ABSTRACT
HIV infection treatment strategies have historically defined effectiveness through measuring patient plasma HIV RNA. While combined antiretroviral therapy (cART) can reduce plasma viral load (pVL) to undetectable levels, the degree that HIV is eliminated from other anatomical sites remains unclear. We investigated the HIV DNA levels in 229 varied autopsy tissues from 20 HIV-positive (HIV+) cART-treated study participants with low or undetectable plasma VL and cerebrospinal fluid (CSF) VL prior to death who were enrolled in the National Neurological AIDS Bank (NNAB) longitudinal study and autopsy cohort. Extensive medical histories were obtained for each participant. Autopsy specimens, including at least six brain and nonbrain tissues per participant, were reviewed by study pathologists. HIV DNA, measured in tissues by quantitative and droplet digital PCR, was identified in 48/87 brain tissues and 82/142 nonbrain tissues at levels >200 HIV copies/million cell equivalents. No participant was found to be completely free of tissue HIV. Parallel sequencing studies from some tissues recovered intact HIV DNA and RNA. Abnormal histological findings were identified in all participants, especially in brain, spleen, lung, lymph node, liver, aorta, and kidney. All brain tissues demonstrated some degree of pathology. Ninety-five percent of participants had some degree of atherosclerosis, and 75% of participants died with cancer. This study assists in characterizing the anatomical locations of HIV, in particular, macrophage-rich tissues, such as the central nervous system (CNS) and testis. Additional studies are needed to determine if the HIV recovered from tissues promotes the pathogenesis of inflammatory diseases, such as HIV-associated neurocognitive disorders, cancer, and atherosclerosis.
IMPORTANCE It is well-known that combined antiretroviral therapy (cART) can reduce plasma HIV to undetectable levels; however, cART cannot completely clear HIV infection. An ongoing question is, “Where is HIV hiding?” A well-studied HIV reservoir is “resting” T cells, which can be isolated from blood products and succumb to cART once activated. Less-studied reservoirs are anatomical tissue samples, which have unknown cART penetration, contain a comparably diverse spectrum of potentially HIV-infected immune cells, and are important since <2% of body lymphocytes actually reside in blood. We examined 229 varied autopsy specimens from 20 HIV+ participants who died while on cART and identified that >50% of tissues were HIV infected. Additionally, we identified considerable pathology in participants' tissues, especially in brain, spleen, lung, lymph node, liver, aorta, and kidney. This study substantiates that tissue-associated HIV is present despite cART and can inform future studies into HIV persistence.
INTRODUCTION
Over 30 years ago, HIV infection and its clinical manifestation, AIDS, emerged as a worldwide epidemic. Since then, significant progress has been achieved in understanding the causes of HIV pathogenesis in the context of drug treatments that extend patients' lives by years or decades. Current combined antiretroviral therapy (cART) regimens encompass a variety of drugs that are focused on inhibiting viral replication, which allows for the recovery of a healthy CD4+ T cell population (1). However, even with carefully monitored cART, pathological trajectories are variable among patients (2–4), as are the adverse effects of therapy (5–10). Complex and difficult-to-measure patient attributes that may affect the efficacy of cART include psychological conditions (e.g., depression and drug or alcohol abuse), adherence to drug treatment requirements, availability of social support systems, and coping strategies (11). In addition, two major problems have emerged in HIV-positive (HIV+) persons with long-term exposure to cART: (i) many patients develop serious “non-AIDS-defining” conditions, including cancer, atherosclerosis, and neurocognitive disorders, at much higher rates than in comparable uninfected populations (12–15), and (ii) the virus is never completely eradicated (16), as interruption in drug therapy results in the rapid rebound of replicating virus in plasma.
Since the introduction of cART, the therapeutic goal of virus eradication has been complicated by the emergence of drug-resistant variants and the inability to prevent viral rebound after cART discontinuation. The persistence of very low levels of HIV in plasma of cART-treated patients has suggested the presence of a cell-based “viral reservoir.” Viral reservoirs contain infected cells that do not release infectious virus (i.e., are latently infected), but can do so following activation, which may occur under a variety of conditions (17, 18). Virus production from latently infected cells is believed to occur in the absence of new rounds of surrounding cell infection (19–21). Currently, HIV latency is primarily attributed to the presence of proviral HIV DNA in resting memory CD4+ T cells (18). These cells can live for long periods, contribute to low-level persistent viremia during cART and viral rebound after treatment interruption, and produce viral variants with escape mutations (17, 22). However, it is important to consider that less than 2% of the total body lymphocyte population resides in peripheral blood (23), suggesting that evaluation of HIV infection in anatomical sanctuary sites is of critical importance, e.g., characterization of compartmentalized HIV in tissue reservoirs such as the brain (24, 25). Further, there is controversy over whether these sanctuary tissues contain significant pools of latent virus in cells other than memory T cells and whether these cells could contribute to viral rebound.
HIV infects a number of different cell types that reside in tissues, including T cells, macrophages, monocytes, astrocytes, microglia, and dendritic cells (26–29). Cellular viral reservoirs may include immature memory T cell populations with stem cell-like properties (CD4+ T memory stem cells) and monocytes/macrophages (30–33). Tissue macrophages, despite being infected at much lower levels than CD4+ T cells (34), are a particularly important pathogenic target of the virus due to the role of macrophages in signaling other immune cells to sites of infection and damage (26, 35–37), which can contribute to HIV-associated comorbidities (34, 36, 38). Moreover, resident tissue macrophages are long-lived and may naturally produce low levels of virus. HIV+ perivascular macrophages and macrophage-like microglia (39, 40) in the brain have been associated with the emergence of antiretroviral drug resistance (41, 42) and HIV-associated neurocognitive disorders (HAND) (43, 44). HIV+ patients may also harbor viral populations in cerebrospinal fluid (CSF) that are genetically distinct from virus in the blood (45–50) and that exhibit characteristics of macrophage/microglia tropism (51). Thus, anatomical sites and/or cell types that harbor viral populations with low replication potential may contribute to the formation of a viral reservoir during primary infection (52, 53). Yet the degree to which cART clears HIV from anatomical tissue reservoirs is unknown, and the relationship between persistent HIV in tissues and tissue damage has never been carefully examined.
Here, we describe extensive background, clinical, and pathological findings in an HIV-infected longitudinal cohort of 20 participants who received cART and died with undetectable VL in body fluids. A total of 229 tissues from varied anatomical sites were isolated from participants at autopsy, each of which was histologically examined and assayed for the presence of HIV DNA. In an accompanying study (54), we generated env-nef DNA and RNA sequences from anatomical tissues from a subset of these participants in order to determine if HIV was evolving in these sites during cART. The results from these studies provide key insights into the effect of cART on residual HIV infection in tissues.
MATERIALS AND METHODS
General protocols for participants enrolled at the NNAB.
The National Neurological AIDS Bank (NNAB) is a member of the National NeuroAIDS Tissue Consortium (NNTC). The NNTC was founded in 1998 to respond to researchers' need for well-characterized HIV-1-infected (HIV+) human tissues and fluid samples, and to study the mechanisms of HIV-associated neurological diseases (55). The NNAB uses standardized NNTC protocols to assess and classify neurocognitive impairment in living HIV+ participants and to assign neurological and psychiatric diagnoses according to established criteria (55–57). Participants are asked to donate blood and urine samples at each study visit, as well as optional CSF specimens, in accordance with NNTC protocols. In addition, the NNAB site collects data on comorbid systemic diseases in living HIV+ persons, such as cancer, hypertension, lipodystrophy, hyperlipidemia, diabetes, pulmonary, cerebrovascular, cardiac, renal, and other diseases, and samples a wide array of postmortem nonneurological organs and tissues whenever possible.
NNAB participants.
NNAB participants are recruited from the greater Los Angeles, CA, area. An institutional review board (IRB)-approved informed consent is obtained from each participant or his or her legal guardian. Eligibility criteria are as follows. Participants must be aged 18 years or older and agree to participate in study examinations and donation of blood, urine, and (optional) CSF during life and to donate their brain and other tissues for research in the event of their death. HIV+ participants are selected because they have one or more of the following: a CD4+ cell count of <50 cells/mm3, systemic lymphoma or another widespread malignancy, Mycobacterium avium complex infection, wasting with loss of ≥30% of body weight, primary central nervous system (CNS) lymphoma, progressive multifocal leukoencephalopathy, congestive heart failure, chronic renal failure with potential or current need for dialysis, chronic obstructive pulmonary disease, end-stage liver disease, serum albumin of ≤3.2 g/dl, or any condition which, in the opinion of the study physician, is likely to lead to death within the period of study. In 2013, the criteria were amended to recruit a limited number of older (age > 60 years) HIV+ participants. There are no exclusions based on race, ethnicity, language, immigration status, socioeconomic status, gender, substance use, or sexual preference. Most participants are recruited premortem; however, the remains of very recently deceased individuals could be donated by the legal heirs, in which case information is obtained from the guardians and/or by authorized release of medical records.
Cohort information, questionnaires, and general medical assessments.
All study material was collected and coded in accordance with The University of California, Los Angeles, IRB (approval number 10-000525-CR-0005) and NNAB protocols to protect the participant's confidentiality. Consent, study materials, and questionnaires were all administered in a standard format in either English or Spanish. Whenever possible, participants (and/or their legal representatives) consented and were enrolled prior to the participant's death and received the following: serial standardized NNTC neuromedical, neuropsychological, psychiatric, and substance use inventories and examination, a comorbidity questionnaire, blood testing for CD4+ subsets and plasma viral load (pVL), and urine toxicology testing for substance abuse. Participants were also offered optional CSF collection. Most participants were also tested for syphilis and hepatitis C virus (HCV) serology once at entry. In all cases, participants or their legal guardians were asked to sign an IRB-approved release of medical information. Participants were scheduled for serial return visits based in part on their level of illness (sicker participants were seen more often and at shorter intervals) and their availability (several participants were unable to come in for various reasons, e.g., travel or incarceration). Some participants were unable or unwilling to complete the full battery due to their terminal illness or refusal by the participant or guardian. Some participants in this cohort were apparently medically stable HIV+ persons who were not deemed to be candidates for NNAB, specifically because they appeared healthy, but who expired suddenly and unexpectedly and were enrolled by their family immediately after their demise. This group included some cases extremely noteworthy precisely because these HIV+ participants were not deemed to be at high risk for death and had a history of good medical adherence, stable CD4+ cell counts, and persistently low or undetectable plasma viral loads as documented by their personal physicians.
Demographic information was collected for each participant, including date of birth, age at death, gender (male, female, or transgender), ethnicity (Hispanic/Latino or not), race (white, black/African-American, Asian, Native Alaskan/American Indian, Native Hawaiian/Pacific Islander, other or mixed), education in years, highest degree obtained, reading level, and mode(s) of exposure (male-to-male sex [MSM], intravenous drug use [IDU], heterosexual transmission, blood product, health care accident, and perinatal, other, and unknown mode of transmission).
Participants who were examined premortem were asked to complete a standardized medical and neurological history covering comorbidities (e.g., hypertension; cardiac, pulmonary, hepatic, renal, and cerebrovascular diseases; diabetes; hyperlipidemia; lipodystrophy; non-AIDS-defining malignancies; and tobacco smoking), AIDS-defining illnesses, and neurological history (head injury, loss of consciousness, learning disorders, seizures, other neurological diseases, and targeted cognitive, sensory, and motor symptoms). Neurological examinations at the NNAB were performed by two board-certified academic neurologists who specialize in neuro-AIDS. This included administration of the Mini-Mental Status Exam and HIV Dementia Scale (HDS) and a targeted examination of cranial nerves, motor systems, coordination, reflexes, and sensation. Neuropsychological testing was performed in English or Spanish by a trained neuropsychometry technician under the supervision of a board-certified neuropsychologist with expertise in HIV. This included a standardized set of neuropsychological tests; a psychiatric and substance abuse inventory based on the Diagnostic and Statistical Manual for Mental Disorders, 4th edition (DSM-IV) (58; http://www.dsm4.org), the Composite International Diagnostic Interview (CIDI) (59, 60), or the Psychiatric Research Interview for Substance and Mental Disorders (PRISM) (61); and the Beck Depression Inventory—second edition (BDI) (62). The Spanish neuropsychological testing varies slightly from the English version in that the Spanish version of the HDS was scored according to slightly different norms and the Test de Vocabulario en Imagenes Peabody (TVIP) (63) was used to determine verbal ability and reading level (64). There was also an abbreviated “step-down” battery, which is used for participants who are too ill to complete the full battery.
Whenever possible, a urine sample was obtained at each visit to screen for substances of abuse; a blood draw was requested at each visit for complete blood count (CBC), including white blood count, hemoglobin, hematocrit, and platelet count; CD4+ cell subsets; pVL, serum albumin (at entry only); HCV serology (at entry only); and rapid plasma regain evaluation for syphilis serology (at entry only). The remainder of the sample was frozen and stored as plasma, serum, and (nonviable) peripheral blood mononuclear cells (PBMCs). An optional lumbar puncture was requested for all participants who were deemed appropriate (on the basis of the neurological exam, blood work, and any available neuroimaging) and to be at low risk for complications. If collected, CSF was measured for cell count, glucose, total protein, VDRL testing, and CSF HIV load (csfVL); the remaining supernatant was frozen. The nadir absolute CD4+ cell count was defined as the lowest known absolute CD4+ cell count as determined by chart review and/or participant recollection. The duration of known HIV infection was calculated using the reported first date of HIV-seropositive diagnosis and the date of the participant's death. In most cases, the participant did not know the exact date that they acquired HIV infection.
Twenty-patient subset of the NNAB general program.
Twenty participants were identified from the 246-participant NNAB cohort who fit the following criteria: (i) were HIV+, (ii) died while on cART or had only a brief interval before death when they did not take cART, (iii) had documented premortem history of low or undetectable pVL, (iv) had an autopsy within a 24-h period after death (called the postmortem interval [PMI]), and (v) had VL measurements from both postmortem aspiration of cardiac fluid that had been centrifuged to remove debris (note: this is not a true pVL, because true plasma is unobtainable after death) and postmortem CSF (collected by aspiration from the lumbar sac or cerebral ventricle). Aggressive efforts were made to review the participants' medical records to collect additional information, with the emphasis on the participants' cART regimens and adherence, CD4+ subsets, pVL, medical comorbidities, and any clinical evaluations of their neurological or HIV-related illnesses.
The last date of cART intake was estimated for each participant as follows. If the participant expired in a hospital, a nursing facility, or hospice, this information was obtained from the medical record. If the participant expired at home and lived at home with a caregiver or partner, this person was queried about the last cART dose the patient certainly ingested (in such cases, the caregivers usually administered cART medications). If the patient was living independently, we listed the last date they were documented to report taking medications and whether there was any reason to suspect they stopped them before death. The last methods were used mostly for sudden, unexpected deaths of persons who were in outpatient care and deemed to be doing well.
The PMI was calculated as follows. If the participant expired while under direct observation (e.g., in a hospital or hospice), the PMI was calculated as the interval between the exact time of death and the time the brain was removed and placed on ice. If the participant was not observed during the time of death, PMI was calculated from the last time a friend, relative, neighbor, or health care worker contacted the participant until the time the brain was removed and placed on ice.
Postmortem tissue harvesting was performed by an autopsy technician and reviewed by one or more board-certified pathologists with expertise in AIDS. All NNAB tissue samples selected for this particular study were individually cut with fresh, unused surgical blades to avoid cross-contamination of tissues within and among cases. Whenever possible, the minimum organs harvested included the entire brain, the spinal cord, and samples of peripheral nerve, striated muscle, spleen, lymph nodes, and thymus. Most tissue harvests also included samples of heart, lung, renal, and gastrointestinal (GI) tissue, encapsulated lymph nodes primarily from the chest and/or abdominal cavity, and any organ, tissue, or fluid that appeared clinically suspicious or appeared to contain an infectious or malignant lesion.
Whenever possible, the technician aspirated a fluid sample from the cardiac ventricles and postmortem CSF from either the lumbar sac or cerebral ventricles. Brain and other tissues were divided into frozen and/or formalin-fixed samples as described in the NNTC standard protocol (55). For this protocol, all fixed and/or frozen samples from different anatomical tissue samples within and between each case were cut with a new, unused surgical blade, which was then discarded to prevent inadvertent transfer of viral DNA among tissues. For initial histopathological examination of samples, formalin-fixed tissues were embedded in paraffin and processed to hematoxylin and eosin slides as described by Morgello et al. (55). Neuropathological slides were examined by one or more board-certified academic neuropathologists with expertise in AIDS, and systemic tissues were examined by a board-certified anatomical pathologist with specialty in AIDS and in hematology-oncology. Additional stains or other tests were ordered as needed based on the clinical history and initial histopathological findings. Criteria outlined by Budka et al. were used to identify HIV encephalitis and other HIV-related CNS pathologies (65).
ACSR.
The AIDS and Cancer Specimen Resource (ACSR) is a National Cancer Institute-funded program that supplies specimens from HIV+ participants with cancer to investigators interested in AIDS malignancy research. Researchers at the ACSR and NNAB have had a long-term collaboration in evaluating the role of HIV infection in the pathogenesis of HIV-associated dementia (HAD). Over the past 5 to 10 years, when it became clear that AIDS patients were dying of complications from cancer more than HAD, the collaboration expanded to provide samples from participants with HIV infection and cancer to the ACSR. For this study, the ACSR independently verified the tissue diagnoses made by the NNAB and evaluated levels of VL in plasma, CSF, and other various tissues provided by the NNAB. All of the cases described here reside at both the ACSR and the NNAB. The use of these materials is acknowledged to be a product of both NIH-funded programs. This study was approved by the University of California, San Francisco (IRB approval number 13-12020).
HIV detection protocols. (i) qPCR.
Autopsy tissues with validated pathology (n = 229) from 20 patients was used for evaluation of HIV DNA content. Quantitative PCR (qPCR) was performed by extracting DNA from each tissue using a QIAamp DNA microkit (Qiagen, Valencia, CA) as per guidelines stated in the manufacturer's handbook, and DNA was eluted in 20 μl of Tris-EDTA (TE) buffer. The extracted DNA was used to assay for HIV DNA using a quantitative multiplex real-time PCR assay. Two standard curves were generated using a mixture of two plasmids containing a single copy of the gag gene (HXB2; GenBank accession number K03455) and a single copy of the β-globin gene (GenBank accession number AF007546) in 1:10 dilutions starting at 1 × 106 and ending at 1 × 101 copies. All standards and samples were set up in triplicate, with each containing 2× TaqMan Gene Expression master mix (Applied Biosystems, Foster City, CA), 1.25 pmol of gag primers (gag forward, GACATCAAGCAGCCATGCAA, and gag reverse, CTCATCTGGCCTGGTGCAAT), 0.625 pmol of β-globin primers (β-globin forward, TCACTAGCAACCTCAAACAGACACC, and β-globin reverse, AGGGCCTCACCACCAACTTC), 1.25 pmol of each probe (β-globin probe, VIC-CTCCTGAGGAGAAGTCTGCCGTTACTGCC, and Gag probe, 6-carboxyfluorescein [FAM]-ACCATCAATGAGGAAGCTGCAGAATGGGA), 3 μl of the eluted DNA, and water for a final volume of 10 μl. Samples were run in a StepOne real-time PCR system (Applied Biosystems) as follows: 95°C for 15 min, followed by 40 cycles at 95°C for 1 min and at 60°C for 1 min, followed by analyses using the StepOne real-time PCR system software. The copy numbers of each gene within the unknowns were determined against the standard curves generated by the plasmids. The copy numbers were used to calculate HIV DNA copy numbers per cell. OM10.1, a promyelocytic cell line containing approximately one copy of HIV per cell, was used as positive control; HIV-negative DNA and water were used as negative controls.
(ii) ddPCR.
Droplet digital PCR (ddPCR) utilized the primers from our multiplex real-time qPCR assay that assessed the β-globin and gag genes, in which a 111-bp gag segment and 109-bp β-globin gene segment were amplified. Briefly, DNA was extracted using the QIAamp DNA microkit (Qiagen), as per guidelines stated in the manufacturer's handbook, and eluted in 20 μl of TE buffer. The extracted DNA was used in the reaction mixture containing 2× ddPCR supermix for probes (no dUTP), 250 nM each primer, 250 nM each probe, 3 μl of DNA, and H2O for a final volume of 20 μl that was put into the droplet generator cartridge along with 70 μl of droplet reader oil. The droplet generator cartridge was then loaded into the QX200 droplet generator (Bio-Rad, Hercules, CA), and 40 μl of the droplets was transferred into a 96-well plate and loaded into the thermocycler for PCR with the following cycling parameters: 95°C for 10 min, 40 cycles of 95°C for 1 min, and 60°C for 1 min. After PCR, the plate was loaded into the QX200 droplet reader (Bio-Rad, Hercules, CA), which analyzes each individual droplet and determines copy numbers using QuantaSoft software. These numbers were used to calculate HIV DNA copy per million cells. The promyelocytic cell line OM10.1 was used as a positive control; HIV-negative DNA and H2O were used as negative controls. The limit of detection from both approaches was 200 copies per 1 million cells. Paired t tests were performed to determine any significant correlations between qPCR and ddPCR results. Also, paired t tests were performed to determine any significant correlations between ddPCR results and histopathological findings.
(iii) SGS.
In order to test whether the HIV present in tissues could encode functional intact viral proteins that would show evidence for ongoing evolution, RNA and genomic DNA was isolated from selected tissues from five participants. A modified single-genome sequencing (SGS) protocol was used to amplify linked HIV env and nef sequences (66). env and nef alignments were generated, and maximum-likelihood phylogenies and Bayesian time-scaled analysis were used to elucidate sequence diversity/evolution in participant sequence data sets. These methods and expanded results are provided in precise detail in reference 54.
RESULTS
Subject demographics.
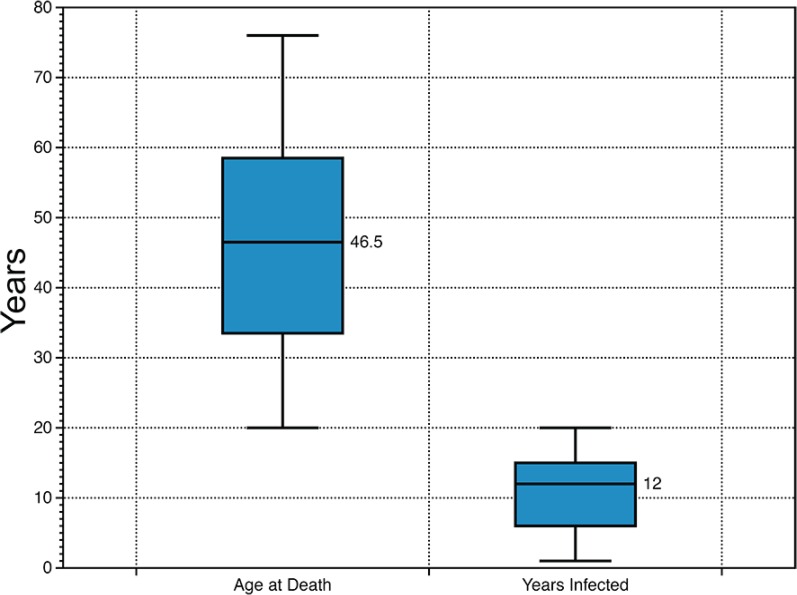
Seventeen male and 3 female HIV+ participants were included in the study (Table 1). Sixteen participants had a PMI of 10 h or less, and the average PMI for the cohort was 7.77 h. The cohort reported a mix of HIV risk factors, with 10 participants likely contracting HIV by MSM, 3 through heterosexual transmission, 3 through IDU, and 1 through an infected blood product; 3 participants reported two or more risk factors. Three participants were enrolled postmortem into the NNAB program. The median age of the participants was 46.5 years, and the median length of known infection was 12 years (Fig. 1).
TABLE 1.
Patient demographics
| Patient identifier | Gender | No. of yrs enrolled in NNAB | Pre- or postmortem enrollmenta | PMIb (h) | No. of years of known HIV infection | Age at death (yrs) | Risk factor(s) |
|---|---|---|---|---|---|---|---|
| 1010 | Male | 5 | Pre | 3.5 | 12 | 66 | MSM |
| 1156 | Male | 0 | Post | 7.5 | 15 | 62 | MSM |
| 2004 | Male | 7 | Pre | 11 | 17 | 57 | MSM |
| 4106 | Male | 2 | Pre | 10 | 18 | 50 | Heterosexual Exposure |
| 4010 | Male | 1 | Pre | 3 | 5 | 76 | MSM |
| 4013 | Male | 2 | Pre | 4.5 | 6 | 42 | IDU/MSM |
| 4124 | Male | 2 | Pre | 19.5 | 16 | 58 | IDU |
| 4129 | Female | 3 | Pre | 4 | 10 | 59 | Heterosexual exposure |
| 4130 | Male | 1 | Pre | 7 | 13 | 59 | Heterosexual exposure |
| 4143 | Male | 1 | Pre | 4 | 5 | 35 | IDU |
| 4149 | Male | 0 | Post | 20.5 | 20 | 51 | MSM |
| 4150 | Male | 0 | Pre | 8 | 12 | 54 | Heterosexual exposure/IDU/MSM |
| 4154 | Male | 0 | Post | 13 | 13 | 39 | MSM |
| 4175 | Male | 1 | Pre | 5 | 1 | 30 | MSM |
| 4179 | Male | 1 | Pre | 5 | 1 | 44 | MSM |
| 5024 | Male | 5 | Pre | 7 | 15 | 39 | Heterosexual exposure/other |
| 5025 | Female | 0 | Pre | 4 | 13 | 69 | Blood product recipient |
| 5095 | Male | 1 | Pre | 8 | 4 | 40 | MSM |
| 6015 | Female | 1 | Pre | 6 | 7 | 30 | IDU |
| 6083 | Male | 0 | Pre | 5 | 6 | 53 | MSM |
Pre, the participant was enrolled in the NNAB prior to death; post, the participant was enrolled after death.
PMI, postmortem interval.
FIG 1.
Cohort age and number of years infected. The box and whisker plot shows the median, upper and lower quartiles and range for the cohort age (left) and number of year infected (right).
Medical histories. (i) Antiretroviral histories.
Detailed treatment history, including last known cART dose, was obtained either from a medical facility or from questioning the caregiver at the time of death and was available for all participants (see Table S1 in the supplemental material). To the best of our ability, we determined that 11 of the participants were on cART until death. Of the remaining participants, four may have stopped their medications within 4 weeks of death and three within 8 weeks of death, and two had their last prescribed cART dose 4 months prior to death. While we have no records to show cART adherence immediately prior to death in these nine participants, compliance is likely due to their low viral loads at autopsy, as HIV rapidly rebounds during treatment interruption (67–69).
(ii) VL and CD4+ cell measurements.
VL data for the cohort at death (in CSF and cardiac aspirates) and the last living pVL measured were obtained for each patient (Table 2). One of the requirements for inclusion in this study was a nondetectable VL in a fluid sample from the cardiac ventricles and postmortem CSF from either the lumbar sac or cerebral ventricles. The limit of detection for premortem CSF and plasma VL varied according to the year that the sample was collected, as these were assayed immediately. In some cases, a low-level HIV infection was identified in the CSF, which may have been due to inadequate CNS penetration efficacy of the cART regimen, CNS mutations, or an independent source of replication in the CNS. For example, participant 6015's last known living pVL was close to 9,000 copies/mm3, 15 months prior to death. While we do not have detailed information concerning her cART intake in the months prior to death, she was prescribed didanosine (ddI), saquinavir, ritonavir, stavudine, and nevirapine 8 weeks prior to death. Because her last CD4+ cell count was 407 cells/mm3 just prior to death, she had no detectable viral load in the cardiac aspirate, and she was administered her medications by a nurse in a nursing facility, adherence was extremely likely. Participants 4179, 5025, and 5095 also retained a low-level HIV infection in CSF at death. Participant 4143 had a low-level residual HIV VL of 258 copies/mm3 in cardiac aspirate at autopsy; however, hospital records indicated that he continued to take cART until 3 days prior to his death from lymphoma.
TABLE 2.
Viral loads prior to and at autopsy
| Subject | csfVL at death (no. of copies/mm3) | pVL at death (no. of copies/mm3)a | Last living pVL (no. of copies/mm3) | Last confirmed cART |
|---|---|---|---|---|
| 1010 | <400 | <40 | <400 | −4 mo |
| 1156 | <40 | <40 | <400 | −2 days |
| 2004 | <40 | <40 | <400 | −0 days |
| 4106 | <40 | Failedb | <400 | −0 days |
| 4010 | <40 | <40 | <400 | −0 days |
| 4013 | <40 | <400 | <400 | −1 year |
| 4124 | <40 | <40 | <400 | −0 days |
| 4129 | <40 | <40 | <40 | −0 days |
| 4130 | <40 | <40 | <40 | −0 days |
| 4143 | <40 | <400 | <40 | −7 days |
| 4149 | <40 | <40 | <40 | −0 days |
| 4150 | <40 | <40 | <40 | −7 days |
| 4154 | <40 | <40 | <40 | −4 days |
| 4175 | <40 | <40 | <40 | −0 days |
| 4179 | <400 | <40 | <40 | −8 days |
| 5024 | <40 | <40 | <40 | −2 mo |
| 5025 | 942 | <40 | <400 | −30 days |
| 5095 | <400 | <40 | <400 | −9 days |
| 6015 | <40 | <40 | <40 | −1 year |
| 6083 | <40 | <40 | <40 | −0 days |
VL obtained from cardiac aspirate at autopsy, not a true pVL.
Assay results not available.
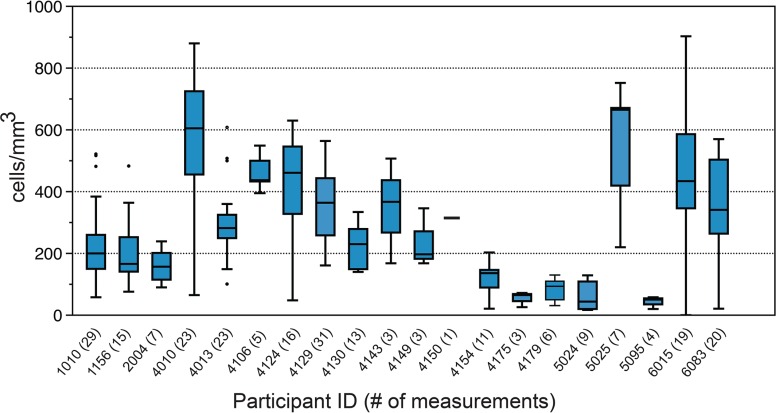
(iii) CD4+ cell measurements (median, range, and outliers) for the participants were low and showed considerable ranges of distribution (Fig. 2), although they should be interpreted with a degree of caution since per study protocols, less healthy participants were seen more often and at shorter intervals; therefore, CD4+ cell count was measured in the participants when it would expectedly be low due to illness, such as during intercurrent infections.
FIG 2.
Available CD4+ cell measurements for study participants. Box-and-whisker plots show the Nadir (lowest value), median, range and highest CD4+ measurements available for each patient in cells/mm3. Participant identifiers are on the x axis, followed by the number of available CD4+ measurements in parentheses.
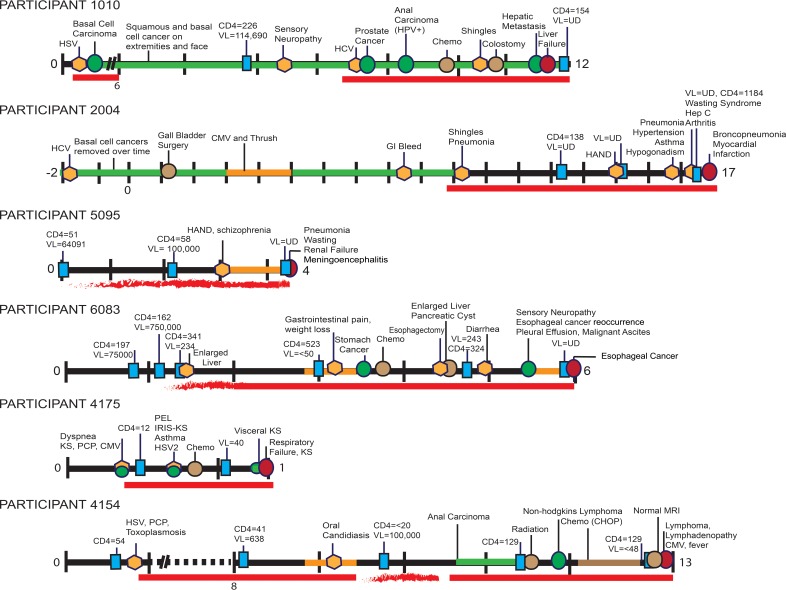
Patient timelines.
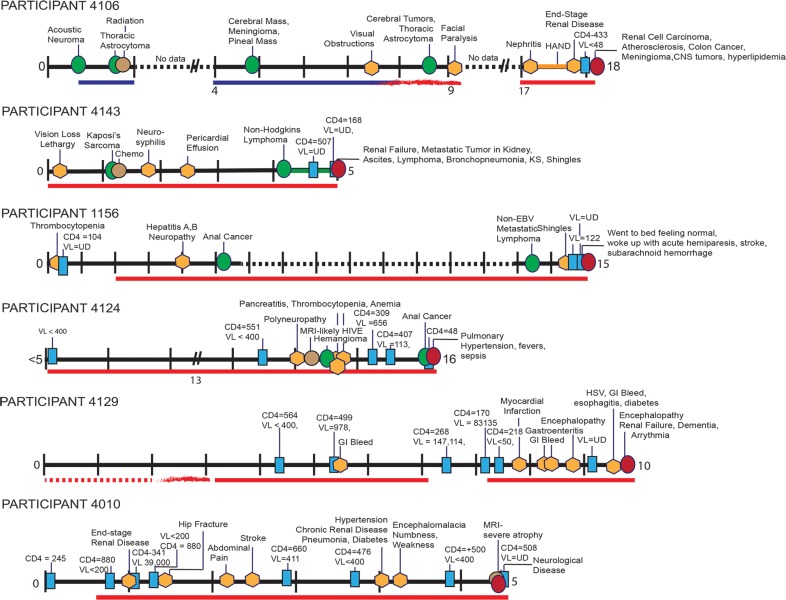
Extensive research was devoted to reconstructing each participant's medical history from the time of HIV diagnosis to death (condensed in Fig. 3 to 5). One clear observation from these data is that all participants presented with numerous serious pathologies during their course of infection despite cART. In some cases, this could be attributed to poor recovery of immune function as indicated by CD4+ cell count, and in other cases this could be ascribed to so-called “non-AIDS-defining” pathologies associated with long-term HIV infection and treatment. Fourteen of the participants in the cohort were diagnosed with at least one type of cancer prior to death, including basal cell carcinoma, prostate cancer, anal carcinoma, stomach cancer, Kaposi's sarcoma (KS; both dermal and visceral), a variety of lymphomas, brain cancers, and lung cancer. Other pathologies observed in the cohort included neurological disorders, miscellaneous infections, and organ diseases.
FIG 3.
Timelines for participants 1010, 2004, 5095, 6083, 4175, and 4154. The timelines highlight major clinical evaluations, procedures, and pathologies diagnosed during the course of each NNAB-participant's HIV infection. Some VL and CD4+ cell measurements are noted (additional measurements are available at the NNAB for each participant). Time “0” indicates the year of HIV diagnoses, with tick marks indicating subsequent years extending until the participant's death. A symbol legend is shown at the bottom of Fig. 5. Abbreviations: VL, viral load (copies per cubic millimeter); CD4+, CD4+ counts (cells per cubic millimeter); UD, undetectable (using the limits available at the time of testing); HSV, herpes simplex virus; HPV, human papillomavirus; Chemo, chemotherapy; CMV, cytomegalovirus; GI, gastrointestinal; HAND, HIV-associated neurocognitive disorders, which encompass ANI (asymptomatic neurocognitive disorder), MND (minor neurocognitive disorder, similar to minor cognitive motor disorder [MCMD]), and HIV-associated dementia (HAD); PCP, Pneumocystis jirovecii pneumonia; PEL, primary effusion lymphoma; IRIS-KS, immune reconstitution inflammatory syndrome complicating Kaposi's sarcoma; HCV, hepatitis C virus; COPD, chronic obstructive pulmonary disease.
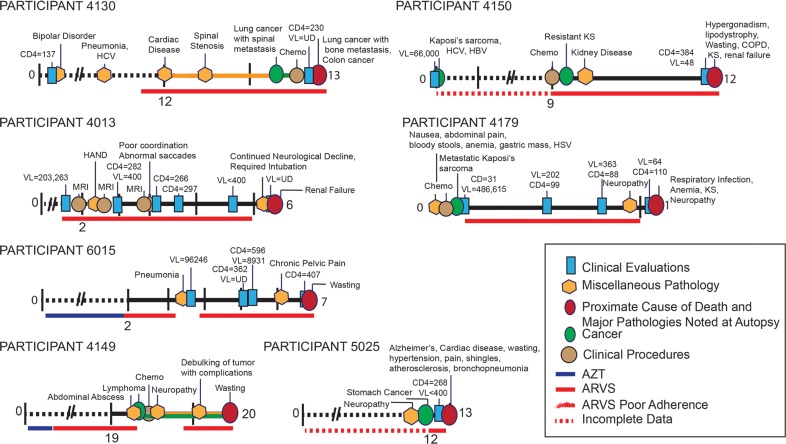
FIG 5.
Timelines for participants 4130, 4013, 6015, and 4149. AZT, azidothymidine; ARVS, antiretrovirals.
FIG 4.
Timelines for participants 4106, 4143, 1156, 4124, 4129, and. 4010. EBV, Epstein-Barr virus; HIVE, HIV-associated encephalitis.
Although cART adherence varied among participants, most were compliant throughout their treatment program. Here we describe the medical histories of three participants with high cART adherence over the course of their HIV infection in order to demonstrate the detailed historical information available for all 20 participants. Participant 2004 (Fig. 3) was an HIV+ male with a reported history of substance abuse and MSM. In the late 1970s he was diagnosed with cutaneous melanoma, which was successfully treated. Over the course of his life he was also diagnosed with and treated for numerous basal cell carcinomas. He was diagnosed with HIV in the mid-1980s and was subsequently treated for thrush and cytomegalovirus (CMV). In the late 1990s he was recruited to the NNAB, and neurocognitive testing indicated a mild neurocognitive disorder. He was treated with lamivudine, indinavir, and nevirapine. He remained on cART until his death (further cART details are provided in Table S1 in the supplemental material). After cART initiation, at least three blood tests indicated an undetectable plasma viral load. One year prior to death he was treated for bacterial pneumonia, hypertension, asthma, and hypogonadism. Subsequently, he developed wasting syndrome and rheumatoid arthritis. His CD4+ cell count was 134 to 239 cells/mm3 during this time and his pVL remained undetectable. At the time of death, he was taking the following cART: lamivudine, abacavir, nevirapine, fosamprenavir, and acyclovir. The participant had no detectable VL in cardiac aspirate or CSF at autopsy, and his last recorded CD4+ cell count was 239 cells/mm3 19 days prior to death. Postmortem pathological observations were as follows: lung abscess with pulmonary edema, severe calcific aortic atherosclerosis with aortic aneurysm, necrotic liver with bile stasis, acute inflammation of the spleen, lymphatic hyperplasia, and focal myocardial fibrosis. Sections of the brain showed reactive astrocytes, abnormally large numbers of corpora amylacea, and CD68+ cells in the white matter. These findings are abnormal but nonspecific. Death was ascribed to multiorgan disease, with acute bronchopneumonia likely the terminal event.
Participant 4154 (Fig. 3) was a male who was diagnosed with HIV infection in his mid-20s, with MSM as a risk factor. His CD4+ cell count was initially measured at 54 cells/mm3. For approximately 8 years after his diagnosis, medical records were unobtainable; however, he self-reported that during this time he was adherent to cART and that he had at least one episode of Pneumocystis jirovecii (formerly carinii) pneumonia (PCP). Eight years later, his cART treatment included tenofovir, lamivudine, and efavirenz. His CD4+ cell count was 41 cells/mm3, and pVL was 638 copies/mm3. While on these medications, his only known complication was oral candida. One and a half years later he stopped cART for a short period and viral rebound ensued (VL > 100,000 copies/mm3), with a corresponding drop in CD4+ cells (CD4+ cells < 20 cells/mm3). After an invasive nonkeratinizing squamous cell anal carcinoma was identified, he resumed cART and his pVL remained nearly undetectable until his death approximately 2 years later. Fifteen months prior to death he was diagnosed with non-Hodgkin's lymphoma and was treated with chemotherapy. After hospitalization for esophageal pain, dysphagia, and fever, he went into hospice care and remained on cART (epzicom, lexiva, norvir, and intravenous [i.v.] foscarnet) until death, a result of diffuse B-cell lymphoma.
Participant 4175 (Fig. 3) was a male with a history of IDU and MSM and who denied any current drug, alcohol, or tobacco use. He was first diagnosed with HIV when he was admitted to the hospital with dyspnea and his CD4+ cell count was 39 cells/mm3. He began taking cART 5 weeks after his initial diagnosis. The participant was also diagnosed with CMV, PCP, and KS (human herpesvirus [HHV-8] positive on biopsy). He was treated with paclitaxel for the KS. Two months later he had a biopsy of a neck mass that was diagnosed as an extracavitary primary effusion lymphoma, and he was treated with EPOCH (etoposide, prednisone, vincritine, cyclophosphamide, and hydroxyldaunorubicin). His course was complicated by immune reconstitution inflammatory syndrome (IRIS), as well as a monoclonal spike on serum protein electrolysis (SPEP), hypothyroidism, asthma, genital herpes, deep vein thrombosis, neutropenia, and thrombocytopenia. Despite cART compliance, the participant's CD4+ cell count remained low. EPOCH chemotherapy continued for several months. Kaposi's sarcoma worsened in his lungs, and he developed a culture-negative pneumonia. Respiratory failure followed and the patient remained on a ventilator for a short period while taking broad-spectrum antibiotics, paclitaxel, and cART until death due to aggressive Kaposi's sarcoma.
Autopsy findings: tissue histology and review of systems.
Histopathology reports revealed numerous tissue abnormalities in tissues from all participants (Table 3); brain, lung, lymph node, spleen, liver, kidney, and aorta tissues were frequently diseased. The overall average number of major tissues with abnormal histology was 7.26 tissues per subject, and the pathologist noted fewer than six abnormal tissues for only one participant (6015). Almost all tissues from participant 4124 demonstrated signs of disease.
TABLE 3.
Histopathological notes and reviews of systemsa
| Subject | Lungs | LNb | Liver | Spleen | Aorta | Kidney | Testis | Heart | Blood vessel | Adrenal | Colon | Stomach | Marrow | Esophagus | Pancreas | Ovary | Muscle | Thyroid |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1010 | X | X | X | X | X | X | X | |||||||||||
| 1156 | X | X | X | X | X | X | ||||||||||||
| 2004 | X | X | X | X | X | X | X | |||||||||||
| 4106 | X | X | X | X | X | X | ||||||||||||
| 4010 | X | X | X | X | X | X | X | X | ||||||||||
| 4013 | X | X | X | X | X | X | X | X | ||||||||||
| 4124 | X | X | X | X | X | X | X | X | X | X | ||||||||
| 4129 | X | X | X | X | X | X | X | X | ||||||||||
| 4130 | X | X | X | X | X | X | X | |||||||||||
| 4143 | X | X | X | X | X | X | X | X | ||||||||||
| 4149 | X | X | X | X | X | X | X | |||||||||||
| 4150 | X | X | X | X | X | X | X | |||||||||||
| 4154 | X | X | X | X | X | X | X | X | X | |||||||||
| 4175 | X | X | X | X | X | X | ||||||||||||
| 4179 | X | X | X | X | X | X | ||||||||||||
| 5024 | X | X | X | X | X | X | X | X | X | |||||||||
| 5025 | X | X | X | X | X | X | X | X | X | |||||||||
| 5095 | X | X | X | X | X | X | X | |||||||||||
| 6015 | X | |||||||||||||||||
| 6083 | X | X | X | X | X | X | X | X | X |
An “X” indicates pathological changes noted in tissues.
LN, lymph nodes.
Clinical notations of pathologies specific to the lungs, lymph nodes, spleen, liver, kidneys, and heart are provided in Table 4. Other organs with pathological findings included the aorta and blood vessels, which in many participants were atherosclerotic. Testes and ovary were atrophied in all cases for which a specimen was available. The adrenals were noted in some cases as exhibiting focal scarring, necrosis, large-cell lymphoma, diffuse large B-cell lymphoma, and metastatic carcinoma. Lower GI tissue was noted in some cases as containing anal condyloma, squamous cell carcinoma, and large cell lymphoma. In the stomach and upper gastrointestinal tract, gastritis, carcinoma and focal ulceration and inflammation were sometimes identified. Additional pathologies included inflammation in the esophagus in participant 4130, lymphoma in the pancreas of participant 4143, diffuse large B-cell lymphoma in the bone marrow of participant 4154, myeloid hyperplasia in the bone marrow of participant 5025, and metastatic carcinoma in the thyroid of participant 5024.
TABLE 4.
Observed pathologies noted in nonbrain tissues
| Pathologies in: | |||||
|---|---|---|---|---|---|
| Lungs | Lymph nodes | Spleen | Liver | Kidneys | Heart |
| Squamous cell carcinoma | Lymphoid hyperplasia | Lymphoid hyperplasia | Metastatic squamous carcinoma | Nephrosclerosis | Coronary atherosclerotic disease |
| Pulmonary congestion and edema | B-cell lymphoma | Inflammation | Hepatic necrosis | Cortical scarring | Myocardial fibrosis |
| Abscesses | Metastatic carcinoma | Congestion | Bile stasis | Glomerular disease | B-cell lymphoma |
| Interstitial pneumonitis | Plasmacytoma | Reduced lymph cells | Centrilobular ischemic change | Renal disease | Metastatic carcinoma |
| Pneumonia | Nodular cirrhosis | Nephro-calcinoma | Ischemic changes | ||
| Diffuse large B-cell lymphoma | Hepatitis | Nephritis | Fibrosis | ||
| Hemorrhage | Steatosis | Bile stasis | Calcification | ||
| Macrophage infiltration | Large cell lymphoma | Autolysis | Luminal narrowing | ||
| Pulmonary congestion | Metastatic carcinoma | Inflammation | |||
| Kaposi's sarcoma | Fatty changes | Large cell lymphoma | |||
| Focal inflammation | Portal fibrosis | B-cell lymphoma | |||
| End-stage degeneration | |||||
Among the many pathologies that were diagnosed during each participant's lifetime, certain underlying pathologies were observed in most participants (Table 5). Cancers were extremely prevalent in the cohort: 14 of the participants were diagnosed with cancer while living, and an additional participant (5095) was identified with renal cell carcinoma, plasmacytoma in lymph nodes, and myeloma upon autopsy. Infections were also common (Fig. 3 to 5 and Table 5). Atherosclerosis was observed at autopsy in numerous participants (Table 5).
TABLE 5.
Patient pathologies at death
| Subject | Tumors | Infection | Atherosclerosis |
|---|---|---|---|
| 1010 | X | X | |
| 1156 | X | X | X |
| 2004 | X | ||
| 4106 | X | X | |
| 4010 | X | ||
| 4013 | X | ||
| 4124 | X | X | |
| 4129 | X | ||
| 4130 | X | ||
| 4143 | X | X | X |
| 4149 | X | X | X |
| 4150 | X | X | |
| 4154 | X | X | |
| 4175 | X | X | X |
| 4179 | X | X | X |
| 5024 | X | ||
| 5025 | X | X | X |
| 5095 | X | X | |
| 6015 | |||
| 6083 | X | X | X |
The brain showed some degree of pathology in all participants (Table 6). Abnormalities ranged from mild gliosis to encephalitis, multiple infarcts, hemorrhage, and cancers. It is of interest to compare the brain pathologies noted at autopsy with the neurological diseases identified in clinical reports (Fig. 3 to 5). For example, participant 5095 was diagnosed with both HIV-associated neurocognitive disorders (HAND) and schizophrenia, and all brain tissues, including spinal cord, exhibited widespread dense leptomeningeal and perivascular polymorphous infiltrate with plasmacytoid features, including multinucleated cells seen in myeloma and neoplastic plasma cell infiltrate. Participant 4124, who had a premortem diagnosis of mild HAND and premortem magnetic resonance imaging (MRI) findings suggestive of HIV encephalitis, exhibited thick blood vessel walls in the brain, perivascular hemosiderin, pink neurons, decreased numbers of Purkinje cells, and a potential early brain infarct. Participant 4013 complained of neurocognitive problems starting in 1997, and although two brain MRI scans from 1997 and mid 1998 were read as normal, in late 1998 a research MRI scan showed diffuse white matter changes and some atrophy. At autopsy, a preterminal subarachnoid hemorrhage was noted along with Alzheimer's type 2 gliosis. The latter refers to a histopathological change in astrocytes characterized by enlarged nuclei, scant cytoplasm, and hyperactive metabolism. Alzheimer's type 2 gliosis (70) is usually in the setting of chronic liver disease with hyperammonemia but can also be seen in other metabolic diseases, such as heavy metal poisoning. Alzheimer's type 2 gliosis is not related to Alzheimer's disease, which is histopathologically characterized by senile plaques and neurofibrillary tangles (71). For reasons that are unknown, and possibly related to comorbid liver dysfunction, Alzheimer type 2 gliosis is a common finding in the NNAB HIV+ cohort. In the subgroup described here, Alzheimer's type 2 gliosis was found in 5 out of 20 brains. However, one elderly participant (5025) did have some histopathological findings suggestive of Alzheimer's disease.
TABLE 6.
CNS pathology at death
| Subject | Pathologies in: |
|
|---|---|---|
| Brain | Spinal cord | |
| 1010 | Alzheimer type 2 gliosis, corpora amylacea | No spinal cord available |
| 1156 | Anoxic-ischemic changes | No significant pathology |
| 2004 | Reactive astrocytes, corpora amylacea, numerous CD68+ cells in white matter | No significant pathology |
| 4106 | Microglial nodule encephalitis, history of neurofibromatosis type 2 with meningioma plaque-like nodule on dura | Neurofibromas |
| 4010 | Focal infarct | No significant pathology |
| 4013 | Alzheimer's type 2 gliosis, subarachnoid hemorrhage due to preterminal event | Focal chronic inflammation of spinal cord and spinal ganglia |
| 4124 | Several areas show thickened blood vessel walls and perivascular hemosiderin. Some areas suspicious for very early infarct. | No significant pathology |
| 4129 | Anoxic-ischemic encephalopathy, Monckeberg medial calcific sclerosis of arteries, possible microinfarcts | No significant pathology |
| 4130 | Focal hemorrhage in anterior basal ganglia with extension into ventricle and subarachnoid space | No significant pathology |
| 4143 | Alzheimer type 2 gliosis | No significant pathology |
| 4149 | Lymphoma | Lymphoma Infiltrate |
| 4150 | Mild acute hypoxic ischemic encephalopathy | No significant pathology |
| 4154 | Focal involvement of leptomeninges by metastatic diffuse B-cell lymphoma | No significant pathology |
| 4175 | No significant pathology, some histologic suggestion of early infarct | No significant pathology |
| 4179 | Aseptic leptomeningitis | Mild focal meningothelial proliferation |
| 5024 | Anoxic ischemic encephalopathy | No significant pathology |
| 5025 | Alzheimer's disease | No significant pathology |
| 5095 | Neoplastic plasma cell infiltrate | Neoplastic plasma cell infiltrate |
| 6015 | Alzheimer type 2 gliosis, multiple cerebral infarcts and/or hemorrhages. Cerebellar Purkinje cell loss and gliosis. | No significant pathology |
| 6083 | Moderate white matter gliosis | No significant pathology |
Presence of HIV. (i) qPCR and ddPCR.
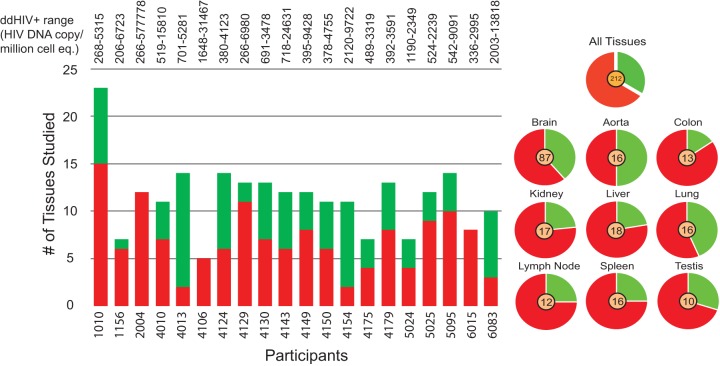
HIV was measured in 229 tissues using both qPCR and ddPCR, including up to 9 nonbrain and 6 brain tissues from each case (see Table S2 in the supplemental material). Only three discordant results (i.e., HIV amplified in one reaction but not the other) were found between the two different HIV DNA quantitation techniques employed, and in each of these cases the HIV copy number was low (<451 copies/million cell equivalents [cp/mi]). Of the 229 tissues tested, HIV DNA was identified in 48/87 brain tissues and 82/142 nonbrain tissues. All participants had at least two HIV+ tissues, and 18 to 100% of the tissues were HIV DNA positive with >200 cp/mi. The low to high HIV DNA percent positivity hierarchy in tissues was aorta (ddPCR range, 266 to 3,861 cp/mi) < lung (ddPCR range, 232 to 5,113 cp/mi) < brain (ddPCR range, 266 to 49,401 cp/mi) < testis (ddPCR range, 268 to 577,778 cp/mi) < lymph node (ddPCR range, 206 to 3,468 cp/mi) <spleen (ddPCR range, 283 to 9,091 cp/mi) < liver (ddPCR range, 518 to 9,722 cp/mi) < kidney (ddPCR range, 416 to 5,315 cp/mi) < colon (ddPCR range, 726 to 23,377 cp/mi) (Fig. 6). The lowest quantifiable HIV copy number measured was in the spleen of participant 4129 (ddPCR = 283 cp/mi), and the highest HIV copy was measured in testis of patient 2004 (ddPCR = 577,778 cp/mi). HIV positivity in the participants was not ubiquitous across their tissue samples; for example, some participants (4130, 4150, and 4010) had a number of HIV+ nonbrain tissues and only one HIV+ brain tissue, whereas participant 4175 had four HIV+ brain tissues and outside the brain, the virus was identified only in his aorta. The finding that all tissues from patient 2004 were HIV+ was interesting, considering our records that indicated high cART adherence until death.
FIG 6.
HIV positivity in cohort autopsy tissues. For each participant, the graph on the left shows the number of tissues assayed along with the number of HIV+ (red) and HIV− (green) tissues identified. On the right, a pie chart is shown for each major tissue assayed for the presence of HIV in the cohort, with the total number of cohort tissues assayed shown in the center.
Results from ddPCR and qPCR were compared to determine any statistical difference between the two techniques. Overall, mean HIV copy numbers for ddPCR and qPCR (5,981.37 and 4,989.47, respectively) were significantly different using a paired t test (P = 0.0130). Due to the consideration that ddPCR is believed to provide improved accuracy (72), we used ddPCR counts to determine their correlation to disease and or factors associated with disease risk (i.e., age or length of known infection) using a paired t test under the assumption that the paired differences were independent and both categories normally distributed. No statistical significance was identified in age category (P = 0.5160) (mean = 52 years; young considered <52 years [mean ddPCR value, 4,481.30; n = 9], old considered ≥52 years [mean ddPCR value, 7,208.71; n = 11]), length of known infection (P = 0.4982) (mean = 10.79 years; short infection considered <10.78 years [mean ddPCR value, 3,904.18; n = 9] and long infection considered ≥10.79 years [mean ddPCR value, 7,880.39; n = 11]), atherosclerosis (P = 0.2860) (mean ddPCR value with atherosclerosis = 7,664.69 [n = 14] mean ddPCR value without atherosclerosis = 2,053.64 [n = 6]), and cancer (P = 0.4336) (mean ddPCR value with tumor = 3,683.15 [n = 15]; mean ddPCR without tumor = 12,876.04 [n = 5]).
(ii) HIV env-nef sequencing and evolutionary analysis.
In order to test if the HIV DNA detected in tissues through ddPCR represented intact HIV genomes, a subset of tissues from five participants was subjected to single-genome sequencing (SGS) of an approximately 3,000-bp region of HIV that contains the HIV env-nef genes. Subsequent to sequencing, extensive genetic analyses were performed to investigate the viral diversity among tissue sites and signatures of replication/evolution. Methods and results from this analysis are presented in detail in reference 54. In brief, env-nef DNA and/or RNA sequences were generated from 54% of the ddPCR HIV+ tissues assayed (n = 19) and at least one tissue for four out of the five participants. The discordance between quantitative PCR and SGS assays is most likely due to the difference in each assay's sensitivity; however, it may be that some tissues contained truncated or defective HIV sequences, which has been previously described for the blood of patients on long-term cART (73, 74).
DISCUSSION
Despite long-term adherence to cART regimens, HIV+ patients continue to harbor replication-competent virus that remains hidden within cellular sanctuary sites (75–77), escaping both immune surveillance and the effects of cART (27, 78). Some of these sites of viral persistence are presently unidentified and prevent complete eradication of the infection. Yet few studies have examined the degree and consequence of HIV infection in anatomical sites other than blood during cART (17, 69, 79, 80), in large part because obtaining tissue samples from living patients is difficult at best. In this study, we collaborated with two NIH-funded tissue banks (NNAB and ACSR) to study over 200 individual postmortem tissues. The efficiency of the study staff involved in minimizing the time between death and tissue collection provided a well-preserved set of characterized tissues, and the extensive records of the NNAB provided comprehensive clinical details for each participant. This unique collection thus allowed us to accurately quantify the amount and anatomical locations of the virus.
Although it has been opined that the viral rebound during structured cART interruption is not harmful, this has been contradicted by the SMART study which demonstrated an increased incidence of opportunistic infection and death in persons who underwent structured treatment interruption compared to those who did not, while not decreasing the adverse effects of antiretroviral therapy (68, 81, 82); however, these and similar studies focused primarily on viral rebound and subsequent viral suppression after cART reinitiation (83). Within this cohort, numerous treatment interruptions, although due to patient adherence and not medically controlled, were recorded and may have contributed to the wide range of subsequent pathologies. Other factors that hypothetically may have contributed to the wide range of pathology included the study inclusion criteria, late diagnosis in some participants, late initiation of treatment in some participants, and concurrent substance use. Clearly, understanding the effects of discontinuous cART on pathology-associated outcomes deserves significantly more research.
Lymphoid tissues are the principal sites of HIV infection and persistence (84, 85) and have been identified as a major site of viral rebound (69). In our cohort, at least four cases existed where we had precise information confirming cART adherence until death, with each case having HIV+ tissues at autopsy. One of these, participant 2004, had quantifiable HIV in every tissue examined and, furthermore, had the tissue with the highest levels of HIV detected out of the entire set of tissues tested (testis; ddPCR value = 577,778 cp/mi). Therefore, we propose that while cART cessation can lead to viral rebound in tissues, it is not the only factor contributing to HIV persistence in anatomical sites. In terms of tissue HIV positivity, it is also important to consider that only a small amount of tissue from each organ was studied, and because HIV presence is not apparently pervasive among tissues in an individual, our results likely greatly underestimate the actual HIV burden in each anatomical site. More broad tissue-based studies such as this could identify the anatomical sites that are the most consistently HIV positive, which would be a great advantage for those studying viral reservoirs.
Many participants were noted to have atherosclerotic vascular disease at autopsy (Table 5). Plaque is macrophage rich and could harbor replication-competent HIV or even protect infected cells from cART targeting (86). In the cases where residual HIV was identified in cancer participants, it may likewise be the case that alternatively activated and anti-inflammatory macrophages, which are linked to certain metastatic processes (87, 88), harbored a low-level HIV infection that migrated to other sites. A recent study elegantly demonstrated that HIV reprograms macrophage migration, resulting in macrophage accumulation in patient tissues, a key step for virus spread and pathogenesis (89). Tumor-associated macrophages express vascular endothelial growth factors (36, 71), and while viral loads in blood and CSF were negative for these participants, an interesting hypothesis is that the lymphatic system, which is more permeable than blood vessels for immune cell migration, may be an alternate migratory pathway for the dispersal of HIV virions or infected immune cells produced by tumors or plaque during cART (87, 90–92). All participants in the study had a degree of brain pathology (Table 6), and most had quantifiable brain HIV DNA at autopsy, suggesting that brain infection or even the damage caused by previous HIV assaults to the brain may significantly contribute to ongoing disease, even the non-brain-associated diseases noted in the cohort. Pre-cART, brain-associated HIV was shown to reinfect the peripheral anatomical sites (93); furthermore, it is well known that many cART regimens fail to penetrate the CNS, and cases of CNS viral escape have been recorded for living patients (66).
A comprehensive understanding of HIV-infected tissue macrophages, to the extent that HIV+ T cell subtypes have been studied, is still needed (26, 78). Previous studies by our group identified unique HIV evolutionary patterns and/or variants that exist in pre-cART patient diseased tissues (94, 95). In these earlier studies, we identified an abundance of HIV recombinants (80), which could arise due to superinfection of long-lived HIV-infected macrophages. Furthermore, Mack et al. (96) found that a large variety of diseased tissues derived from cART-negative autopsy tissues from patients who died with AIDS lymphoma or AIDS dementia contained amplifiable amounts of HIV DNA. In these tissues, p24 antigen staining was localized predominantly to macrophages interspersed in a background of p24-negative lymphocytes; this type of staining was not seen in nondiseased tissues (96). These macrophage p24-rich tissues contained HIV integrated within genetic activation loci. Because persistent macrophage activation is associated with an inhibition of apoptosis, HIV+ macrophages likely have a survival advantage, acting as a continuous source of HIV and serving as a long-term site of viral persistence or viral rebound after cART discontinuation (97). Considering the wide range of macrophage-associated diseases (atherosclerosis, cancer, neurological disease) seen in this particular cohort, therapeutics that target subsets of activated macrophages (98–100) or their associated disease processes by altering host biological pathways is an interesting approach for controlling HIV-associated morbidities and has potential for impacting the brain compartment independent of pVL measurements (101). Additionally, the study raises important questions: Is tissue pathogenesis during HIV infection entirely independent of pVL? What role does host genetics play in the susceptibility to syndromes such as HAND? And, to what extent were the observations in this study due to the weeks or days before death where potentially extensive measures are undertaken to preserve life? Furthermore, while extensive research has focused on the HIV-infected T cell as the primary HIV reservoir, this study suggests that HIV-infected tissue macrophages are an underexplored reservoir during cART.
In summary, after their HIV diagnosis, most participants in this cohort adhered to their prescribed medications over the course of their infection, all developed numerous comorbidities leading to their death, and all were identified as having HIV+ tissues. Tumors and atherosclerosis emerged as major disease processes in this cohort. As a final point, the use of well-defined and documented sources of tissue is likely to advance our knowledge as to how chronic HIV infection may contribute to the disease processes that are currently epidemic in patients on current cART.
Our study combines the attributes of a longitudinal observational study and biobanks with the purpose of supporting diverse, hypothesis-based research projects. Nonetheless, there are acknowledged limitations to our study. First, we depended upon the generous voluntary efforts of our human participants and their caregivers to donate time, data, and tissues. These altruistic individuals may not represent a random or a representative sample of all HIV-infected persons. Due to the focus on tissue collection, the NNAB preferentially selected individuals thought to have serious life-threatening illnesses, which may skew the degree and types of pathology reported in the cohort. Most of our participants did not know the exact date of their HIV infection; we can only measure the time from their HIV diagnosis (which may occur years after a participant first develops symptoms). The participants' ability to successfully access cART and the cART regimens prescribed are subject to the recommendations in force at the time they were diagnosed, as well as the vagaries of the health care system. Many of the participants described herein suffered traumatic medical, psychosocial, and economic adversities during their course, so we were unable to collect a complete set of data at each time point. Some had incomplete personal medical records. Finally, this subset of the entire NNAB cohort was selected in part because their tissues were collected within a relatively short postmortem interval. This is not always feasible, as the participant may expire unexpectedly and at some distance from the study site, there may be the need for a police report, and their families and loved ones deserve a chance to say their last farewells before the decedents remains are removed for dissection. As the vast majority of individuals expire outside normal working hours, harvesting tissues in a timely fashion is labor-intensive and costly. An alternative would be to use an animal model. There are limited models for HIV encephalitis in mice. There is also a primate model that uses simian immunodeficiency virus to induce encephalitis; however, the primates must be sacrificed as soon as they develop AIDS (102), whereas our participants demonstrate the effects of long-term survival with HIV infection treated with cART. There are also a few individuals with HIV who are in generally good health, who die unexpectedly, and whose tissues are harvested. While these cases are very valuable, they tend to lack the detailed neuromedical histories and short PMIs obtained in our cohort, who usually had given premortem consent to donate.
Supplementary Material
ACKNOWLEDGMENTS
S.L.L., D.J.N., R.R., G.B.F., M.S., E.J.S., and M.S.M. were funded by National Institute of Mental Health grant NIH R01 MH100984. E.M., D.L.G., P.B., C.A.S., and M.S.M. were funded by National Cancer Institute grant UM1 CA181255. E.J.S., M.V.S., C.H.H., W.Y., G.M., S.D., N.K., and D.C. were funded by National Institute of Mental Health grant NIMH U24MH100929. S.L.L., D.J.N., and M.S. were funded by National Institute of Neurological Disorders and Stroke grant NS063897.
We are extremely grateful to the NIH funding agencies and to their program officers, as well as to the participants and their caregivers, for providing the resources to support these unique studies.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00674-16.
For a companion article on this topic, see doi:10.1128/JVI.00684-16.
REFERENCES
- 1.Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 2.Fogel GB, Lamers SL, Levine AJ, Valdes-Sueiras M, McGrath MS, Shapshak P, Singer EJ. 2015. Factors related to HIV-associated neurocognitive impairment differ with age. J Neurovirol 21:56–65. doi: 10.1007/s13365-014-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeken JF, Tjen ALA, Rudek MA, Okuliar C, Young M, Little RF, Dezube BJ. 2012. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis 55:1228–1235. doi: 10.1093/cid/cis613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurz M, Burkhalter F, Dickenmann M, Hopfer H, Mayr M, Elzi L, Battegay M. 2015. Acute kidney injury KDIGO stage 2 to 3 in HIV-positive patients treated with cART–a case series over 11 years in a cohort of 1,153 patients. Swiss Med Wkly 145:w14135. [DOI] [PubMed] [Google Scholar]
- 5.Nanavati KA, Fisher SD, Miller TL, Lipshultz SE. 2004. HIV-related cardiovascular disease and drug interactions. Am J Cardiovasc Drugs 4:315–324. doi: 10.2165/00129784-200404050-00004. [DOI] [PubMed] [Google Scholar]
- 6.Grint D, Peters L, Rockstroh JK, Rakmanova A, Trofimova T, Lacombe K, Karpov I, Galli M, Domingo P, Kirk O, Lundgren JD, Mocroft A. 2015. Liver-related death among HIV/hepatitis C virus-co-infected individuals: implications for the era of directly acting antivirals. AIDS 29:1205–1215. doi: 10.1097/QAD.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 7.Samaras K. 2009. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy. J Acquir Immune Defic Syndr 50:499–505. doi: 10.1097/QAI.0b013e31819c291b. [DOI] [PubMed] [Google Scholar]
- 8.Manfredi R, Calza L, Marinacci G, Cascavilla A, Colangeli V, Salvadori C, Martelli G, Appolloni L, Puggioli C, Viale P. 2015. A prospective evaluation of maraviroc administration in patients with advanced HIV disease and multiple comorbidities: focus on efficacy and tolerability issues. Infez Med 23:36–43. [PubMed] [Google Scholar]
- 9.Wang T, Yi R, Green LA, Chelvanambi S, Seimetz M, Clauss M. 9 July 2015. Increased cardiovascular disease risk in the HIV-positive population on ART: potential role of HIV-Nef and Tat. Cardiovasc Pathol doi: 10.1016/j.carpath.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeni PG, Hammer SM, Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, Hirsch MS, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Saag MS, Schechter M, Schooley RT, Thompson MA, Vella S, Volberding PA. 2002. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA Panel. JAMA 288:222–235. doi: 10.1001/jama.288.2.222. [DOI] [PubMed] [Google Scholar]
- 11.Kerr ZY, Miller KR, Galos D, Love R, Poole C. 2013. Challenges, coping strategies, and recommendations related to the HIV services field in the HAART era: a systematic literature review of qualitative studies from the United States and Canada. AIDS Patient Care STDS 27:85–95. doi: 10.1089/apc.2012.0356. [DOI] [PubMed] [Google Scholar]
- 12.Cesarman E. 2013. Pathology of lymphoma in HIV. Curr Opin Oncol 25:487–494. doi: 10.1097/01.cco.0000432525.70099.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong SJ, Song JE, Kim SB, Kim HW, Ku NS, Han SH, Choi JY, Song YG, Cha BS, Kim JM. 2013. Plasma klotho levels were inversely associated with subclinical carotid atherosclerosis in HIV-infected patients receiving combined antiretroviral therapy. AIDS Res Hum Retroviruses 29:1575–1581. doi: 10.1089/aid.2013.0048. [DOI] [PubMed] [Google Scholar]
- 14.Brew BJ, Chan P. 2014. Update on HIV dementia and HIV-associated neurocognitive disorders. Curr Neurol Neurosci Rep 14:468. doi: 10.1007/s11910-014-0468-2. [DOI] [PubMed] [Google Scholar]
- 15.Herndier BG, Kaplan LD, McGrath MS. 1994. Pathogenesis of AIDS lymphomas. AIDS 8:1025–1049. doi: 10.1097/00002030-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Shan L, Siliciano RF. 2013. From reactivation of latent HIV-1 to elimination of the latent reservoir: the presence of multiple barriers to viral eradication. Bioessays 35:544–552. doi: 10.1002/bies.201200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 18.Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. 1995. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med 1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 19.Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho DD. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 20.Redel L, Le Douce V, Cherrier T, Marban C, Janossy A, Aunis D, Van Lint C, Rohr O, Schwartz C. 2010. HIV-1 regulation of latency in the monocyte-macrophage lineage and in CD4+ T lymphocytes. J Leukoc Biol 87:575–588. doi: 10.1189/jlb.0409264. [DOI] [PubMed] [Google Scholar]
- 21.Battistini A, Sgarbanti M. 2014. HIV-1 latency: an update of molecular mechanisms and therapeutic strategies. Viruses 6:1715–1758. doi: 10.3390/v6041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 23.Svicher V, Ceccherini-Silberstein F, Antinori A, Aquaro S, Perno CF. 2014. Understanding HIV compartments and reservoirs. Curr HIV/AIDS Rep 11:186–194. doi: 10.1007/s11904-014-0207-y. [DOI] [PubMed] [Google Scholar]
- 24.Salemi M, Rife B. 2016. Phylogenetics and phyloanatomy of HIV/SIV intra-host compartments and reservoirs: the key role of the central nervous system. Curr HIV Res 14:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O'Brien PE, Harrison LC. 2010. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes 59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell JH, Hearps AC, Martin GE, Williams KC, Crowe SM. 2014. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS 28:2175–2187. doi: 10.1097/QAD.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Churchill M, Nath A. 2013. Where does HIV hide? A focus on the central nervous system. Curr Opin HIV AIDS 8:165–169. doi: 10.1097/COH.0b013e32835fc601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman CM, Wu L. 2009. HIV interactions with monocytes and dendritic cells: viral latency and reservoirs. Retrovirology 6:51. doi: 10.1186/1742-4690-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinman R, Hoffman L, Pope M. 1995. Maturation and migration of cutaneous dendritic cells. J Invest Dermatol 105:2S–7S. [DOI] [PubMed] [Google Scholar]
- 30.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. 2011. A human memory T cell subset with stem cell-like properties. Nat Med 17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, Bondanza A, Bordignon C, Peccatori J, Ciceri F, Lupo-Stanghellini MT, Mavilio F, Mondino A, Bicciato S, Recchia A, Bonini C. 2013. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 121:573–584. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 32.Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, Pereyra F, Zurakowski R, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M. 2014. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med 20:139–142. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A, Abbas W, Herbein G. 2014. HIV-1 latency in monocytes/macrophages. Viruses 6:1837–1860. doi: 10.3390/v6041837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilareski EM, Shah S, Nonnemacher MR, Wigdahl B. 2009. Regulation of HIV-1 transcription in cells of the monocyte-macrophage lineage. Retrovirology 6:118. doi: 10.1186/1742-4690-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mir KD, Mavigner M, Silvestri G. 2012. The myeloid cytokine network in AIDS pathogenesis. Cytokine Growth Factor Rev 23:223–231. doi: 10.1016/j.cytogfr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Oszacki J, Lenczyk M, Lominska K. 1952. Quantitative changes in actidophiles and blood platelets in circulating blood in surgical trauma. Przegl Lek 8:304–308. (In German.) [PubMed] [Google Scholar]
- 37.Herbein G, Varin A. 2010. The macrophage in HIV-1 infection: from activation to deactivation? Retrovirology 7:33. doi: 10.1186/1742-4690-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crowe SM, Westhorpe CL, Mukhamedova N, Jaworowski A, Sviridov D, Bukrinsky M. 2010. The macrophage: the intersection between HIV infection and atherosclerosis. J Leukoc Biol 87:589–598. doi: 10.1189/jlb.0809580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallet MA, Wallet SM, Guiulfo G, Sleasman JW, Goodenow MM. 2010. IFNgamma primes macrophages for inflammatory activation by high molecular weight hyaluronan. Cell Immunol 262:84–88. doi: 10.1016/j.cellimm.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. 2001. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med 193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strain MC, Letendre S, Pillai SK, Russell T, Ignacio CC, Gunthard HF, Good B, Smith DM, Wolinsky SM, Furtado M, Marquie-Beck J, Durelle J, Grant I, Richman DD, Marcotte T, McCutchan JA, Ellis RJ, Wong JK. 2005. Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. J Virol 79:1772–1788. doi: 10.1128/JVI.79.3.1772-1788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smit TK, Brew BJ, Tourtellotte W, Morgello S, Gelman BB, Saksena NK. 2004. Independent evolution of human immunodeficiency virus (HIV) drug resistance mutations in diverse areas of the brain in HIV-infected patients, with and without dementia, on antiretroviral treatment. J Virol 78:10133–10148. doi: 10.1128/JVI.78.18.10133-10148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salemi M, Lamers SL, Yu S, de Oliveira T, Fitch WM, McGrath MS. 2005. Phylodynamic analysis of human immunodeficiency virus type 1 in distinct brain compartments provides a model for the neuropathogenesis of AIDS. J Virol 79:11343–11352. doi: 10.1128/JVI.79.17.11343-11352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamers SL, Salemi M, Galligan DC, Morris A, Gray R, Fogel G, Zhao L, McGrath MS. 2010. Human immunodeficiency virus-1 evolutionary patterns associated with pathogenic processes in the brain. J Neurovirol 16:230–241. doi: 10.3109/13550281003735709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnell G, Spudich S, Harrington P, Price RW, Swanstrom R. 2009. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS Pathog 5:e1000395. doi: 10.1371/journal.ppat.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schnell G, Price RW, Swanstrom R, Spudich S. 2010. Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J Virol 84:2395–2407. doi: 10.1128/JVI.01863-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pillai SK, Pond SL, Liu Y, Good BM, Strain MC, Ellis RJ, Letendre S, Smith DM, Gunthard HF, Grant I, Marcotte TD, McCutchan JA, Richman DD, Wong JK. 2006. Genetic attributes of cerebrospinal fluid-derived HIV-1 env. Brain 129:1872–1883. doi: 10.1093/brain/awl136. [DOI] [PubMed] [Google Scholar]
- 48.Dunfee RL, Thomas ER, Gorry PR, Wang J, Taylor J, Kunstman K, Wolinsky SM, Gabuzda D. 2006. The HIV Env variant N283 enhances macrophage tropism and is associated with brain infection and dementia. Proc Natl Acad Sci U S A 103:15160–15165. doi: 10.1073/pnas.0605513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Falcone EL, Adegbulugbe AA, Sheikh V, Imamichi H, Dewar RL, Hammoud DA, Sereti I, Lane HC. 2013. Cerebrospinal fluid HIV-1 compartmentalization in a patient with AIDS and acute varicella-zoster virus meningomyeloradiculitis. Clin Infect Dis 57:e135–e142. doi: 10.1093/cid/cit356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohagen A, Devitt A, Kunstman KJ, Gorry PR, Rose PP, Korber B, Taylor J, Levy R, Murphy RL, Wolinsky SM, Gabuzda D. 2003. Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. J Virol 77:12336–12345. doi: 10.1128/JVI.77.22.12336-12345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R. 2011. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog 7:e1002286. doi: 10.1371/journal.ppat.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harrington PR, Schnell G, Letendre SL, Ritola K, Robertson K, Hall C, Burch CL, Jabara CB, Moore DT, Ellis RJ, Price RW, Swanstrom R. 2009. Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. AIDS 23:907–915. doi: 10.1097/QAD.0b013e3283299129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sturdevant CB, Joseph SB, Schnell G, Price RW, Swanstrom R, Spudich S. 2015. Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog 11:e1004720. doi: 10.1371/journal.ppat.1004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rose R, Lamers SL, Nolan DJ, Maidji E, Faria NR, Pybus OG, Dollar JJ, Maruniak SA, Mcavoy AC, Salemi M, Stoddart C, Singer EJ, Mcgrath MS. 2016. HIV maintains an evolving and dispersed population in multiple tissues during suppressive combined antiretroviral therapy in individuals with cancer. J Virol 90:8984–8993. doi: 10.1128/JVI.00684-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morgello S, Gelman BB, Kozlowski PB, Vinters HV, Masliah E, Cornford M, Cavert W, Marra C, Grant I, Singer EJ. 2001. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol 27:326–335. doi: 10.1046/j.0305-1846.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 56.Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Hinkin CH, Lazzaretto D, Cherner M, Marcotte TD, Gelman BB, Morgello S, Singer EJ, Grant I, Heaton RK. 2004. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 57.Morgello S, Holzer CE III, Ryan E, Young C, Naseer M, Castellon SA, Frol AB, Atkinson JH, Gelman BB, Grant I, Singer EJ. 2006. Interrater reliability of the Psychiatric Research Interview for Substance and Mental Disorders in an HIV-infected cohort: experience of the National NeuroAIDS Tissue Consortium. Int J Methods Psychiatr Res 15:131–138. doi: 10.1002/mpr.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.American Psychiatric Association. 1994. Diagnostic and statistical manual for mental disorders, 4th ed American Psychiatric Association, Arlington, VA. [Google Scholar]
- 59.Rubio-Stipec M, Bravo M, Canino G. 1991. The Composite International Diagnostic Interview (CIDI): an epidemiologic instrument suitable for using in conjunction with different diagnostic systems in different cultures. Acta Psiquiatr Psicol Am Lat 37:191–204. (In Spanish.) [PubMed] [Google Scholar]
- 60.Kessler RC, Ustun TB. 2004. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res 13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. 1996. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry 153:1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- 62.Beck AT, Steer RA, Ball R, Ranieri W. 1996. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 63.Umbel VM, Pearson BZ, Fernandez MC, Oller DK. 1992. Measuring bilingual children's receptive vocabularies. Child Dev 63:1012–1020. doi: 10.2307/1131250. [DOI] [PubMed] [Google Scholar]
- 64.Levine AJ, Palomo M, Hinkin CH, Valdes-Sueiras M, Lopez E, Mathisen G, Donovan S, Singer EJ. 2011. A comparison of screening batteries in the detection of neurocognitive impairment in HIV-infected Spanish speakers. Neurobehav HIV Med 3:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Budka H, Wiley CA, Kleihues P, Artigas J, Asbury AK, Cho ES, Cornblath DR, Dal Canto MC, DeGirolami U, Dickson D, Epstein LG, Esiri MM, Giangaspero F, Gosztonyi G, Gray F, Griffin JW, Henin D, Iwasaki Y, Janssen RS, Johnson RT, Lantos PL, Lyman WD, McArthur JC, Nagashima K, Peress N, Petito CK, Price RW, Rhodes RH, Rosenblum M, Said G, Scaravilli F, Sharer LR, Vinters HV. 1991. HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol 1:143–152. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 66.Edén A, Fuchs D, Hagberg L, Nilsson S, Spudich S, Svennerholm B, Price RW, Gisslen M. 2010. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis 202:1819–1825. doi: 10.1086/657342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brumme ZL, Dong WW, Yip B, Wynhoven B, Hoffman NG, Swanstrom R, Jensen MA, Mullins JI, Hogg RS, Montaner JS, Harrigan PR. 2004. Clinical and immunological impact of HIV envelope V3 sequence variation after starting initial triple antiretroviral therapy. AIDS 18:F1–F9. doi: 10.1097/00002030-200403050-00001. [DOI] [PubMed] [Google Scholar]
- 68.Neumann AU, Tubiana R, Calvez V, Robert C, Li TS, Agut H, Autran B, Katlama C. 1999. HIV-1 rebound during interruption of highly active antiretroviral therapy has no deleterious effect on reinitiated treatment. Comet Study Group. AIDS 13:677–683. [DOI] [PubMed] [Google Scholar]
- 69.Rothenberger MK, Keele BF, Wietgrefe SW, Fletcher CV, Beilman GJ, Chipman JG, Khoruts A, Estes JD, Anderson J, Callisto SP, Schmidt TE, Thorkelson A, Reilly C, Perkey K, Reimann TG, Utay NS, Nganou Makamdop K, Stevenson M, Douek DC, Haase AT, Schacker TW. 2015. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci U S A 112:E1126–E1134. doi: 10.1073/pnas.1414926112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vinters HV, Kleinschmidt-DeMasters BK. 2008. General pathology of the central nervous system, p 1–56. In Love S, Louis DN, Ellison DW (ed), Greenfield's neuropathology, 8th ed CRC Press, Boca Raton, FL. [Google Scholar]
- 71.Perl DP. 2010. Neuropathology of Alzheimer's disease. Mt Sinai J Med 77:32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strain MC, Lada SM, Luong T, Rought SE, Gianella S, Terry VH, Spina CA, Woelk CH, Richman DD. 2013. Highly precise measurement of HIV DNA by droplet digital PCR. PLoS One 8:e55943. doi: 10.1371/journal.pone.0055943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Temin HM. 1993. Retrovirus variation and reverse transcription: abnormal strand transfers result in retrovirus genetic variation. Proc Natl Acad Sci U S A 90:6900–6903. doi: 10.1073/pnas.90.15.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF. 2013. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abbas W, Herbein G. 2012. Molecular understanding of HIV-1 latency. Adv Virol 2012:574967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alexaki A, Liu Y, Wigdahl B. 2008. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res 6:388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blankson JN, Persaud D, Siliciano RF. 2002. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med 53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- 78.Abbas W, Tariq M, Iqbal M, Kumar A, Herbein G. 2015. Eradication of HIV-1 from the macrophage reservoir: an uncertain goal? Viruses 7:1578–1598. doi: 10.3390/v7041578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aquaro S, Calio R, Balestra E, Bagnarelli P, Cenci A, Bertoli A, Tavazzi B, Di Pierro D, Francesconi M, Abdelahad D, Perno CF. 1998. Clinical implications of HIV dynamics and drug resistance in macrophages. J Biol Regul Homeost Agents 12:23–27. [PubMed] [Google Scholar]
- 80.Lamers SL, Salemi M, Galligan DC, de Oliveira T, Fogel GB, Granier SC, Zhao L, Brown JN, Morris A, Masliah E, McGrath MS. 2009. Extensive HIV-1 intra-host recombination is common in tissues with abnormal histopathology. PLoS One 4:e5065. doi: 10.1371/journal.pone.0005065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sued O, Ambrosioni J, Nicolas D, Manzardo C, Aguero F, Claramonte X, Plana M, Tuset M, Pumarola T, Gallart T, Gatell JM, Miro JM. 2015. Structured treatment interruptions and low doses of IL-2 in patients with primary HIV infection. Inflammatory, virological and immunological outcomes. PLoS One 10:e0131651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lundgren JD, Babiker A, El-Sadr W, Emery S, Grund B, Neaton JD, Neuhaus J, Phillips AN. 2008. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ cell counts and HIV RNA levels during follow-up. J Infect Dis 197:1145–1155. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- 83.Pai NP, Lawrence J, Reingold AL, Tulsky JP. 2006. Structured treatment interruptions (STI) in chronic unsuppressed HIV infection in adults. Cochrane Database Syst Rev 2006:CD006148. doi: 10.1002/14651858.CD006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schacker T, Little S, Connick E, Gebhard-Mitchell K, Zhang ZQ, Krieger J, Pryor J, Havlir D, Wong JK, Richman D, Corey L, Haase AT. 2000. Rapid accumulation of human immunodeficiency virus (HIV) in lymphatic tissue reservoirs during acute and early HIV infection: implications for timing of antiretroviral therapy. J Infect Dis 181:354–357. doi: 10.1086/315178. [DOI] [PubMed] [Google Scholar]
- 85.Reinhart TA, Rogan MJ, Huddleston D, Rausch DM, Eiden LE, Haase AT. 1997. Simian immunodeficiency virus burden in tissues and cellular compartments during clinical latency and AIDS. J Infect Dis 176:1198–1208. doi: 10.1086/514113. [DOI] [PubMed] [Google Scholar]
- 86.Lamers SL, Fogel GB, Singer EJ, Salemi M, Nolan DJ, Huysentruyt LC, McGrath MS. 2012. HIV-1 Nef in macrophage-mediated disease pathogenesis. Int Rev Immunol 31:432–450. doi: 10.3109/08830185.2012.737073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. 2002. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol 161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McGrath MS. 1996. T-cells and macrophages in HIV disease. Clin Rev Allergy Immunol 14:359–366. [DOI] [PubMed] [Google Scholar]
- 89.Vérollet C, Souriant S, Bonnaud E, Jolicoeur P, Raynaud-Messina B, Kinnaer C, Fourquaux I, Imle A, Benichou S, Fackler OT, Poincloux R, Maridonneau-Parini I. 2015. HIV-1 reprograms the migration of macrophages. Blood 125:1611–1622. doi: 10.1182/blood-2014-08-596775. [DOI] [PubMed] [Google Scholar]
- 90.Hong YK, Foreman K, Shin JW, Hirakawa S, Curry CL, Sage DR, Libermann T, Dezube BJ, Fingeroth JD, Detmar M. 2004. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet 36:683–685. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- 91.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. 2007. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lamers SL, Rose R, Ndhlovu LC, Nolan DJ, Salemi M, Maidji E, Stoddart CA, McGrath MS. 16 November 2015. The meningeal lymphatic system: a route for HIV brain migration? J Neurovirol doi: 10.1007/s13365-015-0399-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lamers SL, Gray RR, Salemi M, Huysentruyt LC, McGrath MS. 2011. HIV-1 phylogenetic analysis shows HIV-1 transits through the meninges to brain and peripheral tissues. Infect Genet Evol 11:31–37. doi: 10.1016/j.meegid.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salemi M, Lamers SL, Huysentruyt LC, Galligan D, Gray RR, Morris A, McGrath MS. 2009. Distinct patterns of HIV-1 evolution within metastatic tissues in patients with non-Hodgkins lymphoma. PLoS One 4:e8153. doi: 10.1371/journal.pone.0008153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirszfeldowa H, Lomska J. 1952. Further studies on uroprecipitation and on the other serological reactions in rheumatism. Med Dosw Mikrobiol 4:350–351. (In Polish.) [PubMed] [Google Scholar]
- 96.Mack KD, Jin X, Yu S, Wei R, Kapp L, Green C, Herndier B, Abbey NW, Elbaggari A, Liu Y, McGrath MS. 2003. HIV insertions within and proximal to host cell genes are a common finding in tissues containing high levels of HIV DNA and macrophage-associated p24 antigen expression. J Acquir Immune Defic Syndr 33:308–320. doi: 10.1097/00126334-200307010-00004. [DOI] [PubMed] [Google Scholar]
- 97.Swingler S, Mann AM, Zhou J, Swingler C, Stevenson M. 2007. Apoptotic killing of HIV-1-infected macrophages is subverted by the viral envelope glycoprotein. PLoS Pathog 3:1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jin X, McGrath MS, Xu H. 29 July 2015. Inhibition of HIV expression and integration in macrophages by methylglyoxal-bis-guanylhydrazone. J Virol doi: 10.1128/JVI.01692-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rizzo J, Levine AM, Weiss GR, Pearce T, Kraynak M, Mueck R, Smith S, Von Hoff DD, Kuhn JG. 1996. Pharmacokinetic profile of Mitoguazone (MGBG) in patients with AIDS related non-Hodgkin's lymphoma. Invest New Drugs 14:227–234. doi: 10.1007/BF00210796. [DOI] [PubMed] [Google Scholar]
- 100.Haile WB, Gavegnano C, Tao S, Jiang Y, Schinazi RF, Tyor WR. 2016. The Janus kinase inhibitor ruxolitinib reduces HIV replication in human macrophages and ameliorates HIV encephalitis in a murine model. Neurobiol Dis doi: 10.1016/j.nbd.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lamers SL, Fogel GB, Nolan DJ, McGrath MS, Salemi M. 2014. HIV-associated neuropathogenesis: a systems biology perspective for modeling and therapy. Biosystems 119:53–61. doi: 10.1016/j.biosystems.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.