Abstract
Contrast medium administration is one of the leading causes of acute kidney injury (AKI) in different clinical settings. The aim of the study was to investigate occurrence and predisposing factors of AKI in cirrhotic patients undergoing contrast-enhanced computed tomography (CECT).
Datasets of 1279 consecutively hospitalized cirrhotic patients were retrospectively analyzed. Two hundred forty-nine of 1279 patients (mean age 64 ± 11 years, 165 male) who had undergone CECT were selected on the basis of the availability of serum creatinine (sCr) values evaluated before and after CECT (CECT group). In analogy, 203/1279 cases (mean age 66 ± 10 years, 132 male) who had not undergone CECT and had been tested twice for sCr in 7 days were also included as controls (Control group). AKI network criteria were employed to assess contrast-induced AKI (CI-AKI) development. Apart from lack of narrowed double sCr measurements, additional exclusion criteria were active bacterial infections, nephrotoxic drugs intake, and estimated glomerular filtration rate <30 mL/min.
AKI developed in 22/249 (8.8%) and in 6/203 (3%) of the CECT and the Control groups, respectively (P = 0.01). The multivariate logistic regression analysis showed that AKI was significantly associated with contrast medium administration (odds ratio [OR]: 3.242, 95% confidence interval [CI]: 1.255–8.375; P = 0.015), female sex (OR: 0.339, 95% CI: 0.139–0.827; P = 0.017), and sCr values (OR: 0.124, 95% CI: 0.016–0.975; P = 0.047). In the CECT group, presence of ascites (OR: 2.796, 95% CI: 1.109–7.052; P = 0.029), female sex (OR: 0.192, 95% CI: 0.073–0.510; P = 0.001), and hyperazotemia (OR: 1.018, 95% CI: 1.001–1.037; P = 0.043) correlated with CI-AKI development at multivariate analysis.
CI-AKI is a quite frequent occurrence in cirrhotic patients with female sex, presence of ascites, and hyperazotemia being the predisposing factors.
Keywords: advanced liver disease, computed tomography, contrast-induced acute kidney injury, iodinated contrast medium
1. Introduction
Acute kidney injury (AKI) is a severe complication of several different clinical conditions.[1] Apart from intrinsic renal diseases (i.e., glomerulonephritis, acute tubular necrosis, and interstitial nephritis) or postrenal diseases triggered by an obstruction along the urinary tract, AKI development is mainly related with prerenal azotemia due to an inadequate renal blood supply.[2] Liver cirrhosis “per se” predisposes patients to renal hypoperfusion because of central hypovolemia, arterial hypotension, and the consequent activation of different neurohormonal systems.[3–5]
Indeed, AKI is a quite frequent occurrence in patients with advanced liver disease and it affects about 1/5 of the hospitalized patients with liver cirrhosis.[4] The International Club of Ascites has recently further stressed the association between AKI and cirrhosis, also adopting the definition by the AKI Network which defined AKI as an increase in serum creatinine (sCr) ≥0.3 mg/dL or ≥50% in 2 measurements 48 hours apart.[6,7] An additional and well recognized risk factor of AKI development is the contrast medium administration in patients undergoing computed tomography.[8–10] Since hepatocellular carcinoma (HCC) is an event usually occurring in the course of cirrhosis, cirrhotic patients very often undergo radiological procedures enhanced with the use of contrast media for the early diagnosis of HCC as well as during the follow-up of HCC patients after treatment of cancer lesions.[11] Altogether, the above considerations suggest that cirrhotics might be a subset of patients particularly prone to develop contrast-induced AKI (CI-AKI). However, this aspect has been scarcely evaluated so far.[12–14] The aim of this study was to investigate occurrence and possible predisposing factors of CI-AKI in cirrhotic patients undergoing contrast-enhanced computed tomography (CECT).
2. Patients and methods
One thousand two hundred seventy-nine cirrhotic patients were consecutively hospitalized at the Clinical and Molecular Hepatology Division of the University Hospital of Messina from January 2008 to December 2014. Clinical, biochemical, instrumental, and radiologic data recorded in electronic charts from each of these patients were retrospectively analyzed. According to the scope of the study, 249/1279 patients (mean age 64 ± 11 years, 165 male) who had undergone CECT were selected on the basis of the availability of sCr values evaluated both within 5 days before and 48 hours after CECT (CECT group). In analogy, 203/1279 cases (mean age 66 ± 10 years, 132 male) who had not undergone CECT but had been tested twice for sCr in 7 days during hospitalization were also included in the study as controls (Control group) (Table 1). Eight hundred twenty-seven of 1279 cases excluded from the analysis were patients lacking double sCr checks, patients with an estimated glomerular filtration rate (eGFR) <30 mL/min (calculated by the Modification of Diet in Renal Disease-6 formula),[15] patients with active bacterial infections, patients with history of recent intake of potentially nephrotoxic drugs (i.e., nonsteroidal anti-inflammatory drugs) or history of exposure to contrast medium within 6 months before admission as well as of liver transplantation.
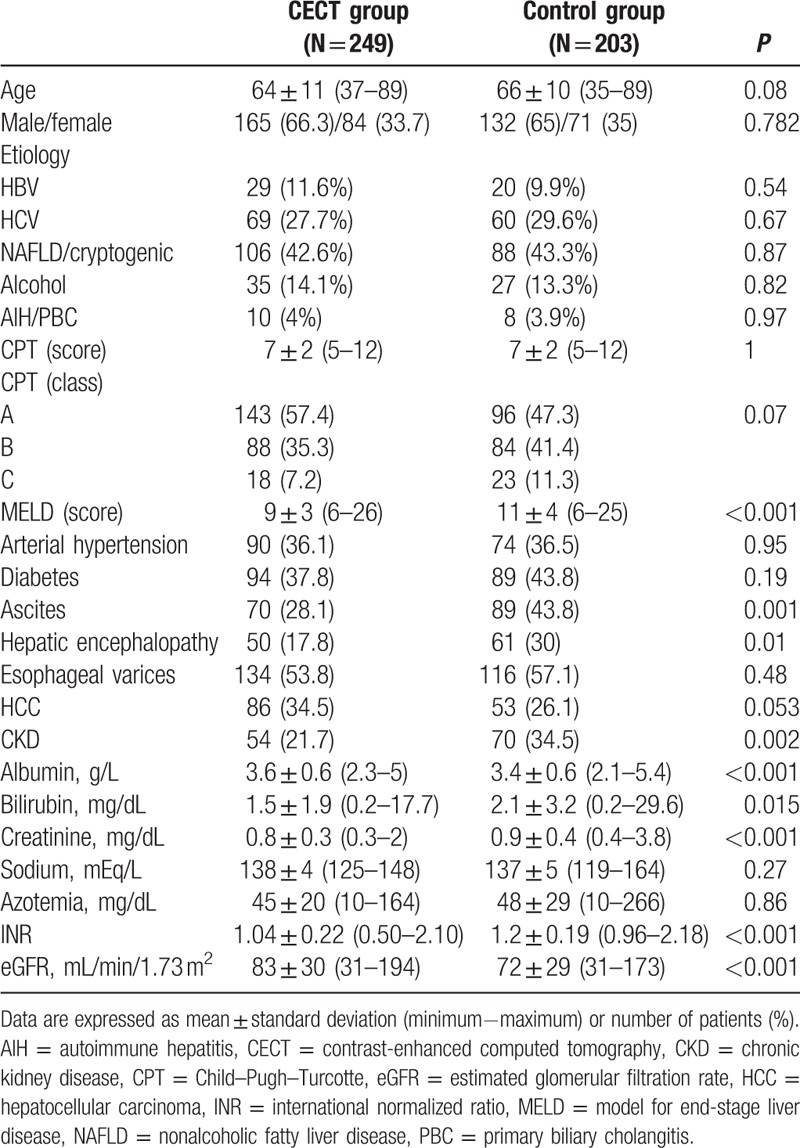
Table 1.
Demographic, clinical, and laboratory data of patients exposed (CECT group) or not exposed (Control group) to contrast medium.
The severity of liver disease was assessed by Child–Pugh–Turcotte (CPT) and model for end-stage liver disease (MELD) scores. The contrast iodine medium used for all CECT was Iopromide (Ultravist®; Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ, USA) (370 mg/mL, intravenously at a dosage of 1.2 mL per kilogram of body weight).
The study was conducted in accordance with the Declaration of Helsinki, and all patients gave their written informed consent to participate in the study.
2.1. Statistical analysis
The numerical data are expressed as mean and standard deviations and the categorical variables as count and percentage. Comparison between CECT and Control groups was performed using 2-sample Student t test and the χ2 test for numerical and for categorical variables, respectively. Variables considered were sex, age, etiology of cirrhosis, CPT score, MELD, eGFR, international normalized ratio, ascites, serum sodium, albumin, bilirubin and creatinine, azotemia, chronic kidney disease (CKD), diabetes, arterial hypertension, HCC, and treatment with diuretics as well as beta-blockers, antihypertensives, and antidiabetics drugs. Univariate logistic regression model was estimated on the cumulative study population (CECT and Control patients) in order to identify predictive factors of CI-AKI occurrence. Variables statistically significant at univariate analysis were then included in the multivariate logistic regression model to identify independent predictive factors of CI-AKI occurrence. Results of univariate and multivariate analyses are reported as P values, odds ratio (OR), and 95% confidence interval (CI).
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22 (IBM Corp., Armonk, NY). A P value less than 0.05 was considered statistically significant.
3. Results
No significant statistical difference was found between the CETC and the Control groups in terms of etiology of the liver disease, CPT classes, presence of HCC, esophageal varices, diabetes, and arterial hypertension (Table 1). In fact, in the CECT group, 98/249 patients (39.4%) had hepatitis B virus (HBV) (29 cases) or hepatitis C virus (HCV) (69 cases) chronic infection, 106 (42.6%) had nonalcoholic fatty liver disease (NAFLD)-related or cryptogenic cirrhosis, 35 (14%) had alcoholic cirrhosis, 10 (4%) had autoimmune liver disease or primary biliary cholangitis (PBC). In the Control group, 80/203 patients (39.4%) had HBV or HCV chronic infection (20 and 60 cases, respectively), 88 (43.3%) had NAFLD or cryptogenic cirrhosis, 27 (13.3%) had alcoholic liver disease, and 8 (3.9%) had autoimmune liver disease or PBC. In the CECT group, 143/249 cases (57.4%) belonged to the CPT class A, 88 (35.3%) to CPT class B, and 18 (7.2%) to CPT class C. In the Control group, 96 cases (47.3%) belonged to the CPT class A, 84 (41.4%) to CPT class B, and 23 (11.3%) to CPT class C. HCC was present in 86 (34.5%) patients in the CECT group and in 53 (26.1%) patients in the Control group. Esophageal varices were detected in 134 (53.8%) patients of the CECT group and in 116 (57.1%) patients of the Control group. Arterial hypertension was present in 90 (36.1%) patients of the CECT group and in 74 (36.5%) patients of the Control group. Ninety-four (37.8%) patients had diabetes in the CECT group, and 89 (43.8%) patients had diabetes in the Control group (Table 1). On the contrary, the CECT group significantly differed from the Control group for presence of ascites (70 vs 89 patients, P < 0.001), hepatic encephalopathy (50 vs 61 patients, P = 0.01), CKD (54 vs 70 patients, P = 0.002), sCr (0.8 ± 0.3 vs 0.9 ± 0.4, P < 0.001), and eGFR (83 ± 30 vs 72 ± 29, P < 0.001) (Table 1).
AKI developed in 22/249 (8.8%) and in 6/203 patients (3%) of the CECT and the Control groups, respectively (P = 0.01). In particular, 20/22 cases (90.9%) had AKI stage 1 and 2/22 (9.1%) AKI stage 2 in the CECT group, whereas 5/6 patients (83.3%) had AKI stage 1 and 1 patient had AKI stage 2 in the Control group.
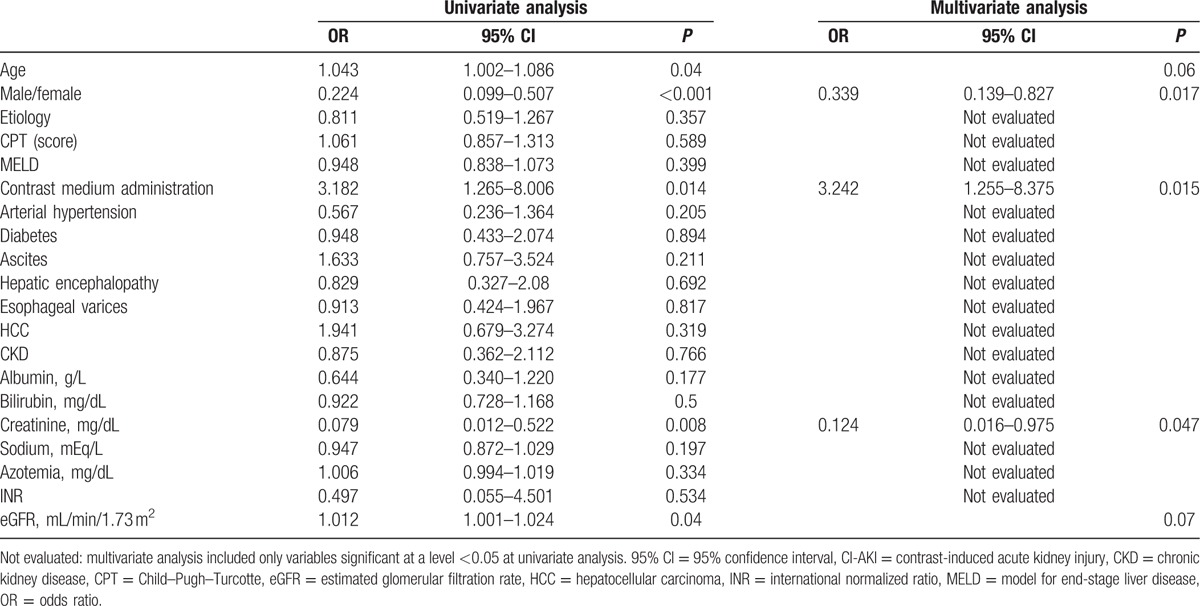
The univariate logistic regression analysis performed on the cumulative population of the CECT and Control groups showed that AKI was significantly associated with contrast medium administration (P = 0.014), female sex (P < 0.001), age (P = 0.04), sCr at baseline (P = 0.008), and eGFR (P = 0.04) (Table 2). At multivariate analysis, the use of contrast medium (OR: 3.242, 95% confidence interval [CI]: 1.255–8.375; P = 0.015), female sex (OR: 0.339, 95% CI: 0.139–0.827; P = 0.017), and baseline sCr (OR: 0.124, 95% CI: 0.016–0.975; P = 0.047) maintained statistical significance (Table 2).
Table 2.
Variables analyzed at univariate and multivariate analyses in the entire study population for possible association with CI-AKI.
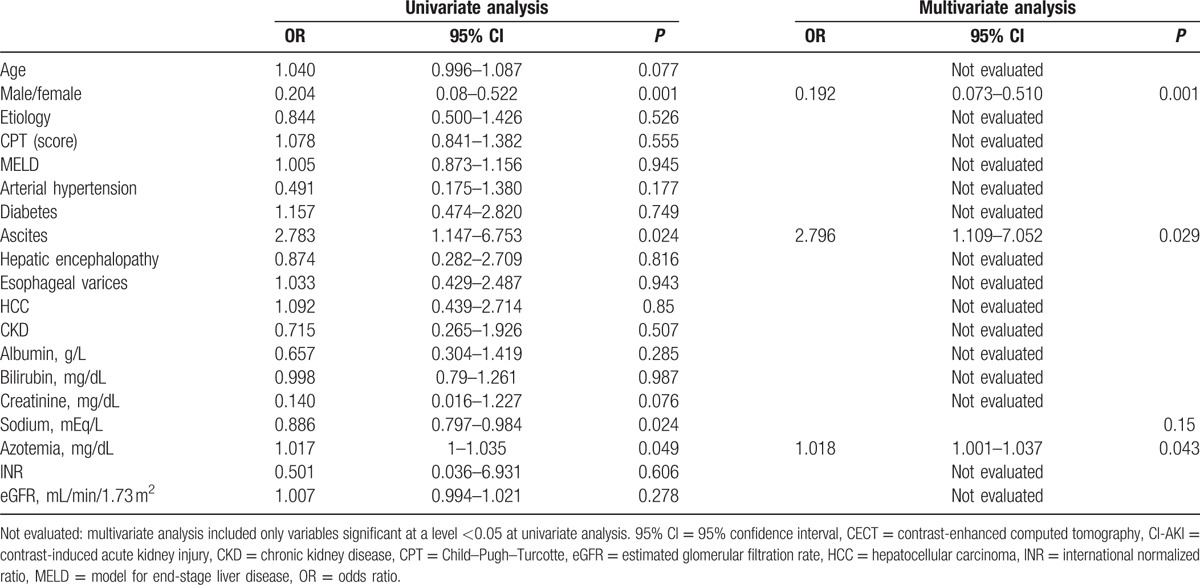
Limiting the analysis to the CECT group, presence of ascites (P = 0.024), female sex (P = 0.001), serum sodium levels (P = 0.024), and azotemia at baseline (P = 0.049) correlated with CI-AKI development at univariate analysis (Table 3). Ascites (OR: 2.796, 95% CI: 1.109–7.052; P = 0.029), female sex (OR: 0.192, 95% CI: 0.073–0.510; P = 0.001), and azotemia at baseline (OR: 1.018, 95% CI: 1.001–1.037; P = 0.043) maintained the statistical significance at multivariate analysis (Table 3).
Table 3.
Variables analyzed at univariate and multivariate analysis in the CECT group for possible association with CI-AKI.
Reevaluation 3 months after discharge from hospital was available in 17/22 and 6/6 patients who developed AKI in the CECT and in the Control groups, respectively. AKI persisted in 10/17 patients (58.8%) and in 1/6 patients (16.6%) in the CECT and the Control groups, respectively. Of note, none of the patients with persistence of worsened kidney function showed signs of liver decompensation.
4. Discussion
Despite the limitations of the retrospective analysis, this study quite clearly indicates that the risk of AKI development in cirrhotics is significantly increased when patients undergo computed tomography with intravenous iodinated contrast. Moreover, although the CI-AKI was not usually severe (stage 1 in 90% of the cases), it seemed to persist over time since the worsening of kidney function was still present 3 months after the CECT execution in the majority of cases. The multivariate analysis revealed that presence of ascites, female sex, and increased levels of azotemia—but not of creatininemia—at baseline were risk factors of CI-AKI development. The fact that ascitic patients may be oversensitive in developing AKI is not surprising considering that kidney dysfunction is a major factor in ascites occurrence and thus advanced liver disease per se may be considered a clinical–pathological condition prone to an acute renal injury.[16,17] It is more difficult to interpret why female sex and hyperazotemia at baseline may favor CI-AKI occurrence. Indeed, previous studies had already reported the association between female sex and CI-AKI.[18–22] In their study focused on liver transplanted patients, Hilmi et al[23] stressed that all the women enrolled were in menopausal age and thus had lost the beneficial protective effects exerted by estrogens against cardiovascular and renal diseases, and would therefore be more exposed to various kidney insults. Of note and in accordance with this hypothesis, all female patients who developed CI-AKI in our study were in menopause. Increased azotemia values in cases with normal creatininemia are usually considered an indicator of hypercatabolic condition and are indeed quite often present in the advanced phases of cirrhosis. Why hyperazotemia at baseline was the only biochemical parameter significantly associated with the subsequent CI-AKI development in our series of patients is not easy to explain. One might speculate a hypothetical involvement of hypercatabolism in favoring renal impairment in these patients, and therefore high azotemia levels may, better than hypercreatininemia, indicate basal conditions of kidney dysfunction that predispose to AKI induced by contrast medium administration.
In conclusion, this study reveals that development of CI-AKI is not a rare event in cirrhotic patients, particularly if in women and/or in a decompensated phase with ascites and hyperazotemia. Renal impairment occurrence is a complication of cirrhosis and a frequent cause of dramatic worsening of the clinical picture and death. The results of this study suggest caution in performing CECT in patients with advanced cirrhosis, and reserving this radiologic approach for cases in which the clinical benefit for the patients of obtaining the information provided by this tool is clear.
Footnotes
Abbreviations: AKI = acute kidney injury, CECT = contrast-enhanced computed tomography, CI = confidence interval, CI-AKI = contrast-induced acute kidney injury, CKD = chronic kidney disease, CPT = Child–Pugh–Turcotte, eGFR = estimated glomerular filtration rate, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, MELD = model for end-stage liver disease, NAFLD = nonalcoholic fatty liver disease, OR = odds ratio, PBC = primary biliary cholangitis, sCr = serum creatinine.
RF and SM have contributed equally to the article.
Grant support: None.
Study design: Retrospective observational.
Author contributions: RF, SM, and GS designed the study and wrote the manuscript together with GR; AA performed the statistical analysis; LV, MSF, and CGG developed and filled in the dataset; GC, CS, and IC followed up the patients; TL and GO collected clinical data; SC and AB performed radiologic procedures in all cases. All the authors contributed to the interpretation of the results and reviewed the manuscript.
The authors have no conflicts of interest to disclose.
References
- 1.Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet 2013; 382:170–179. [DOI] [PubMed] [Google Scholar]
- 2.Stevens PE, Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158:825–830. [DOI] [PubMed] [Google Scholar]
- 3.Betrosian AP, Agarwal B, Douzinas EE. Acute renal dysfunction in liver diseases. World J Gastroenterol 2007; 13:5552–5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology 2008; 48:2064–2077. [DOI] [PubMed] [Google Scholar]
- 5.Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009; 361:1279–1290. [DOI] [PubMed] [Google Scholar]
- 6.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol 2015; 62:968–974. [DOI] [PubMed] [Google Scholar]
- 8.Morcos SK, Thomsen HS, Webb JA. Contrast-media-induced nephrotoxicity: a consensus report. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Eur Radiol 1999; 9:1602–1613. [DOI] [PubMed] [Google Scholar]
- 9.Najjar M, Hamad A, Salameh M, et al. The risk of radiocontrast nephropathy in patients with cirrhosis. Ren Fail 2002; 24:11–18. [DOI] [PubMed] [Google Scholar]
- 10.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis 2002; 39:930–936. [DOI] [PubMed] [Google Scholar]
- 11.European Association For The Study Of The Liver; European Organisation For Research and Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56:908–943. [DOI] [PubMed] [Google Scholar]
- 12.Guevara M, Fernandez-Esparrach G, Alessandria C, et al. Effects of contrast media on renal function in patients with cirrhosis: a prospective study. Hepatology 2004; 40:646–651. [DOI] [PubMed] [Google Scholar]
- 13.Lodhia N, Kader M, Mayes T, et al. Risk of contrast-induced nephropathy in hospitalized patients with cirrhosis. World J Gastroenterol 2009; 15:1459–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safi W, Rauscher I, Umgelter A. Contrast-induced acute kidney injury in cirrhotic patients. A retrospective analysis. Ann Hepatol 2015; 14:895–901. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–470. [DOI] [PubMed] [Google Scholar]
- 16.Montoliu S, Balleste B, Planas R, et al. Incidence and prognosis of different types of functional renal failure in cirrhotic patients with ascites. Clin Gastroenterol Hepatol 2010; 8:616–622. [DOI] [PubMed] [Google Scholar]
- 17.Angeli P, Rodriguez E, Piano S, et al. Acute kidney injury and acute-on-chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut 2015; 64:1616–1622. [DOI] [PubMed] [Google Scholar]
- 18.Patti G, Nusca A, Chello M, et al. Usefulness of statin pretreatment to prevent contrast-induced nephropathy and to improve long-term outcome in patients undergoing percutaneous coronary intervention. Am J Cardiol 2008; 101:279–285. [DOI] [PubMed] [Google Scholar]
- 19.Chong E, Poh KK, Liang S, et al. Comparison of risks and clinical predictors of contrast-induced nephropathy in patients undergoing emergency versus nonemergency percutaneous coronary interventions. J Interv Cardiol 2010; 23:451–459. [DOI] [PubMed] [Google Scholar]
- 20.Senoo T, Motohiro M, Kamihata H, et al. Contrast-induced nephropathy in patients undergoing emergency percutaneous coronary intervention for acute coronary syndrome. Am J Cardiol 2010; 105:624–628. [DOI] [PubMed] [Google Scholar]
- 21.Budano C, Levis M, D’Amico M, et al. Impact of contrast-induced acute kidney injury definition on clinical outcomes. Am Heart J 2011; 161:963–971. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Yang JH, Choi SH, et al. Predictors of outcomes of contrast-induced acute kidney injury after percutaneous coronary intervention in patients with chronic kidney disease. Am J Cardiol 2014; 114:1830–1835. [DOI] [PubMed] [Google Scholar]
- 23.Hilmi IA, Damian D, Al-Khafaji A, et al. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth 2015; 114:919–926. [DOI] [PubMed] [Google Scholar]


