Abstract
Multiple myeloma is a form of plasma cell neoplasm that accounts for approximately 10% of all hematological malignancies. Recently, several novel drugs have been discovered that almost doubled the overall survival of multiple myeloma patients. One of these drugs, the first-in-class proteasome inhibitor bortezomib (Velcade) has demonstrated remarkable response rates in multiple myeloma patients, and yet, currently this disease remains incurable. The major factor undermining the success of multiple myeloma treatment is a rapidly emerging resistance to the available therapy. Thus, the development of stand-alone or adjuvant anti-myeloma agents becomes of paramount importance. Overproduction of intracellular reactive oxygen species (ROS) often accompanies malignant transformation due to oncogene activation and/or enhanced metabolism in tumor cells. As a result, these cells possess higher levels of ROS and lower levels of antioxidant molecules compared to their normal counterparts. Unbalanced production of ROS leads to oxidative stress which, if left unchecked, could be toxic for the cell. In multiple myeloma cells where high rates of immunoglobulin synthesis is an additional factor contributing to overproduction of ROS, further induction of oxidative stress can be an effective strategy to cope with this disease. Here we will review the available data on the role of oxidative stress in the cytotoxicity of proteasome inhibitors and the use of ROS-inducing compounds as anti-myeloma agents.
Graphical abstract
1. Multiple Myeloma
Multiple myeloma arises from oncogenic transformation of plasma cells, a type of white blood cell normally responsible for producing antibodies [1,2]. Multiple myeloma cells accumulate in the bone marrow where they interfere with the production of normal blood cells. The progression of multiple myeloma can be subdivided in stages, which are characterized by the proliferation of a single clone of plasma cells producing homogenous monoclonal (M) protein, also known as abnormal immunoglobulin, and is often assessed by measuring the amount of M protein in the serum [2-4]. The first stage, monoclonal gammopathy of undetermined significance (MGUS), is characterized by less than 30g/l of M protein in the serum or less than 10% plasma cells in the bone marrow and the absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia or bone lesions [3,4]. Smouldering multiple myeloma (SMM), the next stage of the disease, is characterized by a M protein serum concentration of 30g/l or greater and/or 10% or more plasma cells in the bone marrow with no end-organ damage and is thought to precede multiple myeloma [2,5]. Progression to multiple myeloma from SMM occurs in approximately 10% cases a year vs progression to multiple myeloma from MGUS which accounts for approximately 1% of patients annually. Multiple myeloma is characterized by serum concentration of M protein of 30g/l or greater and/or 10% or more plasma cells in the bone marrow accompanied by end-organ damage, which is the indicator for treatment [2].
Plasma cells have the distinct ability to secret antibodies and therefore are committed to massive Ig synthesis, assembly and secretion [6]. In fact, due to this reason, even non-transformed plasma cells undergo substantial endoplasmic and oxidative stresses that are usually associated with the higher rates of protein biosynthesis and secretion [7]. The oncogenic transformation of plasma cells results in even higher protein synthesis rates ultimately leading to exacerbation of the ER stress and oxidative stress [8].
2. Oxidative Stress
Oxidative stress emerges as a result of the excessive production of reactive oxygen species (ROS) or their insufficient elimination by antioxidants [9,10]. The major sources of ROS in the cell include mitochondria (~80%), peroxisomes (where fatty acid oxidation takes part) and endoplasmic reticulum (where the oxidative protein folding occurs [11-13]). ROS detoxification in the cell involves 2 major systems: glutathione (GSH) and thioredoxin (TRX) [14,15]. The GSH system consists of GSH, glutathione peroxidases (GPX) and glutathione s-transferases (GST). GSH, GPX and GST contribute to ROS detoxification by reducing hydrogen peroxides or hydroperoxides using GSH as a substrate [14]. The TXN system includes TXN, thioredoxin reductases (TXNRD) and NADPH [15,16], and functions via reduction of the intracellular disulfides and direct quenching of ROS [16]. ROS detoxification can also occur through superoxide dismutases (SODs) that metabolize superoxide anion (O2−) into oxygen and hydrogen peroxide, and catalases converting hydrogen peroxide into oxygen and water [17].
Cancer cells produce higher amounts of ROS than normal cells due to oncogene activation and/or increased metabolic activity [17-19]. In these cells, elevation of ROS levels has been shown to promote proliferation and enhance motility and invasion [18,19]. Reciprocally, antioxidants have been for a long time considered as tumor suppressing agents [20-24]. However, recently the opposite functions of antioxidants have been reported. Antioxidant N-acetylcysteine (NAC) promoted tumor progression in mouse models of oncogene-induced lung carcinogenesis [20] and increased lymph node metastasis in mouse model of melanoma [25]. Similarly, metastasizing melanoma cells have been shown to undergo metabolic changes leading to resistance to oxidative stress [26], Furthermore, recently it became apparent that tumors utilize several mechanisms of oxidative stress suppression, [27,28] the most common of which is upregulation of NRF2, a key transcriptional activator of antioxidant genes [29,30-32].
Accordingly, overproduction of ROS was identified as an inhibitor of tumor cell proliferation, viability and various transformed phenotypes [20-24;33-36]. The latter effects of ROS caused significant attention to the mechanisms regulating their production and utilization in cancer cells [35,36], and the potential exploitation of these mechanisms in novel antineoplastic agents [37]. As a result, a number of anticancer drugs that directly induce oxidative stress have been approved for treatment of several types of malignancies [37]. However none of these agents are being routinely used for treatment of multiple myeloma.
2.1. Modulators of Oxidative Stress in Multiple Myeloma Patients
The association of multiple myeloma progression with the depletion of antioxidants and the increase of pro-oxidant molecules in patient serum has been described in several reports [38-42]. Estimated serum levels of antioxidants such as superoxide dismutase (SOD1), glutathione peroxidase, catalase and vitamins C and E were lower, whereas serum levels of oxidative stress markers (including malondialdehyd and advanced oxidation protein products) were higher in multiple myeloma patients compared to healthy individuals [38-42]. Accordingly, activities of antioxidants preventing lipid peroxidation such as paraoxonase (PON1) and arylesterase (ARE) were lower in multiple myeloma patient sera [43,44]. Levels of 8-isoprostane, a product of fatty acid peroxidation, were on the contrary higher in multiple myeloma patients [43]. Moreover, decreased activities of ARE and PON1 were proposed as poor prognosis markers for a subgroup of multiple myeloma patients [44]. Additionally, serum levels of scavenger receptor class A member 3 (SCARA3), a protein protecting cells from oxidative stress, inversely correlated with myeloma progression in patients [45]. However, the molecular mechanisms underlying enhanced myelomagenesis under conditions of elevated oxidative stress in patient serum have not been reported.
Only a handful of papers describe genetic events occurring in multiple myeloma cells in the course of its progression that primarily affect intracellular redox status. Thus, overexpression of ACA11, an orphan box H/ACA class small nucleolar RNA (snoRNA) [46] was detected in cells from multiple myeloma patients as a result of t(4;14) chromosomal translocation [46]. Importantly, ACA11 was shown to inhibit oxidative stress in cultured multiple myeloma cells [46]. Additionally, higher SOD1 mRNA levels or epigenetic silencing of glutathione peroxidase 3 gene (GPX3, sensitizes cells to oxidative stress) associated with multiple myeloma progression and poor prognosis [47,48].
2.2. The Role of Oxidative Stress in Cytotoxicity of Proteasome Inhibitors
Discovery of the proteasome inhibitor bortezomib (BTZ) (Velcade) has dramatically improved the overall survival of multiple myeloma patients when used as a single agent or in combination with conventional drugs such as dexamethasone [49,50]. Recently, two more proteasome inhibitors have been approved for treatment of relapsed multiple myelomas including those refractory to BTZ treatment: carfilzomib (CFZ) (Kyprolis) [51] and ixazomib (Ninlaro) [52]. These proteasome inhibitors function via reversible (BTZ, IXZ) or irreversible (CFZ) inhibition of the 20S subunit of the proteasome [49-52].
Proteasome inhibition results in accumulation of unfolded proteins, which trigger endoplasmic reticulum (ER) stress [53,54]. An unresolved ER stress, which is considered a major mediator of the cytotoxicity of proteasome inhibitors, has been shown to cause cell death via multiple pathways including overproduction of reactive oxygen species (ROS) [53,54]. Indeed, oxidative stress has been identified as an important mechanism of BTZ cytotoxicity in myeloma and non-myeloma cells [55,57,58]. For example, ROS generation has been shown to precede the initiation of BTZ-induced apoptotic cascade [59,60]. Furthermore, co-treatment with the antioxidant tiron rescued from BTZ-induced ROS generation and cell death [61,62]. In mantle cell lymphoma patients, high basal antioxidant activity was associated with reduced responsiveness and a worse outcome following BTZ treatment [63].
Oxidative stress resulting from proteasome inhibition has been long considered a byproduct of the ER stress [56]. The enzymatic components involved in ER stress-dependent ROS production include protein disulfide isomerase (PDI) [64], endoplasmic reticulum oxidoreductin (ERO-1) [64], NADPH oxidase complexes [65], and mitochondrial electron transport enzymes [65].
Under normal conditions, ERO-1 and PDI catalyze protein folding by oxidizing the cysteine residues of nascent proteins in order to form correct disulfide bonds [66]. ERO-1 uses a (FAD)-dependent reaction to transfer electrons from PDI to molecular oxygen (O2) [67,68]. Incomplete reduction of oxygen results in the generation of ROS and ER protein-folding induced-oxidative stress [64,65]. Under conditions of ER stress, the activity of these enzymes is increased due to an elevated burden of unfolded proteins, thus higher amounts of ROS are produced as a byproduct, increasing oxidative stress.
Depletion of the glutathione (GSH) buffer system is an additional way by which oxidative stress is increased during times of ER stress [69]. GSH is used in reducing incorrect disulfide bonds, thus the presence of misfolded proteins decreases the GSH/GSSG ratio, altering the redox environment in the ER [70].
ROS produced as a byproduct of protein folding contribute only partially to the generation of oxidative stress during ER stress. Systems involved in calcium homeostasis make up another important component of ROS generation. Thus, oxidative stress can be mediated by Ca2+ leakage from the ER into the cytosol [71]. Ca2+ influences ROS production by the mitochondria via stimulation of the tricarboxylic acid cycle and oxidative phosphorylation, resulting in greater O2 consumption [71-73]. Ca2+ leakage from the ER also results in cytochrome c release from the mitochondria impairing the electron transport system and resulting in altered mitochondrial membrane potential, and as a result, production of ROS [71-73].
In addition, oxidative stress is generated as a by-product of increased mitochondrial respiration, because high levels of ATP are needed to sustain the protein folding machinery, which is a highly ATP-dependent process [74].
There is mounting evidence suggesting that NADPH oxidase complexes (NOX) are important sources of ROS during ER stress [75-79]. The NOX family of enzymes uses oxygen to mediate electron transfer via NADPH, releasing O2− as a by-product [75]. NOX2 [76] and NOX4 [75,77-79] are the only 2 isoforms that have so far been reported to contribute to ER stress. NOX2 causes a CHOP-mediated apoptosis in response to ER stress, and NOX2 deficiency protects mice from tunicamycin-dependent induction of apoptosis in renal cell [76]. NOX4 is induced in human vascular smooth cells during ER stress caused by the oxygenated lipid product, 7-ketocholesterol or tunicamycin [77]. Furthermore, NOX4-associated ROS alters UPR signaling and promotes the activation of RAS [79]. It is noteworthy that in addition to being a byproduct of ER stress, ROS may actually induce ER stress [76,77].
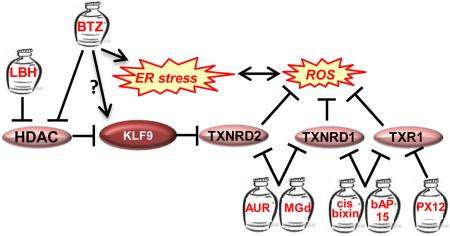
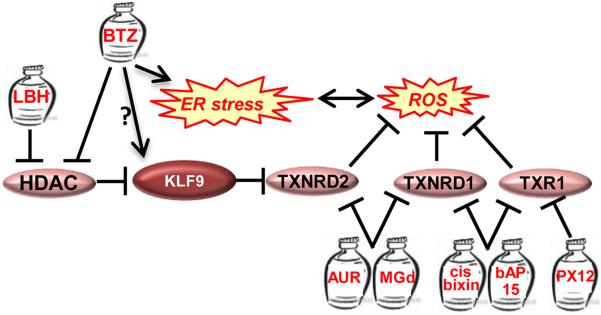
Although oxidative stress and ER stress appear to intertwine, the exact role of oxidative stress in inducing proteasome inhibitor-dependent ER stress is not well-understood. Recently, our group has identified a novel mechanism of BTZ- and CFZ-dependent induction of oxidative stress in multiple myeloma cells. We started with the identification of the transcription regulator Kruppel-like Factor 9 (KLF9) as a mediator of cytotoxicity of BTZ and histone deacetylase (HDAC) inhibitor LBH589 (panobinostat) [80,81]. In the same study we utilized a microarray-based global expression dataset that was generated for multiple myeloma cells collected from patients with relapsed myeloma before treatment with BTZ or high dose dexamethasone [82]. In combination with patient clinical response and survival data, this database serves as a valuable tool for establishing correlations between gene expression profiles and clinical outcome. Thus, we determined that KLF9 levels were significantly elevated in BTZ-responders (with no significance with regard to dexamethasone outcomes) [81]. In tissue culture settings, KLF9 levels in multiple myeloma cells correlated with BTZ-dependent suppression of histone deacetylases 1 and 2 (HDAC1 and HDAC2), and were independently increased by LBH589 [81].
A follow-up study by our group revealed that KLF9 is a ubiquitous inducer of oxidative stress acting via transcriptional repression of the gene for anti-oxidant protein thioredoxin reductase II (TXNRD2) [83]. TXNRD2 is essential for the maintenance of intracellular redox status and suppression of oxidative stress [37].
In a separate report we demonstrated that TXNRD2 levels decrease in multiple myeloma cells treated with BTZ and CFZ, whereas partial transcriptional repression of TXNRD2 in untreated multiple myeloma cells causes oxidative stress, ER stress and death similar to those induced by BTZ or CFZ [84] (Figure 1) [84]. Accordingly, TXNRD2 overexpression suppresses BTZ- or CFZ-induced ER stress and cell death in in vitro, and decreases efficacy of BTZ against multiple myeloma xenografts in immunocompromised mice [84].
Figure 1. Inhibition of Antioxidants in Multiple Myeloma Cells.
See details in the text. Genes are shown by ovals, jars represent drugs. LBH=LBH589, BTZ=bortezomib, AUR=auranofin, MGd= Motexafin gadolinium.
2.3. Oxidative Stress Inducing Agents as Adjuvants for Proteasome Inhibitor Therapy
Tumor cells possess higher basal ROS levels than their normal counterparts and thus may be more vulnerable to the oxidative stress induced by chemotherapeutic agents. Several such agents that directly increase intracellular ROS levels have been already approved for treatment of multiple malignancies including lung, breast ovarian and pancreatic cancers, as well as different forms of leukemias and lymphomas [reviewed in 17]. In multiple myeloma, with the exception of arsenic trioxide (which has demonstrated only limited efficacy [85,86]), no drugs primarily inducing oxidative stress are currently being used in clinic. Yet, data obtained in cultured cells and/or preclinical settings strongly suggest that induction of oxidative stress is a promising myeloma treatment strategy.
Treatment of multiple myeloma cells with selective inhibitor of thioredoxin 1 (TRX1) PX-12 (1-methylpropyl 2-imidazolyl disulfide) or auranofin, a coordinated gold compound that inhibits thioredoxin reductases 1 and 2 (TXNRD1,2) [87], leads to ROS-dependent apoptosis [88]. Similar results were obtained with genetic inhibition of TRX1 or TXNRD1 [88]. Our group demonstrated that depletion of TXNRD2 via shRNA induces death in multiple myeloma cells [84]. Moreover, BTZ-resistant multiple myeloma cells express higher levels of TRX1 and TXNRD2 compared to parental cells [84,88]. Interestingly, auranofin (which has been FDA approved for the treatment of rheumatoid and juvenile arthritis, Felty's syndrome, and psoriatic arthritis [87]) substantially reduced tumor burden and improved survival in a transgenic mouse model of Chronic lymphocytic leukemia [89]. These data suggest that auranofin could be used for treatment of multiple myeloma either as a stand-alone agent or in combination with proteasome inhibitors.
Indeed, combinational treatment of multiple myeloma cells with BTZ and other ROS-inducing agents demonstrated promising results in several studies. Thus, inhibition of the dimerization of the oncoprotein mucin 1 C-terminal subunit (MUC1-C) [90] in multiple myeloma cells with a cell-penetrating peptide GO-203 not only synergized with BTZ via upregulation of intracellular ROS but also re-sensitized BTZ-resistant multiple myeloma cells to BTZ treatment [91]. Similarly, co-treatment of multiple myeloma cells with carfilzomib, a next generation analog of bortezomib, and LBH589 (panobinostat), a novel broad-spectrum inhibitor of HDACs, resulted in ROS-dependent apoptosis [92]. LBH589 has been recently approved for treatment of patients with relapsed and refractory multiple myeloma [80]. Although several mechanisms have been proposed for LBH589-dependent generation of ROS [93-95], it is worth noting that KLF9 has been reported by us previously to mediate LBH589 cytotoxicity in multiple myeloma cells [81] and thus could account, at least in part, for the LBH-dependent oxidative stress in these cells. Several other histone deacetylase inhibitors cooperated or synergized with BTZ in inducing oxidative stress and apoptosis in multiple myeloma cells in vitro, including sodium butyrate and suberoylanilide hydroxamic acid [95], PXD101 (belinostat, Beleodaq), [96], and KD5170 [97].
A number of agents the cytotoxicity of which in multiple myeloma cells depend on the ability to induce oxidative stress have been identified within past fifteen years. These drugs include i)b-AP15 a small molecule inhibitor of the ubiquitin specific peptidase that also possess anti-TXR and anti-TXNRD activities [98]; ii) cis-bixin, a constituent of a plant Bixa Orellana, that also inhibits activity of TXR and TXNRD1 [99]; iii)motexafin gadolinium (Xcytrin), an inhibitor of TXNRD and ribonucleotide reductase [100]; iv)imexon (Amplimexon/NSC-714597), a cyanoaziridine derivative that induces oxidative stress via not completely identified mechanism [101-104]; v) chaetocin, a nonspecific inhibitor of histone methyltransferases [105]; vi)parthenolide, a natural product from the plant Tanacetum parthenium with anti-inflammatory activity [106]. However, further testing is required to assess their efficacy in preclinical models and the ability to cooperate with proteasome inhibitors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myelom. Nat. Rev. Cancer. 2002;2:927–937. doi: 10.1038/nrc952. doi:10.1038/nrc952. [DOI] [PubMed] [Google Scholar]
- 2.Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385:2197–2208. doi: 10.1016/S0140-6736(14)60493-1. doi:10.1016/S0140-6736(14)60493-1. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar SV, Landgren O. Smoldering multiple myeloma. 2015;125:3069–3076. doi: 10.1182/blood-2014-09-568899. doi:10.1182/blood-2014-09-568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Barcelona U. Monoclonal Gammopathy of Undetermined Significance. Evaluation. 2006:2765–2770. [Google Scholar]
- 5.Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance and smouldering multiple myeloma: Emphasis on risk factors for progression. Br. J. Haematol. 2007;139:730–743. doi: 10.1111/j.1365-2141.2007.06873.x. doi:10.1111/j.1365-2141.2007.06873.x. [DOI] [PubMed] [Google Scholar]
- 6.Helmreich E, Kern M, Eisene HN. The secretion of antibody by isolated lymph node cells. J. Biol. Chem. 1961;236:464–473. [PubMed] [Google Scholar]
- 7.Masciarelli S, Sitia R. Building and operating an antibody factory: Redox control during B to plasma cell terminal differentiation. Biochim. Biophys. Acta - Mol. Cell Res. 2008;1783:578–588. doi: 10.1016/j.bbamcr.2008.01.003. doi:10.1016/j.bbamcr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 8.White-Gilbertson S, Hua Y, Liu B. The role of endoplasmic reticulum stress in maintaining and targeting multiple myeloma: a double-edged sword of adaptation and apoptosis. Front. Genet. 2013;4:1–8. doi: 10.3389/fgene.2013.00109. doi:10.3389/fgene.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schieber M, Chandel NS. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. doi:10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong L, Chuang C-C, Wu S, Zuo L. Reactive oxygen species in redox cancer therapy. Cancer Lett. 2015;367:18–25. doi: 10.1016/j.canlet.2015.07.008. doi:10.1016/j.canlet.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. doi:10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 12.Brand MD. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. doi:10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fransen M, Nordgren M, Wang B, Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochim. Biophys. Acta - Mol. Basis Dis. 2012;1822:1363–1373. doi: 10.1016/j.bbadis.2011.12.001. doi:10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Estrela JM, Ortega A, Obrador E. Glutathione in Cancer Biology and Therapy. Crit. Rev. Clin. Lab. Sci. 2006 doi: 10.1080/10408360500523878. doi:10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 15.Nordberg J, Arnér ESJ. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. doi:10.1016/S0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 16.Arnér ESJ. Focus on mammalian thioredoxin reductases - Important selenoproteins with versatile functions. Biochim. Biophys. Acta - Gen. Subj. 2009;1790:495–526. doi: 10.1016/j.bbagen.2009.01.014. doi:10.1016/j.bbagen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Liou M-Y, Storz P. Reactive oxygen species in cancer. 2010 doi: 10.3109/10715761003667554. doi:10.3109/10715761003667554.Reactive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic. Biol. Med. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. doi:10.1016/0891-5849(94)00198-S. [DOI] [PubMed] [Google Scholar]
- 19.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. doi:10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sayin VI, Ibrahim MX, Larsson E, Nilsson J. a, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014;6:221ra15. doi: 10.1126/scitranslmed.3007653. doi:10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 21.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. doi:10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol. 2013;3:120144. doi: 10.1098/rsob.120144. doi:10.1098/rsob.120144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai J, Niu X, Chen Y, Hu Q, Shi G, Wu H, Wang J, Yi J. Emodin-induced generation of reactive oxygen species inhibits RhoA activation to sensitize gastric carcinoma cells to anoikis. Neoplasia. 2008;10:41–51. doi: 10.1593/neo.07754. doi:10.1593/neo.07754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otsubo C, Otomo R, Miyazaki M, Matsushima-Hibiya Y, Kohno T, Iwakawa R, Takeshita F, Okayama H, Ichikawa H, Saya H, Kiyono T, Ochiya T, Tashiro F, Nakagama H, Yokota J, Enari M. TSPAN2 is involved in Cell Invasion and Motility during Lung Cancer Progression. Cell Rep. 2014;7:527–538. doi: 10.1016/j.celrep.2014.03.027. doi:10.1016/j.celrep.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Le Gal K, Ibrahim MX, Wiel C, Sayin VI, Akula MK, Karlsson C, Dalin MG, Akyürek LM, Lindhal P, Nilsson J, Bergo MO. Antioxidants can increase melanoma metastasis in mice. 2015;7:1–7. doi: 10.1126/scitranslmed.aad3740. doi:10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 26.Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBarardinis RJ, Morrison SJ. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015 doi: 10.1038/nature15726. doi:10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu F, Fu Y, Meyskens FL., Jr. MiTF regulates cellular response to reactive oxygen species through transcriptional regulation of APE-1/Ref-1. J Invest Dermatol. 2009;129:422–31. doi: 10.1038/jid.2008.255. doi: 10.1038/jid.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park HJ, Carr JR, Wang Z, Nogueira V, Hay N, Tyner AL, Lau LF, Costa RH, Raychaudhuri P. FoxM1, a critical regulator of oxidative stress during oncogenesis. EMBO J. 2009;28:2908–18. doi: 10.1038/emboj.2009.239. doi: 10.1038/emboj.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itoh K, Ishii T, Wakabayashi N, Yamamoto M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic Res. 1999;31:319–324. doi: 10.1080/10715769900300881. doi: 10.1080/10715769900300881. [DOI] [PubMed] [Google Scholar]
- 30.Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–9. doi: 10.1016/j.redox.2012.10.001. doi:10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo NJ, Kim HR, Kim YR, An CH, Lee SH. Somatic mutations of the KEAP1 gene in common solid cancers. Histopathology. 2012;60:943–52. doi: 10.1111/j.1365-2559.2012.04178.x. doi: 10.1111/j.1365-2559.2012.04178.x. [DOI] [PubMed] [Google Scholar]
- 32.Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, Watanabe S, Ando J, Iwadate M, Yamamoto M, Lee MS, Tanaka K, Komatsu M. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol. 2011;193:275–84. doi: 10.1083/jcb.201102031. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klaunig JE, Kamendulis LM. The Role of Oxidative Stress in Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. doi:10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 34.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. doi:10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 35.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J. Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. doi:10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castaldo PMS, Freitas J, Conchinha N. The Tumorigenic Roles of the Cellular REDOX Regulatory Systems. Oxid. Med. Cell. Longev. 2015;2016 doi: 10.1155/2016/8413032. doi:10.1155/2016/8413032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. doi:10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 38.Cieslar P, Másová L, Scheiner T, Rysavá J, Krízová P, Danzigová Z, et al. Oxidative stress and platelet function in multiple myeloma and renal insufficiency: clinical relations of different tests. Thromb. Res. 2002;105:277–83. doi: 10.1016/s0049-3848(02)00003-8. doi:10.1016/S0049-3848(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 39.Sharma A, Tripathi M, Satyam A, Kumar L. Study of antioxidant levels in patients with multiple myeloma. Leuk. Lymphoma. 2009;50:809–815. doi: 10.1080/10428190902802323. doi:10.1080/10428190902802323. [DOI] [PubMed] [Google Scholar]
- 40.Gangemi S, Allegra A, Alonci A, Cristani M, Russo S, Speciale A, et al. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm. Res. 2012;61:1063–1067. doi: 10.1007/s00011-012-0498-7. doi:10.1007/s00011-012-0498-7. [DOI] [PubMed] [Google Scholar]
- 41.Mehdi W, Zainulabdeen J, Mehde A. Investigation of the antioxidant status in multiple myeloma patients: effects of therapy. Asian Pac J Cancer Prev. 2013;14:3663–3667. doi: 10.7314/apjcp.2013.14.6.3663. 2013;14(6)3663-7. [DOI] [PubMed] [Google Scholar]
- 42.Smirnova OV, Titova NM, Elmanova NG. The Relationship between the Pro-Oxidant and Antioxidant System Status of Patients with Multiple Myeloma and the Disease Stage. 2014;157:375–379. doi: 10.1007/s10517-014-2570-5. [DOI] [PubMed] [Google Scholar]
- 43.Faridvand Y, Oskuyi AE, Khadem-Ansari MH. Serum 8-isoprostane levels and paraoxonase 1 activity in patients with stage I multiple myeloma. Redox Rep. Commun. Free Radic. Res. doi: 10.1179/1351000215Y.0000000034. doi: 10.1179/1351000215Y.0000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellidag HY, Eren E, Aydin O, Yıldırım M, Sezer C, Yilmaz N. Multiple myeloma: relationship to antioxidant esterases. Med. Princ. Pract. 2014;23:18–23. doi: 10.1159/000355826. doi:10.1159/000355826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown CO, Schibler J, Fitzgerald MP, Singh N, Salem K, Zhan F, Goel A. Scavenger receptor class A member 3 (SCARA3) in disease progression and therapy resistance in multiple myeloma. Leuk. Res. 2013;37:963–9. doi: 10.1016/j.leukres.2013.03.004. doi:10.1016/j.leukres.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu L, Su MY, Maggi LB, Lu L, Mullins C, Crosby S, Huang G, Chng WJ, Vij R, Tomasson MH. Multiple myeloma-associated chromosomal translocation activates orphan snoRNA ACA11 to suppress oxidative stress. J. Clin. Invest. 2012;122:2793–806. doi: 10.1172/JCI63051. doi:10.1172/JCI63051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salem K, McCormick ML, Wendlandt E, Zhan F, Goel A. Copper-zinc superoxide dismutase-mediated redox regulation of bortezomib resistance in multiple myeloma. Redox Biol. 2014;4C:23–33. doi: 10.1016/j.redox.2014.11.002. doi:10.1016/j.redox.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaiser MF, Johnson DC, Wu P, Walker BA, Brioli A, Mirabella F, Wardell CP, Melchor L, Davies FE, Morgan GJ. Global methylation analysis identifies prognostically important epigenetically inactivated tumor suppressor genes in multiple myeloma. Blood. 2013;122:219–26. doi: 10.1182/blood-2013-03-487884. doi:10.1182/blood-2013-03-487884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Field-Smith A, Morgan GJ, Davies FE. Bortezomib (Velcadetrade mark) in the Treatment of Multiple Myeloma. Ther. Clin. Risk Manag. 2006;2:271–279. doi: 10.2147/tcrm.2006.2.3.271. doi:10.2147/tcrm.2006.2.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2005;352:2487–98. doi: 10.1056/NEJMoa043445. doi:10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 51.Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825. doi: 10.1182/blood-2012-05-425934. doi:10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar SK, Bensinger WI, Zimmerman TM, Reeder CB, Berenson JR, Berg D, et al. Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed / refractory multiple myeloma. Blood. 2014;124:1047–1055. doi: 10.1182/blood-2014-01-548941. doi:10.1182/blood-2014-01-548941.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obeng EA, Carlson LM, Gutman DM, W.J.H., Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4917. doi: 10.1182/blood-2005-08-3531. doi:10.1182/blood-2005-08-3531.Supported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee A-H, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9946–9951. doi: 10.1073/pnas.1334037100. doi:10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maharjan S, Oku M, Tsuda M, Hoseki J, Sakai Y. Mitochondrial impairment triggers cytosolic oxidative stress and cell death following proteasome inhibition. Sci. Rep. 2014;4:5896. doi: 10.1038/srep05896. doi:10.1038/srep05896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fribley A, Zeng Q, Wang C. Proteasome Inhibitor PS-341 Induces Apoptosis through Induction of Endoplasmic Reticulum Stress-Reactive Oxygen Species in Head and Neck Squamous Cell Carcinoma Cells. Society. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. doi:10.1128/MCB.24.22.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parekh S, Weniger M. a., Wiestner A. New molecular targets in mantle cell lymphoma. Semin. Cancer Biol. 2011;21:335–346. doi: 10.1016/j.semcancer.2011.09.008. doi:10.1016/j.semcancer.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goel A, Spitz DR, Weiner GJ. Manipulation of cellular redox parameters for improving therapeutic responses in B-cell lymphoma and multiple myeloma. J. Cell. Biochem. 2012;113:419–425. doi: 10.1002/jcb.23387. doi:10.1002/jcb.23387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pérez-Galán P, Roue G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–264. doi: 10.1182/blood-2005-05-2091. doi:10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 60.Ling Y-H, Liebes L, Zou Y, Perez-Soler R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J. Biol. Chem. 2003;278:33714–33723. doi: 10.1074/jbc.M302559200. doi:10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- 61.Llobet D, Eritja N, Encinas M, Sorolla A, Yeramian A, Schoenenberger JA, et al. Antioxidants block proteasome inhibitor function in endometrial carcinoma cells. Anticancer. Drugs. 2008;19:115–124. doi: 10.1097/CAD.0b013e3282f24031. doi:10.1097/CAD.0b013e3282f24031. [DOI] [PubMed] [Google Scholar]
- 62.Yang RA, Jinming, Su Yingjun Antioxidants tiron and N-acetyl-L cysteine differentially mediate apoptosis in melanoma cells via a reactive oxygen species-independent NF-KB pathway. Free Radic. Biol. Med. 2007;9:1369–1380. doi: 10.1016/j.freeradbiomed.2007.01.036. doi 10.1016/j.freeradbiomed.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.A. Weniger Marc, Rizzatti Edgar G, Perez-Galan Patricia, Liu Delong, Wang Qiuyan, Munson Peter J., Raghavachari Nalini, White Therese, Tweito Megan, M., Kieron Dunleavy, Ye Yihong, Wilson Wyndham, H., Wiestner Treatment-induced oxidative stress and cellular antioxidant capacity determine response to bortezomib in mantle cell lymphoma. Clin. Cancer Res. 2011;15:5101–5112. doi: 10.1158/1078-0432.CCR-10-3367. doi: 10.1158/1078-0432.CCR-10-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos Celio X, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid. Redox Signal. 2009;10:2409–2427. doi: 10.1089/ars.2009.2625. doi: 10.1089/ARS.2009.2625. [DOI] [PubMed] [Google Scholar]
- 65.Cadenas E, Davies KJA. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. doi:10.1016/S0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 66.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. doi:10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frand AR, Kaiser CA. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell. 1998;1:161–170. doi: 10.1016/s1097-2765(00)80017-9. doi:10.1016/S1097-2765(00)80017-9. [DOI] [PubMed] [Google Scholar]
- 68.Pollard MG, Travers KJ, Weissman JS. Ero1p: A Novel and Ubiquitous Protein with an Essential Role in Oxidative Protein Folding in the Endoplasmic Reticulum. Mol. Cell. 1998;1:171–182. doi: 10.1016/s1097-2765(00)80018-0. doi:10.1016/S1097-2765(00)80018-0. [DOI] [PubMed] [Google Scholar]
- 69.Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. doi:10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 70.Haynes CM, Titus EA, Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell. 2004;15:767–776. doi: 10.1016/j.molcel.2004.08.025. doi:10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 71.Yan Y, Wei C, Zhang W, Cheng H, Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol. Sin. 2006;27:821–826. doi: 10.1111/j.1745-7254.2006.00390.x. doi:10.1111/j.1745-7254.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 72.Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:REDOXD1500102. doi: 10.1016/j.redox.2015.08.010. doi:10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brookes PS, Yoon YS, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. doi:10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 74.Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. doi:10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. doi:10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 76.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 2008;181:1129–39. doi: 10.1083/jcb.200709049. doi:10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell Biol. 2010;191:1113–25. doi: 10.1083/jcb.201006121. doi:10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pedruzzi E, Guichard C, Ollivier V, Fay M, Prunet C, Marie J, et al. NAD(P)H Oxidase Nox-4 Mediates 7-Ketocholesterol-Induced Endoplasmic Reticulum Stress and Apoptosis in Human Aortic Smooth Muscle Cells. Mol. Cell. Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. doi:10.1128/MCB.24.24.10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu R-F, Ma Z, Liu Z, Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol. Cell. Biol. 2010;30:3553–3568. doi: 10.1128/MCB.01445-09. doi:10.1128/MCB.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laubach JP, Moreau P, San-Miguel JF, Richardson PG. Panobinostat for the Treatment of Multiple Myeloma. Clin. Cancer Res. 2015;21:4767–4773. doi: 10.1158/1078-0432.CCR-15-0530. doi:10.1158/1078-0432.CCR-15-0530. [DOI] [PubMed] [Google Scholar]
- 81.Mannava S, Zhuang D, Nair JR, Bansal R, Wawrzyniak JA, Zucker SN, et al. KLF9 is a novel transcriptional regulator of bortezomib- and LBH589-induced apoptosis in multiple myeloma cells. Blood. 2012;119:1450–8. doi: 10.1182/blood-2011-04-346676. doi:10.1182/blood-2011-04-346676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mulligan G, Mitsiades C, Bryant B, Zhan F, Chng WJ, Roels S, et al. Gene expression pro ling and correlation with outcome in clinical trials of the proteasome inhibitor bortezomib. Gene Expr. 2007;109:3177–3188. doi: 10.1182/blood-2006-09-044974. doi:10.1182/blood-2006-09-044974. [DOI] [PubMed] [Google Scholar]
- 83.Zucker SN, Fink EE, Bagai A, Mannava S, Bianchi-Smiraglia A, Bogner PN, Wawrzyniak JA, Foley C, Leonova KI, Grimm MJ, Moparthy K, Ionov Y, Wang J, Liu S, Sexton S, Kandel ES, Bakin AV, Zhang Y, Kaminski N, Segel BH, Nikiforov MA. Nrf2 amplifies oxidative stress via induction of Klf9. Mol. Cell. 2014;53:916–928. doi: 10.1016/j.molcel.2014.01.033. doi: 10.1016/j.molcel.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fink EE, Mannava S, Bagati A, Bianchi-Smiraglia A, Nair JR, Moparthy K, et al. Mitochondrial thioredoxin reductase regulates major cytotoxicity pathways of proteasome inhibitors in multiple myeloma cells. Leukemia. 2015:1–8. doi: 10.1038/leu.2015.190. doi:10.1038/leu.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Munshi NC, Tricot G, Desikan R, a Badros M, Zangari a Toor, et al. Clinical activity of arsenic trioxide for the treatment of multiple myeloma. Leuk. Off. J. Leuk. Soc. Am. Leuk. Res. Fund, U.K. 2002;16:1835–1837. doi: 10.1038/sj.leu.2402599. doi:10.1038/sj.leu.2402599. [DOI] [PubMed] [Google Scholar]
- 86.Röllig C, Illmer T. The efficacy of arsenic trioxide for the treatment of relapsed and refractory multiple myeloma: A systematic review. Cancer Treat. Rev. 2009;35:425–430. doi: 10.1016/j.ctrv.2009.04.007. doi:10.1016/j.ctrv.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 87.Madeira JM, Gibson DL, Kean WF, Klegeris A. The biological activity of auranofin: implications for novel treatment of diseases. Inflammopharmacology. 2012;20:297–306. doi: 10.1007/s10787-012-0149-1. doi:10.1007/s10787-012-0149-1. [DOI] [PubMed] [Google Scholar]
- 88.Raninga PV, Di Trapani G, Vuckovic S, Bhatia M, Tonissen KF. Inhibition of thioredoxin 1 leads to apoptosis in drug-resistant multiple myeloma. 2015;6 doi: 10.18632/oncotarget.3795. doi: 10.18632/oncotarget.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fiskus W, Saba N, Shen M, Ghias M, Liu J, Gupta SD, et al. Auranofin Induces Lethal Oxidative and Endoplasmic Reticulum Stress and Exerts Potent Preclinical Activity against Chronic Lymphocytic Leukemia. Cancer Res. 2014;74:2520–2532. doi: 10.1158/0008-5472.CAN-13-2033. doi:10.1158/0008-5472.CAN-13-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yin L, Kosugi M, Kufe D. Inhibition of the MUC1-C oncoprotein induces multiple myeloma cell death by down-regulating TIGAR expression and depleting NADPH. Blood. 2012;119:810–6. doi: 10.1182/blood-2011-07-369686. doi:10.1182/blood-2011-07-369686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yin L, Kufe T, Avigan D, Kufe D. Targeting MUC1-C is synergistic with bortezomib in downregulating TIGAR and inducing ROS-mediated myeloma cell death. Blood. 2014;123:2997–3006. doi: 10.1182/blood-2013-11-539395. doi:10.1182/blood-2013-11-539395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao L, Gao M, Yang G, Tao Y, Kong Y, Yang R, et al. Synergistic Activity of Carfilzomib and Panobinostat in Multiple Myeloma Cells via Modulation of ROS Generation and ERK1/2. Biomed Res. Int. 2015;2015:459052. doi: 10.1155/2015/459052. doi:10.1155/2015/459052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bruzzese F, Pucci B, Milone MR, Ciardiello C, Franco R, Chianese MI, et al. Panobinostat synergizes with zoledronic acid in prostate cancer and multiple myeloma models by increasing ROS and modulating mevalonate and p38-MAPK pathways. Cell Death Dis. 2013;4:e878. doi: 10.1038/cddis.2013.406. doi:10.1038/cddis.2013.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hedrick E, Crose L, Linardic CM, Safe S. Histone Deacetylase Inhibitors Inhibit Rhabdomyosarcoma by Reactive Oxygen Species-Dependent Targeting of Specificity Protein Transcription Factors. Mol. Cancer Ther. 2015:2143–2154. doi: 10.1158/1535-7163.MCT-15-0148. doi:10.1158/1535-7163.MCT-15-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pei X-Y, Dai Y, Grant S. Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clin. Cancer Res. 2004;10:3839–52. doi: 10.1158/1078-0432.CCR-03-0561. doi:10.1158/1078-0432.CCR-03-0561. [DOI] [PubMed] [Google Scholar]
- 96.Feng R, Oton A, Mapara MY, Anderson G, Belani C, Lentzsch S. The histone deacetylase inhibitor, PXD101, potentiates bortezomib-induced anti-multiple myeloma effect by induction of oxidative stress and DNA damage. Br. J. Haematol. 2007;139:385–97. doi: 10.1111/j.1365-2141.2007.06772.x. doi:10.1111/j.1365-2141.2007.06772.x. [DOI] [PubMed] [Google Scholar]
- 97.Feng R, Ma H, Hassig C. a, Payne JE, Smith ND, Mapara MY, et al. KD5170, a novel mercaptoketone-based histone deacetylase inhibitor, exerts antimyeloma effects by DNA damage and mitochondrial signaling. Mol. Cancer Ther. 2008;7:1494–505. doi: 10.1158/1535-7163.MCT-08-0183. doi:10.1158/1535-7163.MCT-08-0183. [DOI] [PubMed] [Google Scholar]
- 98.Wang X, Stafford W, Mazurkiewicz M, Fryknäs M, Brjnic S, Zhang X, et al. The 19S Deubiquitinase inhibitor b-AP15 is enriched in cells and elicits rapid commitment to cell death. Mol. Pharmacol. 2014;85:932–45. doi: 10.1124/mol.113.091322. doi:10.1124/mol.113.091322. [DOI] [PubMed] [Google Scholar]
- 99.Tibodeau JD, Isham CR, Bible KC. Annatto constituent cis-bixin has selective antimyeloma effects mediated by oxidative stress and associated with inhibition of thioredoxin and thioredoxin reductase. Antioxid. Redox Signal. 2010;13:987–97. doi: 10.1089/ars.2009.2896. doi:10.1089/ars.2009.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Evens AM, Lecane P, Magda D, Prachand S, Singhal S, Nelson J, et al. Motexafin gadolinium generates reactive oxygen species and induces apoptosis in sensitive and highly resistant multiple myeloma cells. Blood. 2005;105:1265–1273. doi: 10.1182/blood-2004-03-0964. doi:10.1182/blood-2004-03-0964. [DOI] [PubMed] [Google Scholar]
- 101.Dvorakova K, Payne CM, Tome ME, Briehl MM, McClure T, Dorr RT. Induction of oxidative stress and apoptosis in myeloma cells by the aziridine-containing agent imexon. Biochem. Pharmacol. 2000;60:749–758. doi: 10.1016/s0006-2952(00)00380-4. doi:10.1016/S0006-2952(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 102.Dvorakova K, Waltmire CN, Payne CM, Tome ME, Briehl MM, Dorr RT. Induction of mitochondrial changes in myeloma cells by imexon. Blood. 2001;97:3544–3551. doi: 10.1182/blood.v97.11.3544. doi:10.1182/blood.V97.11.3544. [DOI] [PubMed] [Google Scholar]
- 103.Dvorakova K, Payne CM, Tome ME, Briehl MM, Vasquez M. a, Waltmire CN, et al. Molecular and cellular characterization of imexon-resistant RPMI8226/I myeloma cells. Mol. Cancer Ther. 2002;1:185–195. doi: 10.1371/journal.pone.0053945. [PubMed] [Google Scholar]
- 104.Tavenner BE, Dragovich T, Coon A, Powis G. Expression : Evidence for an Oxidative Stress Response. 2014;13:3388–3394. doi: 10.1158/1078-0432.CCR-06-0873. doi:10.1158/1078-0432.CCR-06-0873.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Isham CR, Tibodeau JD, Jin W, Xu R, Timm MM, Bible KC. Chaetocin: A promising new antimyeloma agent with in vitro and in vivo activity mediated via imposition of oxidative stress. Blood. 2007;109:2579–2588. doi: 10.1182/blood-2006-07-027326. doi:10.1182/blood-2006-07-027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang W, Adachi M, Kawamura R, Sakamoto H, Hayashi T, Ishida T, et al. Parthenolide-induced apoptosis in multiple myeloma cells involves reactive oxygen species generation and cell sensitivity depends on catalase activity. Apoptosis. 2006;11:2225–35. doi: 10.1007/s10495-006-0287-2. doi:10.1007/s10495-006-0287-2. [DOI] [PubMed] [Google Scholar]