Sleep is usually considered a whole-brain phenomenon in which neuronal regulatory circuits impose sleep on the brain. This paradigm has its origins in the historically important work of Viennese neurologist Constantin von Economo, who found that people who suffered from brain infections that damaged the anterior hypothalamus slept less. The finding was a turning point in sleep research, as it suggested that sleep was a consequence of active processes within the brain. This stood in stark contrast to the ideas of renowned St. Petersburg physiologist Ivan Pavlov, who believed that sleep resulted from the passive withdrawal of sensory input. Although the withdrawal of sensory input remains recognized as playing a role in sleep initiation, there is now much evidence supporting the idea that neuronal and glial activity in the anterior hypothalamus leads to the inhibition of multiple excitatory neuronal networks that project widely throughout the brain.
But we also know from millions of stroke cases that cause brain damage and from experimentally induced brain damage in animal models that, regardless of where a lesion occurs in the brain, including the anterior hypothalamus, all humans or animals that survive the brain damage will continue to sleep. Further, a key question remains inadequately answered: How does the hypothalamus know to initiate sleep? Unless one believes in the separation of mind and brain, then, one must ask: What is telling the hypothalamus to initiate sleep? If an answer is found, it leads to: What is telling the structure that told the hypothalamus? This is what philosophers call an infinite regress, an unacceptable spiral of logic.
For these reasons, 25 years ago the late Ferenc Obál Jr. of A. Szent-Györgyi Medical University in Szeged, Hungary, and I (J.K.) began questioning the prevailing ideas of how sleep is regulated. The field needed answers to fundamental questions. What is the minimum amount of brain tissue required for sleep to manifest? Where is sleep located? What actually sleeps? Without knowing what sleeps or where sleep is, how can one talk with any degree of precision about sleep regulation or sleep function? A new paradigm was needed.
What is sleep?
There is no direct measure of sleep, and no single measure is always indicative of sleep. Quiescent behavior and muscle relaxation usually occur simultaneously with sleep but are also found in other circumstances, such as during meditation or watching a boring TV show. Sleep is thus defined in the clinic and in experimental animals using a combination of multiple parameters that typically correlate with sleep.
The primary tool for assessing sleep state in mammals and birds is the electroencephalogram (EEG). High-amplitude delta waves (0.5–4 Hz) are a defining characteristic of the deepest stage of non–rapid eye movement (non-REM) sleep. However, similar waves are evident in adolescents who hyperventilate for a few seconds while wide awake. Other measures used to characterize sleep include synchronization of electrical activity between EEG electrodes and the quantification of EEG delta wave amplitudes. Within specific sensory circuits, the cortical electrical responses induced by sensory stimulation (called evoked response potentials, or ERPs) are higher during sleep than during waking. And individual neurons in the cerebral cortex and thalamus display action potential burst-pause patterns of firing during sleep.
Using such measures, researchers have shown that different parts of the mammalian brain can sleep independently of one another. Well-characterized sleep regulatory substances, or somnogens, such as growth hormone releasing hormone (GHRH) and tumor necrosis factor α (TNF-α), can induce supranormal EEG delta waves during non-REM sleep in the specific half of the rat brain where the molecules were injected. Conversely, if endogenous TNF-α or GHRH production is inhibited, spontaneous EEG delta waves during non-REM sleep are lower on the side receiving the inhibitor. A more natural example of sleep lateralization is found in the normal unihemispheric sleep of some marine mammals. (See “Who Sleeps?” on page 28.)
Much smaller parts of the brain also exhibit sleep-like cycles. As early as 1949, Kristian Kristiansen and Guy Courtois at McGill University and the Montreal Neurological Institute showed that, when neurons carrying input from the thalamus and surrounding cortical tissue are surgically severed, clusters of neurons called cerebral cortical islands will alternate between periods of high-amplitude slow waves that characterize sleep and low-amplitude fast waves typical of waking, independently of surrounding tissue.1 This suggests that sleep is self-organizing within small brain units.
In 1997, Ivan Pigarev of the Russian Academy of Sciences in Moscow and colleagues provided more-concrete evidence that sleep is a property of local networks. Measuring the firing patterns of neurons in monkeys’ visual cortices as the animals fell asleep while performing a visual task, they found that some of the neurons began to stop firing even while performance persisted. Specifically, the researchers found that, within the visual receptive field being engaged, cells on the outer edges of the field stopped firing first. Then, as the animal progressed deeper into a sleep state, cells in more-central areas stopped firing. This characteristic spatial distribution of the firing failures is likely a consequence of network behavior. The researchers thus concluded that sleep is a property of small networks.2
More recently, David Rector at Washington State University and colleagues provided support for the idea of locally occurring sleep-like states. In a series of experiments, they recorded electrical activity from single cortical columns using a small array of 60 electrodes placed over the rat somatosensory cortex. The sensory input from individual facial whiskers maps onto individual cortical columns. As expected, ERPs in the cortical columns induced by twitching a whisker were higher during sleep than during waking. But looking at the activity of individual columns, the researchers observed that they could behave somewhat independently of each other. When a rat slept, most—but not all—of the columns exhibited the sleep-like high-amplitude ERPs; during waking, most—but not all—of the columns were in a wake-like state. Interestingly, the individual cortical columns also exhibited patterns that resembled a sleep rebound response: the longer a column was in the wake-like state, the higher the probability that it would soon transition into a sleep-like state.3
To test how cortical-column state can affect whole-animal behavior, Rector and his team trained rats to lick a sucrose solution upon the stimulation of a single whisker, then characterized the whisker’s cortical-column state. If the column receiving input from the stimulated whisker was in a wake-like state (low-magnitude ERP), the rats did not make mistakes. But if the column was in the sleep-like state (high-magnitude ERP), the animals would fail to lick the sucrose when stimulated and would sometimes lick it even when their whisker was not flicked.4 Even though the animal was awake, if a cortical column receiving stimulation was asleep, it compromised the animal’s performance. These experiments indicate that even very small neuronal networks sleep and that the performance of learned behavior can depend on the state of such networks.
CHARACTERIZING SLEEP
Sleep-like patterns of neural activity are apparent not just at the level of the whole brain, but also in isolated neural circuits. Researchers have even documented sleep-like behavior in cultures of glial and neural cells. By increasing the number of electrophysiological measurements we use to characterize sleep states, the homology between sleep-like states in culture and sleep in intact animals becomes stronger.
WHOLE BRAIN
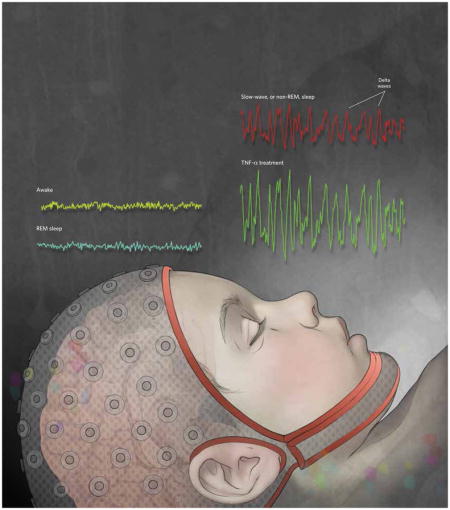
Mammalian sleep is characterized by several stages, typically measured using an electroencephalogram (EEG), which involves the recording of brain activity from an array of electrodes on the scalp. Rapid-eye movement (REM) sleep, the stage during which vivid dreams occur, is characterized by EEG waves similar to those observed during waking. High-amplitude delta waves (0.5–4 Hz) occur at the deepest stage of non-REM, or slow-wave, sleep. Both the presence and amplitude of these delta waves are used to characterize sleep in whole animals. When treated with the somnogen tumor necrosis factor α (TNF-α), the brain produces higher-amplitude delta waves, indicating a deeper stage of sleep.
HALF BRAIN
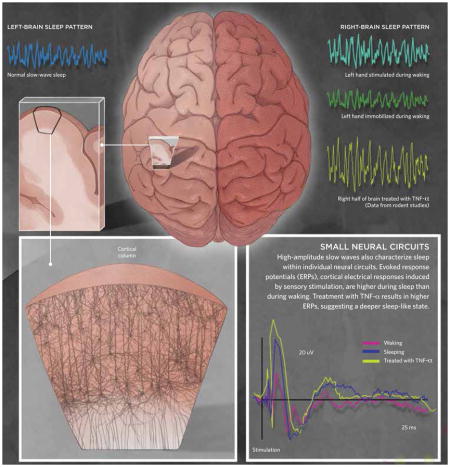
Research has also yielded evidence that the brain’s two hemispheres can sleep somewhat independently of each other. When a person holds a vibrating wand in the left hand during waking, for example, he stimulates only the right side of the somatosensory cortex, and in subsequent sleep, the right side of the brain exhibits higher amplitude EEG slow waves than the left side, indicating greater sleep intensity. Conversely, if a person’s left arm is immobilized during waking, amplitudes of EEG slow waves from the right side of the brain are lower than the left side during subsequent sleep. These half-brain measurements indicate that local sleep depth is a function of activity during waking. Moreover, rodent studies have shown that TNF-α treatment to only half of the brain will invoke higher than normal delta waves during sleep only in that hemisphere.
SLEEP IN VITRO
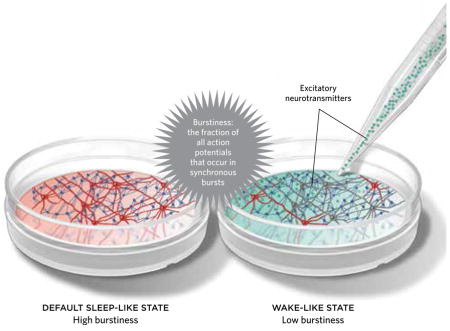
Neurons co-cultured with glial cells display patterns of action potentials and slow (delta) waves, suggesting that small neural networks can and do sleep, even outside of the body. In culture, neurons fire in bursts, and slow-wave electrical activity is synchronized while in a default sleep-like state. However, if the culture is stimulated with electricity or excitatory neurotransmitters, delta-wave amplitude and the neurons’ synchrony, or burstiness, are reduced, suggesting that the culture “wakes up.” Conversely, the addition of TNF-α, a sleep-inducing agent, increases burstiness and the amplitudes of delta waves.
Sleep in a dish
Given that sleep can manifest in relatively small brain regions, perhaps it should not be too surprising that co-cultures of neurons and glia possess many of the electrophysiological sleep phenotypes that are used to define sleep in intact animal brains. During sleep, cortical and thalamic neurons display bursts of action potentials lasting about 500 ms, followed by periods of hyperpolarization lasting about the same length of time. The synchronization of this firing pattern across many neurons is thought to generate the EEG activity characteristic of delta-wave sleep, and undisturbed co-cultures of glia and neurons display periodic bursts of action potentials, suggesting that the culture default state is sleep-like. In contrast, if neuronal and glia networks are stimulated with excitatory neurotransmitters, the culture’s “burstiness”—the fraction of all action potentials found within bursts—is reduced, indicating a transition to a wake-like state. Treatment of co-cultures with excitatory neurotransmitters also converts their gene expression profile from a spontaneous sleep-like pattern to a wake-like pattern.5
Cell cultures also respond to sleep-inducing agents similarly to whole organisms. If a neuronal and glial culture is treated with TNF-α, the synchronization and amplitudes of slow-wave electrical activity increase, indicating a deeper sleep-like state. Moreover, ERPs are of greater magnitude after cultures are treated with TNF-α than during the sleep-like default state, suggesting that the somnogen induces a deeper sleep-like state in vitro as it does in vivo.6
Researchers have even studied the developmental pattern of such sleep phenotypes, using multielectrode arrays to characterize network activity throughout the culture, and the emergence of network properties follows a similar time course as in intact mouse pups. Spontaneous action potentials occur during the first few days in culture, but network emergent properties are not evident until after about 10 days. Then, synchronization of electrical potentials begins to emerge, and the network’s slow waves begin to increase in amplitude. If the cultures are electrically stimulated, slow-wave synchronization and amplitudes are reduced, suggesting the networks wake up. This is followed by rebound-enhanced slow-wave synchronization and amplitudes the next day, suggesting sleep homeostasis is also a characteristic of cultured networks.6
Clearly, even small neural networks can exhibit sleep-like behavior, in a dish or in the brain. But the question remains: What is driving the oscillations between sleep- and wake-like states?
Sleep emerges
In the intact brain, communication among neurons and between neurons and other cells is ever changing. Bursts of action potentials trigger the release of multiple substances and changes in gene expression, both of which alter the efficacy of signal transmission. For instance, neural or glial activity induces the release of ATP into the local extracellular space. Extracellular ATP, in turn, induces changes in the expression of TNF-α and other somnogens known to induce a sleep-like state. Because these effects take place in the immediate vicinity of the cell activity, they target sleep to local areas that were active during prior wakefulness.
In 1993, Obál and I (J.K.) proposed that sleep is initiated within local networks as a function of prior activity.7 The following year, Derk-Jan Dijk and Alex Borbely of the University of Zurich provided support for this idea when they had volunteers hold hand vibrators in one hand during waking to stimulate one side of the somatosensory cortex. In subsequent sleep, the side of the brain that received input from the stimulated hand exhibited greater sleep intensity, determined from amplitudes of EEG slow waves, than the opposite side of the brain. And in 2006, Reto Huber, then at the University of Wisconsin, showed that if an arm is immobilized during waking, amplitudes of EEG slow waves from the side of the brain receiving input from that arm are lower in subsequent sleep.
These experiments indicate that local sleep depth is a function of the activity of the local network during waking—an idea that has been confirmed by multiple human and animal studies. Moreover, local network state oscillations strongly indicate that sleep is initiated within local networks such as cortical columns. But how do the states of a population of small networks translate into whole-animal sleep?
Small local clusters of neurons and glia are loosely connected with each other via electrophysiological and biochemical signaling, allowing for constant communication between local networks. Steven Strogatz of Cornell University showed that dynamically coupled entities, including small neuronal circuits, will synchronize with each other spontaneously without requiring direction by an external actor. Synchronization of loosely coupled entities occurs at multiple levels of complexity in nature from intact animals to molecules—for example, birds flocking, or the transition from water to ice. The patterns generated by bird flocking, or the hardness of ice, are called emergent properties.
We, Obál, and our colleagues proposed that whole-brain sleep is an emergent property resulting from the synchronization of local neuronal network states.7,8,9 This would explain why sleep continues to occur after brain damage: because the remaining local circuits will spontaneously synchronize with each other. This view also allows one to easily envision variations in the depth or degree of sleep and waking because it allows for some parts of the brain to be in sleep-like states while other areas are in wake-like states, just as Rector observed. These independent states of local networks may account for sleep inertia, the minutes-long period upon awakening of poor cognitive performance and fuzzy-mindedness, and may also play a role in the manifestation of dissociated states such as sleepwalking. Most importantly, this paradigm frees sleep regulation from the dualism trap of mind/brain separation: top-down imposition of state is not required for the initiation of local state oscillations or for subsequent whole-organism sleep to ensue.
Our theory is also consistent with the modulation of sleep and wakefulness by sleep regulatory circuits such as those in the hypothalamus. For example, if interleukin-1, a sleep regulatory substance, is applied locally to the surface of the rat cortex, it induces local high-amplitude EEG slow waves indicative of a greater local depth of sleep.10 The responses induced by interleukin-1 in the cortex enhanced neuronal activity in anterior hypothalamic sleep regulatory areas.11 That hypothalamic neuronal activity likely provides information on local sleep- and wake-like states occurring in the cortex to the hypothalamus, where it can modulate the orchestration of the sleep initiated within the smaller brain units.
Finally, our ideas may inform the study of how sleep influences the formation of memories. A fundamental problem a living brain faces is the incorporation of new memories and behaviors while conserving existing ones. We know that cell activity enhances neuronal connectivity and the efficacy of neurotransmission within active circuits, a phenomenon that has been posited to be a mechanism by which memories are formed and solidified. By themselves, however, these use-dependent mechanisms would lead to unchecked growth of connectivity (in response to activity patterns) and positive feedback (since increased connectivity leads to reuse), ultimately resulting in a rigid, non-plastic network.7 Instead, we suggest that biochemical mechanisms—specifically, the use-dependent expression of genes involved in sleep regulation and memory—induce oscillations, representing local wake- and sleep-like states, which serve to stabilize and preserve brain plasiticity.7
For more than a century, researchers have struggled to understand how sleep works and what it does. Perhaps this lack of answers stems from a fundamental misconception about what sleeps. By thinking about sleep in smaller units, such as individual networks in the brain, hopefully the field will start to understand what exactly is going on during this enigmatic—but very common—phenomenon.
Contributor Information
JAMES M. KRUEGER, Regents professor of neuroscience at Washington State University
SANDIP ROY, Associate professor of electrical engineering at Washington State University.
References
- 1.Kristiansen K, Courtois G. Rhythmic electrical activity from isolated cerebral cortex. Electroen Clin Neuro. 1949;1:265–72. [PubMed] [Google Scholar]
- 2.Pigarev IN, et al. Evidence for asynchronous development of sleep in cortical areas. Neuroreport. 1997;8:2557–60. doi: 10.1097/00001756-199707280-00027. [DOI] [PubMed] [Google Scholar]
- 3.Rector DM, et al. Local functional state differences between rat cortical columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Krueger JM, et al. Sleep: A synchrony of cell activity-driven small network states. European J Neurosci. 2013;38:2199–09. doi: 10.1111/ejn.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinard V, et al. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J Neurosci. 2012;32:12506–17. doi: 10.1523/JNEUROSCI.2306-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jewett KA, et al. Tumor necrosis factor enhances the sleep-like state and electrical stimulation induces a wake-like state in co-cultures of neurons and glia. European J Neurosci. 2015;42:2078–90. doi: 10.1111/ejn.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krueger JM, Obál F. A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 8.Krueger JM, et al. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–19. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy S, et al. A network model for activity-dependent sleep regulation. J Theor Biol. 2008;253:462–68. doi: 10.1016/j.jtbi.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuda T, et al. Interleukin-1 beta has a role in cerebral cortical state-dependent electroencephalographic slow-wave activity. Sleep. 2005;28:177–84. doi: 10.1093/sleep/28.2.177. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda K, et al. Unilateral cortical application of interleukin-1β (IL1β) induces asymmetry in Fos- and IL1β-immunoreactivity: Implications for sleep regulation. Brain Res. 2007;1131:44–59. doi: 10.1016/j.brainres.2006.11.051. [DOI] [PubMed] [Google Scholar]


