Abstract
Magnesium (Mg)-based biodegradable materials are promising candidates for the new generation of implantable medical devices, particularly cardiovascular stents and orthopaedic implants. Mg-based cardiovascular stents represent the most innovative stent technology to date. However, these products still do not fully meet clinical requirements with regards to fast degradation rates, late restenosis, and thrombosis. Thus various surface coatings have been introduced to protect Mg-based stents from rapid corrosion and to improve biocompatibility. Similarly, different coatings have been used for orthopaedic implants, e.g., plates and pins for bone fracture fixation or as an interference screw for tendon-bone or ligament-bone insertion, to improve biocompatibility and corrosion resistance. Metal coatings, nanoporous inorganic coatings and permanent polymers have been proved to enhance corrosion resistance; however, inflammation and foreign body reactions have also been reported. By contrast, biodegradable polymers are more biocompatible in general and are favoured over permanent materials. Drugs are also loaded with biodegradable polymers to improve their performance. The key similarities and differences in coatings for Mg-based stents and orthopaedic implants are summarized.
Keywords: Biocompatibility, Biodegradable materials, Coatings, Drug elution, Magnesium alloys
Introduction
Magnesium (Mg) is one of the lightest metals, exhibiting good mechanical properties, biodegradability, and biocompatibility [1,2], and has thus received great attention in the field of percutaneous coronary intervention (PCI) [3] and orthopaedic applications [4,5]. The main applications of Mg-based implantable medical devices currently include cardiovascular stents, bone fixation plates and pins, and screws for tendon-bone or ligament-bone insertions. The nature of their biodegradability makes Mg alloys look promising in implant applications because there is no need for secondary surgery to remove the implants [6]. Unfortunately, due to low corrosion resistance, many problems including hydrogen elution and decreasing mechanical strength prior to the healing of the surgical regions have also arisen during in vivo studies [7,8].
To prevent rapid corrosion, various surface modification techniques have been used [9,10]. Among them, the application of coatings has been documented as one of the most effective [11]. In addition to corrosion prevention, coatings can also provide a drug reservoir for Mg-based biomedical implants. Many coating technologies have been developed for Mg alloys, including inorganic coatings, metal coatings, metallic oxide coatings, metallic hydroxide coatings, chemical conversion coatings, nanoporous inorganic coatings, and polymer coatings [11–17]. This paper reviews the various coating techniques applied to Mg alloy device scaffolds and also determines the role that coatings play in stent functionality and orthopaedic implants. The differences and similarities of coatings used in stents and orthopaedic implants are also addressed.
Metal, metallic oxide, and metallic hydroxide coatings
Metal coatings
Titanium (Ti) implantation has been shown to improve the corrosion resistance of AZ91 alloy [18]. The vapour deposition of aluminium (Al) has been applied to Mg-based alloys and has been shown to decrease the degradation rate [19]. The downside of this Al deposition, however, is its low biocompatibility. Al has also shown signs of corroding in sodium chloride (NaCl) solution, an outcome that does not suggest efficiency for an implant coating material [13]. Therefore further analysis of other more effective materials is needed for a better understanding of deposited metal coatings that produce low toxicity values when implanted. Gold was also investigated as a coating for Mg alloy in another patent [20]. However, others workers have demonstrated that stents coated with gold increase the risk of restenosis [21].
Metallic oxide and metallic hydroxide coatings
A thin film of metallic oxide can provide an interface with vascular milieu for a stent as well as enhancing its biocompatibility [22]. Therefore some metallic oxides, such as titanium dioxide and zirconium oxide, were coated on stents to improve their performance. A titanium-nitrideoxide coating was investigated to reduce neointimal hyperplasia. Compared with stainless steel, two stents coated with different titanium-nitride-oxide coatings showed better biocompatibility and reduced neointimal area [23]. Another study investigated converting metallic polycrystalline oxides into an amorphous oxide to increase the corrosion resistance of stents. The results indicated that an amorphous oxide-coated stent was safer and more biocompatible [24]. Earlier research suggests that nickel (Ni)–Ti stents may have a native oxide layer. By an electropolishing, heat treatment and passivation process, the deformed native oxide layer on a Ni–Ti stent can be removed and a new uniform oxide layer will form. These processes improved the corrosion resistance of Ni–Ti stents due to the uniformity of the oxide layer grown on the stent surface [25]. Zirconium oxide [26], iridium oxide [27], and noble metal oxides [28] have also been reported in patents as coatings for stents. Another patent reported a multilayer metal and metallic oxide coating for a stent: the inner metallic layer was a noble metal or alloy and the outer layer was iridium oxide [29].
The simplest method of generating a coating on an Mg sample is to simply expose it to the environment (air and water). This process, called passivation, exposes the sample to atmospheric humidity at a level sufficient to create a Mg hydroxide layer on the outer surface; continuing to store the sample in air creates an additional, beneficial, carbonate layer. Oxide layers usually provide better corrosion protection than hydroxide layers. The Mg(OH)2 layer actually increases in thickness on the implant surface over time, whereas the MgO layer stays at a relatively constant thickness, but can be increased through thermal treatment [30]. Also, alkaline solution treatment was also believed to create a layer of Mg(OH)2, MgCO3, and MgO on the surface of Mg alloys [14].
Chemical conversion coating
Chemical conversion coating involves taking the surface of the metal implant material and converting it into the desired coating via a chemical or electrochemical process. In the past, the process was performed to create chromate layers because of its ability to provide effective corrosion resistance. However harmful environmental outcomes arise from the use of chromium (Cr) in chemical conversion baths, therefore a Cr substitute must be found [31].
Metal phosphate compounds as a possible replacement were investigated. The results of Chen et al [12] suggested that the performance of these metal phosphate layers were significantly dependent on the pre-treatments used to make the layers more or less functional. For biomedical applications, research shows that two potential coating materials, fluoride-based layers and calcium phosphates, can be applied.
Zhang et al [169] explored the preparation of calcium phosphate coatings on an Mg–1.0Ca alloy using electrochemical deposition. Enhanced corrosion resistance was observed in Hank’s solutions. The thickness and morphology of the coating had a significant effect on the corrosion behaviour of this Mg alloy. Another investigation showed that calcium phosphate precipitation could be controlled by an anodization and autoclaving process [33].
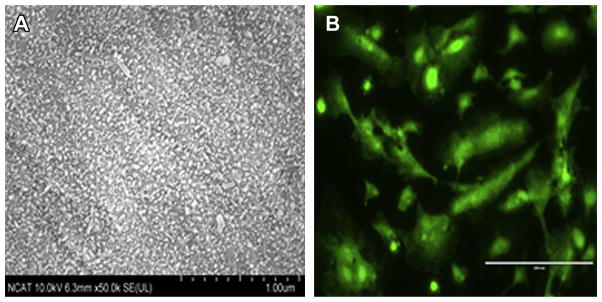
A MgF2 suspension was also synthesized to prevent corrosion of the Mg alloy by Waltz et al [34] via a plasma suspension spraying process. Li et al [35] studied the corrosion resistance and cytotoxicity of MgF2-coated Mg–1Ca alloy by a vacuum evaporation deposition method. The results indicated that MgF2-coated samples had much lower degradation rate than uncoated samples. Moreover, the MgF2 coating induced calcium phosphate deposition on Mg–1Ca alloys, which may promote bone cell growth. Pereda et al [36] attempted to inhibit the corrosion of pure Mg by fluoride treatments and their results showed that different conditions could form different films on Mg. At 0.1 M fluoride solution treatment, KMgF3 was present on the surface, whereas an MgF2 film was observed at 1 M fluoride solution treatment. Another study investigated the biocompatibility of fluoride-coated Mg–Ca alloys in a subcutaneous mouse model. No visible inflammation reaction or broad proliferative effect was observed, indicating sufficient biocompatibility of fluoride-coated Mg alloys [37]. Studies from our group also showed that fluoride-coated Mg–rare earth element alloys had much better corrosion resistance, endothelial attachment, growth, and proliferation (Fig. 1).
Figure 1.
(A) Scanning electron microscopy image of fluoride coating morphologies on a magnesium alloy; scale bar = 10.0 μm. (B) Endothelialization on the same magnesium alloy surface coated with fluoride; scale bar = 10.0 μm.
Nanoporous inorganic coatings
Nanoporous materials have a large surface area and surface modifications at the nanoscale level can increase biocompatibility [38]. Similarly, properties of nanoporous coatings can be easily adjusted by the manipulation of surface properties based on the specific application [39].
Hydroxyapatite
Hydroxyapatite (HA) is one of the main inorganic components of bone and teeth [40,41]. HA and some calcium phosphate compounds are used as coatings because of their biocompatibility and bioactivity [42]. Various HA and calcium phosphate coatings are summarized in Table 1 [41,44,48,55,108,150–161]. Calcium phosphate combined with zoledronate was used as a bone substitute. In vitro experiments on an unfractured rabbit bone indicated that calcium phosphate loaded with zoledronate decreased the area resorbed compared with calcium phosphate without zoledronate [43]. Fluorine-doped hydroxyapatite (FHA) coating is porous and loose and can ensure the long stability of an Mg alloy implant. However, different electrodeposition coating processes can have an effect on the corrosion resistance of FHA. For example, FHA coatings processed by a pulse reverse current had better microstructure and corrosion resistance than coatings processed by traditional cathodic processes [44].
Table 1.
Hydroxyapatite and calcium phosphate coated magnesium and magnesium alloys.
| Reference | Mg/Mg alloy | HA/calcium phosphate-related compounds | In vitro/in vivo tests | Results |
|---|---|---|---|---|
| Chen et al. [150] | Mg | HA–Mg(OH)2 | Electrochemical and immersion tests | Corrosion not completely stopped, but moderated rapid corrosion |
| Bornapour et al. [151] | Mg–0.5Sr | HA and Mg(OH)2 (formed by degradation in SBF) | Immersion tests, cytotoxicity evaluation, in vivo test in dog | Formation of an Sr-substituted HA layer in SBF; no thrombosis during 3-week implantation |
| Wen et al. [152] | AZ31 | HA | Electrochemical test, immersion test | Alkaline-treated HA more stable; Ca–P–Mg deposition inhibited further corrosion |
| Zhang et al. [153] | Mg–Al, Mg–Ca | Calcium phosphate | Electrochemical test, immersion test | Coated samples had a higher free corrosion potential, lower corrosion current densities, and lower hydrogen elution rate |
| Jamesh et al. [154] | CP-Mg | HA | Potentiodynamic polarization tests, EIS studies | Three-fold charge transfer resistance increase in coated CP-Mg; improved corrosion-protective ability |
| Wu et al. [155] | AZ91D | Calcium phosphate/chitosan | Immersion test in PBS | Percentage of Ca(OH)2 in deposited layers influenced conversion rate and composition |
| Hiromoto and Tomozawa [156] | AZ31 | HA | Immersion test, polarization test | Reduced Mg2+ ion release and corrosion current density |
| Abdal-hay [108] | AZ31 | HA–PLLA | In vitro degradation test, electrochemical corrosion test, mechanical properties test, cell viability assay | Improved performance for high corrosion rate |
| Feng and Han [157] | ZK60A | Calcium polyphosphate | Immersion test, electrochemical test | Enhanced corrosion resistance |
| Xu et al. [55] | Mg–Mn–Zn | Calcium phosphate | In vitro cell test, in vivo study | Enhanced cytocompatibility |
| Gao et al. [48] | Mg–Zn–Ca | Nano HA | Bonding strength test, electrochemical test, immersion test | Corrosion current density of coated alloys decreased; good corrosion resistance |
| Bakhsheshi-Rad et al. [158] | Mg–Ca–Zn | Nano-HA/MgF2; DCPD/MgF2 | Electrochemical test, immersion test | Enhanced polarization resistance and corrosion potential of coated alloys |
| Meng et al. [44] Wang et al. [159] |
Mg–Zn–Ca Mg–Zn–Ca |
Fluorine-doped HA Ca-deficient HA |
Electrochemical test, immersion test Coating adhesion test, electrochemical test, SSRT test |
PRC coating higher corrosion resistance, lower corrosion rate, compared with TED coating. Increased Ecorr value of coated alloys; delayed corrosion of coated alloys |
| Rojaee et al. [47] | AZ91 | Nano HA | In vitro bioactivity evaluation, electrochemical test | Higher corrosion resistance of coated alloys |
| Jo et al. [160] | Mg | HA, MgF2 | Immersion test, in vitro cell test, in vivo test | Improved corrosion resistance and bioactivity of coated Mg |
| Zhang et al. [41] | AZ91D | Calcium phosphate/chitosan | Scratch test, immersion test | Optimized fabrication parameters; enhanced corrosion protection |
| Niu et al. [161] | Mg–Nd–Zn–Zr | Brushite | Immersion test, electrochemical test, cytotoxicity evaluation, in vitro cell adhesion test, haemolysis test, in vivo test | Enhanced corrosion test; reduced haemolysis; produced less gas; good surface bioactivity |
DCPD = dicalcium phosphate dehydrate; EIS = electrochemical impedance spectroscopy; HA = hydroxyapatite; PBS = phosphate-buffered solution; PLLA = poly-l-lactic acid; PRC = pulse reverse current; SBF = simulated body fluid; SSRT = slow strain rate tensile; TED = traditional cathodic process.
Micro-arc oxidation (MAO) technology is widely used in surface modification. It has also been well investigated as a coating on Mg alloys. Tang et al [45] compared the electrophoresis deposition (EPD) technique with MAO on Mg alloys to develop surface coatings for orthopaedic applications. Both in vitro and in vivo tests indicated that the EPD technique produced better corrosion resistance than MAO. Another study explored the effect of MAO coatings on Mg–Ca alloys. MAO treatment enhanced the corrosion resistance and biocompatibility of the Mg–Ca alloy and the corrosion resistance increased with voltage [46]. Some researchers are trying to apply MAO technology to HA coatings. The role that the MAO usually plays is to create a porous coating and then HA or another calcium phosphate based coating is adhered to the MAO coating. A three-layer coating was used to delay the corrosion behaviour of Mg alloy AZ91. The inner layer was an MgF2 conversion coating and the intermediate layer was produced by MAO with nanostructured hydroxyapatite as the outer layer. Such coated alloys had enhanced corrosion resistance as well as good cell adhesion in vitro [47]. Gao et al [48] developed a two-layer HA coating to enhance Mg alloy corrosion resistance and biocompatibility. The inner layer of coating was produced by MAO technology with the HA coating adhered to it as an outer layer. Electrochemical tests showed that the corrosion potential of coated Mg alloys increased by 161 mV. Only one layer of MAO coating was necessary to protect pure Mg and the coated Mg had superior corrosion resistance to pure Mg [49].
Because of structural and constituted similarities, most HA and calcium phosphate compound coatings have been applied in orthopaedics. Mg has been associated with the mineralization of calcified bones and teeth [50]. Moreover, research has shown that Mg ions can improve bone cell adhesion on the surface of implants [51] and HA can promote bone cell adhesion and proliferation [52,53]. Also, the mechanical properties of Mg alloys are more similar to those of bone than other alloys, thus decreasing stress-shielding effects [7]. Therefore, HA-coated Mg alloys are ideal biomaterials for orthopaedic applications and have been widely investigated to prove this idea [8,51,54,55]. HA coatings applied to cardiovascular stents have also been reported. Costa et al [56] studied HA coatings on stainless-steel stents loaded with low-dose sirolimus. Clinical trials demonstrated the antiproliferative effect and biocompatibility of HA. No patient had obvious neointimal hyperplasia during the trial. Another study of drug-eluting stents with HA coatings was conducted to evaluate platelet activation and deposition. The results showed that in an ex vivo model, a cobalt–Cr alloy coated with HA did not increase platelet reactivity and adhesion in human blood compared with a bare metal stent, demonstrating the biocompatibility of HA [57]. However, some work has indicated that calcium phosphate might cause vascular calcification and cell mineralization [58–61].
Sol–gel processed coatings
Sol–gel application is a process in which inorganic precursors undergo various reactions to form a three-dimensional molecular network [62]. Sol–gel coatings can form a dense barrier to protect corrosive metal substrates and can be used to synthesize coatings with controlled properties [63]. Several attempts to produce inorganic coatings by the sol–gel method have been reported. Different sol–gel films were coated on Mg alloys, including titanium dioxide [64] and a methyltriethoxysilane–tetraethoxysilane mixture [65]; both showed good corrosion resistance.
Polymer coatings
Polymer coatings can be used to enhance corrosion resistance and the abrasion and wear properties of Mg alloys [66]. Polymer coatings can also provide mechanical support or serve as a drug vehicle for controlled release [67].
Natural polymers
Compared with synthesized polymers, natural polymers have much higher biocompatibility [68]. Some natural polymers, such as collagen [69–71] and chitosan [72–76] have been used as coatings for stents and demonstrated to have good biocompatibility. Collagen coatings have also been used in orthopaedic implants for bone regeneration [77]. A novel tripolymer composed of collagen, RGD (Arg-Gly-Asp) peptide, and chondroitin sulfate was produced to coat Ti implants to enhance bone healing [78]. Chitosan is also a widely used coating for orthopaedic tissue-engineering materials [79,80]. Some peptides, such as GFOGER (glycine-phenylalanine- hydroxyproline-glycine-glutamate-arginine), have also been investigated in orthopaedic tissue healing or bone repair [81,82]. However, both collagen and chitosan may cause an immunological response [83] and activate complement and blood coagulation [84]. Another natural polymer, bacterial cellulose (BC), because of its high mechanical strength, high water content, and good biocompatibility [85], has been widely used in vascular grafts or as a vascular replacement [86–89], in wound healing [90] and in tissue-engineering scaffolds [91,92]. Composites formed by BC mixed with other substances, e.g., poly(vinyl alcohol), as a coating for stents was reported in a patent [93]. BC was also used as a tablet coating for a drug-release system [94]. Moreover, as a biodegradable coating, the end degradable product of BC is glucose, which is non-toxic to the body. These studies suggest that BC has a great potential as a biodegradable coating for drug-eluting stents (DES) and orthopaedic implant applications; more work is needed to explore such possibilities.
Synthesized polymers
Most polymer coatings in cardiovascular and orthopaedic applications are synthesized polymers. This is a result of the easily altered properties of synthesized polymers through the manipulation of the synthesis condition or other modifications. For polymers to be used as coatings in these applications, the prevention of rapid corrosion with good biocompatibility and controlled drug release are of great interest. Table 2 [9,104,113,121,163–168] summarizes the synthesized polymers used for Mg and Mg alloy coatings.
Table 2.
Synthesized polymer coatings for magnesium and magnesium alloys.
| Reference | Mg/Mg alloys | Polymers |
|---|---|---|
| Xu and Yamamoto [104] | Mg | PLLA, PCL |
| Chen et al. [9] | Mg | PLLA, PCL |
| Li et al. [113] | Mg–6Zn | PLGA |
| Lu et al. [162] | AZ81 | PLLA, PLGA |
| Zomorodian et al. [163] | AZ31 | PEI, diethylene triamine, HA |
| Scharnagl et al. [121] | AZ31 | PEI |
| Truong et al. [164] | Mg–Mn alloy | Polypyrrole |
| Yfantis et al. [165] | AZ31 | Polyacrylic–polypyrrole |
| Wang et al. [166] | Mg–Zn–Mn | PTMC |
| Liu et al. [167] | WE43 | Chitosan, PSS, polyelectrolyte |
| Adden [168] | Mg + rare earth elements | Polyphosphazene |
PLLA = poly-l-lactic acid; PCL = poly(ε-caprolactone); PLGA = poly(lactide-co-glycolide); PEI = poly(ether imide); PTMC = poly(1,3-trimethylene carbonate); PSS = poly(styrene sulfonate).
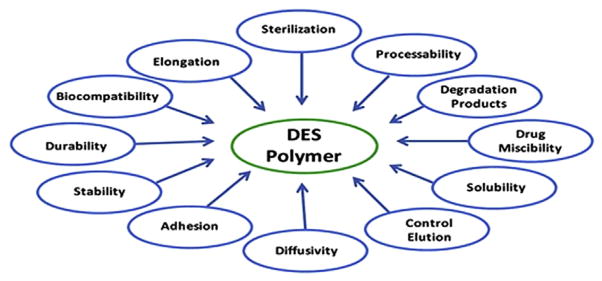
Synthesized polymers used in DES and orthopaedic implants can be permanent or biodegradable. Permanent polymers can allow controlled drug release and remain after the drug is completely released [95,96]. Polymer properties affecting DES performance are summarized in Fig. 2[112]. The polymers used in the first and second generations of DES are permanent. They can reduce angiographic restenosis and demonstrate effectiveness in PCI. However, tests show that they will cause late stent thrombosis [97]. Moreover, long-stay polymers can cause inflammatory reactions [98]. To solve the problems that permanent polymers introduce, various kinds of biodegradable polymers, such as poly-l-lactic acid (PLLA) [99] and poly(lactide-co-glycolide) (PLGA) [100,101], were synthesized and tested; both had good chemical properties, low immunogenicity and toxicity, and predictable biodegradation kinetics [102].
Figure 2.
Polymer properties affecting drug-eluting stent (DES) performance [112].
PLLA is a common biodegradable polymer with good mechanical properties and biocompatibility. Moreover, the end biodegradable product can be removed by body fluids and then metabolized by the liver and kidneys [103]. Xu and Yamamoto [104] compared PLLA with another biodegradable polymer, poly(ε-caprolactone) (PCL) as a coating to protect Mg. The results showed that PLLA had a better adhesion strength with Mg substrates than PCL and that more cells were more proliferative on PLLA. Interestingly, the early performance of PLLA-coated DES was similar to a bare metal stent, though the long-term performance was not studied [105]. In vitro dynamic degradation of pure Mg with PLLA and PCL coatings showed that PCL had better corrosion resistance in modified simulated body fluid solution than PLLA [9]. Wong et al [106] reported a polymer fabricated by PCL and dichloromethane to enhance the performance of AZ91 alloy in orthopaedic applications. The results demonstrated improved corrosion resistance and good cell biocompatibility. Gollwitzer et al [107] showed that a PLLA coating for orthopaedic implants based on three different alloys had good stability. Abdal-hay et al [108] studied an HA-doped PLLA coating with respect to the bioactivity and corrosion behaviour of AZ 31 alloy as an orthopaedic implant; the coated samples showed a better biocompatibility and bending strength.
PLGA has been used in drug-delivery systems [109,110] and in tissue engineering for decades [102,111]. It can be hydrolysed in vivo by breaking ester linkages into lactic and glycolic acids, which are non-toxic [112]. In vitro degradation tests on PLGA as a coating for Mg–6Zn alloy indicated that it corroded slower and was more suitable for cell attachment than bare Mg–6Zn alloy [113]. Another study compared in vitro degradation with in vivo changes for PLGA-coated DES. The coating degradation rate was similar in both in vitro and in vivo tests. However, polymer degradation in a real vascular bed may give different results [114]. Ostrowski et al [115] used various concentrations of PLGA to control the thickness of coatings in orthopaedic applications. Although PLLA and PLGA were well investigated and demonstrated acceptable biocompatibility, some tests reported foreign body reactions with PLLA [116,117] and PLGA [118].
Poly(ether imide) (PEI) has good mechanical properties and is stable at high temperatures and has therefore been explored as a coating for Mg alloys. da Conceicao and coworkers [119,120] and Scharnagl et al [121] studied PEI as a coating for the Mg alloy AZ31. Thin layers of PEI showed high resistance to corrosion when exposed to a 3.5% NaCl solution. This could be due to the formation of Mg polyamate, which increased the corrosion impedance of the PEI coating.
Coatings and biocompatibility
Although coatings can usually enhance the corrosion resistance of Mg-based implants, sometimes the coating material itself may cause a chronic inflammatory response, especially for permanent polymer coatings [122]. A coating with good biocompatibility should not produce obvious foreign body reactions, blood coagulation, nor inflammation [123]. The physical and chemical properties of coatings determine their biocompatibility. Surface properties such as surface ligands [32], molecular chirality [124], surface patterns [125], surface roughness [126], and chemical coatings [127,128] can regulate cell behaviour significantly, thus having an effect on biocompatibility. Moreover, composites formed by combining coatings and other chemicals could enhance biocompatibility. For example, BC combined with gelatin has a better bioactivity and biocompatibility than BC alone [129]. Composite coatings produced by the sol–gel process showed a higher compatibility than coatings produced by MAO [130]. In DES, drugs of the “limus” family [57,95,99,131] and paclitaxel [95,132,133] can help inhibit smooth muscle cell proliferation. Another approach to improve the in vivo performance of stents is to promote endothelialization. Vascular endothelial growth factor can be loaded on to coatings to simulate endothelialization [98,123]. In orthopaedic implants, antibiotics are loaded on to coatings to reduce inflammation and infection [134,135].
Coatings for controlled drug release
In addition to enhanced biocompatibility and corrosion resistance, coatings can also be used to control the release rate of drugs. Some inorganic coatings, such as aluminium oxide, have been applied in drug-delivery systems. Aluminium oxide mixed with PLLA and PMMA (polymethyl methacrylate) has been investigated as a coating for drug release [112,136]. Nanoporous aluminium oxide, as a drug carrier, has also been reported [137,138]. A chitosan- and PLGA-coated titanium oxide nanotube to control drug release and enhance osteoblast adhesion was explored. Depending on the thickness of the polymers, reduced burst release (from 77% to >20%) and extended overall release (from 4 days to 30 days) were observed [139]. Kikuchi and Okano [140] reviewed pulsatile control of drug release using hydrogels. Coatings can provide a reservoir for drugs and controlled drug release. Sustained drug release is essential in preventing an inflammation response and reducing late restenosis. The drug release kinetics of various DES samples was similar. Many DES samples had a burst release at an early stage (24–36 hours) and then a sustained release for at least 30 days, followed by reduced neointimal hyperplasia and restenosis [112,141]. It was found that the morphology of the coating surface had no effect on the amount of drug released [142]. PLLA, a widely used biodegradable polymer coating in DES, is also a good candidate for controlled drug release. Some factors, such as the solvent removal rate [143], the matrix coated on PLLA [144], and microsphere processing parameters [145], were explored for a PLLA microsphere. Copolymers of PLGA and mPEG [monomethoxy poly(- ethyleneglycol)] nanoparticles was also investigated for polymer degradation and drug release [146].
The most common mechanisms of drug release are diffusion and degradation [147,148]. For diffusion, the coating acts as a rate control membrane. Degradation- controlled drug release is based on the degradation of the polymer that covered the drug reservoir. It has been shown that porosity and size had effects on the drug release mechanisms of PLGA [149].
Similarities and differences in the coatings used in stents and orthopaedic implants
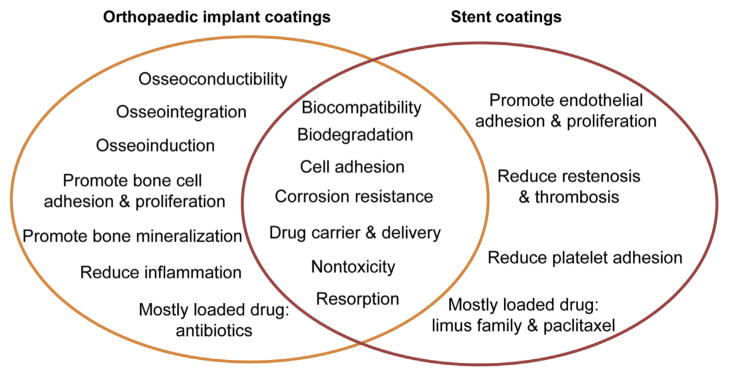
The main purpose of the coatings used in Mg-based implants is to prevent rapid corrosion and improve the biocompatibility of the implants. Most surface modification methods can be used in both applications. However, the surface modification methods may vary because of the different cell types the coatings may interface with. For stents, biodegradable polymers with good biocompatibility and ideal controlled drug release profiles are of great interest. Although some biodegradable polymers have been reported in orthopaedic applications, the most commonly investigated coatings are still HA and calcium phosphate compounds because of their structural and constituted similarities to bone. In fact, HA-coated Mg alloys have been well studied and display good biocompatibility in orthopaedic applications. However, they may not be a good choice for DES because of potential vascular calcification. Drug-eluting orthopaedic implants based on Mg alloys have not been well explored to date. The differences and similarities in coatings for orthopaedic implants and stents are summarized in Fig. 3.
Figure 3.
Differences and similarities: coating purposes and functions for stents and orthopaedic implants.
Conclusion
Mg-based biomaterials have a great potential in cardiovascular and orthopaedic applications due to their biodegradability, biocompatibility, and appropriate mechanical properties. However, they also have the limitation of low corrosion resistance and suboptimal biocompatibility. Coating technology is one of the leading approaches used to overcome these problems.
Surface coatings played an important role in the development of stents. Stent technology emerged in the 1980s and developed rapidly from bare metal stents to coated DES stents. Mg-based stents represent the latest generation of biodegradable stents and offer appealing features in clinical applications. There have been several clinical trials with promising outcomes on such Mg-based stents. Coatings on Mg-based stents can vary from metal and inorganic coatings to biodegradable coatings. Among these, biodegradable polymer coatings with drug-eluting features might be a better choice because of their advanced biocompatibility and capability to reduce late restenosis compared with other coatings. In the future design of coatings for Mg-based stents, novel biodegradable polymers or copolymers should be explored to further enhance biocompatibility with sustained control of drug release. Moreover, new drugs that can inhibit smooth muscle cell proliferation and reduce neointimal hyperplasia while promoting endothelialization are preferred.
In orthopaedic applications, HA and calcium phosphate compounds have many advantages over other coatings, such as structural and constituted similarity, and promoting bone cell adhesion and proliferation. There are fewer reports on orthopaedic implants with drug-eluting features. It would be interesting to use drug-eluting orthopaedic implants with controlled release to achieve an even better healing process.
Acknowledgments
The authors acknowledge funding from the National Institute of Health (SC2NS082475) and support from the National Science Foundation Engineering Research Center–Revolutionizing Metallic Biomaterials (ERC–RMB) (NSF-0812348) at North Carolina Agricultural and Technical State University, Greensboro, NC, USA.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
References
- 1.Eliezer D, Aghion E, Froes F. Magnesium science, technology, and applications. Adv Perform Mater. 1998;5:201–12. [Google Scholar]
- 2.Witte F, Hort N, Vogt C, Cohen S, Kainer K, Willumeit R, et al. Degradable biomaterials based on magnesium corrosion. Curr Opin Solid State Mater Sci. 2008;12:63–72. [Google Scholar]
- 3.Zeng R, Dietzel W, Witte F, Hort N, Blawert C. Progress and challenge for magnesium alloys as biomaterials. Adv Eng Mater. 2008;10:B3–14. [Google Scholar]
- 4.Reifenrath J, Bormann D, Meyer-Lindenberg A. Magnesium alloys as promising degradable implant materials in orthopaedic research. Czerwinski F, editor. [accessed 21.02.14];Magnesium alloys—corrosion and surface treatments. 2011 :94–108. Available from: http://www.intechopen.com/books/magnesiumalloys-corrosion-and-surface-treatments/magnesium-alloys-as-promising-degradable-implant-materials-in-orthopaedic-research.
- 5.Seal CK, Vince K, Hodgson MA. Biodegradable surgical implants based on magnesium alloys – a review of current research. IOP Conf Ser Mater Sci Eng. 2009;4:012011. [Google Scholar]
- 6.Song G. Control of biodegradation of biocompatible magnesium alloys. Corrosion Sci. 2007;49:1696–701. [Google Scholar]
- 7.Staiger MP, Pietak AM, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27:1728–34. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A, Wirth CJ, et al. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26:3557–63. doi: 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Song Y, Zhang S, Li J, Zhao C, Zhang X. Interaction between a high purity magnesium surface and PCL and PLA coatings during dynamic degradation. Biomed Mater. 2011;6:025005. doi: 10.1088/1748-6041/6/2/025005. [DOI] [PubMed] [Google Scholar]
- 10.Zhao N, Zhu D. Application of Mg-based alloys for cardiovascular stents. Int J Biomed Eng Technol. 2013;12:382–98. [Google Scholar]
- 11.Gray JE, Luan B. Protective coatings on magnesium and its alloys–a critical review. J Alloys Compounds. 2002;336:88–113. [Google Scholar]
- 12.Chen X, Birbilis N, Abbott TB. Review of corrosion-resistant conversion coatings for magnesium and its alloys. Corrosion. 2011;67:D1–16. [Google Scholar]
- 13.Hornberger H, Virtanen S, Boccaccini AR. Biomedical coatings on magnesium alloys – a review. Acta Biomater. 2012;8:2442–55. doi: 10.1016/j.actbio.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Tang J, Zhang P, Li Y, Wang J, Lai Y, et al. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: a general review. J Biomed Mater Res B Appl Biomater. 2012;100:1691–701. doi: 10.1002/jbm.b.32707. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Cheng Y, Zheng YF, Zhang X, Xi TF, Wei SC. Surface characteristics and corrosion behaviour of WE43 magnesium alloy coated by SiC film. Appl Surf Sci. 2012;258:3074–81. [Google Scholar]
- 16.Li M, Cheng Y, Zheng YF, Zhang X, Xi TF, Wei SC. Plasma enhanced chemical vapor deposited silicon coatings on Mg alloy for biomedical application. Surf Coat Technol. 2013;228(Suppl 1):S262–5. [Google Scholar]
- 17.Ye CH, Zheng YF, Wang SQ, Xi TF, Li YD. In vitro corrosion and biocompatibility study of phytic acid modified WE43 magnesium alloy. Appl Surf Sci. 2012;258:3420–7. [Google Scholar]
- 18.Liu C, Xin Y, Tian X, Zhao J, Chu PK. Corrosion resistance of titanium ion implanted AZ91 magnesium alloy. J Vac Sci Technol A. 2007;25:334. [Google Scholar]
- 19.Christoglou C, Voudouris N, Angelopoulos GN, Pant M, Dahl W. Deposition of aluminium on magnesium by a CVD process. Surf Coat Technol. 2004;184:149–55. [Google Scholar]
- 20.Hossainy SFA, Roorda WE, Michal ET. Rate limiting barriers for implantable devices and methods for fabrication thereof. 20030104028 A1. US patent. 2003
- 21.Kastrati A, Schomig A, Dirschinger J, Mehilli J, von Welser N, Pache J, et al. Increased risk of restenosis after placement of gold-coated stents: results of a randomized trial comparing gold-coated with uncoated steel stents in patients with coronary artery disease. Circulation. 2000;101:2478–83. doi: 10.1161/01.cir.101.21.2478. [DOI] [PubMed] [Google Scholar]
- 22.Palmaz JC. Intravascular stents: tissue-stent interactions and design considerations. Am Roentgen Ray Soc. 1993;160:613–8. doi: 10.2214/ajr.160.3.8430566. [DOI] [PubMed] [Google Scholar]
- 23.Windecker S, Mayer I, De Pasquale G, Maier W, Dirsch O, De Groot P, et al. Stent coating with titanium-nitride-oxide for reduction of neointimal hyperplasia. Circulation. 2001;104:928–33. doi: 10.1161/hc3401.093146. [DOI] [PubMed] [Google Scholar]
- 24.Shih C-C, Lin S-J, Chung K-H, Chen Y-L, Su Y-Y. Increased corrosion resistance of stent materials by converting current surface film of polycrystalline oxide into amorphous oxide. J Biomed Mater Res. 2000;52:323–32. doi: 10.1002/1097-4636(200011)52:2<323::aid-jbm11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 25.Trepanier C, Tabrizian M, Yahia L’H, Bilodeau L, Piron D. Effect of modification of oxide layer on NiTi stent corrosion resistance. J Biomed Mater Res. 1998;43:433–40. doi: 10.1002/(sici)1097-4636(199824)43:4<433::aid-jbm11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Davidson JA. Zirconium oxide and zirconium nitride coated stents. 5649951 A. US patent. 1997
- 27.Alt E, Stotts L. Vascular and endoluminal stents with iridium oxide coating. 5980566 A. US patent. 1999
- 28.Scheuermann T, Stehling L. Metal stent with surface layer of noble metal oxide and method of fabrication. 7402173 B2. US patent. 2008
- 29.Alt E. Vascular and endoluminal stents with improved coatings. 6099561 A. US patent. 2000
- 30.Santamaria M, Di Quarto F, Zanna S, Marcus P. Initial surface film on magnesium metal: a characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS) Electrochim Acta. 2007;53:1314–24. [Google Scholar]
- 31.Umehara H, Takaya M, Terauchi S. Chrome-free surface treatments for magnesium alloy. Surf Coat Technol. 2003;169–170:666–9. [Google Scholar]
- 32.Lai Y, Xie C, Zhang Z, Lu W, Ding J. Design and synthesis of a potent peptide containing both specific and non-specific cell-adhesion motifs. Biomaterials. 2010;31:4809–17. doi: 10.1016/j.biomaterials.2010.02.064. [DOI] [PubMed] [Google Scholar]
- 33.Hiromoto S, Shishido T, Yamamoto A, Maruyama N, Somekawa H, Mukai T. Precipitation control of calcium phosphate on pure magnesium by anodization. Corrosion Sci. 2008;50:2906–13. [Google Scholar]
- 34.Waltz F, Swider MA, Hoyer P, Hassel T, Erne M, Möhwald K, et al. Synthesis of highly stable magnesium fluoride suspensions and their application in the corrosion protection of a magnesium alloy. J Mater Sci. 2011;47:176–83. [Google Scholar]
- 35.Li N, Li YD, Wang YB, Li M, Cheng Y, Wu YH, et al. Corrosion resistance and cytotoxicity of a MgF2 coating on biomedical Mg-1Ca alloy via vacuum evaporation deposition method. Surf Interf Anal. 2013;45:1217–22. [Google Scholar]
- 36.Pereda MD, Alonso C, Burgos-Asperilla L, del Valle JA, Ruano OA, Perez P, et al. Corrosion inhibition of powder metallurgy Mg by fluoride treatments. Acta Biomater. 2010;6:1772–82. doi: 10.1016/j.actbio.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Misra RD, Nune C, Pesacreta TC, Somani MC, Karjalainen LP. Interplay between grain structure and protein adsorption on functional response of osteoblasts: ultrafine-grained versus coarse-grained substrates. J Biomed Mater Res A. 2013;101:1–12. doi: 10.1002/jbm.a.34105. [DOI] [PubMed] [Google Scholar]
- 38.Roszek B, de Jong WH, Geertsma RE. Welfare and Sports RIVM Report. 2005. Nanotechnology in medical applications: state-of-the-art in materials and devices. Department of Pharmaceutical Affairs and Medical Technology of the Dutch Ministry of Health; pp. 1–123. [Google Scholar]
- 39.Gultepe E, Nagesha D, Sridhar S, Amiji M. Nanoporous inorganic membranes or coatings for sustained drug delivery in implantable devices. Adv Drug Deliv Rev. 2010;62:305–15. doi: 10.1016/j.addr.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Abdel-Aal EA, Dietrich D, Steinhaeuser S, Wielage B. Electrocrystallization of nanocrystallite calcium phosphate coatings on titanium substrate at different current densities. Surf Coat Technol. 2008;202:5895–900. [Google Scholar]
- 41.Zhang J, Dai C-S, Wei J, Wen Z-H. Study on the bonding strength between calcium phosphate/chitosan composite coatings and a Mg alloy substrate. Appl Surf Sci. 2012;261:276–86. [Google Scholar]
- 42.Huang Y, Qu Y, Yang B, Li W, Zhang B, Zhang X. In vivo biological responses of plasma sprayed hydroxyapatite coatings with an electric polarized treatment in alkaline solution. Mater Sci Eng C. 2009;29:2411–6. [Google Scholar]
- 43.Josse S, Faucheux C, Soueidan A, Grimandi G, Massiot D, Alonso B, et al. Chemically modified calcium phosphates as novel materials for bisphosphonate delivery. Adv Mater. 2004;16:1423–7. [Google Scholar]
- 44.Meng EC, Guan SK, Wang HX, Wang LG, Zhu SJ, Hu JH, et al. Effect of electrodeposition modes on surface characteristics and corrosion properties of fluorine-doped hydroxyapatite coatings on Mg–Zn–Ca alloy. Appl Surf Sci. 2011;257:4811–6. [Google Scholar]
- 45.Tang J, Wang J, Xie X, Zhang P, Lai Y, Li Y, et al. Surface coating reduces degradation rate of magnesium alloy developed for orthopaedic applications. J Orthop Translation. 2013;1:41–8. [Google Scholar]
- 46.Gu XN, Li N, Zhou WR, Zheng YF, Zhao X, Cai QZ, et al. Corrosion resistance and surface biocompatibility of a microarc oxidation coating on a Mg–Ca alloy. Acta Biomater. 2011;7:1880–9. doi: 10.1016/j.actbio.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 47.Rojaee R, Fathi M, Raeissi K. Electrophoretic deposition of nanostructured hydroxyapatite coating on AZ91 magnesium alloy implants with different surface treatments. Appl Surf Sci. 2013;285:664–73. [Google Scholar]
- 48.Gao JH, Guan SK, Chen J, Wang LG, Zhu SJ, Hu JH, et al. Fabrication and characterization of rod-like nano-hydroxyapatite on MAO coating supported on Mg–Zn–Ca alloy. Appl Surf Sci. 2011;257:2231–7. [Google Scholar]
- 49.Zhao L, Cui C, Wang Q, Bu S. Growth characteristics and corrosion resistance of micro-arc oxidation coating on pure magnesium for biomedical applications. Corrosion Sci. 2010;52:2228–34. [Google Scholar]
- 50.Wiesmann H-P, Tkotz T, Joos U, Zierold K, Stratmann U, Szuwart T, et al. Magnesium in newly formed dentin mineral of rat incisor. J Bone Miner Res. 1997;12:380–3. doi: 10.1359/jbmr.1997.12.3.380. [DOI] [PubMed] [Google Scholar]
- 51.Zreiqat H, Howlett CR, Zannettino A, Evans P, Schulze-Tanzil G, Knabe C, et al. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J Biomed Mater Res. 2002;62:175–84. doi: 10.1002/jbm.10270. [DOI] [PubMed] [Google Scholar]
- 52.Cai Y, Liu Y, Yan W, Hu Q, Tao J, Zhang M, et al. Role of hydroxyapatite nanoparticle size in bone cell proliferation. J Mater Chem. 2007;17:3780–7. [Google Scholar]
- 53.Shi Z, Huang X, Cai Y, Tang R, Yang D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009;5:338–45. doi: 10.1016/j.actbio.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 54.Shadanbaz S, Dias GJ. Calcium phosphate coatings on magnesium alloys for biomedical applications: a review. Acta Biomater. 2012;8:20–30. doi: 10.1016/j.actbio.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Xu L, Pan F, Yu G, Yang L, Zhang E, Yang K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials. 2009;30:1512–23. doi: 10.1016/j.biomaterials.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Costa JR, Jr, Abizaid A, Costa R, Feres F, Tanajura LF, Abizaid A, et al. 1-year results of the hydroxyapatite polymer-free sirolimus-eluting stent for the treatment of single de novo coronary lesions: the VESTASYNC I trial. JACC Cardiovasc Intervent. 2009;2:422–7. doi: 10.1016/j.jcin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Alviar CL, Tellez A, Wang M, Potts P, Smith D, Tsui M, et al. Low-dose sirolimus-eluting hydroxyapatite coating on stents does not increase platelet activation and adhesion ex vivo. J Thromb Thrombolysis. 2012;34:91–8. doi: 10.1007/s11239-012-0696-8. [DOI] [PubMed] [Google Scholar]
- 58.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–70. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 59.Giachelli CM. Vascular calcification: in vitro evidence for the role of inorganic phosphate. J Am Soc Nephrol. 2003;14:300S–4S. doi: 10.1097/01.asn.0000081663.52165.66. [DOI] [PubMed] [Google Scholar]
- 60.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:e10–7. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 61.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–67. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 62.Caruso RA, Antonietti M. Sol–gel nanocoating: an approach to the preparation of structured materials. Chem Mater. 2001;13:3272–82. [Google Scholar]
- 63.Lamaka SV, Montemor MF, Galio AF, Zheludkevich ML, Trindade C, Dick LF, et al. Novel hybrid sol–gel coatings for corrosion protection of AZ31B magnesium alloy. Electrochim Acta. 2008;53:4773–83. [Google Scholar]
- 64.Hu J, Zhang C, Cui B, Bai K, Guan S, Wang L, et al. In vitro degradation of AZ31 magnesium alloy coated with nano TiO2 film by sol–gel method. Appl Surf Sci. 2011;257:8772–7. [Google Scholar]
- 65.Supplit R, Koch T, Schubert U. Evaluation of the anticorrosive effect of acid pickling and sol–gel coating on magnesium AZ31 alloy. Corrosion Sci. 2007;49:3015–23. [Google Scholar]
- 66.Yang J, Cui F, Lee IS. Surface modifications of magnesium alloys for biomedical applications. Ann Biomed Eng. 2011;39:1857–71. doi: 10.1007/s10439-011-0300-y. [DOI] [PubMed] [Google Scholar]
- 67.Byrne R, Joner M, Kastrati A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol. 2009;57:567–84. [PubMed] [Google Scholar]
- 68.Zhao N, Zhu D. Bioscaffolds development for small–diameter vascular grafts. Int J Biomed Engin Technol. 2013;12:113–29. [Google Scholar]
- 69.Buirge AW, Buscemi PJ, Burmeister PH. Stent with collagen. 6391052 B2. US patent. 2002
- 70.Chen MC, Liang HF, Chiu YL, Chang Y, Wei HJ, Sung HW. A novel drug-eluting stent spray-coated with multi-layers of collagen and sirolimus. J Control Release. 2005;108:178–89. doi: 10.1016/j.jconrel.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 71.Lin Q, Ding X, Qiu F, Song X, Fu G, Ji J. In situ endothelialization of intravascular stents coated with an anti-CD34 antibody functionalized heparin-collagen multilayer. Biomaterials. 2010;31:4017–25. doi: 10.1016/j.biomaterials.2010.01.092. [DOI] [PubMed] [Google Scholar]
- 72.Chen MC, Liu CT, Tsai HW, Lai WY, Chang Y, Sung HW. Mechanical properties, drug eluting characteristics and in vivo performance of a genipin-crosslinked chitosan polymeric stent. Biomaterials. 2009;30:5560–71. doi: 10.1016/j.biomaterials.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 73.Lauto A, Ohebshalom M, Esposito M, Mingin J, Li PS, Felsen D, et al. Self-expandable chitosan stent design and preparation. Biomaterials. 2001;22:1869–74. doi: 10.1016/s0142-9612(00)00371-9. [DOI] [PubMed] [Google Scholar]
- 74.Lin C-H, Lin J-C, Chen C-Y, Cheng C-Y, Lin X-Z, Wu J-J. Feasibility evaluation of chitosan coatings on polyethylene tubing for biliary stent applications. J Appl Polym Sci. 2005;97:893–902. [Google Scholar]
- 75.Minami Y, Kaneda H, Inoue M, Ikutomi M, Morita T, Nakajima T. Endothelial dysfunction following drug-eluting stent implantation: a systematic review of the literature. Int J Cardiol. 2013;165:222–8. doi: 10.1016/j.ijcard.2012.03.084. [DOI] [PubMed] [Google Scholar]
- 76.Thierry B, Merhi Y, Silver J, Tabrizian M. Biodegradable membrane-covered stent from chitosan-based polymers. J Biomed Mater Res A. 2005;75:556–66. doi: 10.1002/jbm.a.30450. [DOI] [PubMed] [Google Scholar]
- 77.Rammelt S, Schulze E, Bernhardt R, Hanisch U, Scharnweber D, Worch H, et al. Coating of titanium implants with type-I collagen. J Orthop Res. 2004;22:1025–34. doi: 10.1016/j.orthres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 78.Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials. 2006;27:5561–71. doi: 10.1016/j.biomaterials.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 79.Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–90. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 80.Gu XN, Zheng YF, Lan QX, Cheng Y, Zhang ZX, Xi TF, et al. Surface modification of an Mg-1Ca alloy to slow down its biocorrosion by chitosan. Biomed Mater. 2009;4:044109. doi: 10.1088/1748-6041/4/4/044109. [DOI] [PubMed] [Google Scholar]
- 81.Reyes CD, Petrie TA, Burns KL, Schwartz Z, Garcia AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28:3228–35. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wojtowicz AM, Shekaran A, Oest ME, Dupont KM, Templeman KL, Hutmacher DW, et al. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials. 2010;31:2574–82. doi: 10.1016/j.biomaterials.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petersen N, Gatenholm P. Bacterial cellulose-based materials and medical devices: current state and perspectives. Appl Microbiol Biotechnol. 2011;91:1277–86. doi: 10.1007/s00253-011-3432-y. [DOI] [PubMed] [Google Scholar]
- 84.Amiji MM. Surface modification of chitosan membranes by complexation-interpenetration of anionic polysaccharides for improved blood compatibility in hemodialysis. J Biomater Sci Polym Ed. 1997;8:281–98. doi: 10.1163/156856296x00309. [DOI] [PubMed] [Google Scholar]
- 85.Helenius G, Backdahl H, Bodin A, Nannmark U, Gatenholm P, Risberg B. In vivo biocompatibility of bacterial cellulose. J Biomed Mater Res A. 2006;76:431–8. doi: 10.1002/jbm.a.30570. [DOI] [PubMed] [Google Scholar]
- 86.Andrade FK, Costa R, Domingues L, Soares R, Gama M. Improving bacterial cellulose for blood vessel replacement: functionalization with a chimeric protein containing a cellulose-binding module and an adhesion peptide. Acta Biomater. 2010;6:4034–41. doi: 10.1016/j.actbio.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 87.Charpentier PA, Maguire A, Wan W-K. Surface modification of polyester to produce a bacterial cellulose-based vascular prosthetic device. Appl Surf Sci. 2006;252:6360–7. [Google Scholar]
- 88.Fink H, Hong J, Drotz K, Risberg B, Sanchez J, Sellborn A. An in vitro study of blood compatibility of vascular grafts made of bacterial cellulose in comparison with conventionally- used graft materials. J Biomed Mater Res A. 2011;97A:52–8. doi: 10.1002/jbm.a.33031. [DOI] [PubMed] [Google Scholar]
- 89.Klemm D, Schumann D, Udhardt U, Marsch S. Bacterial synthesized cellulose—artificial blood vessels for microsurgery. Prog Polym Sci. 2001;26:1561–603. [Google Scholar]
- 90.Czaja WK, Young DJ, Kawecki M, Brown RM., Jr The future prospects of microbial cellulose in biomedical applications. Biomacromolecules. 2007;8:1–12. doi: 10.1021/bm060620d. [DOI] [PubMed] [Google Scholar]
- 91.Luo H, Xiong G, Hu D, Ren K, Yao F, Zhu Y, et al. Characterization of TEMPO-oxidized bacterial cellulose scaffolds for tissue engineering applications. Mater Chem Phys. 2013;143:373–9. [Google Scholar]
- 92.Zaborowska M, Bodin A, Backdahl H, Popp J, Goldstein A, Gatenholm P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010;6:2540–7. doi: 10.1016/j.actbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 93.Wan W-K, Millon L. Poly(vinyl alcohol)-bacterial cellulose nanocomposite. 20050037082 A1. US patent. 2005
- 94.Amin M, Abadi A, Ahmad N, Katas H, Jamal J. Bacterial cellulose film coating as drug delivery system physicochemical, thermal and drug release properties. Sains Malaysiana. 2012;41:561–8. [Google Scholar]
- 95.Kim S-J, Kim T-H, Choi J-W, Kwon IK. Current perspectives of biodegradable drug-eluting stents for improved safety. Biotechnol Bioproc Eng. 2012;17:912–24. [Google Scholar]
- 96.Kukreja N, Onuma Y, Daemen J, Serruys PW. The future of drug-eluting stents. Pharm Res. 2008;57:171–80. doi: 10.1016/j.phrs.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 97.Wijns W. First-generation drug-eluting stents..and beyond. Drug Saf. 2009;32:771–3. doi: 10.2165/11316600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 98.Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333–42. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 99.Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia HMG, et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results form multiple imaging methods. Lancet. 2009;373:897–910. doi: 10.1016/S0140-6736(09)60325-1. [DOI] [PubMed] [Google Scholar]
- 100.Carlyle WC, McClain JB, Tzafriri AR, Bailey L, Zani BG, Markham PM, et al. Enhanced drug delivery capabilities from stents coated with absorbable polymer and crystalline drug. J Control Release. 2012;162:561–7. doi: 10.1016/j.jconrel.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Finkelstein A. Local drug delivery via a coronary stent with programmable release pharmacokinetics. Circulation. 2003;107:777–84. doi: 10.1161/01.cir.0000050367.65079.71. [DOI] [PubMed] [Google Scholar]
- 102.Liu X, Won Y, Ma PX. Surface modification of interconnected porous scaffolds. J Biomed Mater Res A. 2005;74:84–91. doi: 10.1002/jbm.a.30367. [DOI] [PubMed] [Google Scholar]
- 103.He Y, Wang W, Ding J. Effects of L-lactic acid and D,L-lactic acid on viability and osteogenic differentiation of mesenchymal stem cells. Chin Sci Bull. 2013;58:2404–11. [Google Scholar]
- 104.Xu L, Yamamoto A. Characteristics and cytocompatibility of biodegradable polymer film on magnesium by spin coating. Colloids Surf B Biointerfaces. 2012;93:67–74. doi: 10.1016/j.colsurfb.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 105.Ormiston JA, Webster MW, Armstrong G. First-in-human implantation of a fully bioabsorbable drug-eluting stent: the BVS poly-L-lactic acid everolimus-eluting coronary stent. Catheter Cardiovasc Interv. 2007;69:128–31. doi: 10.1002/ccd.20895. [DOI] [PubMed] [Google Scholar]
- 106.Wong HM, Yeung KW, Lam KO, Tam V, Chu PK, Luk KD, et al. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials. 2010;31:2084–96. doi: 10.1016/j.biomaterials.2009.11.111. [DOI] [PubMed] [Google Scholar]
- 107.Gollwitzer H, Thomas P, Diehl P, Steinhauser E, Summer B, Barnstorf S, et al. Biomechanical and allergological characteristics of a biodegradable poly (D, L-lactic acid) coating for orthopaedic implants. J Orthop Res. 2005;23:802–9. doi: 10.1016/j.orthres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 108.Abdal-hay A, Barakat NAM, Lim JK. Hydroxyapatite-doped poly(lactic acid) porous film coating for enhanced bioactivity and corrosion behavior of AZ31 Mg alloy for orthopedic applications. Ceramics Int. 2013;39:183–95. [Google Scholar]
- 109.Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)- based drug delivery systems –a review. Int J Pharm. 2011;415:34–52. doi: 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 110.Siepmann J, Elkharraz K, Siepmann F, Klose D. How autocatalysis accelerates drug release from PLGA-based microparticles: a quantitative treatment. Biomacromolecules. 2005;6:2312–9. doi: 10.1021/bm050228k. [DOI] [PubMed] [Google Scholar]
- 111.Pan Z, Ding J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interf Focus. 2012;2:366–77. doi: 10.1098/rsfs.2011.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parker T, Dave V, Falotico R. Polymers for drug eluting stents. Curr Pharm Des. 2010;16:3978–88. doi: 10.2174/138161210794454897. [DOI] [PubMed] [Google Scholar]
- 113.Li JN, Cao P, Zhang XN, Zhang SX, He YH. In vitro degradation and cell attachment of a PLGA coated biodegradable Mg–6Zn based alloy. J Mater Sci. 2010;45:6038–45. [Google Scholar]
- 114.Xi T, Gao R, Xu B, Chen L, Luo T, Liu J, et al. In vitro and in vivo changes to PLGA/sirolimus coating on drug eluting stents. Biomaterials. 2010;31:5151–8. doi: 10.1016/j.biomaterials.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 115.Ostrowski NJ, Lee B, Roy A, Ramanathan M, Kumta PN. Biodegradable poly(lactide-co-glycolide) coatings on magnesium. J Mater Sci Mater Med. 2013;24:85–96. doi: 10.1007/s10856-012-4773-5. [DOI] [PubMed] [Google Scholar]
- 116.Cutright DE, Hunsuck EE. Tissue reaction to the biodegradable polylactic acid suture. Oral Surg Oral Med Oral Pathol. 1971;31:134–9. doi: 10.1016/0030-4220(71)90044-2. [DOI] [PubMed] [Google Scholar]
- 117.Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, et al. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26:147–55. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 118.Bostman O. Osteolytic changes accompanying degradation of absorbable fracture fixation implants. Bone Joint J. 1991;73-B:679–82. doi: 10.1302/0301-620X.73B4.1649195. [DOI] [PubMed] [Google Scholar]
- 119.Conceicao TF, Scharnagl N, Blawert C, Dietzel W, Kainer KU. Corrosion protection of magnesium alloy AZ31 sheets by spin coating process with poly(ether imide) [PEI] Corrosion Sci. 2010;52:2066–79. [Google Scholar]
- 120.da Conceicao TF, Scharnagl N, Dietzel W, Kainer KU. On the degradation mechanism of corrosion protective poly(ether imide) coatings on magnesium AZ31 alloy. Corrosion Sci. 2010;52:3155–7. [Google Scholar]
- 121.Scharnagl N, Blawert C, Dietzel W. Corrosion protection of magnesium alloy AZ31 by coating with poly(ether imides) (PEI) Surf Coat Technol. 2009;203:1423–8. [Google Scholar]
- 122.Byrne RA, Iijima R, Mehilli J, Pinieck S, Bruskina O, Schomig A, et al. Durability of antirestenotic efficacy in drugeluting stents with and without permanent polymer. J Am Coll Cardiol Cardiovasc Interv. 2009;2:291–9. doi: 10.1016/j.jcin.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 123.Werner C, Maitz MF, Sperling C. Current strategies towards hemocompatible coatings. J Mater Chem. 2007;17:3376–84. [Google Scholar]
- 124.Yao X, Hu Y, Cao B, Peng R, Ding J. Effects of surface molecular chirality on adhesion and differentiation of stem cells. Biomaterials. 2013;34:9001–9. doi: 10.1016/j.biomaterials.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 125.Yao X, Peng R, Ding J. Cell-material interactions revealed via material techniques of surface patterning. Adv Mater. 2013;25:5257–86. doi: 10.1002/adma.201301762. [DOI] [PubMed] [Google Scholar]
- 126.Hasebe T, Ishimaru T, Kamijo A, Yoshimoto Y, Yoshimura T, Yohena S, et al. Effects of surface roughness on antithrombogenicity of diamond-like carbon films. Diamond Relat Mater. 2007;16:1343–8. [Google Scholar]
- 127.Hasebe T, Shimada A, Suzuki T, Matsuoka Y, Saito T, Yohena S, et al. Fluorinated diamond-like carbon as antithrombogenic coating for blood-contacting devices. J Biomed Mater Res A. 2006;76:86–94. doi: 10.1002/jbm.a.30512. [DOI] [PubMed] [Google Scholar]
- 128.Virtanen S. Biodegradable Mg and Mg alloys: corrosion and biocompatibility. Mater Sci Eng B. 2011;176:1600–8. [Google Scholar]
- 129.Wang J, Wan YZ, Luo HL, Gao C, Huang Y. Immobilization of gelatin on bacterial cellulose nanofibers surface via cross-linking technique. Mater Sci Eng C. 2012;32:536–41. [Google Scholar]
- 130.Gao JH, Shi XY, Yang B, Hou SS, Meng EC, Guan FX, et al. Fabrication and characterization of bioactive composite coatings on Mg–Zn–Ca alloy by MAO/sol–gel. J Mater Sci Mater Med. 2011;22:1681–7. doi: 10.1007/s10856-011-4349-9. [DOI] [PubMed] [Google Scholar]
- 131.Steffel J, Latini RA, Akhmedov A, Zimmermann D, Zimmerling P, Luscher TF, et al. Rapamycin, but not FK-506, increases endothelial tissue factor expression: implications for drug-eluting stent design. Circulation. 2005;112:2002–11. doi: 10.1161/CIRCULATIONAHA.105.569129. [DOI] [PubMed] [Google Scholar]
- 132.Kamath KR, Barry JJ, Miller KM. The Taxus drug-eluting stent: a new paradigm in controlled drug delivery. Adv Drug Deliv Rev. 2006;58:412–36. doi: 10.1016/j.addr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 133.Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation. 2004;109:1942–7. doi: 10.1161/01.CIR.0000127110.49192.72. [DOI] [PubMed] [Google Scholar]
- 134.Kalicke T, Schierholz J, Schlegel U, Frangen TM, Koller M, Printzen G, et al. Effect on infection resistance of a local antiseptic and antibiotic coating on osteosynthesis implants: an in vitro and in vivo study. J Orthop Res. 2006;24:1622–40. doi: 10.1002/jor.20193. [DOI] [PubMed] [Google Scholar]
- 135.Simchi A, Tamjid E, Pishbin F, Boccaccini AR. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomed Nanotechnol Biol Med. 2011;7:22–39. doi: 10.1016/j.nano.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 136.Vallet-Regi M, Granado S, Arcos D, Gordo M, Cabanas MV, Ragel CV, et al. Preparation, characterization, and in vitro release of ibuprofen from Al2O3/PLA/PMMA composites. J Biomed Mater Res A. 1998;39:423–8. doi: 10.1002/(sici)1097-4636(19980305)39:3<423::aid-jbm11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 137.Gong D, Yadavalli V, Paulose M, Pishko M, Grimes CA. Controlled molecular release using nanoporous alumina capsules. Biomed Microdevices. 2003;5:75–80. [Google Scholar]
- 138.Kapoor S, Hegde R, Bhattacharyya AJ. Influence of surface chemistry of mesoporous alumina with wide pore distribution on controlled drug release. J Control Release. 2009;140:34–9. doi: 10.1016/j.jconrel.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 139.Gulati K, Ramakrishnan S, Aw MS, Atkins GJ, Findlay DM, Losic D. Biocompatible polymer coating of titania nanotube arrays for improved drug elution and osteoblast adhesion. Acta Biomater. 2012;8:449–56. doi: 10.1016/j.actbio.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 140.Kikuchi A, Okano T. Pulsatile drug release control using hydrogels. Adv Drug Deliv Rev. 2002;54:53–77. doi: 10.1016/s0169-409x(01)00243-5. [DOI] [PubMed] [Google Scholar]
- 141.Wu P, Grainger DW. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials. 2006;27:2450–67. doi: 10.1016/j.biomaterials.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 142.Swanson TE, Cheng X, Friedrich C. Development of chitosanvancomycin antimicrobial coatings on titanium implants. J Biomed Mater Res A. 2011;97:167–76. doi: 10.1002/jbm.a.33043. [DOI] [PubMed] [Google Scholar]
- 143.Chung T-W, Huang Y-Y, Liu Y-Z. Effects of the rate of solvent evaporation on the characteristics of drug loaded PLLA and PDLLA microspheres. Int J Pharm. 2001;212:161–9. doi: 10.1016/s0378-5173(00)00574-3. [DOI] [PubMed] [Google Scholar]
- 144.Chiou SH, Wu WT, Huang YY, Chung TW. Effects of the characteristics of chitosan on controlling drug release of chitosan coated PLLA microspheres. J Microencapsul. 2001;18:613–25. doi: 10.1080/02652040010019497. [DOI] [PubMed] [Google Scholar]
- 145.Liggins RT, Burt HM. Paclitaxel loaded poly(L-lactic acid) (PLLA) microspheres. II. The effect of processing parameters on microsphere morphology and drug release kinetics. Int J Pharm. 2004;281:103–6. doi: 10.1016/j.ijpharm.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 146.Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Karydas AG, Ithakissios DS. PLGA–mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J Control Release. 2002;79:123–35. doi: 10.1016/s0168-3659(01)00530-2. [DOI] [PubMed] [Google Scholar]
- 147.Acharya G, Park K. Mechanisms of controlled drug release from drug-eluting stents. Adv Drug Deliv Rev. 2006;58:387–401. doi: 10.1016/j.addr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 148.Commandeur S, Van Beusekom HMM, Van Der Giessen WJ. Polymers, drug release, and drug-eluting stents. J Interv Cardiol. 2006;19:500–6. doi: 10.1111/j.1540-8183.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 149.Klose D, Siepmann F, Elkharraz K, Krenzlin S, Siepmann J. How porosity and size affect the drug release mechanisms from PLGA-based microparticles. Int J Pharm. 2006;314:198–206. doi: 10.1016/j.ijpharm.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 150.Chen XB, Birbilis N, Abbott TB. A simple route towards a hydroxyapatite–Mg(OH)2 conversion coating for magnesium. Corrosion Sci. 2011;53:2263–8. [Google Scholar]
- 151.Bornapour M, Muja N, Shum-Tim D, Cerruti M, Pekguleryuz M. Biocompatibility and biodegradability of Mg–Sr alloys: the formation of Sr-substituted hydroxyapatite. Acta Biomater. 2013;9:5319–30. doi: 10.1016/j.actbio.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 152.Wen C, Guan S, Peng L, Ren C, Wang X, Hu Z. Characterization and degradation behavior of AZ31 alloy surface modified by bone-like hydroxyapatite for implant applications. Appl Surf Sci. 2009;255:6433–8. [Google Scholar]
- 153.Zhang C, Zeng R, Liu C, Gao J. Comparison of calcium phosphate coatings on Mg–Al and Mg–Ca alloys and their corrosion behavior in Hank’s solution. Surf Coat Technol. 2010;204:3636–40. [Google Scholar]
- 154.Jamesh M, Kumar S, Sankara Narayanan TSN. Electrodeposition of hydroxyapatite coating on magnesium for biomedical applications. J Coat Technol Res. 2011;9:495–502. [Google Scholar]
- 155.Wu C, Wen Z, Dai C, Lu Y, Yang F. Fabrication of calcium phosphate/chitosan coatings on AZ91D magnesium alloy with a novel method. Surf Coat Technol. 2010;204:3336–47. [Google Scholar]
- 156.Hiromoto S, Tomozawa M. Hydroxyapatite coating of AZ31 magnesium alloy by a solution treatment and its corrosion behavior in NaCl solution. Surf Coat Technol. 2011;205:4711–9. [Google Scholar]
- 157.Feng A, Han Y. The microstructure, mechanical and corrosion properties of calcium polyphosphate reinforced ZK60A magnesium alloy composites. J Alloys Compounds. 2010;504:585–93. [Google Scholar]
- 158.Bakhsheshi-Rad HR, Idris MH, Abdul-Kadir MR. Synthesis and in vitro degradation evaluation of the nano-HA/MgF2 and DCPD/MgF2 composite coating on biodegradable Mg–Ca–Zn alloy. Surf Coat Technol. 2013;222:79–89. [Google Scholar]
- 159.Wang HX, Guan SK, Wang X, Ren CX, Wang LG. In vitro degradation and mechanical integrity of Mg–Zn–Ca alloy coated with Ca-deficient hydroxyapatite by the pulse electrodeposition process. Acta Biomater. 2010;6:1743–8. doi: 10.1016/j.actbio.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 160.Jo JH, Kang BG, Shin KS, Kim HE, Hahn BD, Park DS, et al. Hydroxyapatite coating on magnesium with MgF(2) interlayer for enhanced corrosion resistance and biocompatibility. J Mater Sci Mater Med. 2011;22:2437–47. doi: 10.1007/s10856-011-4431-3. [DOI] [PubMed] [Google Scholar]
- 161.Niu J, Yuan G, Liao Y, Mao L, Zhang J, Wang Y, et al. Enhanced biocorrosion resistance and biocompatibility of degradable Mg–Nd–Zn–Zr alloy by brushite coating. Mater Sci Eng C Mater Biol Appl. 2013;33:4833–41. doi: 10.1016/j.msec.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 162.Lu P, Fan H, Liu Y, Cao L, Wu X, Xu X. Controllable biodegradability, drug release behavior and hemocompatibility of PTX-eluting magnesium stents. Colloids Surf B Biointerfaces. 2011;83:23–8. doi: 10.1016/j.colsurfb.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 163.Zomorodian A, Garcia MP, Moura e Silva T, Fernandes JC, Fernandes MH, Montemor MF. Corrosion resistance of a composite polymeric coating applied on biodegradable AZ31 magnesium alloy. Acta Biomater. 2013;9:8660–70. doi: 10.1016/j.actbio.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 164.Truong V-T, Lai PK, Moore BT, Muscat RF, Russo MS. Corrosion protection of magnesium by electroactive polypyrrole/paint coatings. Synth Met. 2000;110:7–15. [Google Scholar]
- 165.Yfantis A, Paloumpa I, Schmeiber D, Yfantis D. Novel corrosion-resistant films for Mg alloys. Surf Coat Technol. 2002;151–152:400–4. [Google Scholar]
- 166.Wang J, He Y, Maitz MF, Collins B, Xiong K, Guo L, et al. A surface-eroding poly(1,3-trimethylene carbonate) coating for fully biodegradable magnesium-based stent applications: toward better biofunction, biodegradation and biocompatibility. Acta Biomater. 2013;9:8678–89. doi: 10.1016/j.actbio.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 167.Liu P, Pan X, Yang W, Cai K, Chen Y. Improved anticorrosion of magnesium alloy via layer-by-layer self-assembly technique combined with micro-arc oxidation. Mater Lett. 2012;75:118–21. [Google Scholar]
- 168.Adden N. Implant of a biocorrodable magnesium alloy and having a coating of a biocorrodable polyphosphazene. 20090048660 A1. US patent. 2009
- 169.Zhang CY, Zeng RC, Chen RS, Liu CL, Gao JC. Preparation of calcium phosphate coatings on Mg-1. 0Ca alloy. Transactions of Nonferrous Metals Society of China. 2010;20:s655–9. [Google Scholar]