Supplemental Digital Content is available in the text
Keywords: family history, history taking, hypertension, incidence, risk factors
Abstract
Although a family history (FH) of hypertension is a risk factor for the development of hypertension, only a few studies have investigated in detail the impact of individual components of an FH on incident hypertension. We investigated the impact of individual components and their combinations on the presence or development of hypertension considering obesity, smoking habits, physical activity, and other metabolic parameters.
Studied were 12,222 Japanese individuals without hypertension (n = 9,766) and with hypertension (n = 2,456) at the baseline examination. The presence or incidence of hypertension during 5 years after a baseline examination was assessed by the presence of systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or a self-reported history of clinician-diagnosed hypertension. In this prospective study, the odds ratio for incident hypertension was 1.39 (95% confidence interval [CI], 1.22, 1.59) for individuals with any FH of hypertension compared with those without such an FH. Individuals with an FH of hypertension in both parents and one or more grandparents had an odds ratio of 3.05 (95% CI 1.74, 5.36) for hypertension compared with those without an FH of hypertension. FH was associated with incident hypertension independently of other modifiable risk factors such as obesity, smoking, physical inactivity, hyperglycemia, hyperuricemia, and hypertriglyceridemia.
A parental history of hypertension was an essential component within an FH for incident hypertension. FH of hypertension over two generations with both parents affected was the most important risk factor for incident hypertension. Although an FH is not a modifiable risk factor, modifying other risk factors could contribute to reducing the risk of hypertension even among individuals with a family history of hypertension.
1. Introduction
A family history (FH) of hypertension is a well-established risk factor for the development of hypertension.[1,2] In spite of this, only a few studies have investigated the impact of details of FH on the development of hypertension. A cross-sectional study showed that the prevalence of hypertension was 1.29-fold higher among individuals with than without an FH of hypertension.[3] Another cross-sectional study in Japan suggested an association between the number of parents and siblings with hypertension and the incidence of hypertension.[4] However, the quantitative influence of individual components of an FH such as for hypertension in either one or both parents, grandparents, or siblings or their combinations on incident hypertension is unclear. It is also well established that many environmental and metabolic factors such as smoking, sedentary behavior, obesity, hyperglycemia, hyperuricemia, or dyslipidemia cause hypertension.[5–7] Nevertheless, whether the impact of these predictors on the development of hypertension is affected by an FH of hypertension is unknown. Therefore, we investigated the effect of each element of an FH of hypertension on the presence of hypertension in a cross-sectional study and evaluated the impact of each element of an FH of hypertension on incident hypertension in a longitudinal study of Japanese men and women. We also investigated the combined effects of FH and other risk factors on the development of hypertension to clarify possible interactions among them on incident hypertension.
2. Methods and Methods
2.1. Study population
The Toranomon Hospital Health Management Center study included a cohort consisting mainly of apparently healthy Japanese government employees who had annual examinations for routine health screening and also some members of the general public. All participants were interviewed at each examination to obtain information on demographic characteristics, health-related habits, and medical history including an FH of hypertension. We investigated 12,357 individuals who underwent a baseline health examination during the period from 1997 to 2007. Information was obtained on blood pressure measurements, a self-reported history of medical treatment for hypertension, or the use of antihypertensive medication at the baseline examination and at a reexamination 5 years after (2002–2012) the baseline examination. Among the 12,357 individuals, we excluded those with missing data on self-reported lifestyle characteristics (n = 135). We evaluated those with hypertension (n = 2456) and without hypertension (n = 9766) at the baseline examination in a cross-sectional study (Supplemental Figure 1). Then, we performed a longitudinal study that included a total of 9766 individuals (6773 men and 2993 women) aged 20 to 82 years without hypertension at the baseline examination (Supplemental Figure 1). The study protocol followed the Japanese Government's Ethical Guidelines Regarding Epidemiological Studies in accordance with the Declaration of Helsinki and was reviewed by the Institutional Review Board at Toranomon Hospital.[8]
2.2. Clinical and other measurements
Height and weight were measured without shoes or heavy clothing, and body mass index (BMI) was calculated using the following formula: weight (kg)/height (m2). At the time of the health examinations, after a brief period of rest, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in the morning in either arm using a sphygmomanometer (Omron Corporation, Kyoto, Japan) with the participants in a sitting position according to our medical checkup rules. Blood pressure was measured once in most participants, but up to 3 measurements at 1 to 2 minute intervals were made in participants who had hypertensive or prehypertensive SBP and DBP values. The lowest reading was used in the analysis that assessed the incidence of hypertension. Blood samples were collected after an overnight fast, and measurements were made using an automatic clinical chemistry analyzer (Hitachi, LABOSPECT 008, Tokyo, Japan).[8] HbA1c was assessed by high-performance liquid chromatography (Tosoh, Tokyo, Japan). The value for HbA1c (%) was estimated as the National Glycohemoglobin Standardization Program equivalent value (%), which was calculated by the formula HbA1c (%) = 1.02 × HbA1c (Japan Diabetes Society) (%) + 0.25 (%).[9]
An FH of hypertension among grandparents, parents, or siblings and smoking habits of the participants (never, former, and current) and presence or absence of physical activity were assessed by a questionnaire.
2.3. Definitions
Hypertension was defined by SBP ≥140 mm Hg, DBP ≥90 mm Hg, or a self-reported history of clinician-diagnosed hypertension. A positive FH of hypertension was defined according to the presence of hypertension in at least 1 grandparent, parent, or sibling. Participants were classified according to a BMI <18.5, 18.5–22.9, 23.0–27.4, and ≥27.5 kg/m2 based on the classification of the World Health Organization.[10] We defined smoking habits as follows: “never,” having never smoked; “former,” having smoked in the past but not currently; and “current,” using tobacco at present. Physical activity was defined as the performance of any physical activity for 30 minutes or more per occasion at least once a week. Fasting plasma glucose (FPG) ≥126 mg/dL, HbA1c ≥6.5%, uric acid (UA) ≥7.0 mg/dL, and triglycerides (TG) ≥150 mg/dL were considered to be high.
2.4. Statistical analysis
Categorical variables were expressed as numbers and percentages and were compared with Chi-square tests. Continuous variables were expressed as mean ± SD and were compared with Student t tests. A logistic regression analysis was performed to calculate odds ratios (ORs) and their 95% confidence interval (CI) for the presence or development of hypertension. We used crude ORs in the analysis of the relationship between well-known risk factors for hypertension and its presence or development with regard to the baseline examination for the cross-sectional study and for the longitudinal study. Then, we investigated the impact of each detail within an FH on the presence or development of hypertension. Covariates included age, sex, BMI, smoking habits, physical activity, FPG, UA, and TG. In order to evaluate the interactions between the presence or the absence of an FH of hypertension and each risk factor such as BMI, smoking habit, physical activity, FPG, HbA1c, UA, and TG on the development of hypertension, logistic regression analysis was performed according to the presence or absence of an FH of hypertension. All statistical analyses were performed with IBM SPSS Statistics 19. Statistical significance was defined as P < 0.05.
3. Results
Characteristics of the total study population with hypertension (n = 2456) and without hypertension (n = 9766) at the baseline examination are shown in Table 1 and Supplemental Table 1. Of the 9766 individuals, 3594 (36.8%) had at least 1 family member with a history of hypertension. During the 5-year follow-up, 1124 individuals (11.5%) developed hypertension. As shown in Table 1 both SBP and DBP were significantly though very slightly higher in individuals with an FH of hypertension compared with those without.
Table 1.
Baseline characteristics of study participants without hypertension.
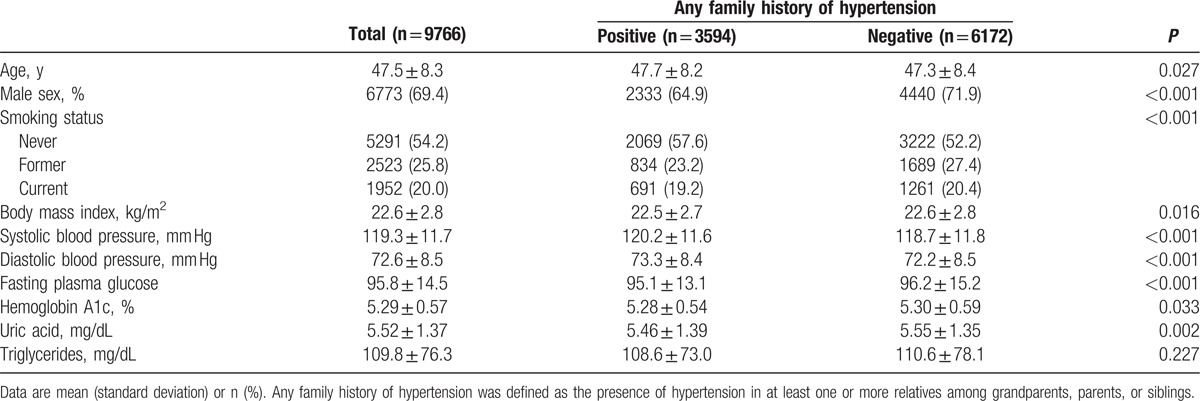
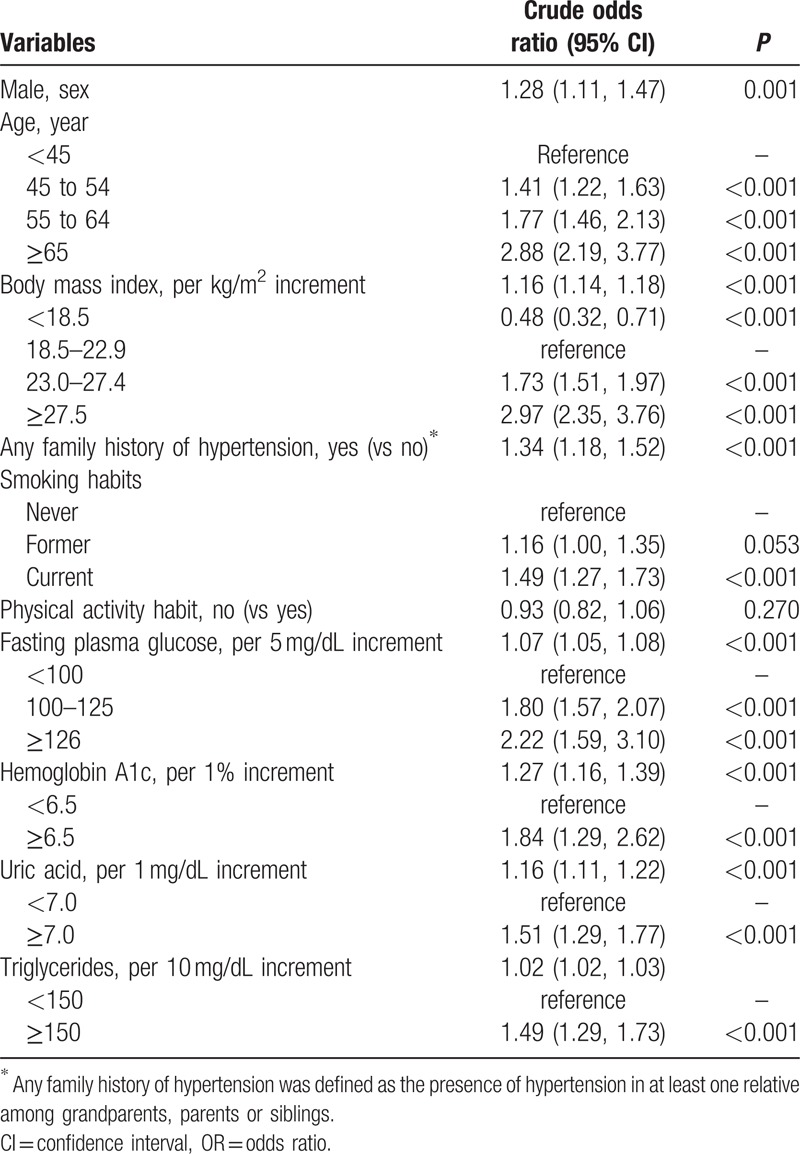
Table 2 and Supplemental Table 2, show the crude ORs of various risk factors for the presence or development of hypertension. The OR for developing hypertension in individuals with any FH of hypertension was 1.34 (95% CI 1.18, 1.52) compared with those without any FH of hypertension. The ORs for hypertension for BMI 23.0 to 27.4 and BMI ≥27.5 kg/m2 compared to the OR for BMI 18.5 to 22.9 were 1.73 (1.51, 1.97) and 2.97 (1.51, 1.97), respectively. Current smoking and high values for FPG, HbA1c, UA, and TG were also significantly associated with incident hypertension, whereas a physical activity habit was not significantly associated with hypertension (Table 2).
Table 2.
Crude ORs of various risk factors for developing hypertension by univariate regression model.
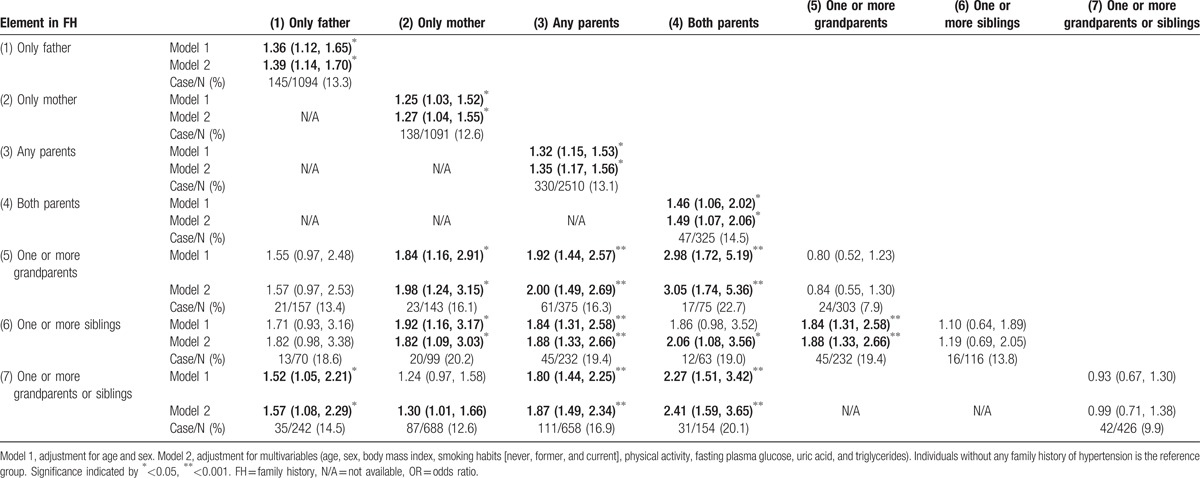
The association between the presence of hypertension at the baseline examination and individual components of an FH of hypertension and their combinations are shown in Supplemental Tables 3 and 4. Parental history of hypertension was the most important component for the presence of hypertension. Having only 1 or more siblings or only 1 or more grandparents with hypertension also increased the risk of the presence of hypertension. These components further enhanced the risk of the presence of hypertension in individuals with an FH of hypertension in any parent. A difference between the risk of hypertension with an FH of hypertension in only the father or only the mother FH was not detected.
The impacts of individual components of an FH of hypertension and their combinations on the incidence of hypertension are shown in Table 3 and Supplemental Table 5. The multivariate adjusted OR for hypertension for individuals with any FH of hypertension compared with the reference group (i.e., individuals without any FH) was 1.39 (1.22, 1.59). Individuals with a parental or biparental FH of hypertension had ORs of 1.45 (1.27, 1.66) or 1.76 (1.35, 2.29) for incident hypertension, respectively, regardless of the presence or absence of any other FH of hypertension (Supplemental Table 5). Individuals with an FH of hypertension in at least 1 of parent but in no other relative had an OR of 1.35 (1.17, 1.56) for hypertension compared with the reference category. Individuals with 1 or more grandparents or 1 or more siblings with an FH of hypertension did not have a significantly increased risk of hypertension. Individuals with an FH of hypertension in both parents and 1 or more grandparents or 1 or more siblings had an OR of 2.41 (1.59, 3.65) for hypertension compared with those without any FH of hypertension. Individuals with an FH of hypertension in both parents and 1 or more siblings or in both parents and 1 or more grandparents had a 1.88 (1.33, 2.26) or 3.05 (1.74, 5.36) times increased risk of hypertension, respectively, compared to the reference group.
Table 3.
Age-, sex-adjusted, and multivariate ORs for incident hypertension according to each element in FH.
Generally, FH is considered to enhance other risk factors in an additive manner. Figure 1 shows the interaction between an FH of hypertension and BMI, smoking habits, physical activity, FPG, HbA1c, UA, or TG for the development of hypertension. Individuals with any FH of hypertension and BMI 23.0 to 27.4 or BMI ≥27.5 had an OR of 2.44 (2.02, 2.96) or 3.99 (2.69, 5.92), respectively, for developing hypertension compared with those without any FH and BMI 18.5 to 22.9. Compared with never smokers without any FH of hypertension, current smokers with any FH of hypertension had a 1.69 (95% CI 1.33, 2.15) times increased risk of the development of hypertension. Physically inactive individuals with any FH of hypertension had an OR of 1.35 (95% CI 1.15, 4.62) for developing hypertension compared with those who were physically active and without such an FH. Other risk factors for the development of hypertension such as hyperglycemia, hyperuricemia, and hypertriglyceridemia were all enhanced moderately by the presence of an FH of hypertension (Fig. 1).
Figure 1.
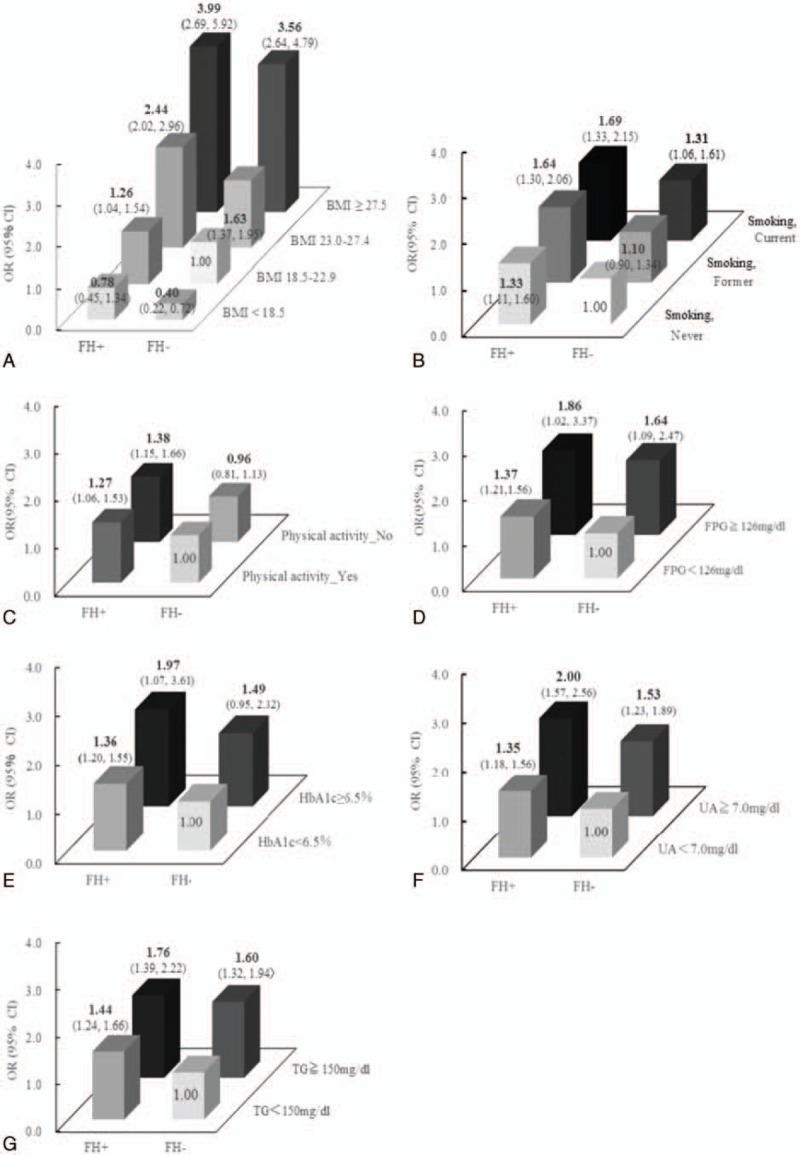
The interactions between family history of hypertension and body mass index, smoking habits, fasting plasma glucose, hemoglobin A1c, uric acid, or triglycerides for the development of hypertension. ORs (95% CI) for incident hypertension are adjusted for age and sex. Each P value for interaction of family history and body mass index, smoking habit, physical activity, fasting plasma glucose, hemoglobin A1c, uric acid, or triglycerides were 0.394, 0.701, 0.339, 0.597, 0.945, 0.853, and 0.083, respectively. BMI = body mass index, CI = confidence interval, FH+ = individuals with any family history of hypertension, FH− = individuals without any family history of hypertension, FPG = fasting plasma glucose, HbA1c = hemoglobin A1c, OR = odds ratio, TG = triglycerides, UA = uric acid.
4. Discussion
An FH of hypertension was associated with its presence or development independently of other established predictors. Especially, individuals with an FH of hypertension in both parents and 1 or more grandparents had a 3.05-fold higher risk of the development of hypertension than those with no FH of hypertension. Our findings indicated that an FH of hypertension over 2 generations, that is, involving both parents and one or more grandparents, was the most important component for predicting hypertension.
In our prospective study, both SBP and DBP were significantly elevated in those with an FH of hypertension. However, the difference was too small to predict future occurrence of hypertension, and blood pressure at baseline seemed to be only minimally affected by an FH of hypertension. Similarly, though statistically significant, the differences in BMI, FPG, HbA1c, and UA between those with and without an FH of hypertension were very small, suggesting that these parameters were not largely affected by such an FH.
Among predictors of hypertension, previous studies have shown that an FH of hypertension is not necessarily a very strong predictor compared to other major factors such as age or obesity,[11–14] which was also confirmed by our results. Moreover, a recent study conducted in Switzerland showed that screening of children with a parental history of hypertension may not be a substantially better strategy to predict hypertension in children than universal screening.[15] However, as a predictor of hypertension in comparison with other factors, an FH of hypertension had an impact similar to almost a 2-unit increase in BMI, a 1% increase in HbA1c, or current smoking. Therefore, future studies are needed to clarify this issue.
A cross-sectional study in Sri Lanka showed that the ORs for the presence of hypertension in those with an FH of hypertension in parents, grandparents, or siblings were 1.28, 1.34, or 1.27, respectively.[3] However, the study did not show the impact of individual components of an FH, such as a risk to those with a parental history of hypertension but without an FH of hypertension in other family members. In our study, we analyzed all of these individual FH components separately to elucidate individual and combined risks.
A previous Japanese study compared the risk of hypertension in participants with an FH in both parents and in those without a parental history. Compared with individuals without a parental history, those with an FH of hypertension in both parents had an OR of 1.67 for the development of hypertension.[14] However, this study did not consider whether the participants also had an FH of hypertension in a grandparent or sibling when assessing the impact of a parental FH of hypertension. In contrast, our reference group was comprised of individuals without any FH of hypertension, which enabled us to assess the impact of a parental FH of hypertension alone. In fact, the ORs for the risk of hypertension in individuals with any FH, an FH involving both parents and 1 or more grandparents, or involving both parents and 1 or more siblings compared with the reference group were all higher than in the previous study, indicating that not only an FH involving both parents but also 1 or more grandparents or 1 or more siblings are important for the development of hypertension.
To the best of our knowledge, this is the 1st study to investigate the independent effects on incident hypertension of various components of an FH of hypertension. Results showed that individuals with a parental FH of hypertension have a significantly increased risk of hypertension compared with those without any FH of hypertension regardless of an FH of hypertension in other relatives. Moreover, individuals with any parental history of hypertension have a similar OR for the development of hypertension to those with any FH of hypertension, suggesting that a parental FH plays a major part in the impact of an FH of hypertension. An FH of hypertension in 1 or more grandparents or 1 or more siblings but not in a parent did not significantly increase the risk of hypertension but did enhance the effect of a parental history. An FH of hypertension in both parents and 1 or more siblings has a small influence on the development of hypertension, whereas an FH of hypertension in both parents and 1 or more grandparents is the strongest predictor of the incidence of hypertension. This indicates that an FH of hypertension over 2 generations involving both parents is the most important component for predicting hypertension. Although mitochondrial deoxyribonucleic acid (DNA) was suggested to be related to hypertension, and only the mother can pass down the mitochondrial DNA to offspring, a difference between an FH of hypertension in only the mother or only the father was not detected. In addition, the difference between an FH of hypertension in only the mother and any group and an FH of hypertension in only the father and any group was not detected. That is, mitochondrial DNA might have little impact on the development of hypertension. Unfortunately, since we could not obtain detailed information on the FH of grandparents, future studies are needed to assess those issues.
A parental history of hypertension was significantly associated with hypertension at the baseline examination. An FH of hypertension in 1 or more grandparents or in 1 or more siblings did not significantly increase the risk of hypertension in the prospective study, whereas it was associated with the presence of hypertension in the cross-sectional study. The OR in our cross-sectional study of individuals with any FH putting them at risk for hypertension compared with the reference group was higher than that of the prospective study. These discrepancies between results of the cross-sectional study and longitudinal study may result from the higher number of individuals in the cross-sectional study who had an FH of hypertension in either 1 or more grandparents or 1 or more siblings at the baseline examination. In addition, the follow-up period in the longitudinal study was only 5 years, which may not have been sufficient for the development of hypertension from baseline. Thus, if the follow-up period had been longer, the incidence of hypertension might have been increased. Conversely, since the mean age of participants in the cross-sectional study was 47.5 years, these participants might have developed hypertension over a period exceeding that of the 5-year follow-up in the longitudinal study.
As shown previously, obesity,[16] smoking,[17] physical inactivity,[7] hyperglycemia,[16] hyperuricemia,[18] and hypertriglyceridemia[16] were also independently associated with incident hypertension. We clarified whether each of those factors was affected by an FH of hypertension. The interactions between an FH of hypertension and each of those factors were not significant, suggesting that their association was not synergistic but additive, which also indicates that the influence of an FH of hypertension could be considerably weakened by improvement of other modifiable risk factors.
In this study, we did not consider the effect of sodium intake, which is an established risk factor for hypertension.[19] Takase et al[20] showed that every 1 g/day increase in sodium intake is associated with a 1.09 times greater risk of hypertension. The OR of any pattern of FH of hypertension examined in our study was almost equal to an increase in sodium intake of 4 g/day. Similarly, the OR in individuals with an FH of hypertension in both parents and 1 or more grandparents was equivalent to an increase of sodium intake of 13 g/day. Although lifestyle modification is always necessary to prevent hypertension regardless of the presence or the absence of an FH, our findings suggested that it could be difficult to cancel out the risk of an FH involving a high concentration of family members by just lowering daily sodium intake.
An FH has been used as a simple and inexpensive surrogate marker for genetic factors. Genome-wide association studies revealed many susceptibility genes for the incidence of hypertension.[21–24] It is known that a single susceptibility gene increases disease risk only 1.2 to 1.3 times. Individuals who have a combination of the highest risk genotype have about twice as high a risk of developing hypertension than those with a combination of the lowest risk genotypes.[25] This risk corresponded to the OR in individuals with the highest concentration of an FH of hypertension in our study. Although the effects of the intrafamily living environment are involved in an FH but not a genotype, according to our results an evaluation of FH could be as sensitive as the current most advanced genetic test for predicting incident hypertension.
The strengths of the present study were its prospective design as well as cross-sectional design and one of the largest sample sizes in this type of study. This enabled us to separate individual components of the FH in detail in assessing the impact of an FH on incident hypertension. We could also clarify the interaction between FH and other well-known risk factors for hypertension.
Our study had several limitations. First, although the incidence of hypertension can be considered similar to the results of a previous study in Japan,[26] this value was lower than that in Western countries.[27] Therefore, the study results might be limited to an ethnic Japanese population. Further studies are needed to confirm these results in other ethnic groups. Second, FH was assessed by a simple questionnaire and was therefore self-reported. We could not deny the possibility of recall bias and misclassification errors. However, high sensitivity was shown for reporting an FH of hypertension. Bochud et al[28] reported that when history (in siblings) could be evaluated, sensitivity and specificity of a history were 90% and 55% for hypertension, respectively. Third, we did not evaluate waist circumference at baseline, which might have provided more information about abdominal fat accumulation than BMI measurements. Thus, in obese individuals, ORs for hypertension might have been over- or underestimated. Fourth, our study included participants who underwent a routine health examination, and these individuals might pay more attention to their health than those who do not take such examinations.[29,30]
5. Conclusion
In conclusion, our findings demonstrated that a quantitative evaluation of an FH of hypertension according to individual components is possible and essential to evaluate the risk of hypertension. An FH of hypertension over 2 generations that included both parents was a strong risk factor for hypertension. Although an FH of hypertension is a quite independent risk factor for incident hypertension, its effect could be weakened by improvement of modifiable risk factors.
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank the late Professor and Director Kinori Kosaka, who established the foundation and framework of this project and was always the foremost pillar of spiritual support of the Toranomon Hospital Health Management Center Study project. The authors also thank the Ministry of Health, Labour and Welfare, Japan for the support, and Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science for the grant to HS and YH.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, DBP = diastolic blood pressure, DNA = deoxyribonucleic acid, FH = family history, FPG = fasting plasma glucose, HbA1c = hemoglobin A1c, OR = odds ratio, SBP = systolic blood pressure, TG = triglycerids, UA = uric acid.
Funding/support: This work is financially supported, in part, by the Ministry of Health, Labour and Welfare, Japan. HS and YH are recipients of a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
The sponsors had no role in the design and conduct of the study.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Wei Q, Sun J, Huang J, et al. Prevalence of hypertension and associated risk factors in Dehui City of Jilin Province in China. J Hum Hypertens 2015; 29:64–68. [DOI] [PubMed] [Google Scholar]
- 2.Katz EG, Stevens J, Truesdale KP, et al. Interactions between obesity, parental history of hypertension, and age on prevalent hypertension: the People's Republic of China Study. Asia Pac J Public Health 2012; 24:970–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranasinghe P, Cooray DN, Jayawardena R, et al. The influence of family history of hypertension on disease prevalence and associated metabolic risk factors among Sri Lankan adults. BMC Public Health 2015; 15:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tozawa M, Oshiro S, Iseki C, et al. Family history of hypertension and blood pressure in a screened cohort. Hypertens Res 2001; 24:93–98. [DOI] [PubMed] [Google Scholar]
- 5.Bhadoria AS, Kasar PK, Toppo NA, et al. Prevalence of hypertension and associated cardiovascular risk factors in Central India. J Family Community Med 2014; 21:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dogan N, Toprak D, Demir S. Hypertension prevalence and risk factors among adult population in Afyonkarahisar region: a cross-sectional research. Anadolu Kardiyol Derg 2012; 12:47–52. [DOI] [PubMed] [Google Scholar]
- 7.Beunza JJ, Martinez-Gonzalez MA, Ebrahim S, et al. Sedentary behaviors and the risk of incident hypertension: the SUN Cohort. Am J Hypertens 2007; 20:1156–1162. [DOI] [PubMed] [Google Scholar]
- 8.Heianza Y, Kodama S, Arase Y, et al. Role of body mass index history in predicting risk of the development of hypertension in Japanese individuals: Toranomon Hospital Health Management Center Study 18 (TOPICS 18). Hypertension 2014; 64:247–252. [DOI] [PubMed] [Google Scholar]
- 9.Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012; 3:39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157–163. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Yang Z, Xiao J, et al. Association between family history risk categories and prevalence of diabetes in Chinese population. PLoS One 2015; 10:e0117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira GF, Oliveira TR, Ikejiri AT, et al. Prevalence of hypertension and associated factors in an indigenous community of central Brazil: a population-based study. PLoS One 2014; 9:e86278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JW, Lim NK, Baek TH, et al. Anthropometric indices as predictors of hypertension among men and women aged 40–69 years in the Korean population: the Korean Genome and Epidemiology Study. BMC Public Health 2015; 15:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada K, Tamakoshi K, Yatsuya H, et al. Association between parental histories of hypertension, diabetes and dyslipidemia and the clustering of these disorders in offspring. Prev Med 2006; 42:358–363. [DOI] [PubMed] [Google Scholar]
- 15.Bloetzer C, Paccaud F, Burnier M, et al. Performance of parental history for the targeted screening of hypertension in children. J Hypertens 2015; 33:1167–1173. [DOI] [PubMed] [Google Scholar]
- 16.Tozawa M, Iseki K, Iseki C, et al. Impact of multiple risk factor clustering on the elevation of blood pressure. Hypertens Res 2002; 25:811–816. [DOI] [PubMed] [Google Scholar]
- 17.Halperin RO, Gaziano JM, Sesso HD. Smoking and the risk of incident hypertension in middle-aged and older men. Am J Hypertens 2008; 21:148–152. [DOI] [PubMed] [Google Scholar]
- 18.Nagahama K, Inoue T, Iseki K, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res 2004; 27:835–841. [DOI] [PubMed] [Google Scholar]
- 19.Du S, Batis C, Wang H, et al. Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in China. Am J Clin Nutr 2014; 99:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takase H, Sugiura T, Kimura G, et al. Dietary sodium consumption predicts future blood pressure and incident hypertension in the Japanese normotensive general population. J Am Heart Assoc 2015; 4:e001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy D, Ehret GB, Rice K, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet 2009; 41:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newton-Cheh C, Johnson T, Gateva V, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 2009; 41:666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang HC, Liang YJ, Wu YL, et al. Genome-wide association study of young-onset hypertension in the Han Chinese population of Taiwan. PLoS One 2009; 4:e5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiura Y, Tabara Y, Kokubo Y, et al. A genome-wide association study of hypertension-related phenotypes in a Japanese population. Circ J 2010; 74:2353–2359. [DOI] [PubMed] [Google Scholar]
- 25.Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011; 478:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohmori S, Kiyohara Y, Kato I, et al. Alcohol intake and future incidence of hypertension in a general Japanese population: the Hisayama study. Alcohol Clin Exp Res 2002; 26:1010–1016. [DOI] [PubMed] [Google Scholar]
- 27.Shimbo D, Muntner P, Mann D, et al. Association of left ventricular hypertrophy with incident hypertension: the multi-ethnic study of atherosclerosis. Am J Epidemiol 2011; 173:898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bochud M, Burnier M, Paccaud F, et al. Patients’ sibling history was sensitive for hypertension and specific for diabetes. J Clin Epidemiol 2004; 57:497–501. [DOI] [PubMed] [Google Scholar]
- 29.Heianza Y, Kato K, Kodama S, et al. Risk of the development of Type 2 diabetes in relation to overall obesity, abdominal obesity and the clustering of metabolic abnormalities in Japanese individuals: does metabolically healthy overweight really exist? The Niigata Wellness Study. Diabet Med 2015; 32:665–672. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai M, Nakamura K, Miura K, et al. Family history of diabetes, lifestyle factors, and the 7-year incident risk of type 2 diabetes mellitus in middle-aged Japanese men and women. J Diabetes Investig 2013; 4:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


