Supplemental Digital Content is available in the text
Keywords: adjuvant treatment, intraoperative radiotherapy, overall survival, pancreatic cancer
Abstract
To assess prognostic benefits of intraoperative electron beam radiation therapy (IOERT) in patients with nonmetastatic locally advanced pancreatic cancer (LAPC) and evaluate optimal adjuvant treatment after IOERT.
A retrospective cohort study using prospectively collected data was conducted at the Cancer Hospital of the Chinese Academy of Medical Sciences, China National Cancer Center.
Two hundred forty-seven consecutive patients with nonmetastatic LAPC who underwent IOERT between January 2008 and May 2015 were identified and included in the study. Overall survival (OS) was calculated from the day of IOERT. Prognostic factors were examined using Cox proportional hazards models. The 1-, 2-, and 3-year actuarial survival rates were 40%, 14%, and 7.2%, respectively, with a median OS of 9.0 months. On multivariate analysis, an IOERT applicator diameter < 6 cm (hazards ratio [HR], 0.67; 95% confidence interval [CI], 0.47–0.97), no intraoperative interstitial sustained-release 5-fluorouracil chemotherapy (HR, 0.46; 95% CI, 0.32–0.66), and receipt of postoperative chemoradiotherapy followed by chemotherapy (HR, 0.11; 95% CI, 0.04–0.25) were significantly associated with improved OS. Pain relief after IOERT was achieved in 111 of the 117 patients, with complete remission in 74 and partial remission in 37. Postoperative complications rate and mortality were 14.0% and 0.4%, respectively. Nonmetastatic LAPC patients with smaller size tumors could achieve positive long-term survival outcomes with a treatment strategy incorporating IOERT and postoperative adjuvant treatment.
Chemoradiotherapy followed by chemotherapy might be a recommended adjuvant treatment strategy for well-selected cases. Intraoperative interstitial sustained-release 5-fluorouracil chemotherapy should not be recommended for patients with nonmetastatic LAPC.
1. Introduction
Pancreatic cancer is the fourth leading cause of cancer-related deaths worldwide.[1] Surgical resection is the major curative treatment, however, it provides survival benefits only for patients with small and localized pancreatic tumors. Approximately 30% of pancreatic cancer patients present with locally advanced, unresectable nonmetastatic disease have median survival times ranging from 5 to 11 months.[2,3] Currently, no standard treatment has been established for these patients.[4] While radiotherapy has been largely adopted to treat nonmetastatic locally advanced pancreatic cancer (LAPC), the optimal treatment strategy has not been clearly defined.[4] Several studies suggested that external beam radiotherapy (EBRT) alone was insufficient for controlling bulky tumors without damaging the surrounding normal tissues.[5,6]
Intraoperative electron beam radiation therapy (IOERT) is characterized as use of a single high dose of radiotherapy during the operation focused on the tumor without damaging surrounding healthy tissues.[7] However, some opponents claim that IOERT does not result in greater effectiveness in terms of overall survival (OS) when used in conjunction with surgery.[8] IOERT for nonmetastatic LAPC was initiated at the China National Cancer Center in 2008 and since then 247 patients have been treated by IOERT. To the best of our knowledge, this cohort is the largest to date in the IOERT literature for patients with nonmetastatic LAPC.
The aim of present study was to assess long-term survival, safety, and prognostic factors for OS among patients with nonmetastatic LAPC who received IOERT as part of their treatment at China National Cancer Center. We also sought prognostic factors to aid in the selection of optimal adjuvant treatment after IOERT for those patients.
2. Patients and methods
2.1. Patient selection
A total of 247 consecutive patients with nonmetastatic, histologically confirmed LAPC who underwent IOERT at the China National Cancer Center between January 2008 and May 2015 were identified and included in the study. The prospective database tracks data on patient anthropometrics, demographics, clinical history, past medical history, smoking and alcohol consumption, family history, comorbidities, diagnostic tests, tumor characteristics, therapeutic interventions, complications, pathologic data, and outcomes. All data were backed up by source documents and the accuracy of the data entered into the database was periodically reviewed. All study procedures were approved by the Institutional Review Board at the China National Cancer Center.
2.2. Surgery
When surgery was commenced, the patients underwent gross examination for metastatic lesions. Histologically and/or cytologically proof of pancreatic cancer was obtained for all 247 tumors either by incisional biopsy and/or needle biopsy of the primary tumors. In the case of locally advanced cancer, ultrasound examination was performed for diagnosis of invasion of portal vein or major arteries. Patients with tumor invasion to the major arteries, including the common hepatic, superior mesenteric, and celiac arteries, were confirmed having advanced unresectable pancreatic cancer. Accompanying procedures were often performed before surgical closure: gastrojejunostomy (70 patients; 28.3%); cholecystojejunostomy (5 patients; 2%), gastrojejunostomy plus choledochojejunostomy (14 patients; 5.7%), or gastrojejunostomy plus cholecystojejunostomy (125 patients; 50.6%).
2.3. IOERT
After palliative surgical procedures, IOERT was delivered using the Mobetron linear accelerator (Intraop Medical Corporation, Sunnyvale, CA). The maximum electron energy was 12 MeV. Several Asian studies such as Nagoya University performed IOERT for LAPC using 10 to 12 MeV energy electron beam.[9] Because of the concern of potential toxicity of high-dose irradiation, we employed 12 MeV electron energy. The surgeon and radiation oncologist assessed the extent of disease at operation and a cylindric applicator of appropriate size was selected to cover the tumor comfortably within the field, usually with a 1-cm margin around the pancreatic mass. Cone sizes were selected to deliver an average dose of 14 Gy (range from 10 to 20 Gy) to a field that included the primary tumor and a margin of 1 to 2 cm covering the regional lymph nodes (mean applicator diameter, 6.0 cm; range, 4–10 cm). Because of the potential toxicity of high-dose irradiation, we have employed IOERT at 10 to 20 Gy combined with adjuvant treatment. The cone size, treatment setup, and immobilization were selected to treat the target volume while minimizing exposure of adjacent normal tissue.
2.4. Interstitial sustained-release 5-fluorouracil chemotherapy
Of the 247 patients, 101 underwent intraoperative interstitial chemotherapy using Sinofuan sustained-release 5-fluorouracil implants (Xiansheng Corporation, Nanjing, China). 500 mg/m2 of sustained-release 5-fluorouracil particles were implanted into the tumor after administration of IOERT.
2.5. Adjuvant therapy
Postoperative adjuvant therapy (excluding intraoperative interstitial chemotherapy) was recommended for all patients underwent IOERT. However, some patients declined adjuvant therapy due to various reasons. Among 194 patients (78.5%) with documented postoperative adjuvant therapy information, 67 patients did not receive any type of adjuvant therapy; 49 patients received concurrent chemoradiation therapy: 4- to 6-field intensity-modulated radiation therapy (IMRT) 95% planning gross tumor volume (PGTV) 36 to 40 Gy in 1.8 to 2 Gy fractions, concurrent capecitabine (at a dose of 1600 mg/m2 per day) or gemcitabine (at a dose of 1000 mg/m2 for 3 of every 4 weeks), followed by maintenance chemotherapy, typically capecitabine or gemcitabine; 29 patients received concurrent chemoradiation therapy only: 4- to 6-field IMRT 95% PGTV 36 to 40 Gy in 1.8 to 2 Gy fractions, concurrent capecitabine (at a dose of 1600 mg/m2 per day) or gemcitabine (at a dose of 1000 mg/m2 for 3 of every 4 weeks); 37 patients received capecitabine or gemcitabine-based chemotherapy only; 12 patients received EBRT only: 4- to 6-field IMRT 95% PGTV 36 to 40 Gy in 1.8 to 2 Gy fractions.
2.6. Follow-up
During the period of treatment patients were examined weekly. After completion of treatment they were followed-up every 2 to 4 weeks for the first 3 months and every 3 months afterwards until death. Follow-up included physical examination, complete blood count, hepatic function, serum tumor marker assessment, chest and abdominopelvic computed tomography imaging. Information on pain relief was collected at 4 weeks after IOERT through in-person interview.
2.7. IOERT toxicity
Early radiation-related toxicity was recorded using the Nation Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Late radiation-related toxicity was recorded using the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer Late Radiation Morbidity Scoring Schema.
2.8. Statistics
OS was the primary endpoint. Dates of death from any cause were obtained from medical records and the China National Citizen Identity Information Center. OS was counted from the date of operation and was estimated by the Kaplan–Meier method. Secondary endpoints were local progression-free survival (LPFS), and distant metastasis-free survival (DMFS). LPFS and DMFS were determined by imagine or biopsy. Times were measured relative to treatment initiation dates and censored at dates of last follow-up when applicable. The third endpoint was pain relief. The pain relief was categorized as complete remission, partial remission, and no remission.
Prognostic factors for OS were evaluated at the univariate level by the log-rank test and at the multivariate level by Cox proportional hazards models. Adjustments for several variables, such as age, gender, smoking, alcohol consumption, and family history, did not result in material changes in the observed associations therefore they were not included in the final Cox model. All tests were 2-sided and performed using Software version 9.3 (SAS Institute, Inc., Cary, NC). A P-value < 0.05 was defined as statistical significance.
3. Results
3.1. Demographics, comorbidities, tumor and treatment characteristics
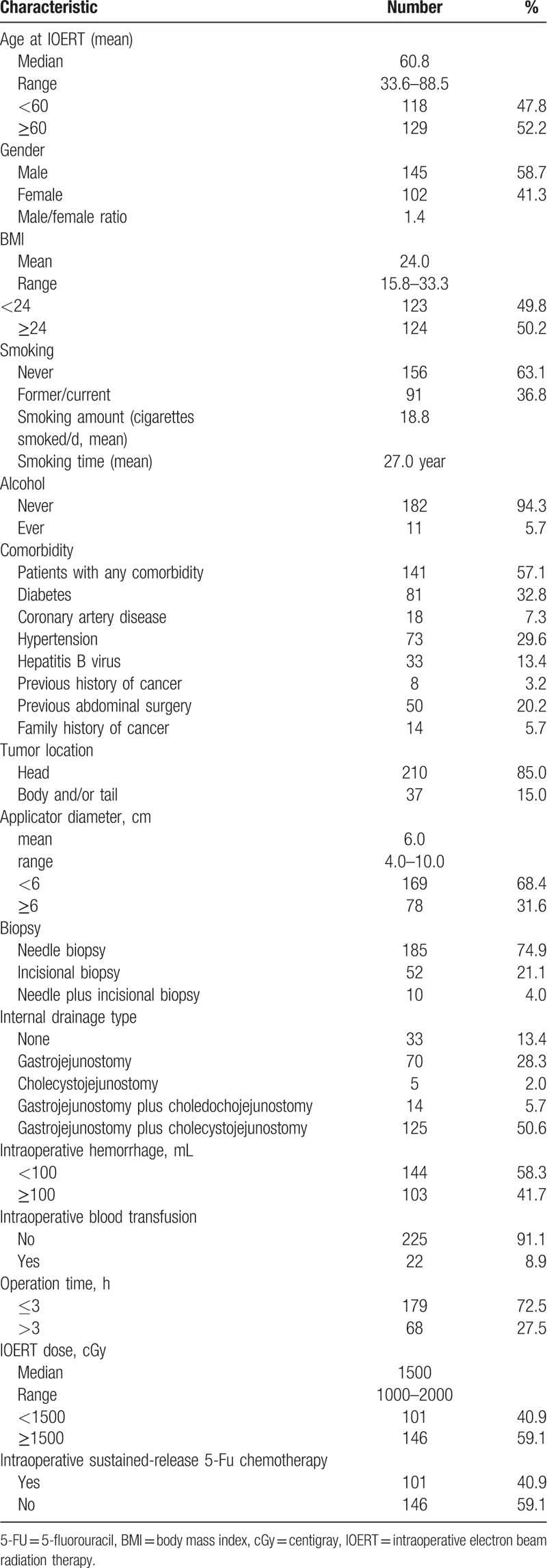
A total of 247 consecutive patients who underwent IOERT were identified and included in the study (Table 1). The median age of the patients was 60.8 years. Among the 247 patients, 141 had 1 or more comorbidities. The primary tumor was located in the head of the pancreas in 210 patients (85%) and in the body and tail in the other 37 patients (15%). The median applicator diameter was 6.0 cm. Table 1 summarizes operations, IOERT, intraoperative sustained-release 5-fluorouracil chemotherapy characteristics, and adjuvant treatment regimens information.
Table 1.
Patient, tumor, and treatment characteristics.
3.2. OS, LPFS, and DMFS
Over a median follow-up period of 10.1 months, the 1-, 2-, and 3-year actuarial survival rates of all 217 patients were 40.0% (95% confidence interval [CI], 32.9–47.1%), 14.0% (95% CI, 8.7–19.3%), and 7.2% (95% CI, 2.9–11.5%), respectively. Only 2 patients survived for >5 years: 1 patient died of liver metastasis at 6.1 years, and 1 patient was alive at the time of last follow-up. The median OS was 9.0 months (95% CI, 7.6–10.4 months) (Fig. 1).
Figure 1.
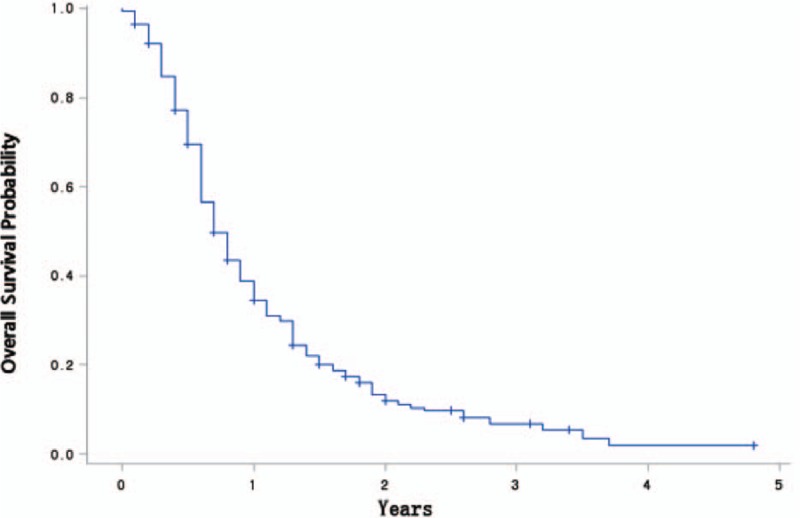
Overall survival is shown among patients with nonmetastatic LAPC who were treated with IOERT (n = 217).
Among 205 patients (83.0%) for whom post-IOERT disease status follow-up information was available, 152 (74.1%) had documented disease progression at the time of last follow-up, with 82 patients (40.0%) demonstrating local disease progression and 125 patients (61.0%) demonstrating distant metastasis. The 1-, 2-, and 3-year LPFS rates were 51.3% (95% CI, 41.3–58.7%), 40.1% (95% CI, 29.6–61.3%), and 34.6% (95% CI, 19.7–43.7%), respectively. The 1-, 2-, and 3-year DMFS rates were 39.3% (95% CI, 31.6–47.1%), 23.4% (95% CI, 17.7–33.4%), and 11.9% (95% CI, 10.6–27.6%), respectively. The median times to local progression and distant metastasis were 8.3 and 7.7 months, respectively.
3.3. Univariate and multivariate analysis of prognostic factors for OS
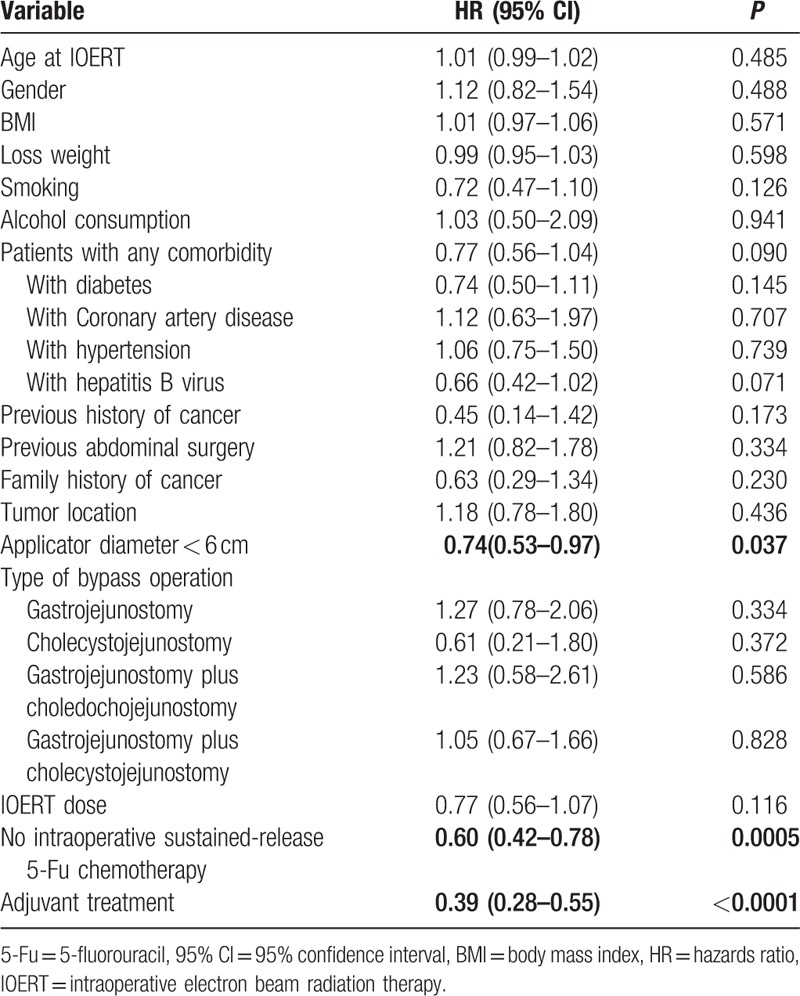
On univariate analysis (Table 2), an IOERT applicator diameter < 6 cm (hazards ratio [HR], 0.74; 95% CI, 0.53–0.97 [P = 0.037]), no intraoperative sustained-release 5-fluorouracil chemotherapy (HR, 0.60; 95% CI, 0.42–0.78 [P = 0.0005]), and receipt of adjuvant treatment (HR, 0.39; 95% CI, 0.28–0.55 [P < 0.0001]) were associated with improved OS. Age, gender, body mass index (BMI), smoking history, alcohol consumption history, presence of any comorbidity, presence of diabetes, tumor location, type of bypass operation, and IOERT dose were not significantly associated with OS.
Table 2.
Univariate prognostic factor analysis for overall survival (n = 205).
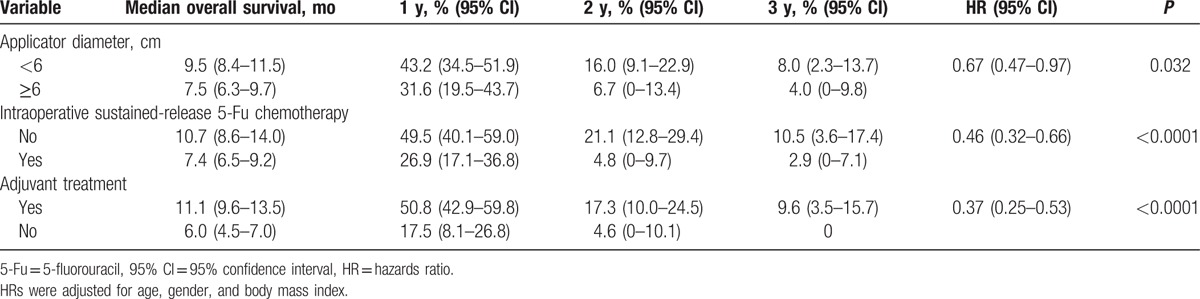
On multivariate analysis, an IOERT applicator diameter < 6 cm (HR, 0.59; 95% CI, 0.43–0.76 [P = 0.049]), no intraoperative sustained-release 5-fluorouracil chemotherapy (HR, 0.41; 95% CI, 0.33–0.44 [P = 0.0009]), and receipt of adjuvant treatment (HR, 0.38; 95% CI, 0.27–0.54 [P < 0.0001]) were independently associated with improved OS (Table 3). Among the 57 patients with all 3 prognostic factors, the median OS was 14.0 months (95% CI, 9.6–19.1 months), with 1-, 2-, and 3-year OS rates of 62.8% (95% CI, 49.5–76.0%), 26.9% (95% CI, 13.3–40.4%), and 16.8% (95% CI, 4.4–29.2%), respectively. We also analyzed the prognostic factors for LPFS and found similar results as OS (Supplementary Table 1).
Table 3.
Multivariate prognostic factor analysis for overall survival.
3.4. OS according to adjuvant treatment modality
To explore optimal adjuvant treatment strategy after IOERT, we performed subset survival analysis of the 197 patients in the present study cohort who had available adjuvant treatment follow-up information.
On univariate analysis, an IOERT applicator diameter < 6 cm (HR, 0.69; 95% CI, 0.49–0.98 [P = 0.031]), no intraoperative sustained-release 5-fluorouracil chemotherapy (HR, 0.56; 95% CI, 0.38–0.73 [P = 0.0006]), and receipt of postoperative chemotherapy (HR, 0.48; 95% CI, 0.31–0.76 [P = 0.002]), or postoperative chemoradiotherapy (HR, 0.50; 95% CI, 0.28–0.88 [P = 0.017]), or postoperative chemoradiotherapy followed by chemotherapy (HR, 0.26; 95% CI, 0.17–0.40 [P < 0.0001]) were associated with improved OS.
On multivariate analysis (Table 4), an IOERT applicator diameter < 6 cm (HR, 0.60; 95% CI, 0.46–0.77 [P = 0.048]), no intraoperative sustained-release 5-fluorouracil chemotherapy (HR, 0.51; 95% CI, 0.37–0.71 [P = 0.0009]), and receipt of postoperative chemotherapy (HR, 0.50; 95% CI, 0.31–0.80 [P = 0.0037]), or postoperative chemoradiotherapy (HR, 0.42; 95% CI, 0.23–0.75 [P = 0.0038]), or postoperative chemoradiotherapy followed by chemotherapy (HR, 0.24; 95% CI, 0.15–0.38 [P < 0.0001]) were independently associated with improved OS. Among the 20 patients with IOERT applicator diameter < 6 cm, no intraoperative sustained-release 5-fluorouracil chemotherapy, and receipt of postoperative chemoradiotherapy followed by chemotherapy, the median OS was 20.0 months (95% CI, 13.5–31.7 months), with 1-, 2-, and 3-year OS rates of 85.0% (95% CI, 69.4–100.0%), 42.5% (95% CI, 20.3–64.7%), and 28.3% (95% CI, 6.5–50.2%), respectively.
Table 4.
Subset multivariate prognostic factor analysis for overall survival among patients with documented adjuvant therapy information.
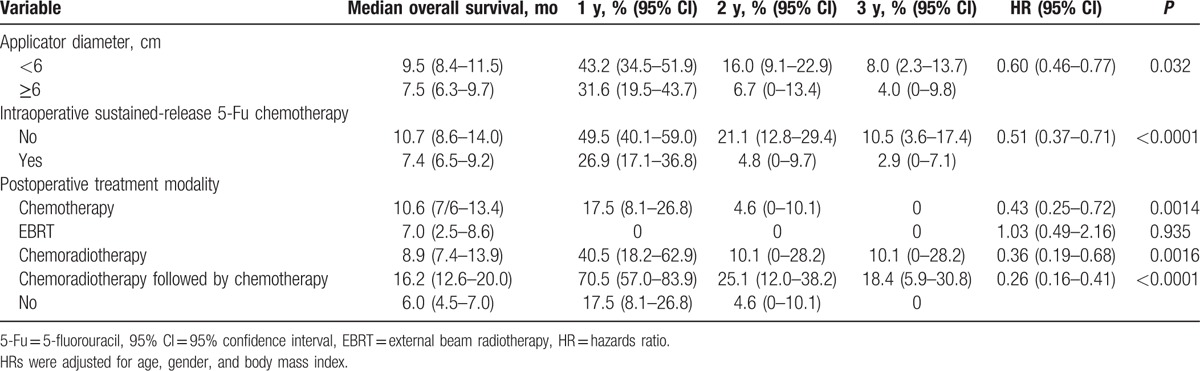
As shown in Fig. 2, the longest OS was observed in patients who had an adjuvant treatment of chemoradiotherapy followed by chemotherapy (16.2 months, 95% CI, 12.6–20.0 months [P < 0.0001]), with 1-, 2-, and 3-year OS rates of 70.5% (95% CI, 57.0–83.9%), 25.1% (95% CI, 12.0–38.2%), and 18.4% (5.9–30.8%), respectively.
Figure 2.
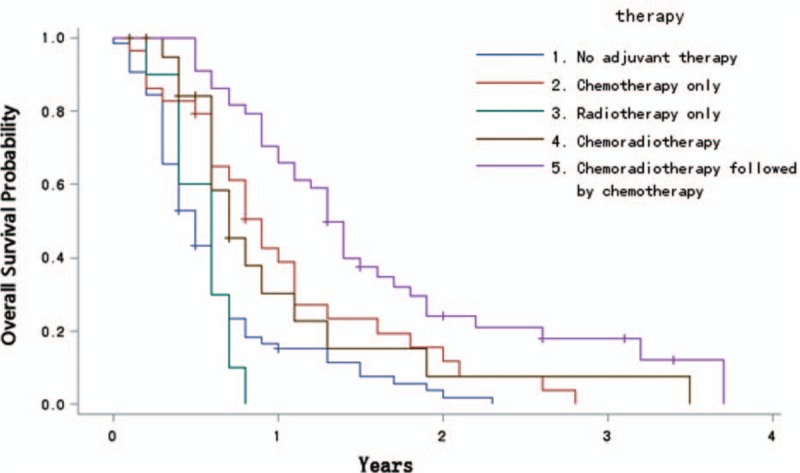
Overall survival according to adjuvant treatment modality (n = 197).
3.5. Pain relief
One hundred seventeen patients (47.4%) presented abdominal and/or back pain before IOERT. Pain was relieved after IOERT in 111 of the 117 patients (94.9%), with complete remission in 74, partial remission in 37, and no remission in 6. An increased rate of complete remission of abdominal/back pain was observed for patients who received IOERT with dose ≥1500 cGy compared to that of those with dose < 1500 cGy (70.0% vs 61.0%), though the difference was not statistically significant (P = 0.330). No significant difference in complete remission of abdominal/back pain was detected among 5 adjuvant treatment groups (data are not shown).
3.6. IOERT toxicity
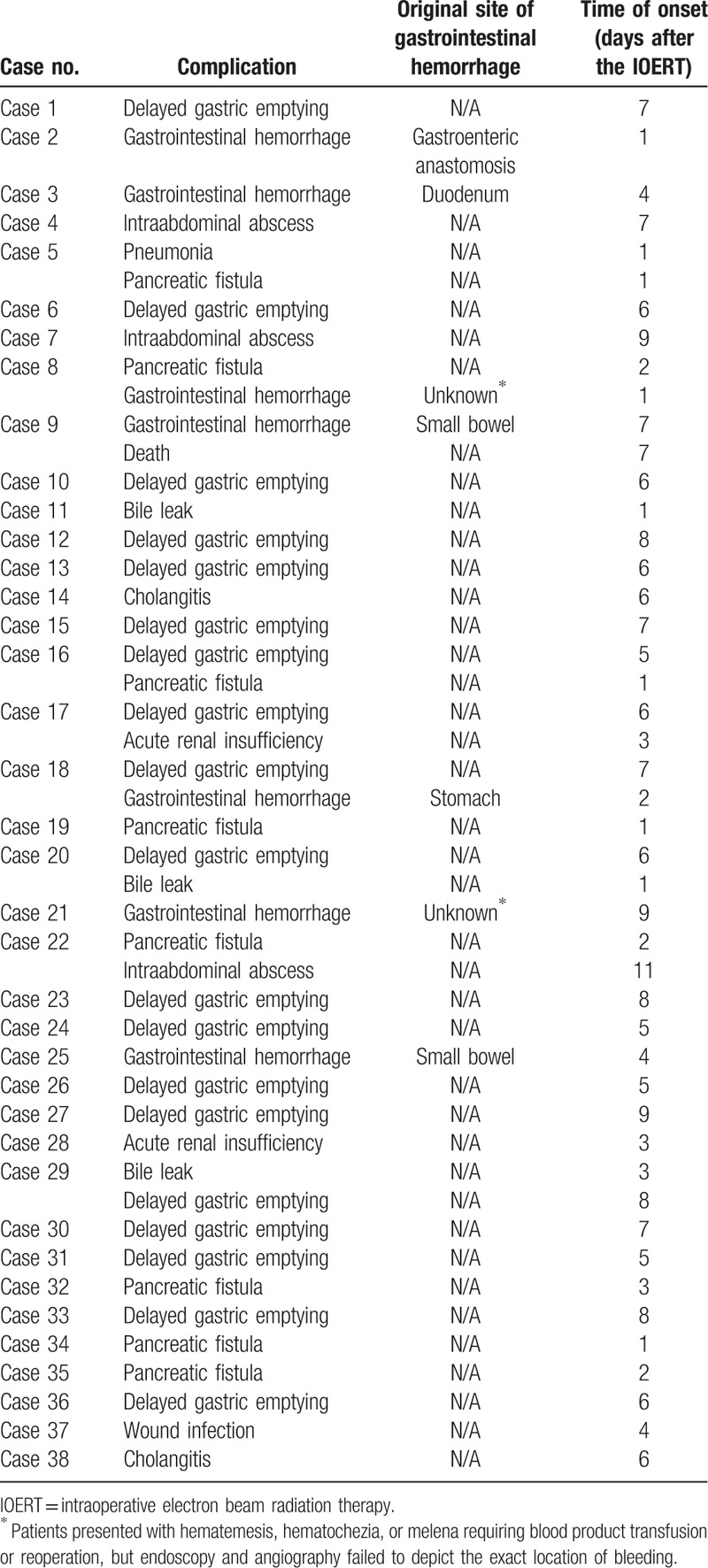
Mortality was 0.4%, accounting for 1 patient who died postoperatively of gastrointestinal hemorrhage. One or more postoperative complications occurred in 15.4% of the patients following IOERT (Table 5). Delayed gastric empty was the most frequent postoperative complication (19 patients, 7.7%). Pancreatic and biliary fistula were noted in 8 patients (3.2%) and 3 patients (1.2%), respectively. Gastrointestinal hemorrhage was observed in 7 patients (2.8%). Dosage of IOERT did not show a significant association with postoperative complications.
Table 5.
IOERT toxicity.
4. Discussion
Several studies have evaluated IOERT in the treatment of patients with LAPC, even though the optimal protocol has not yet been established.[10–13] Considering the potential toxicity of high-dose irradiation, we took IOERT at 10 to 20 Gy combined with adjuvant therapy. In the present study, an IOERT applicator diameter that was a surrogate for tumor less than 6 cm in size was associated with improved OS. While the mechanisms underlying this phenomenon remained unclear, a possible explanation was that OS might be prolonged if good local control rates of smaller tumor size could be achieved.
The investigators of Massachusetts General Hospital reported the results of IOERT in the treatment of 194 patients with LAPC.11 The 1-, 2-, and 3-year survival rates in their study were 49%, 16%, and 6%, respectively. Median OS was 12.0 months.[11] In a multiinstitution analysis conducted in 2011 in 144 patients with LAPC who were treated with IOERT with or without EBRT and/or chemotherapy, Ogawa and his team[12] reported a median OS of 10.5 months, a 2-year OS rate of 15%, and a 2-year local control rate of 45%. On the other hand, Okamoto and his group reported a median OS of 10.8 months with 1- and 3-year OS rates of 57% and 10%, respectively, in a single-institution analysis implemented in 2004 in 65 patients with LAPC who were submitted to treatment with IOERT and EBRT. The median OS in the present study was 9.0 months that was slightly shorter than previous reports.[11–13] This could be due to that less patients in our cohort received pre- or post-IOERT treatment compare to patients in previous studies (65.5% for present cohort vs 97% for Massachusetts General Hospital's study and 79.2% for Ogawa's report).
In the present study, we found that patients who had smaller tumors that did not receive intraoperative interstitial sustained-release 5-fluorouracil chemotherapy, and received postoperative chemoradiotherapy followed by chemotherapy achieved best long-term outcomes, with a median OS of 20.0 months and a 3-year OS rate of 28.3%. The results support the current emphasis on systemic treatment of patients with LAPC.[14] It is now generally agreed that IOERT alone is inferior to IOERT plus EBRT in the treatment of LAPC.[15] Moreover, the role of concurrent chemoradiotherapy has received increasing attention since the results of the 1985 analysis by Kalser et al, demonstrating that chemoradiotherapy might be a viable option for pancreatic cancer.[16]
Since 2008, China National Cancer Center has adopted the protocol of IOERT without pre-IOERT treatment. It is interesting to note that the median OS of 16.2 months noted among the patients in the present study who received IOERT plus postoperative chemoradiotherapy followed by chemotherapy is comparable to the 17.6-month median OS reported by Cai et al in their 2013 study demonstrating improved OS among patients with LAPC who received pre-IOERT and post-IOERT maintenance chemotherapy, independent of radiosensitizing chemotherapy given concurrently with EBRT. Although upfront EBRT of patients with nonmetastatic LAPC has been attracting increasing attention, long-term outcomes remain unclear.[8,11,14] A retrospective study of 27 patients at the Mayo Clinic suggested that the sequence of full-dose EBRT before IOERT may be more appropriate because it allows better patient selection compared to IOERT followed by postoperative high-dose EBRT.[17] However, the limited sample size made it difficult to adequately adjust for all potential confounding factors. To our knowledge, there are few randomized studies comparing neoadjuvant treatment before IOERT with IOERT followed by postoperative adjuvant treatment for nonmetastatic LAPC; future well-designed clinical trials on this issue are needed.
To the best of our knowledge, our study was the first to investigate a prognostic role of the intraoperative interstitial sustained-release 5-fluorouracil chemotherapy in patients with nonmetastatic LAPC. Few China health insurance systems reimburse intraoperative interstitial sustained-release 5-fluorouracil chemotherapy, and patients have to pay for it themselves. Due to financial reason, only 101 patients were treated with intraoperative interstitial sustained-release 5-fluorouracil chemotherapy. Evidence shows that a majority of tumors do not respond to the treatment because drugs lack the ability to penetrate to the tumor interstitium,[18] suggesting that developing a dosage form capable to concentrating the drug close to the tumor site without too wide a distribution is crucial.[19] Local delivery of chemotherapeutic drugs is recognized as a potential method of delivering a drug to the target site with minimal systemic exposure. Smith et al[20] reported that intratumoral chemotherapy with a sustained-release drug delivery system could inhibit growth of human pancreatic cancer xenografts. The present study, however, found that LAPC patients who were treated with intraoperative interstitial sustained-release 5-fluorouracil chemotherapy had worse OS compare to those without intraoperative interstitial sustained-release 5-fluorouracil chemotherapy. The mechanisms underlying this phenomenon were unclear. It was possible that the sustained-release 5-fluorouracil particles were implanted in relatively shallow sites of pancreatic cancer due to technical difficulty. Additionally, the risk of causing iatrogenic metastasis by implantation of 5-fluorouracil particles might also be responsible for this phenomenon.
For pancreatic cancer, it is believed that relief of patients’ symptoms and abdominal and back pain is a main goal for palliation and improving patients’ quality of life. Overall, pain relief after IOERT was significant. Of the 117 patients experiencing pain before IOERT, pain relief after IOERT was achieved in 111 patients (94.9%). Because of the remarkable analgesic effect of IOERT, analgesic drug use could be decreased after IOERT and the patients’ quality of life were improved to some extent.[21] A previous study investigating the advantages and palliative effectiveness of IOERT in LAPC patients provided similar outcomes: pain improved in 88.8% of their cases, with complete remission in 55.5%, and partial remission in 33.3%.[22]
It was believed that radiotherapy-related complications often occur in patients who received high dose radiotherapy.[22,23] Tokyo Metropolitan Komagome Hospital investigators demonstrated that the dose of IOERT ranging from 20 to 25 Gy was considered to contribute to the improvement of prognosis without causing serious side effects.[24] Consistent with previous studies,[22,25,26] our results demonstrated that postoperative and IOERT-related toxicity rates were acceptable. Since 2008, we have employed IOERT at 10 to 20 Gy combined with adjuvant treatment and the dosage of IOERT did not show a significant association with postoperative complications.
Strengths and limitations should be considered when interpreting the study results. The present study was a retrospective cohort study using prospectively collected data, which minimized potential differential information bias. Our study, for the first time, reported that intraoperative interstitial sustained-release 5-fluorouracil chemotherapy had a harmful effect on the prognosis for nonmetastatic LAPC. While we had the largest number of the patients to date, the sample size was modest therefore chance cannot be ruled out for some of the significant findings. Patients who were estimated as resectable disease and restaged as unresectable disease during the surgery might have different outcomes compared to patients who were initially diagnosed as unresectable disease. Unfortunately, our study failed to record this information. We were unable to assess pain relief using a quantitative index such as a visual analog scale (VAS). Moreover, we did not collect Eastern Cooperative Oncology Group (ECOG) performance status during treatment. Finally, this was a single-institutional analysis with significant treatment heterogeneity.
5. Conclusion
The present study confirmed that nonmetastatic LAPC patients with small size tumors could achieve good long-term survival outcomes with a treatment strategy incorporating IOERT and postoperative adjuvant treatment. Chemoradiotherapy followed by chemotherapy might be a recommended adjuvant treatment strategy for well-selected cases. Intraoperative interstitial sustained-release 5-fluorouracil chemotherapy should not be recommended for patients with nonmetastatic LAPC. IOERT was well tolerated and could alleviate pain in most cases. The positive findings in our study need to be verified in randomized clinical trials.
Supplementary Material
Footnotes
Abbreviations: 5-Fu = 5-fluorouracil, CI = confidence interval, DMFS = distant metastasis-free survival, EBRT = external beam radiotherapy, HR = hazards ratio, IMRT = intensity-modulated radiation therapy, IOERT = intraoperative electron beam radiation therapy, LAPC = locally advanced pancreatic cancer, LPFS = local progression-free survival, OS = overall survival, PGTV = planning gross tumor volume, VAS = visual analog scale.
Funding: This work was supported by National Natural Science Foundation of China (no. 81401947), Beijing Nova Program (no. xxjh2015A090), and Cancer Hospital of the Chinese Academy of Medical Sciences (no. LC2015L11).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249. [DOI] [PubMed] [Google Scholar]
- 2.Huguet F, Goodman KA, Azria D, et al. Radiotherapy technical considerations in the management of locally advanced pancreatic cancer: American-French consensus recommendations. Int J Radiat Oncol Biol Phys 2012; 83:1355–1364. [DOI] [PubMed] [Google Scholar]
- 3.Royal RE, Wolff RA, Crane CH. De Vita VTJ, Lawrence TS, Rosenberg SA. Cancer of the pancreas. Principles and Practice of Oncology 8th ed.Philadelphia: Lippincott Williams & Wilkins; 2008. 1086–1123. [Google Scholar]
- 4.Huguet F, Girard N, Guerche CS, et al. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol 2009; 27:2269–2277. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa H, Suzuki Y, Nakayama Y, et al. Intraoperative radiotherapy and bypass surgery for unresectable pancreatic cancer. Hepatogastroenterology 2000; 47:1151–1155. [PubMed] [Google Scholar]
- 6.Whittington R, Dobelbower RR, Mohiuddin M, et al. Radiotherapy of unresectable pancreatic carcinoma: a six year experience with 104 patients. Int J Radiat Oncol Biol Phys 1981; 7:1639–1644. [DOI] [PubMed] [Google Scholar]
- 7.Evans DB, Abbruzzese JL, Rich TA. De Vita VTJ, Hellmann S, Rosenberg SA. Cancer of the pancreas. Cancer, Principles and Practice of Oncology 5th ed.Philadelphia: Lippincott Williams & Wilkins; 1997. 1054–1087. [Google Scholar]
- 8.Ruano-Ravina A, Almazán Ortega R, Guedea F. Intraoperative radiotherapy in pancreatic cancer: a systematic review. Radiother Oncol 2008; 87:318–325. [DOI] [PubMed] [Google Scholar]
- 9.Nagai S, Fujii T, Kodera Y, et al. Prognostic implications of intraoperative radiotherapy for unresectable pancreatic cancer. Pancreatology 2011; 11:68–75. [DOI] [PubMed] [Google Scholar]
- 10.Abe M, Shibamoto Y, Ono K, et al. Intraoperative radiation therapy for carcinoma of the stomach and pancreas. Front Radiat Ther Oncol 1991; 29:258–269. [DOI] [PubMed] [Google Scholar]
- 11.Cai S, Hong TS, Goldberg SI. Updated long-term outcomes and prognostic factors for patients with unresectable locally advanced pancreatic cancer treated with intraoperative radiotherapy at the Massachusetts General Hospital, 1978 to 2010. Cancer 2013; 119:4196–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa K, Karasawa K, Ito Y, et al. Intraoperative radiotherapy for unresectable pancreatic cancer: a multi-institutional retrospective analysis of 144 patients. Int J Radiat Oncol Biol Phys 2011; 80:111–118. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto A, Matsumoto G, Tsuruta K. Intraoperative radiation therapy for pancreatic adenocarcinoma: the Komagome hospital experience. Pancreas 2004; 28:296–300. [DOI] [PubMed] [Google Scholar]
- 14.NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. Available at nccn.org/professionals/physician_gls/pdf/pancreatic.pdf Accessed November 21, 2012 [Google Scholar]
- 15.Shibamoto Y, Nishimura Y, Abe M. Intraoperative radiotherapy and hyperthermia for unresectable pancreatic cancer. Hepatogastroenterology 1996; 43:326–332. [PubMed] [Google Scholar]
- 16.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009; 27:1806–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garton GR, Gunderson LL, Nagorney DM, et al. High dose preoperative external beam and intraoperative irradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 1993; 27:1153–1157. [DOI] [PubMed] [Google Scholar]
- 18.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer 2006; 6:583–592. [DOI] [PubMed] [Google Scholar]
- 19.Dubernet C, Fattal E, Couvreur P. Wise D. Nanoparticulate controlled release systems for cancer therapy. Handbook of Pharmaceutical Controlled Release Technology. New York: Marcel Dekker; 2000. 287–300. [Google Scholar]
- 20.Smith JP, Stock E, Orenberg EK, et al. Intratumoral chemotherapy with a sustained-release drug delivery system inhibits growth of human pancreatic cancer xenografts. Anticancer Drugs 1995; 6:717–726. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Ren ZG, Ma NY, et al. Intensity modulated radiotherapy for locally advanced and metastatic pancreatic cancer: a mono-institutional retrospective analysis. Radiat Oncol 2015; 10:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma HB, Di ZL, Wang XJ, et al. Effect of intraoperative radiotherapy combined with external beam radiotherapy following internal drainage for advanced pancreatic carcinoma. World J Gastroenterol 2004; 10:1669–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egawa S, Tsukiyama I, Akine Y, et al. Control of after effects due to intraoperative radiotherapy. Gan No Rinsho 1990; 36:815–820. [PubMed] [Google Scholar]
- 24.Tanaka Y, Takeshita N, Niwa K, et al. Studies on treatment results and prognostic factors of intraoperative radiotherapy in pancreatic carcinoma. Nippon Igaku Hoshasen Gakkai Zasshi 1989; 49:614–621. [PubMed] [Google Scholar]
- 25.Schwarz RE, Smith DD, Keny H, et al. Impact of intraoperative radiation on postoperative and disease-specific outcome after pancreatoduodenectomy for adenocarcinoma: a propensity score analysis. Am J Clin Oncol 2003; 26:16–21. [DOI] [PubMed] [Google Scholar]
- 26.Willett CG, Del Castillo CF, Shih HA, et al. Long-term results of intraoperative electron beam irradiation (IOERT) for patients with unresectable pancreatic cancer. Ann Surg 2005; 241:295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


