Abstract
The relationship between homocysteine levels and glaucoma has been questioned in previous studies without conclusive results. In the current study, we assessed the relationship between homocysteine levels and intraocular pressure which is one of the main factors in the development of glaucoma in men and women.
A retrospective cross-sectional analysis of a database from a screening center in Israel which assessed 11,850 subjects, within an age range 20 to 80 years. The relationship between homocysteine and intraocular pressure has been investigated by comparing intraocular pressure in subjects with elevated and normal homocysteine and by comparing homocysteine levels in subjects with elevated and normal intraocular pressure. In addition, we compared the levels of homocysteine in subjects with and without a confirmed diagnosis of glaucoma.
The mean IOP (±SD) in subjects with normal homocysteine levels(≤15 μmol/L) was 13.2 ± 2.3 mm Hg and 13.4 ± 2.4 mm Hg in those with high homocysteine levels (>15 μmol/L) (P < 0.008, 95% confidence interval [CI] 0.3–0.09).Nonetheless, after multivariate adjustment for age, gender, vitamin B12, and folic acid statistical significance was no longer demonstrated (P = 0.37). Mean homocysteine levels (±SD) in subjects with normal intraocular pressure of ≤ 21 mm Hg was 11.7 ± 5.5 μmol/L and 12.09 ± 3.43 μmol/L in those with elevated intraocular pressure (P = 0.4, 95%CI 1.1–1.8). Mean homocysteine levels (±SD) in subjects with glaucoma were 11.2 ± 3.5 μmol/L compared to 11.7 ± 5.5 μmol/L in subjects without glaucoma and normal intraocular pressure ≤ 21 mm Hg (P = 0.4, 95% CI 1.2–2.1).
The current study displays no clinical correlation between the homocysteine level and the intraocular pressure. Homocysteine may not be used as a predictive parameter to recognize those subjects prone to develop elevated intraocular pressure.
Keywords: glaucoma, homocysteine (Hcy), intraocular pressure (IOP)
1. Introduction
Glaucoma is a major healthcare-associated morbidity worldwide. Approximately 60 million people are affected by glaucomatous optic neuropathy, while three-quarters out of these cases are open-angle glaucoma.[1] It is estimated that around 8.4 million people worldwide are blind as the result of glaucoma, and actually, after cataracts, glaucoma is the second leading cause of blindness worldwide[1,2] and the main reason for blindness among African Americans.[3,4] The major risk factors for developing open-angle glaucoma include age, black race, family history, and elevated intraocular pressure (IOP).[5,6] Other risk factors include hypertension,[7] diabetes mellitus,[8] hypothyroidism,[9] vitamin B12 deficiency, and folic acid deficiency. A large body of literature describes the association between elevated IOP and both the development and progression of open-angle glaucoma.[11–13] Indeed, treatment of glaucoma primarily focuses on lowering IOP.[14]
Homocysteine (Hcy) is an amino acid which serves as an intermediate in methionine metabolism to cysteine.[15] Hyperhomocysteinemia refers to elevated plasma levels of Hcy. It could result either from genetic defects in enzymes involved in its metabolism,[16] nutritional deficiencies in vitamins cofactors such as vitamin B12 and folic acid or from other factors including chronic medical conditions[18] and drugs[19]. Both elevated Hcy levels and glaucoma were found to be related to increased risk of vascular disease[17,20–22] as well as chronic kidney disease.[23,24,25]
The rational to investigate the relationship between Hcy and glaucoma is based upon studies which demonstrated glaucoma to be related with increased risk of cardiovascular diseases[26–28] and upon studies that showed a correlation between elevated Hcy levels and glaucoma, especially the pseudoexfoliative subtype in comparison to primary open-angle glaucoma and normotensive glaucoma.[29–31] Nonetheless, studies issuing this relationship yielded conflicting results. In their meta-analysis, Xu et al[32] pointed to a possible relationship between elevated Hcy levels and open-angle glaucoma yet the investigated studies included a small number of subjects and suffered from significant between-study heterogenicity. As elevated IOP is a major risk factor in the development of glaucoma, a question arises concerning a possible relationship between Hcy and IOP as well.
In the current study, we investigated the relationship between Hcy levels and IOP in both men and women in a large cohort from Israel.
2. Subjects and methods
2.1. Study population and design
A large health database was analyzed from a screening center at the Rabin Medical Center in Israel between the years 2000–2013. The population that attends the center for screening comprises subjects sent by their employers for an annual medical examination. The subjects attending the center include men and nonpregnant women within an age range of 20 to 80 years .Hospitalized patients are not examined. Each subject undergoes a thorough medical history evaluation and a complete physical examination along with a broad series of blood and urine tests, a chest x-ray, an electrocardiogram, an exercise stress test, and a respiratory function test. Subjects may return once a year for a repeat investigation. For the purpose of this study, we used data from each subject's most recent visit. All subjects were evaluated by an experienced ophthalmologist who performed a full ophthalmology examination including Goldmann applanation tonometry. Measurements of IOP were performed in the sitting position between 8:00 am and 11:00 am. In this study, we used the IOP measurement of the right eye for assessment. We categorized IOP as <21 mm Hg or >21 mm Hg.[33] Diagnosis of glaucoma has been retrieved from the diagnosis registry key written by the ophthalmologist on the day of the examination. Hcy levels were defined as normal if measured 15 μmol/L or less and elevated if measured higher than 15 μmol/L.[34] For the determination of plasma homocysteine, fresh blood samples were immediately cooled on ice and protected from light. The plasma was separated by cold centrifugation and kept in refrigeration (2–8°C) until assessment. Assessment was performed once or twice weekly on the Abbott Axsym system using a Fluorescence Polarization immunoassay technology.
We have examined the relationship between Hcy and IOP, first by comparing IOP in subjects with elevated and normal Hcy levels and thereafter by comparing Hcy levels in subjects with elevated and normal IOP. For this purpose, subjects with glaucoma or those undergoing surgery for glaucoma were excluded from the study. In addition, we compared the levels of Hcy in subjects with and without a confirmed diagnosis of glaucoma. The study was approved by the Helsinki Ethics committee of the Rabin Medical Center.
2.2. Statistical analysis
Student's t-test and Chi-square test for continuous and categorical variables, respectively, were used to compare the means of baseline characteristics of men and women. The Pearson correlation index was used to assess the correlation between IOP and Hcy. Hcy levels were compared in subjects with normal versus elevated IOP and in subjects with diagnosis of glaucoma versus subjects without the diagnosis of glaucoma using Student's t-test. Multivariate adjusted analysis of variance (ANOVA) was used to assess the difference between the mean (95% confidence interval [CI]) IOP in subjects with elevated Hcy levels compared to the mean IOP measurement in the normal Hcy levels reference group. Parameters included in the multivariate analysis were Hcy, age, gender, vitamin B12, and folic acid. All analyses were conducted using SAS 9.4.
3. Results
3.1. Baseline characteristics
From 2000 to 2013, data on11,850 subjects were recorded and included all the data relevant for this assessment. The clinical characteristics of the subjects are presented in Table 1. The mean age of the study sample was 47.0 ± 9.5 years, 68% were males and 32% females. Mean IOP was higher in men than in women; 13.4 ± 2.5 vs 13.0 ± 2.2 mm Hg respectively (P < 0.001). There were no statistically significant differences between males and females as to their age and smoking pattern. Men had significantly higher levels of body-mass index (BMI), low-density-lipoprotein (LDL) cholesterol, and triglycerides and higher rates of hypertension and diabetes mellitus (P < 0.001 for all). In contrast, thyroid stimulation hormone (TSH), high-density-lipoprotein (HDL) cholesterol, vitamin B12 and folic acid serum levels were significantly higher in females (P < 0.001).
Table 1.
Baseline characteristics of the study population (N = 11,850).
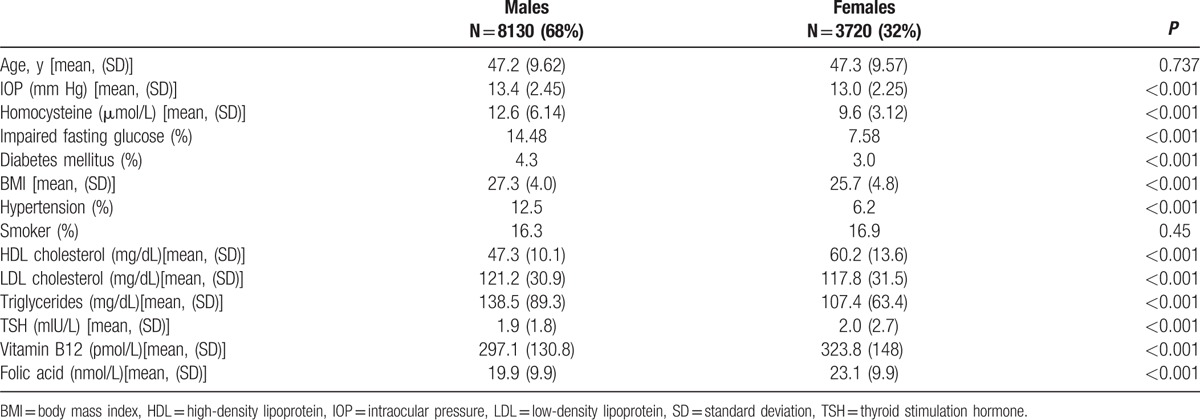
3.2. Relationship between homocysteine and IOP in subjects without glaucoma
There was a positive correlation between IOP and Hcy, r = 0.033, P < 0.001. A scatter plot summarizes the results (Fig. 1). Mean IOP (±standard deviation [SD]) in subjects with elevated Hcy levels (>15 μmol/L) was 13.4 ± 2.4 mm Hg, which was statistically significantly higher compared to those with normal Hcy levels 13.2 ± 2.3 mm Hg (P < 0.008). Nonetheless, after multivariate adjustment, statistical significance was no longer demonstrated for Hcy (P = 0.37) and the only factors influencing IOP were age and gender (P < 0.001 for both) (Table 2). Hcy levels were also compared between subjects with normal and elevated IOP (Fig. 2). In univariate analysis, there was no statistically significant difference between both groups, with the mean Hcy levels (±SD) in subjects with normal IOP being 11.7 ± 5.5 μmol/L compared to 12.09 ± 3.43 μmol/L in those with elevated IOP (P = 0.4).
Figure 1.
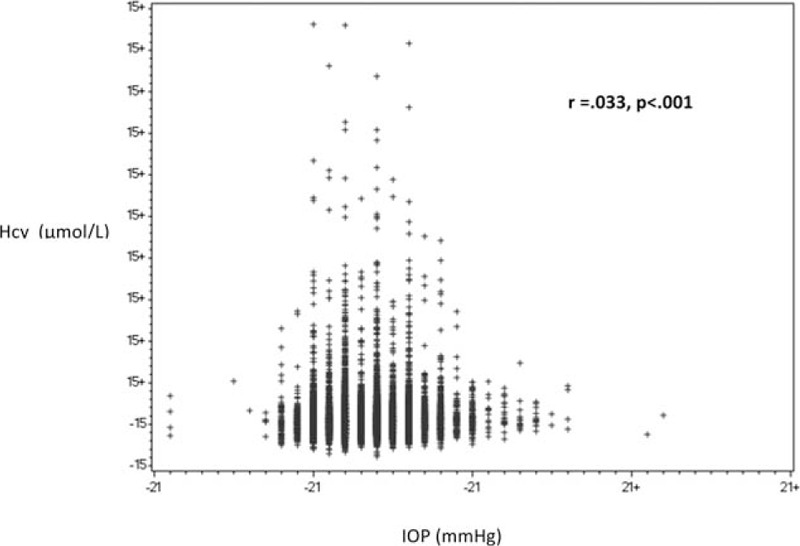
Relationship between homocysteine and intraocular pressure in subjects without glaucoma. Hcy = homocysteine, IOP = intraocular pressure.
Table 2.
Multivariate analysis of the relationship between homocysteine and intraocular pressure in subjects without glaucoma.
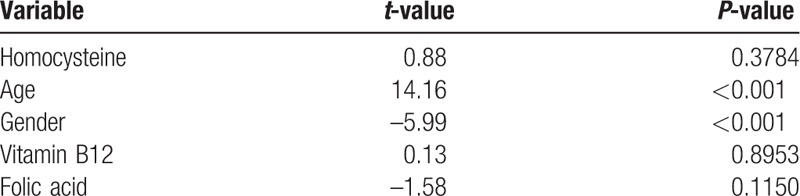
Figure 2.
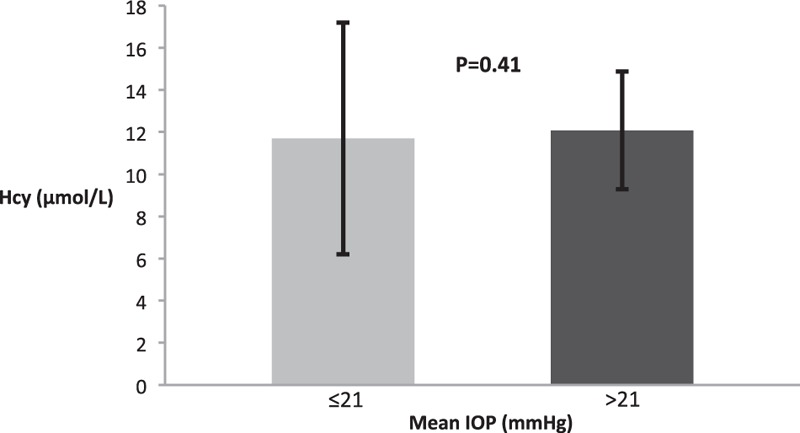
Homocysteine in subjects with normal and elevated intraocular pressure. Hcy = homocysteine, IOP = intraocular pressure.
3.3. Relation between homocysteine and glaucoma
Several previous cross-sectional and longitudinal studies[29–32] have investigated a possible correlation between Hcy and glaucoma yielding conflicting results. Therefore, in our cohort, we also examined the relationship between Hcy and glaucoma. As shown in Fig. 3, in univariate analysis, there was no statistically significant difference in the mean Hcy level in subjects with diagnosis of glaucoma compared to those without glaucoma and normal IOP (11.2 ± 3.5 μmol/L and 11.7 ± 5.5 μmol/L, respectively; P = 0.4, 95% CI 1.2–2.1).
Figure 3.
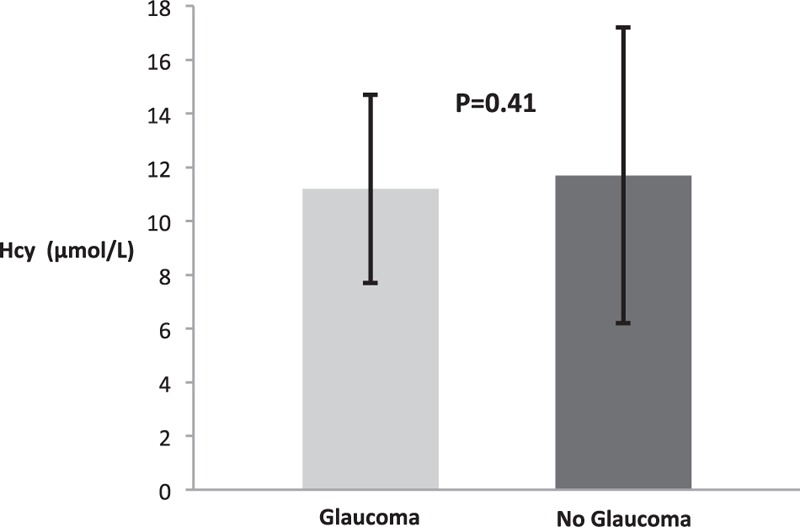
Homocysteine in subjects with and without glaucoma. Hcy = homocysteine, IOP = intraocular pressure.
4. Discussion
As glaucoma is a leading cause for blindness worldwide, efforts should be taken in order to minimize and recognize in advance risk factors which contribute to its development including elevated IOP. Several metabolic factors thought to be related to the development of elevated IOP, including Hcy levels, have been investigated in the past. In the current large cross sectional study, 11,850 subjects attending a screening examination center in Israel were evaluated for the relationship between IOP and Hcy levels in both males and females.
We analyzed this relationship in several aspects. We studied IOP levels in subjects with normal and elevated Hcy levels, as well as Hcy levels in subjects with normal and elevated IOP. It is important to emphasize that in both cases subjects with the diagnosis of glaucoma were excluded, since those subjects were already treated with IOP lowering medications which could alter the effect of Hcy on IOP. Although the level of IOP in subjects with elevated Hcy levels was minimally elevated compared to subjects with normal Hcy levels, the levels in both groups were almost identical in absolute numbers and it was fair to conclude that it probably has no clinical weight. Indeed after multivariate adjustment for age, gender, vitamin B12 and folic acid, there was no statistical difference between the groups. Similarly, no statistical difference has been demonstrated comparing Hcy levels among subjects with normal and elevated IOP.
Furthermore, we also examined Hcy levels in those subjects with confirmed diagnosis of glaucoma compared to those without glaucoma and normal IOP levels and found no statistical significant difference between the two groups.
Our findings imply that there is no clinical significant relation between IOP and Hcy levels; hence, Hcy may not be used as a predictive parameter for elevated IOP. So far, the relationship between IOP and Hcy has not been investigated directly except for 1 small-scaled study of Chang et al[35] who examined the association between IOP and several metabolic parameters, among them Hcy. In their study, no significant correlation between Hcy and IOP has been demonstrated. Nonetheless, the study was limited by small sample size and the usage of noncontact tonometry which is less accurate measurement technique of the IOP.
Our study is notable for the large number of subjects assessed, that is, 11,850 subjects, with complete data sets. The population of our study largely represents a western population and includes a broad range of ages. Possible gender differences were taken into account and all analyses were performed separately for males and females. As results were the same for both males and females, we have presented the results combined for both genders. We performed regression analysis for vitamin B12 and folic acid, commonly associated with Hcy. Roedl et al[10] investigated the levels of folic acid and vitamin B12 and their associations with Hcy in subjects with pseudoexfoliation syndrome (PEXG), which is a common subtype of glaucoma. All subjects with PEXG had an elevated IOP of >21 mm Hg. Folate and vitamin B12 levels were found to be significantly decreased and associated with elevated Hcy levels in subjects with PEXG. Their conclusion was that PEXG is associated with B-vitamin deficiency and hyperhomocysteinemia and that further investigations are necessary to evaluate the causal relationship of these results. Our results imply whenever folate, vitamin B12, and Hcy are risk factors for glaucoma, their pathogenic mechanism might not be mediated via increased IOP. In relation to PEXG, other mechanism aside from elevated IOP, such as the contribution of Hcy in the progressive build-up of abnormal extracellular fibrils in ocular and extraocular tissues, were proposed.[29,31]
Our study has some limitations. Data from this study were drawn from a selected population that attends an examination center. This center is funded by employers and hence may not be generalizable to nonemployed populations. Furthermore, our study is cross-sectional and hence causality cannot be determined.
Although our study focuses on IOP, we also issued the question regarding glaucoma and its relation to Hcy levels. As shown above, no statistically significant difference was found in Hcy levels between subjects with and without glaucoma. Nonetheless, the sample size of those subjects with glaucoma was quite small (N = 42) and data regarding the precise type of glaucoma was lacking. Specifically in respect to the correlation between glaucoma and Hcy, further larger scale studies should take place.
In summary, this large cohort of subjects displayed no clinical correlation between Hcy and IOP. We conclude that Hcy should not be used as a predictive parameter to recognize those subjects prone to develop elevated IOP. The question still remains as to the association between Hcy and glaucoma via other pathogenic mechanisms.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, Hcy = homocysteine, HDL = high-density lipoprotein, IOP = intraocular pressure, PEXG = Pseudoexfoliation syndrome, SD = standard deviation, TSH = thyroid stimulation hormone.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Colin CPF. Epidemiology of glaucoma: what's new? Can J Ophthalmol 2012; 47:223–226. [DOI] [PubMed] [Google Scholar]
- 2.Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ 2004; 82:887–888. [PMC free article] [PubMed] [Google Scholar]
- 3.Yih-Chung TXL, Tien YW, Harry A, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014; 121:2081–2090. [DOI] [PubMed] [Google Scholar]
- 4.Alfred SJM, Joanne K. Racial differences in the cause-specific prevalence of blindness in east Baltimore. NEJM 1991; 325:1412–1417. [DOI] [PubMed] [Google Scholar]
- 5.Young HKJH, Markus HK, Wallace LM. Primary open-angle glaucoma. NEJM 2009; 360:1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monika ACWD, Roger CW. Incidence of glaucomatous visual field loss: a ten-year follow-up from the Rotterdam Study. Ophthalmology 2010; 117:1705–1712. [DOI] [PubMed] [Google Scholar]
- 7.Di ZJC, Myung HK, Eliseo G. The association of blood pressure and primary open-angle glaucoma: a meta-analysis. Am J Ophthalmol 2014; 158: 615-627 e619. [DOI] [PubMed] [Google Scholar]
- 8.Di ZJC, Myung HK, David SF, et al. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology 2015; 122:72–78. [DOI] [PubMed] [Google Scholar]
- 9.Christopher AGGMJ, Sandre FM, Paul PL, et al. Hypothyroidism and the development of open-angle glaucoma in a male population. Ophthalmology 2004; 111:1649–1652. [DOI] [PubMed] [Google Scholar]
- 10.Roedl JBBS, Reulbach U. Vitamin deficiency and hyperhomocysteinemia in pseudoexfoliation glaucoma. J Neural Transm 2007; 114:571–575. [DOI] [PubMed] [Google Scholar]
- 11.Ekstrom C. Risk factors for incident open-angle glaucoma: a population-based 20-year follow-up study. Acta Ophthalmol 2012; 90:316–321. [DOI] [PubMed] [Google Scholar]
- 12.Nemesure BHR, Hennis A, Wu SY, et al. Incident open-angle glaucoma and intraocular pressure. Ophthalmology 2007; 114:1810–1815. [DOI] [PubMed] [Google Scholar]
- 13.Martínez BCCB, Nicolela MT, McCormick TA, et al. Intraocular pressure and progression of glaucomatous visual field loss. Am J Ophthalmol 2000; 129:302–308. [DOI] [PubMed] [Google Scholar]
- 14.Robert NWTA, Felipe AM. The pathophysiology and treatment of glaucoma: a review. JAMA 2014; 311:1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henk JBYS. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis 2011; 34:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jürgen GUH, Marion B, Heike S, et al. The role of genetic factors in the development of hyperhomocysteinemia. Clin Chem Lab Med 2003; 41:1427–1434. [DOI] [PubMed] [Google Scholar]
- 17.Eva LSY, Malcolm JA. Homocysteine lowering with folic acid and B vitamins in vascular disease. NEJM 2006; 354:1567–1577. [DOI] [PubMed] [Google Scholar]
- 18.Min-Chun CS-LH, Lance ED. Serum homocysteine level is positively associated with chronic kidney disease in a Taiwan Chinese population. J Nephrol 2014; 27:299–305. [DOI] [PubMed] [Google Scholar]
- 19.Cyrus DMK, Dennis BM, Vivian F. Drugs affecting homocysteine metabolism impact on cardiovascular risk. Drugs 2002; 62:605–616. [DOI] [PubMed] [Google Scholar]
- 20.Jean-Charles FMC, Erik SGS, John JP, et al. New risk factors for atherosclerosis and patient risk assessment. Circulation 2004; 109 (23 suppl 1):III15–III19. [DOI] [PubMed] [Google Scholar]
- 21.Aaron RFFJN, Paul GM. Prospective study of coronary heart disease incidence in relation to fasting total homocysteine, related genetic polymorphisms, and B vitamins. The Atherosclerosis Risk in Communities (ARIC) study. Circulation 1998; 98:204–210. [DOI] [PubMed] [Google Scholar]
- 22.Jeganathan VS, Wong TY, Foster PJ, et al. Peripheral artery disease and glaucoma: the Singapore Malay Eye Study. Arch Ophthalmol 2009; 127:888–893. [DOI] [PubMed] [Google Scholar]
- 23.Amos LEC, Micha L, Elad G, et al. Elevated serum homocysteine is a predictor of accelerated decline in renal function and chronic kidney disease: a historical prospective study. Eur J Int Med 2014; 25:951–955. [DOI] [PubMed] [Google Scholar]
- 24.Nongpiur ME, Wong TY, Sabanayagam C, et al. Chronic kidney disease and intraocular pressure: the Singapore Malay Eye Study. Ophthalmology 2010; 117:477–483. [DOI] [PubMed] [Google Scholar]
- 25.Djordjevic-Jocic J, Cukuranovic R, Mitic B, et al. Ocular and systemic factors associated with glaucoma in chronic kidney disease patients. Int Urol Nephrol 2014; 46:2191–2198. [DOI] [PubMed] [Google Scholar]
- 26.Kim YHJS, Nam GE. High intraocular pressure is associated with cardiometabolic risk factors in South Korean men: Korean National Health and Nutrition Examination Survey, 2008–2010. Eye 2014; 28:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sungmin YYC, Chan-Won K. Intraocular pressure and coronary artery calcification in asymptomatic men and women. Br J Ophthalmol 2015; 99:932–936. [DOI] [PubMed] [Google Scholar]
- 28.Dustin DFCE, Lynn EH. Ocular pseudoexfoliation and cardiovascular disease: a National Cross-Section Comparison Study. N Am J Med Sci 2012; 4:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colin ICIG, Paul RH, Stuart LG. Plasma homocysteine, MTHFR gene mutation, and open-angle glaucoma. J Glaucoma 2009; 18:73–78. [DOI] [PubMed] [Google Scholar]
- 30.Christopher WRDB, Udo R. Plasma homocysteine levels in patients with normal tension glaucoma. J Glaucoma 2010; 19:576–580. [DOI] [PubMed] [Google Scholar]
- 31.Tongabay CSS, Erdinc A. Serum homocysteine, vitamin B 12 and folic acid levels in different types of glaucoma. BMC Ophthalmol 2006; 6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu F, Zhao X, Zeng Si-M, et al. Homocysteine, B vitamins, methylenetetrahydrofolate reductase gene, and risk of primary open-angle glaucoma: a meta-analysis. Ophthalmology 2012; 119:2493–2499. [DOI] [PubMed] [Google Scholar]
- 33.Pedro BMA, Francisa GB. Predictive value of tonometry with Tono-pen XL in primary care. Brit J Gen Pract 2007; 57:653–654. [PMC free article] [PubMed] [Google Scholar]
- 34.Raj SBDW, Killian R. Homocysteine: update on a new risk factor. Cleve Clin J Med 1997; 64:543–549. [DOI] [PubMed] [Google Scholar]
- 35.Chang YCLJ, Wang LC, Chen HM, et al. Association of intraocular pressure with the metabolic syndrome and novel cardiometabolic risk factors. Eye 2010; 24:1037–1043. [DOI] [PubMed] [Google Scholar]


