Abstract
Both vertebral body wedging and disc wedging are found in ankylosing spondylitis (AS) patients with thoracolumbar kyphosis. However, their relative contribution to thoracolumbar kyphosis is not fully understood. The objective of this study was to compare different contributions of vertebral and disc wedging to the thoracolumbar kyphosis in AS patients, and to analyze the relationship between the apical vertebral wedging angle and thoracolumbar kyphosis.
From October 2009 to October 2013, a total of 59 consecutive AS patients with thoracolumbar kyphosis with a mean age of 38.1 years were recruited in this study. Based on global kyphosis (GK), 26 patients with GK < 70° were assigned to group A, and the other 33 patients with GK ≥ 70° were included in group B. Each GK was divided into disc wedge angles and vertebral wedge angles. The wedging angle of each disc and vertebra comprising the thoracolumbar kyphosis was measured, and the proportion of the wedging angle to the GK was calculated accordingly. Intergroup and intragroup comparisons were subsequently performed to investigate the different contributions of disc and vertebra to the GK. The correlation between the apical vertebral wedging angle and GK was calculated by Pearson correlation analysis. The duration of disease and sex were also recorded in this study.
With respect to the mean disease duration, significant difference was observed between the two groups (P < 0.01). The wedging angle and wedging percentage of discs were significantly higher than those of vertebrae in group A (34.8° ± 2.5° vs 26.7° ± 2.7°, P < 0.01 and 56.6% vs 43.4%, P < 0.01), whereas disc wedging and disc wedging percentage were significantly lower than vertebrae in group B (37.6° ± 7.0° vs 50.1° ± 5.1°, P < 0.01 and 42.7% vs 57.3%, P < 0.01). The wedging of vertebrae was significantly higher in group B than in group A (50.1° ± 5.1° vs 26.7° ± 2.7°, P < 0.01). Additionally, correlation analysis revealed a significant correlation between the apical vertebral wedging angle and GK (R = 0.850, P = 0.001).
Various disc and vertebral wedging exist in thoracolumbar kyphosis secondary to AS. The discs wedging contributes more to the thoracolumbar kyphosis in patients with GK < 70° than vertebral wedging, whereas vertebral wedging is more conducive to the thoracolumbar kyphosis in patients with GK ≥ 70°, indicating different biomechanical pathogenesis in varied severity of thoracolumbar kyphosis secondary to AS.
Keywords: ankylosing spondylitis, disc, thoracolumbar kyphosis, vertebral wedging, wedging
1. Introduction
Ankylosing spondylitis (AS) is a systematic rheumatic disorder, which is characterized clinically by pain and stiffness of the back, and radiologically by arthritic changes in the sacroiliac joints and the entire spine.[1,2] The global incidence of AS is 0.25% to 0.45%, and is 0.3% in China.[3,4] The chronic inflammation leads to progressive ossification of the spinal ligaments and facet joints. Advanced stages of AS are often associated with thoracolumbar kyphosis resulting in sagittal imbalance and impairment of the ability to look straight ahead, which dramatically restricts patient's activities of daily living.[5–7] Thoracolumbar kyphosis is an exaggerated outward curvature of the thoracic kyphosis and decreased lumbar lordosis, resulting in a rounded upper back. Additionally, AS patients may experience a cosmetic deformity and psychological complication. More importantly, AS patients with thoracolumbar kyphosis cannot lie down at full length, and the compression of the abdominal viscera by the inferior margins of the ribs may cause intra-abdominal complications.
It has been increasingly recognized that the fixed thoracolumbar kyphosis is the result of wedging of vertebrae and intervertebral discs,[8] and the sum of deformities of the vertebrae and wedging of the discs is also correlated with thoracolumbar kyphosis.[9] In general, ventral wedging (ventral height less than dorsal height) of the vertebral bodies and intervertebral discs increases the kyphosis angle, whereas dorsal wedging (ventral height greater than dorsal height) decreases it. Increased ventral wedging of the vertebral bodies is believed to be conducive to the increase in the kyphosis angle in AS patients.[10–13] In 2001, Geusens et al[8] reported that deformities of the thoracic vertebrae occur frequently and, together with wedging of the thoracic discs, contribute significantly to fixed hyperkyphosis secondary to AS. Similarly, in 2006, Vosse et al[14] also found that wedging of thoracic vertebrae was an independent significant contributory factor to hyperkyphosis in AS patients. However, they did not investigate the relative contributions of the vertebral bodies and discs to kyphosis in the whole spine.
To the best of our knowledge, although both discs and vertebrae are determinants of thoracolumbar kyphosis secondary to AS, the relative contributions of the vertebral bodies and discs to kyphosis have not been well addressed. Hence, we conducted this retrospective radiographic study of patients with AS to investigate the difference of contribution to the thoracolumbar kyphosis between vertebral and disc wedging, and to analyze the relationship between the apical vertebral wedging angle and thoracolumbar kyphosis.
2. Materials and methods
This is a retrospective comparative study approved by the institutional review board of our hospital. The date and reference number of IRB was 2011–07–29 and 2011–05–02. A total of 81 consecutive AS patients who underwent surgical correction at our institution from October 2009 to October 2013 were retrospectively reviewed. The inclusion criteria were: the diagnosis of AS according to the modified New York criteria,[15] age at surgery ≥ 20 years, and availability of full-length lateral standing spine radiographs. The exclusion criteria were: a coronal curve of > 10° in anteroposterior radiographs of the entire spine, patients had previous spinal or hip surgery, and the presence of pathological spinal fractures or pseudarthrosis in X-ray radiographs. Twenty-two AS patients were excluded from this study (age < 20 years: 4 patients, 4.94%; previous spinal or hip surgery: 4 patients, 4.94%; coronal curve of >10°: 2 patients, 2.47%; pathological spinal fractures or pseudarthrosis: 12 patients, 14.81%). Finally, 59 AS patients (51 males and 8 females), with a mean age of 38.1 years (range, 21–63 years), were recruited. According to the severity of thoracolumbar kyphosis, patients were divided into 2 groups. Group A with a global kyphosis (GK) < 70°, and group B with a GK ≥ 70°.[16,17]
The standing lateral radiographs were reviewed to measure the following parameters: the GK,[16] represented by the angle between the superior endplate of the maximally tilted upper end vertebra and the inferior endplate of the maximally tilted lower end vertebra on the full-length lateral radiographs, the angle is positive if the curve is kyphotic and is negative if the curve is lordotic; the vertebral wedge was defined as the angle between the superior and inferior endplates of each vertebra in the curve to evaluate the spine deformity; and the intervertebral disc wedge was calculated from the difference between the superior endplate of the interior vertebral body and the inferior endplate of the superior vertebral body to assess the disc wedging deformity (Fig. 1),[18] and the proportion of the wedging angle to the GK was calculated accordingly. An independent observer performed the measurements of all parameters. Each parameter was measured 3 times with the use of surgimap software (Nemaris, New York, NY) and the average values were calculated in both groups. Intergroup and intragroup comparisons were subsequently performed to investigate the different contributions of disc and vertebra to the GK. The correlation between the apical vertebral wedging angle and GK was calculated by Pearson correlation analysis. The duration of disease and sex were also recorded in this study.
Figure 1.
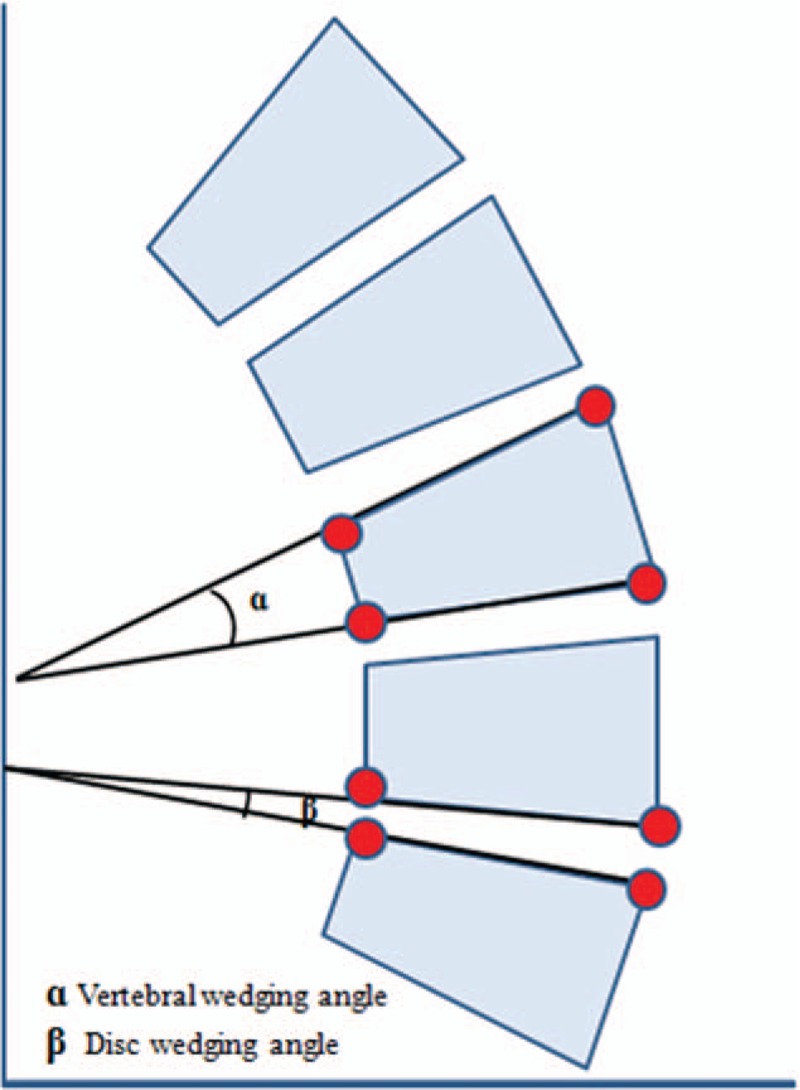
The vertebral wedging angle (ɑ) is the angle between the upper endplate and lower endplate of the vertebra. The intervertebral disc wedge (β) is defined as the angular difference between the inferior endplate of the upper vertebra and superior endplate of the lower vertebra.
The data were analyzed using SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL). The vertebral and intervertebral wedging angle as a proportion of the thoracolumbar kyphosis within groups were compared with t-test to see if one of the measures is statistically different than 50%, and the difference in each radiographic parameter between group A and group B was analyzed with an unpaired t-test. The correlation between the apical vertebral wedging angle and GK was performed by Pearson correlation analysis. For all these statistical methods, significance was defined as a P value of less than 0.05.
3. Results
A total of 59 AS patients (51 males and 8 females), with a mean age of 38.1 years (range, 21–63 years), were enrolled in this study. None of the participants had missing data for each variable of interest. According to the severity of thoracolumbar kyphosis, patients were divided into two groups (< 70°: Group A; ≥ 70°: Group B). Group A was composed of 2 females and 24 males with an average age of 31.3 ± 7.0 years (range, 21–46 years), and Group B consisted of 6 females and 27 males with a mean age of 43.5 ± 7.5years (range, 30–63 years). GK was 61.6 ± 2.7° in group A and 87.7 ± 9.6° in group B (P < 0.01). The mean disease duration of AS was 11.9 ± 3.3 years (range, 6–16 years) in group A versus 19.4 ± 3.4 years (range, 13–26 years) in group B. In terms of age and sex distribution, no significant difference was found between group A and Group B (P > 0.05), but a significant longer mean disease duration was observed in group B (P < 0.01).
Significant difference was found between vertebral and intervertebral disc wedging degrees in the same GK group in AS patients (P < 0.01). In group A, the degree and percentage of vertebral wedging were 26.7 ± 2.7° and 43.4%, respectively, while the wedging angle (percentage) of intervertebral disc was 34.8 ± 2.5° (56.6%). In Group B, the total vertebral wedging angle (percentage) was 50.1 ± 5.1° (57.3%) whereas intervertebral disc wedging accounted for only 37.6 ± 7.0° (42.7%) of the overall difference (Table 1). The degree of vertebral wedging angle was significantly higher than intervertebral disc wedging in group B (Fig. 2A), whereas intervertebral disc wedging was significantly greater than vertebral wedging in group A (Fig. 2B).
Table 1.
Radiological data of all patients.

Figure 2.
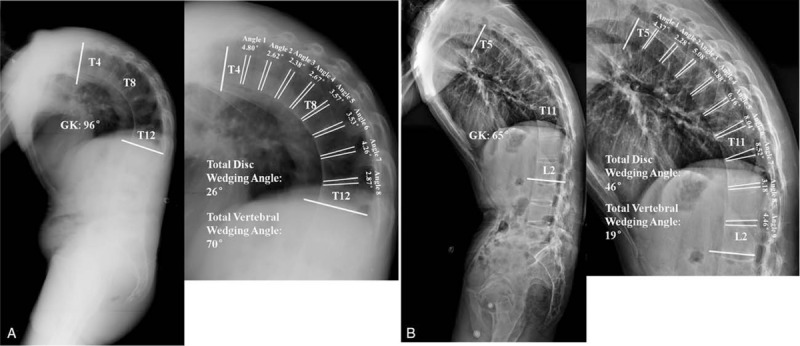
(A) A 43-year-old male ankylosing spondylitis patient with global kyphosis of 96°. The thoracolumbar kyphosis extended from T4 to T12 with the apex at T8. The degree and percentage of vertebral wedging were 70° and 73%, respectively. The total disc wedging angle (percentage) was only 26° (27%). (B) A 38-year-old male ankylosing spondylitis patient with a thoracolumbar kyphosis of 65° from T5 to L2. The apical vertebra was located at T11. Radiographic measurement showed that the wedging angle (percentage) of vertebrae was 19° (29%), whereas the total disc wedging angle and percentage were 46° and 71%, respectively.
The proportion of vertebral wedging in GK was 43.4% in group A and 57.3% in group B. The wedging of vertebrae was significantly higher in group B than in group A (50.1 ± 5.1° vs 26.7 ± 2.7°, P < 0.01) (Table 1). The apical vertebral body wedging was significantly larger in group B than in group A (19.2° ± 4.4° vs 7.3° ± 1.2°, P < 0.01). The correlation analysis also demonstrated that apical vertebral wedging angle was significantly positively associated with GK in AS patients (R = 0.850, P = 0.001) (Table 2).
Table 2.
The correlation between the apical vertebral wedging angle and GK in ankylosing spondylitis patients.

4. Discussion
AS is a chronic inflammatory disease of uncertain etiology that primarily affects the sacroiliac joints and axial skeleton, leading to progressive thoracolumbar kyphosis.[19,20] Due to the thoracolumbar kyphosis, the line of gravity typically lies anterior to the vertebrae, imposing mechanical loads on the anterior aspect of the vertebral bodies.[21] The cumulative effects of these loads may result in progression of thoracolumbar kyphosis.[22] Therefore, AS patients in advanced stages are prone to fracture due to the mechanical effects and loss of spinal mobility.[23–25] It has been increasingly recognized that the vertebral deformities and discal wedging are related to the ankylosing nature of the disease.[10] Although the shape characteristics of the thoracolumbar vertebral bodies and their role in determining kyphotic curvature have received considerable focus, detailed information regarding the contribution of the intervertebral discs has not yet been reported. Geusens et al[8] asserted that thoracic but not lumbar vertebral deformities, together with wedging of the thoracic discs, are related to an increase of occiput to wall distance (OWD) in AS patients. However, the exact anatomical changes of the vertebral bodies and intervertebral discs associated with fixed thoracolumbar kyphosis are still poorly understood.
The results of our study demonstrated that the degree of the fixed thoracolumbar kyphosis in AS is correlated with a combination of deformations of the intervertebral discs and, to a higher degree, of wedging of the vertebral bodies when GK ≥ 70°. This finding suggested that vertebral body wedging only develops following increased thoracolumbar kyphosis. Moreover, the significant correlations of the apical verteral wedging angle with GK also indicated that vertebral deformity is more likely to occur in thoracolumbar region in AS patients with more severe kyphosis. In group A, the prevalence of wedged discs in AS patients with GK < 70° was significantly higher compared to those with GK ≥ 70°, suggesting that besides vertebral deformities, wedging of the intervertebral discs contributes independently to thoracolumbar kyphosis in AS patients. In addition, the absence of correlation between vertebral and disc wedging indicated that different pathophysiological processes might be involved in the occurrence of vertebral and discal deformities in AS patients.
Three limitations of this study need to be addressed. First, the sample size is relatively small due to the strict inclusion and exclusion criteria. Second, this study is a retrospective analysis with no follow-up of the same patients throughout progression of their deformity. As such, we are assuming exchangeability between patients in Group A and Group B, which may not necessarily be true. Third, detailed analysis of the disc and vertebral wedging changes in thoracic spine and lumbar spine should be investigated separately. Despite the limitations, to our knowledge, this is the first study demonstrating the relative contributions of vertebral and disc wedging to the thoracolumbar kyphosis in the whole spine of AS patients.
Intervertebral discs, which contribute to the stabilization and flexibility of the spinal column, consist of 3 basic structures, the nucleus pulposus, annulus fibrosus, and cartilage endplates.[26] Disc tissue contains water, proteoglycan, and collagen. Physiologically, disc water and glycoprotein levels decrease with the inflammations, fibrosis, and calcifications appearing.[27] Generally, AS is characterized by spinal inflammation, and therefore many authors have focused on the inflammatory etiology of intervertebral discitis. Recent MRI studies and previous pathological investigations proved that the primary target of the immune response is at the cartilage/vertebral endplates of the intervertebral disc in AS patients.[28] Romanus et al[29] described that marginal erosions of the anterior vertebral corners related to inflammation of the anterior annulus fibrosus occur in AS patients. The anterior erosion becomes enclosed by a rim of sclerosis and further healing results in the formation of syndesmophytes, finally resulting in a ventral intervertebral disc wedging and complete ankylosed spinal segment.[30] This inflammatory mechanism could explain the higher percentage of disc wedging compared with vertebral wedging (56.6% vs 43.4%, P < 0.01) in AS patients with a significant shorter disease duration in group A (GK < 70°). Hence, we confirmed that the intervertebral disc is wedged but not the vertebral body when the thoracolumbar kyphosis deformity is initiating, due to the increased inflammation of vertebral endplates of the intervertebral disc.
Specifically, Sambrook et al[31] pointed out that wedging of the vertebrae contributing to thoracolumbar kyphosis is independent of the modified Stoke Ankylosing Spondylitis Spine Scores (mSASSS). Increased thoracolumbar kyphosis can, therefore, be regarded not only as a clinical consequence but also as an indicator of the presence of vertebral wedging in AS.[14,32] In a series of 50 AS patients in Geusens et al's study,[22] the prevalence of vertebral deformities was higher in patients with hyperkyphosis compared with patients without hyperkyphosis (45% vs 8%, P = 0.01). In the present study, as thoracolumbar kyphosis was more severe in group B, it is not difficult to understand why these patients had a larger proportion of vertebral wedging. The increased proportion of vertebral wedging in AS patients with hyperkyphosis may be ascribed to an anterior shift in body mass that effectively increases the moment arm between the spine and the superincumbent body mass.[33]
Possible confounders of the relationship between wedging and thoracolumbar kyphosis are the duration of disease and sex, as thoracolumbar kyphosis may increase with duration of disease,[34] and AS is a predominantly male disease with a more serious course in male than in female patients.[12] In our study, the percentage of thoracolumbar vertebral deformities increased significantly with the duration of disease, reflecting that the duration of disease contribute to the risk for vertebral deformities. However, no significant correlation was found between sex and severity of kyphosis (P > 0.05). It may be due to the small sample size.
Our results suggest that patients with AS start out with an inflammatory process that causes disc wedging, leading to a minor GK. After a long duration of disease, the vertebral body wedging starts to occur and creates a worsening GK. Our findings might open new perspectives for treatment of AS, which not only aim at prevention of inflammation-associated disc wedging in the early stage but also at prevention of the vertebral body wedging at later stage.
More importantly, the clinical relevance of this study lies in other 2 aspects. First, many authors advocated that pedicle subtraction osteotomy (PSO) is preferred to be performed at the apical region of thoracolumbar kyphosis to obtain a maximal correction. Besides the level of the osteotomy, the vertebral wedging angle may be another influencing factor of the amount of correction. If the vertebral wedging of the apex is large, the mean amount of correction at the apical vertebra may decrease due to less decancellation of the vertebral body (Fig. 3).[35] Second, the underlying active inflammation should not be ignored after correction surgery. Small disc wedging angle (well-maintained disc height) in AS patients may indicate the incompletely calcified disc, which may cause the loss of correction in the uninstrumented area. Hence, close follow-up is required.
Figure 3.
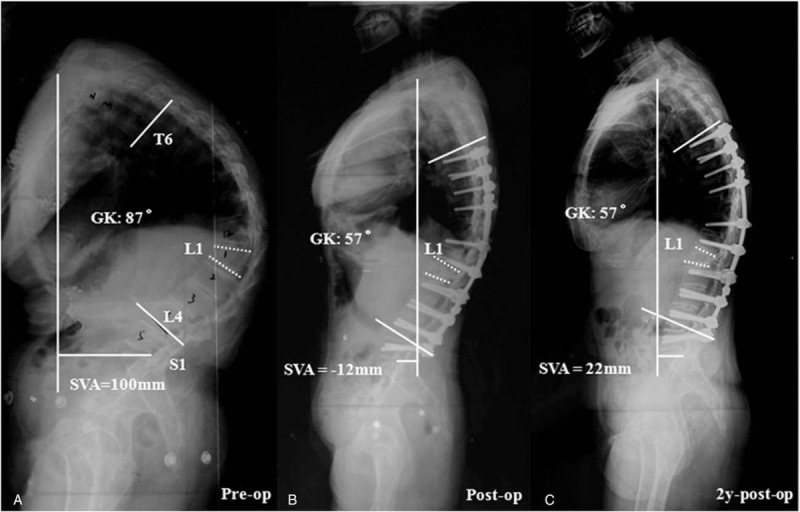
(A) A 44-year-old female ankylosing spondylitis patient with a global kyphosis (GK) of 87°. Preoperative X-ray showed that the apical vertebra was located at L1, the vertebral wedging angle was 25°, and the sagittal vertical axis (SVA) was 100 mm. (B) The GK and SVA were corrected to 57° and −12 mm, respectively, after L1 pedicle subtraction osteotomy (PSO); however, there was still some residual kyphosis due to the less decancellation from the apical vertebra (L1). (C) Two-year postoperative lateral radiograph demonstrated that the global sagittal alignment had been well maintained (GK = 57°, SVA = 22 mm).
5. Conclusion
In AS patients with thoracolumbar kyphosis, vertebral deformities occur frequently and, together with discal wedging, contribute significantly to thoracolumbar kyphosis. The thoracolumbar kyphosis results mainly from an increase in intervertebral disc wedging in patients with GK < 70°, whereas vertebrae wedging contributes more to the thoracolumbar kyphosis when GK ≥ 70°. These data are important for understanding the different pathophysiological processes in varied severity of thoracolumbar kyphosis in AS patients.
Footnotes
Abbreviations: AS = ankylosing spondylitis, GK = global kyphosis, mSASSS = modified Stoke Ankylosing Spondylitis Spine Scores, OWD = occiput to wall distance, PSO = pedicle subtraction osteotomy.
Ethical review committee statement: This study was approved by the ethical committee of Nanjing Drum Tower Hospital. The date and reference number of IRB was 2011–07–29 and 2011–05-02.
Authors’ contribution: HL carried out the study, and drafted the manuscript. YQ and BPQ conceived of the study. HL participated in the radiographic measuring and performed the statistical analysis. YW participated in the design of the study. BW, YY, and ZZZ participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Funding/support: One of the authors (Bang-ping Qian) has received funding from the National Natural Science Foundation of China (81372009), the Fourth Period of “333” High-level Personnel Training Program of Jiangsu Province, and the Maternity and Child Health Care Research Program of Jiangsu Province (F021353).
The remaining authors have no conflicts of interest to disclose.
References
- 1.Lu ML, Tsai TT, Lai PL, et al. A retrospective study of treating thoracolumbar spine fractures in ankylosing spondylitis. Eur J Orthop Surg Traumatol 2014; 24:S117–123. [DOI] [PubMed] [Google Scholar]
- 2.El-Sharkawi MM, Koptan WM, El-Miligui YH, et al. Comparison between pedicle subtraction osteotomy and anterior corpectomy and plating for correcting post-traumatic kyphosis: a multicenter study. Eur Spine J 2011; 20:1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007; 369:1379–1390. [DOI] [PubMed] [Google Scholar]
- 4.Liao ZT, Li C, Gu JR. Epidemiology of spondyloarthritis in Asian countries and regions. Curr Rheumatol Rev 2008; 4:87–90. [Google Scholar]
- 5.Qian BP, Wang XH, Qiu Y, et al. The influence of closing-opening wedge osteotomy on sagittal balance in thoracolumbar kyphosis secondary to ankylosing spondylitis: a comparison with closing wedge osteotomy. Spine 2012; 37:1415–1423. [DOI] [PubMed] [Google Scholar]
- 6.Mehdian H, Arun R, Aresti NA. V-Y vertebral body osteotomy for the treatment of fixed sagittal plane spinal deformity. Spine J 2015; 15:771–776. [DOI] [PubMed] [Google Scholar]
- 7.Qian BP, Qiu Y, Wang B, et al. Pedicle subtraction osteotomy through pseudarthrosis to correct thoracolumbar kyphotic deformity in advanced ankylosing spondylitis. Eur Spine J 2012; 21:711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geusens P, Vosse D, van der Heijde D, et al. High prevalence of thoracic vertebral deformities and discal wedging in ankylosing spondylitis patients with hyperkyphosis. J Rheumatol 2001; 28:1856–1861. [PubMed] [Google Scholar]
- 9.Goh S, Price RI, Leedman PJ, et al. The relative influence of vertebral body and intervertebral disc shape on thoracic kyphosis. Clin Biomech 1999; 14:439–448. [DOI] [PubMed] [Google Scholar]
- 10.Averns HL, Oxtoby J, Taylor HG, et al. Radiological outcome in ankylosing spondylitis: use of the Stoke Ankylosing Spondylitis Spine Score (SASSS). Br J Rheumatol 1996; 35:373–376. [DOI] [PubMed] [Google Scholar]
- 11.Geusens P, Vosse D, van der Linden S, et al. Osteoporosis and vertebral fractures in ankylosing spondylitis. Curr Opin Rheumatol 2007; 19:335–339. [DOI] [PubMed] [Google Scholar]
- 12.Ghozlani I, Ghazi M, Nouijai A, et al. Prevalence and risk factors of osteoporosis and vertebral fractures in patients with ankylosing spondylitis. Bone 2009; 44:772–776. [DOI] [PubMed] [Google Scholar]
- 13.Jun JB, Joo KB, Her MY, et al. Femoral bone mineral density is associated with vertebral fractures in patients with ankylosing spondylitis: a cross-sectional study. J Rheumatol 2006; 33:1637–1641. [PubMed] [Google Scholar]
- 14.Vosse D, van der Heijde D, Landewe R, et al. Determinants of hyperkyphosis in patients with ankylosing spondylitis. Ann Rheum Dis 2006; 65:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984; 27:361–368. [DOI] [PubMed] [Google Scholar]
- 16.Qian BP, Jiang J, Qiu Y, et al. The presence of a negative sacral slope in patients with ankylosing spondylitis with severe thoracolumbar kyphosis. J Bone Joint Surg Am 2014; 96:e188. [DOI] [PubMed] [Google Scholar]
- 17.Qian BP, Wang XQ, Qiu Y, et al. An exon polymorphism of programmed cell death 1 gene is associated with both the susceptibility and thoracolumbar kyphosis severity of ankylosing spondylitis in a Chinese Han population. J Orthop Sci 2013; 18:514–518. [DOI] [PubMed] [Google Scholar]
- 18.Modi HN, Suh SW, Song HR, et al. Differential wedging of vertebral body and intervertebral disc in thoracic and lumbar spine in adolescent idiopathic scoliosis - A cross sectional study in 150 patients. Scoliosis 2008; 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Zhang Y, Zhao Y, et al. Radiologic and clinical outcomes comparison between single- and two-level pedicle subtraction osteotomies in correcting ankylosing spondylitis kyphosis. Spine J 2015; 15:290–297. [DOI] [PubMed] [Google Scholar]
- 20.Qian BP, Jiang J, Qiu Y, et al. Radiographical predictors for postoperative sagittal imbalance in patients with thoracolumbar kyphosis secondary to ankylosing spondylitis after lumbar pedicle subtraction osteotomy. Spine 2013; 38:E1669–1675. [DOI] [PubMed] [Google Scholar]
- 21.Bruno AG, Anderson DE, D’Agostino J, et al. The effect of thoracic kyphosis and sagittal plane alignment on vertebral compressive loading. J Bone Miner Res 2012; 27:2144–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geusens P, Lems WF. Osteoimmunology and osteoporosis. Arthritis Res Ther 2011; 13:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathews M, Bolesta MJ. Treatment of spinal fractures in ankylosing spondylitis. Orthopedics 2013; 36:e1203–1208. [DOI] [PubMed] [Google Scholar]
- 24.Vosse D, Landewe R, van der Heijde D, et al. Ankylosing spondylitis and the risk of fracture: results from a large primary care-based nested case-control study. Ann Rheum Di 2009; 68:1839–1842. [DOI] [PubMed] [Google Scholar]
- 25.Robinson Y, Sanden B, Olerud C. Increased occurrence of spinal fractures related to ankylosing spondylitis: a prospective 22-year cohort study in 17,764 patients from a national registry in Sweden. Patient Saf Surg 2013; 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poole AR. Biologic markers and disc degeneration. J Bone Joint Surg Am 2006; 88:72–75. [DOI] [PubMed] [Google Scholar]
- 27.Resorlu M, Gokmen F, Resorlu H, et al. Association between apparent diffusion coefficient and intervertebral disc degeneration in patients with ankylosing spondylitis. Int J Clin Exp Med 2015; 8:1241–1246. [PMC free article] [PubMed] [Google Scholar]
- 28.Maksymowych WP. Ankylosing spondylitis--at the interface of bone and cartilage. J Rheumatol 2000; 27:2295–2301. [PubMed] [Google Scholar]
- 29.Romanus R, Yden S. Destructive and ossifying spondylitic changes in rheumatoid ankylosing spondylitis (pelvo-spondylitis ossificans). Acta Orthop Scand 1952; 22:88–99. [DOI] [PubMed] [Google Scholar]
- 30.Bron JL, de Vries MK, Snieders MN, et al. Discovertebral (Andersson) lesions of the spine in ankylosing spondylitis revisited. Clin Rheumatol 2009; 28:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook PN, Geusens P. The epidemiology of osteoporosis and fractures in ankylosing spondylitis. Ther Adv Musculoskelet Dis 2012; 4:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinigaglia L, Varenna M, Girasole G, et al. Epidemiology of osteoporosis in rheumatic diseases. Rheum Dis Clin North Am 2006; 32:631–658. [DOI] [PubMed] [Google Scholar]
- 33.Briggs AM, van Dieen JH, Wrigley TV, et al. Thoracic kyphosis affects spinal loads and trunk muscle force. Physical therapy 2007; 87:595–607. [DOI] [PubMed] [Google Scholar]
- 34.Maksymowych WP, Gooch KL, Wong RL, et al. Impact of age, sex, physical function, health-related quality of life, and treatment with adalimumab on work status and work productivity of patients with ankylosing spondylitis. J Rheumatol 2010; 37:385–392. [DOI] [PubMed] [Google Scholar]
- 35.Chen IH, Chien JT, Yu TC. Transpedicular wedge osteotomy for correction of thoracolumbar kyphosis in ankylosing spondylitis: experience with 78 patients. Spine 2001; 26:E354–360. [DOI] [PubMed] [Google Scholar]


