconomic position by each measure (i.e., paternal occupation or parental educational level) on mean percent methylation. Adolescent overweight was controlled by weighting only, while “high” traditional family role and any alcohol or cigarette use were also included in the outcome model as adjustment variables.
A 1%-point higher HSD11B2 promoter methylation was associated with lower weight, total cholesterol, and low-density lipoprotein cholesterol as well as 12% greater odds (odds ratio = 1.1, 95% confidence interval: 1.0, 1.3) of having any low birth weight offspring ( Supplementary Data ). Each 1%-point higher NR3C1 methylation was associated with 0.3 mm Hg higher (95% confidence interval: 0.1, 0.5) diastolic blood pressure ( Supplementary Data ). No significant association was found for ABCA1 , LEP , or INS-IGF2 .
In exploratory mediation analyses, we used multivariable regression and marginal structural models to estimate the direct and indirect effects of early life socioeconomic position on adult outcomes using our 2 models: 1) adjusted for continuous methylation only, partitioning all indirect effects to the methylation pathway (“early programming”) and 2) adjusted for methylation and life-course measures (“cumulative effects”). We estimated indirect effects using the product of coefficients approach ( 55 , 56 ) and bootstrapped confidence intervals. We explored whether for methylation, the 10th, 50th, 75th, and 95th percentiles were “functional” thresholds, in the sense that they mediated a relationship between socioeconomic position and adult phenotype. Although we observed an overall association between maternal education on metabolic syndrome and low birth weight ( Supplementary Data ), we did not find any evidence for mediation ( Supplementary Data ).
DISCUSSION
Overall, we found some evidence for a positive association between early life socioeconomic position and adult peripheral blood DNA methylation at ABCA1 and HSD11B2 . We also found associations between HSD11B2 methylation and several adult cardiometabolic and pregnancy outcomes. Moreover, associations were fairly consistent across models, suggesting that measured life-course trajectory did not substantially explain the relationship between early life and adult methylation and supporting an early programming hypothesis. To our knowledge, this study is the first to examine these associations among young adult women while comparing different life-course causal models.
Although studies of early life environment commonly assess methylation globally ( 20–22 , 57 ), several have investigated candidate genes. Appleton et al. ( 39 ) found an association between maternal ≥ high school attainment and a 9%-point higher (β = 8.8; P < 0.05; n = 444) maternal-side placental HSD11B2 methylation, adjusted for maternal age, prepregnancy BMI, race, infant sex, and birth weight. Using similar adjustments, we found a positive association between maternal education and adult female HSD11B2 peripheral blood methylation. Our findings complement several studies extending associations with HSD11B2 methylation to offspring tissues ( 40–42 ), supporting the plausibility of a persistent effect of offspring early life environment. Importantly, reduced HSD11B2 activity may be related to hypertension ( 58 ), and a study of HSD11B2 methylation in adult peripheral blood found that essential hypertension was associated with a 20% higher promoter methylation ( 59 ). Although we found several associations between increased HSD11B2 methylation and adult phenotype, they did not include blood pressure. However, it is unclear thus far if peripheral blood methylation itself is causal, as expressions in renal and vascular cell walls are more functionally implicated in inflammatory pathways ( 58 ). Consequently, it is possible that observed associations with peripheral blood methylation may be noncausal proxies for systemic changes in methylation produced in early development ( 8–10 ).
Nonetheless, whether such changes persist into adulthood is a separately important question, as recent investigations have suggested that early differences in peripheral blood DNA methylation related to intrauterine experience may revert to “normal” levels by late childhood ( 60 ). Even without a direct functional role, adult candidate gene DNA methylation in peripheral blood can serve as an easily assayed biomarker for early social disadvantage. However, alternative explanations are possible, such as the tendency of offspring to have environments similar to those of their parents. Our study attempted to clarify whether or not differences in adult peripheral blood DNA methylation can be explained by life-course events, or whether they are independently related to early life environment. The concordance between models supports early programming and suggests that life-course events do not completely explain observed associations. Interestingly, although we found consistent associations between HSD11B2 methylation and maternal education across models accounting for life-course factors, we did not find as strong evidence for an association with occupational class or years of paternal education (Tables 3 and 4 ). While there are several potential explanations for this, including the role of the mother in child rearing, the findings are at least consistent with an in utero exposure hypothesis ( 61 ).
We also found evidence for a positive association between paternal occupational class and adult methylation at ABCA1 (0.5%-points per level). Tobi et al. ( 33 ) found a similar association (0.7%-points, relative to unexposed siblings) between early gestation famine exposure and ABCA1 methylation in older adults (∼58 years). Unlike Tobi et al. ( 33 ), we did not find associations with INS-IGF2 or LEP methylation. It is possible that famine exposure may influence adult methylation through different pathways than socioeconomic position ( 12 , 19 , 33 ) or that they may be more sensitive to life-course influences ( 62 ). Although it has been hypothesized that ABCA1 may be related to risk of myocardial infarction, our study was in line with that of Talens et al. ( 24 ) in finding no substantial association with cardiometabolic phenotype.
Finally, we found little evidence for mediation by methylation. This may be due to several reasons including the aforementioned potential for noncausal methylation differences, the cross-sectional nature of our biomarkers, and the weak relationships between early life socioeconomic position and adult outcomes in our sample. Additionally, a mediating role of methylation in other regions and tissues cannot be excluded ( 29 ). Importantly, the exploratory analysis illustrates the difficulty of drawing conclusions about potential mediation strictly from binary associations between methylation and either exposures or outcomes ( 30 ).
There are several notable strengths to our study. First, we used state-of-the-art methods to quantify adult methylation at candidate genes previously related to early life conditions. Additionally, our study of 613 women is one of the largest to relate early life environment and candidate gene methylation, enabling us to detect small differences. Finally, our study was conducted among a population with extensive information on maternal demographics and pregnancy course, as well as offspring biomarkers at 32 years ( 36 ). Additionally, this population has a unique context for studying intergenerational health. Since 1948, Israel has had large influxes of immigrants (47% of mothers in our study) driven by religious and political pressures and exposed to multiple wars ( 36 , 63 ). In contrast, it has developed rapidly, with female education averaging 16 years and a gross domestic product per capita exceeding $33,000 (US dollars) ( 64 ). Parental adversity may operate intergenerationally though persistent alterations in DNA methylation ( 22 , 27 , 28 ). Consequently, recent improvements in material conditions may mask susceptibility to morbidity and mortality ( 65 ). We show that various ways of adjusting for offspring life-course dynamics, including time-dependent confounding by health selection or risk behavior adoption, do little to change associations between early life socioeconomic position and adult methylation.
Several technical limitations to our study deserve mention. First, no replications of polymerase chain reaction amplification were performed ( 44 ). Because observed methylation may vary across amplification runs ( 66 ), we cannot exclude chance findings or the possibility that this imprecision prevented detection of other relationships. We note again, however, that DNA methylation at our target genes was tightly regulated. The interquartile range for HSD11B2 methylation was 1.75%-points (4.5%–6.25%) with a majority of the data within 1 standard deviation (2.1%). Additionally, we were unable to quantify cell composition. We attempted application of an adjustment method developed for epigenome-wide association studies ( 67 ). Due to the limited number of CpG units ( n = 50), however, we found associations between number of maternal years of education and methylation at individual CpG units to be weak and dependent on modeling parameters, also implying that associations may only be at the aggregate level. Consequently, any observed differences in methylation may be mediated by differences in cell compositions of the samples. This is relevant as inflammation, and therefore peripheral cell composition, is likely to be an important mediator of socioeconomic position-cardiometabolic health relationships ( 16 , 68 , 69 ).
Relatedly, we did not conduct any primary analysis of CpG site-specific associations. There are 3 reasons for this: First, we wished to remain consistent with prior candidate gene studies from which we derived primer regions ( 17 , 18 , 24 , 25 , 33 , 39–43 ; Supplementary Data ). Second, we wished to provide a straightforward analysis in the candidate gene setting, which often is an adjunct to exploratory epigenome-wide association studies ( 60 ). Third, given the dearth of literature identifying target CpGs, the small number of CpGs available for an agnostic approach would be underpowered to survive necessary multiple testing corrections. As it is, if we had chosen to correct for testing across 4 related socioeconomic position measures, only the marginal structural model-estimated association between maternal education and HSD11B2 ( P = 0.008) would fall under a Bonferroni P = 0.0125. Overall, these limitations reemphasize the need for replication (both within and between studies) and consideration of other sequencing techniques that may improve detection of small means and differences in candidate gene methylation ( 43 , 66 ).
Additionally, caution is needed when interpreting and generalizing our findings. Israeli women are required to serve in the military following completion of 12 years of compulsory education. Women may defer service if they are admitted to university or other training opportunity, identify as ultra-Orthodox, or are pregnant. As a result, approximately half of all women serve in the military ( 36 ) and differ from those who don't serve with respect to education, religiosity, and childbearing. Although our models account for these factors, the life-course dynamics of Israeli women may be unique.
In summary, we found some evidence of early life socioeconomic position-candidate gene methylation associations among young adult women. Specifically, we found similar associations using various causal models to account for life-course trajectory, supporting an early programming hypothesis. We also found that methylation of identified genes to be associated with adult cardiometabolic phenotype. However, exploratory mediation analyses were inconclusive, highlighting the challenging nature of epigenetic mediation analyses ( 30 ). Nonetheless, we believe that the approach we have described can add to the understanding of life-course methylation dynamics when applied to existing cohorts where blood samples are limited or in follow-up studies to epigenome-wide association studies.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, University of Washington School of Public Health, Seattle, Washington (Jonathan Y. Huang, Ali Rowhani-Rahbar, Daniel A. Enquobahrie); Institute for Health and Social Policy, McGill University, Montreal, Quebec, Canada (Jonathan Y. Huang); School of Social Work, University of Washington, Seattle, Washington (Amelia R. Gavin); Department of Statistics, University of Washington, Seattle, Washington (Thomas S. Richardson); New York Academy of Medicine, New York, New York (David S. Siscovick); and Braun School of Public Health, Hebrew University-Hadassah Medical Center, Jerusalem, Israel (Hagit Hochner, Yechiel Friedlander).
This work was supported by the University of Washington Global Women, Adolescents, and Children Integrated Health Seed Grant 2013–2014; Israeli Science Foundation grants 1252/07 and 552/12; National Institutes of Health grants T32HD052462, K01HL103174, and R01HL088884; and Canadian Institutes of Health Research operating grant 115214, “Ethics, Social Determinants of Health, and Health Equity: Integrating Theory and Practice,” although no direct funding was received or set aside for the writing of this paper.
We thank Dr. Andres Houseman for his advice regarding cell-mixture adjustment and the use of the RefFreeEWAS R package.
An earlier version of this work was presented at the Society for Pediatric and Perinatal Epidemiology 28th Annual Meeting and the Society for Epidemiologic Research 48th Annual Meeting on June 15 and 18, 2015, Denver, Colorado, respectively.
Conflict of interest: none declared.
REFERENCES
- 1. Galobardes B , Lynch JW , Smith GD . Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review . J Epidemiol Community Health . 2008. ; 625 : 387 – 390 . [DOI] [PubMed] [Google Scholar]
- 2. Johnson RC , Schoeni RF . Early-life origins of adult disease: national longitudinal population-based study of the United States . Am J Public Health . 2011. ; 10112 : 2317 – 2324 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nandi A , Glymour MM , Kawachi I et al. . Using marginal structural models to estimate the direct effect of adverse childhood social conditions on onset of heart disease, diabetes, and stroke . Epidemiology . 2012. ; 232 : 223 – 232 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harville EW , Boynton-Jarrett R , Power C et al. . Childhood hardship, maternal smoking, and birth outcomes: a prospective cohort study . Arch Pediatr Adolesc Med . 2010. ; 1646 : 533 – 539 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gavin AR , Hill KG , Hawkins JD et al. . The role of maternal early-life and later-life risk factors on offspring low birth weight: findings from a three-generational study . J Adolesc Health . 2011. ; 492 : 166 – 171 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sauerbrun-Cutler M-T , Segars JH . Do in utero events contribute to current health disparities in reproductive medicine . Semin Reprod Med . 2013. ; 315 : 325 – 332 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reynolds RM . Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis—2012 Curt Richter Award Winner . Psychoneuroendocrinology . 2013. ; 381 : 1 – 11 . [DOI] [PubMed] [Google Scholar]
- 8. Gluckman PD , Hanson MA , Cooper C et al. . Effect of in utero and early-life conditions on adult health and disease . N Engl J Med . 2008. ; 3591 : 61 – 73 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gluckman PD , Hanson MA , Buklijas T et al. . Epigenetic mechanisms that underpin metabolic and cardiovascular diseases . Nat Rev Endocrinol . 2009. ; 57 : 401 – 408 . [DOI] [PubMed] [Google Scholar]
- 10. Reynolds RM , Jacobsen GH , Drake AJ . What is the evidence in humans that DNA methylation changes link events in utero and later life disease . Clin Endocrinol (Oxf) . 2013. ; 786 : 814 – 822 . [DOI] [PubMed] [Google Scholar]
- 11. Dominguez-Salas P , Moore SE , Baker MS et al. . Maternal nutrition at conception modulates DNA methylation of human metastable epialleles . Nat Commun . 2014. ; 5 : 3746 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heijmans BT , Tobi EW , Stein AD et al. . Persistent epigenetic differences associated with prenatal exposure to famine in humans . Proc Natl Acad Sci U S A . 2008. ; 10544 : 17046 – 17049 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lumey LH , Terry MB , Delgado-Cruzata L et al. . Adult global DNA methylation in relation to pre-natal nutrition . Int J Epidemiol . 2012. ; 411 : 116 – 123 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turan N , Ghalwash MF , Katari S et al. . DNA methylation differences at growth related genes correlate with birth weight: a molecular signature linked to developmental origins of adult disease . BMC Med Genomics . 2012. ; 5 : 10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoffmann A , Spengler D . The lasting legacy of social stress on the epigenome of the hypothalamic-pituitary-adrenal axis . Epigenomics . 2012. ; 44 : 431 – 444 . [DOI] [PubMed] [Google Scholar]
- 16. Stringhini S , Polidoro S , Sacerdote C et al. . Life-course socioeconomic status and DNA methylation of genes regulating inflammation . Int J Epidemiol . 2015. ; 444 : 1320 – 1330 . [DOI] [PubMed] [Google Scholar]
- 17. Mulligan CJ , D'Errico NC , Stees J et al. . Methylation changes at NR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight . Epigenetics . 2012. ; 78 : 853 – 857 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Radtke KM , Ruf M , Gunter HM et al. . Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor . Transl Psychiatry . 2011. ; 1 : e21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tobi EW , Slagboom PE , van Dongen J et al. . Prenatal famine and genetic variation are independently and additively associated with DNA methylation at regulatory loci within IGF2/H19 . PLoS One . 2012. ; 75 : e37933 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Essex MJ , Boyce WT , Hertzman C et al. . Epigenetic vestiges of early developmental adversity: childhood stress exposure and DNA methylation in adolescence . Child Dev . 2013. ; 841 : 58 – 75 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borghol N , Suderman M , McArdle W et al. . Associations with early-life socio-economic position in adult DNA methylation . Int J Epidemiol . 2012. ; 411 : 62 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tehranifar P , Wu H-C , Fan X et al. . Early life socioeconomic factors and genomic DNA methylation in mid-life . Epigenetics . 2013. ; 81 : 23 – 27 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lam LL , Emberly E , Fraser HB et al. . Factors underlying variable DNA methylation in a human community cohort . Proc Natl Acad Sci U S A . 2012. ; 109 ( suppl 2 ): 17253 – 17260 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Talens RP , Jukema JW , Trompet S et al. . Hypermethylation at loci sensitive to the prenatal environment is associated with increased incidence of myocardial infarction . Int J Epidemiol . 2012. ; 411 : 106 – 115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Obermann-Borst SA , Heijmans BT , Eilers PHC et al. . Periconception maternal smoking and low education are associated with methylation of INSIGF in children at the age of 17 months . J Dev Orig Health Dis . 2012. ; 35 : 315 – 320 . [DOI] [PubMed] [Google Scholar]
- 26. Matthews SG , Phillips DIW . Minireview: transgenerational inheritance of the stress response: a new frontier in stress research . Endocrinology . 2010. ; 1511 : 7 – 13 . [DOI] [PubMed] [Google Scholar]
- 27. Wells JCK . Maternal capital and the metabolic ghetto: an evolutionary perspective on the transgenerational basis of health inequalities . Am J Hum Biol . 2010. ; 221 : 1 – 17 . [DOI] [PubMed] [Google Scholar]
- 28. Drake AJ , Seckl JR . Transmission of programming effects across generations . Pediatr Endocrinol Rev . 2011. ; 92 : 566 – 578 . [PubMed] [Google Scholar]
- 29. Szyf M . The genome- and system-wide response of DNA methylation to early life adversity and its implication on mental health . Can J Psychiatry . 2013. ; 5812 : 697 – 704 . [DOI] [PubMed] [Google Scholar]
- 30. Richmond RC , Timpson NJ , Sørensen TI . Exploring possible epigenetic mediation of early-life environmental exposures on adiposity and obesity development . Int J Epidemiol . 2015. ; 444 : 1191 – 1198 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaluski DN , Chinich A , Leventhal A et al. . Overweight, stature, and socioeconomic status among women—cause or effect: Israel National Women's Health Interview Survey, 1998 . J Gend Specif Med . 2001. ; 44 : 18 – 24 . [PubMed] [Google Scholar]
- 32. Lanza HI , Grella CE , Chung PJ . Does adolescent weight status predict problematic substance use patterns . Am J Health Behav . 2014. ; 385 : 708 – 716 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tobi EW , Lumey LH , Talens RP et al. . DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific . Hum Mol Genet . 2009. ; 1821 : 4046 – 4053 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perroud N , Paoloni-Giacobino A , Prada P et al. . Increased methylation of glucocorticoid receptor gene ( NR3C1 ) in adults with a history of childhood maltreatment: a link with the severity and type of trauma . Transl Psychiatry . 2011. ; 1 : e59 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harris A , Seckl J . Glucocorticoids, prenatal stress and the programming of disease . Horm Behav . 2011. ; 593 : 279 – 289 . [DOI] [PubMed] [Google Scholar]
- 36. Hochner H , Friedlander Y , Calderon-Margalit R et al. . Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study . Circulation . 2012. ; 12511 : 1381 – 1389 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harlap S , Davies AM , Deutsch L et al. . The Jerusalem Perinatal Study cohort, 1964–2005: methods and a review of the main results . Paediatr Perinat Epidemiol . 2007. ; 213 : 256 – 273 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lawrence GM , Shulman S , Friedlander Y et al. . Associations of maternal pre-pregnancy and gestational body size with offspring longitudinal change in BMI . Obesity (Silver Spring) . 2014. ; 224 : 1165 – 1171 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Appleton AA , Armstrong DA , Lesseur C et al. . Patterning in placental 11-B hydroxysteroid dehydrogenase methylation according to prenatal socioeconomic adversity . PLoS One . 2013. ; 89 : e74691 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marsit CJ , Maccani MA , Padbury JF et al. . Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome . PLoS One . 2012. ; 73 : e33794 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jensen Peña C , Monk C , Champagne FA . Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain . PLoS One . 2012. ; 76 : e39791 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conradt E , Lester BM , Appleton AA et al. . The roles of DNA methylation of NR3C1 and 11β-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior . Epigenetics . 2013. ; 812 : 1321 – 1329 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oberlander TF , Weinberg J , Papsdorf M et al. . Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene ( NR3C1 ) and infant cortisol stress responses . Epigenetics . 2008. ; 32 : 97 – 106 . [DOI] [PubMed] [Google Scholar]
- 44. Ehrich M , Nelson MR , Stanssens P et al. . Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry . Proc Natl Acad Sci U S A . 2005. ; 10244 : 15785 – 15790 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li L-C , Dahiya R . MethPrimer: designing primers for methylation PCRs . Bioinformatics . 2002. ; 1811 : 1427 – 1431 . [DOI] [PubMed] [Google Scholar]
- 46. Cohen E , Krause I , Fraser A et al. . Hyperuricemia and metabolic syndrome: lessons from a large cohort from Israel . Isr Med Assoc J . 2012. ; 1411 : 676 – 680 . [PubMed] [Google Scholar]
- 47. Huang JY , Gavin AR , Richardson TS et al. . Are early-life socioeconomic conditions directly related to birth outcomes? Grandmaternal education, grandchild birth weight, and associated bias analyses . Am J Epidemiol . 2015. ; 1827 : 568 – 578 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gluckman PD , Hanson MA . Maternal constraint of fetal growth and its consequences . Semin Fetal Neonatal Med . 2004. ; 95 : 419 – 425 . [DOI] [PubMed] [Google Scholar]
- 49. Baron-Epel O , Haviv A , Garty N et al. . Who are the sedentary people in Israel? A public health indicator . Isr Med Assoc J . 2005. ; 711 : 694 – 699 . [PubMed] [Google Scholar]
- 50. Isralowitz R , Reznik A . Binge drinking and risk taking behavior among adolescent females in Israel . J Child Adolesc Psychiatr Nurs . 2015. ; 284 : 175 – 179 . [DOI] [PubMed] [Google Scholar]
- 51. McMunn A , Bartley M , Hardy R et al. . Life course social roles and women's health in mid-life: causation or selection . J Epidemiol Community Health . 2006. ; 606 : 484 – 489 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heijmans BT , Mill J . Commentary: the seven plagues of epigenetic epidemiology . Int J Epidemiol . 2012. ; 411 : 74 – 78 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jablonka E . Epigenetic epidemiology . Int J Epidemiol . 2004. ; 335 : 929 – 935 . [DOI] [PubMed] [Google Scholar]
- 54. Moodie EEM , Stephens DA . Marginal structural models: unbiased estimation for longitudinal studies . Int J Public Health . 2011. ; 561 : 117 – 119 . [DOI] [PubMed] [Google Scholar]
- 55. Imai K , Keele L , Tingley D . A general approach to causal mediation analysis . Psychol Methods . 2010. ; 154 : 309 – 334 . [DOI] [PubMed] [Google Scholar]
- 56. VanderWeele TJ , Robinson WR . On the causal interpretation of race in regressions adjusting for confounding and mediating variables . Epidemiology . 2014. ; 254 : 473 – 484 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suderman M , Borghol N , Pappas JJ et al. . Childhood abuse is associated with methylation of multiple loci in adult DNA . BMC Med Genomics . 2014. ; 7 : 13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ferrari P . The role of 11β-hydroxysteroid dehydrogenase type 2 in human hypertension . Biochim Biophys Acta . 2010. ; 180212 : 1178 – 1187 . [DOI] [PubMed] [Google Scholar]
- 59. Friso S , Pizzolo F , Choi SW et al. . Epigenetic control of 11 beta-hydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension . Atherosclerosis . 2008. ; 1992 : 323 – 327 . [DOI] [PubMed] [Google Scholar]
- 60. Sharp GC , Lawlor DA , Richmond RC et al. . Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon Longitudinal Study of Parents and Children . Int J Epidemiol . 2015. ; 444 : 1288 – 1304 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Richmond RC , Al-Amin A , Smith GD et al. . Approaches for drawing causal inferences from epidemiological birth cohorts: a review . Early Hum Dev . 2014. ; 9011 : 769 – 780 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Talens RP , Christensen K , Putter H et al. . Epigenetic variation during the adult lifespan: cross-sectional and longitudinal data on monozygotic twin pairs . Aging Cell . 2012. ; 114 : 694 – 703 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kleinhaus K , Harlap S , Perrin M et al. . Prenatal stress and affective disorders in a population birth cohort . Bipolar Disord . 2013. ; 151 : 92 – 99 . [DOI] [PubMed] [Google Scholar]
- 64. US Central Intelligence Agency . The world factbook—Israel . https://www.cia.gov/library/publications/the-world-factbook/geos/is.html . Updated December 18, 2015. Accessed January 11, 2016 .
- 65. Martorell R , Zongrone A . Intergenerational influences on child growth and undernutrition . Paediatr Perinat Epidemiol . 2012. ; 26 ( suppl 1 ): 302 – 314 . [DOI] [PubMed] [Google Scholar]
- 66. Coolen MW , Statham AL , Gardiner-Garden M et al. . Genomic profiling of CpG methylation and allelic specificity using quantitative high-throughput mass spectrometry: critical evaluation and improvements . Nucleic Acids Res . 2007. ; 3518 : e119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Houseman EA , Molitor J , Marsit CJ . Reference-free cell mixture adjustments in analysis of DNA methylation data . Bioinformatics . 2014. ; 3010 : 1431 – 1439 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Beach SR , Lei MK , Brody GH et al. . Higher levels of protective parenting are associated with better young adult health: exploration of mediation through epigenetic influences on pro-inflammatory processes . Front Psychol . 2015. ; 6 : 676 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Richmond RC , Simpkin AJ , Woodward G et al. . Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC) . Hum Mol Genet . 2015. ; 248 : 2201 – 2217 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.