Abstract
A straightforward argument is presented to calculate the number of different major histocompatibility complex (MHC) molecules in an individual that maximizes the probability of mounting immune responses against a large number of foreign peptides. It is assumed that increasing the number of MHC molecules per individual, n, has three different effects: (i) it increases the number of foreign peptides that can be presented; (ii) it increases the number of different T-cell receptors (TCRs) positively selected in the thymus; but (iii) it reduces the number of TCRs by negative selection. The mathematical analysis shows that n = 1/f maximizes the number of different TCRs that pass through positive and negative selection and that n = 2/f maximizes the probability to mount immune responses against a large fraction of foreign peptides. Here f is the fraction of TCRs deleted by one MHC molecule. Both results depend on approximations that are discussed in the paper. The model presented has implications for our understanding of the evolutionary forces acting on the MHC.
Full text
PDF
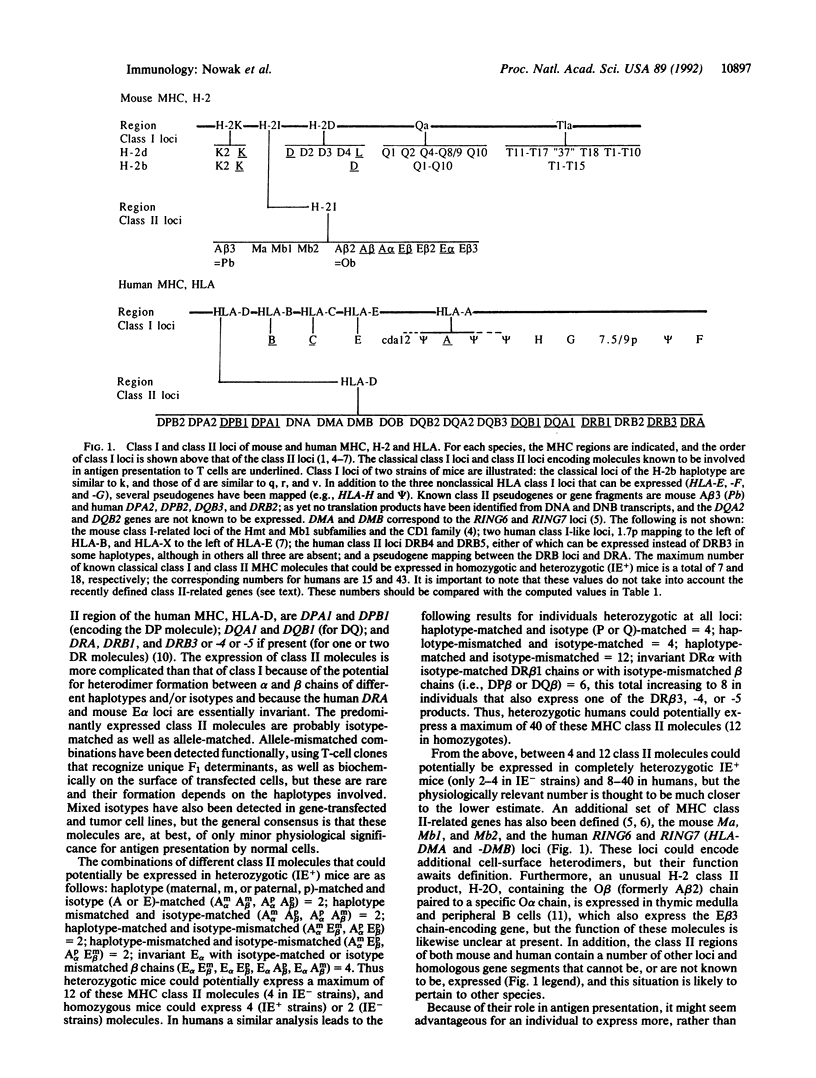


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. J. Killer cells reactive to altered-self antigens can also be alloreactive. Proc Natl Acad Sci U S A. 1977 May;74(5):2094–2098. doi: 10.1073/pnas.74.5.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer J. G., Marsh S. G., Albert E. D., Bodmer W. F., Dupont B., Erlich H. A., Mach B., Mayr W. R., Parham P., Sasazuki T. Nomenclature for factors of the HLA system, 1990. Hum Immunol. 1991 Jul;31(3):186–194. doi: 10.1016/0198-8859(91)90025-5. [DOI] [PubMed] [Google Scholar]
- Cho S. G., Attaya M., Monaco J. J. New class II-like genes in the murine MHC. Nature. 1991 Oct 10;353(6344):573–576. doi: 10.1038/353573a0. [DOI] [PubMed] [Google Scholar]
- Dent A. L., Matis L. A., Hooshmand F., Widacki S. M., Bluestone J. A., Hedrick S. M. Self-reactive gamma delta T cells are eliminated in the thymus. Nature. 1990 Feb 22;343(6260):714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- Ford W. L., Simmonds S. J., Atkins R. C. Early cellular events in a systemic graft-vs.-host reaction. II. Autoradiographic estimates of the frequency of donor lymphocytes which respond to each Ag-B-determined antigenic complex. J Exp Med. 1975 Mar 1;141(3):681–696. doi: 10.1084/jem.141.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs H., Orr H. T. HLA non-A,B,C class I genes: their structure and expression. Immunol Res. 1990;9(4):265–274. doi: 10.1007/BF02935526. [DOI] [PubMed] [Google Scholar]
- Karlsson L., Surh C. D., Sprent J., Peterson P. A. A novel class II MHC molecule with unusual tissue distribution. Nature. 1991 Jun 6;351(6326):485–488. doi: 10.1038/351485a0. [DOI] [PubMed] [Google Scholar]
- Kelly A. P., Monaco J. J., Cho S. G., Trowsdale J. A new human HLA class II-related locus, DM. Nature. 1991 Oct 10;353(6344):571–573. doi: 10.1038/353571a0. [DOI] [PubMed] [Google Scholar]
- Lawlor D. A., Zemmour J., Ennis P. D., Parham P. Evolution of class-I MHC genes and proteins: from natural selection to thymic selection. Annu Rev Immunol. 1990;8:23–63. doi: 10.1146/annurev.iy.08.040190.000323. [DOI] [PubMed] [Google Scholar]
- Lechler R. I., Lombardi G., Batchelor J. R., Reinsmoen N., Bach F. H. The molecular basis of alloreactivity. Immunol Today. 1990 Mar;11(3):83–88. doi: 10.1016/0167-5699(90)90033-6. [DOI] [PubMed] [Google Scholar]
- Milligan G. N., Flaherty L., Braciale V. L., Braciale T. J. Nonconventional (TL-encoded) major histocompatibility complex molecules present processed viral antigen to cytotoxic T lymphocytes. J Exp Med. 1991 Jul 1;174(1):133–138. doi: 10.1084/jem.174.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Gefter M. L. Interactions between immunogenic peptides and MHC proteins. Annu Rev Immunol. 1991;9:527–565. doi: 10.1146/annurev.iy.09.040191.002523. [DOI] [PubMed] [Google Scholar]
- Stroynowski I. Molecules related to class-I major histocompatibility complex antigens. Annu Rev Immunol. 1990;8:501–530. doi: 10.1146/annurev.iy.08.040190.002441. [DOI] [PubMed] [Google Scholar]
- Swat W., Ignatowicz L., von Boehmer H., Kisielow P. Clonal deletion of immature CD4+8+ thymocytes in suspension culture by extrathymic antigen-presenting cells. Nature. 1991 May 9;351(6322):150–153. doi: 10.1038/351150a0. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Ragoussis J., Campbell R. D. Map of the human MHC. Immunol Today. 1991 Dec;12(12):443–446. doi: 10.1016/0167-5699(91)90017-n. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Blyth JL NOWELL P. C. Quantitative studies on the mixed lymphocyte interaction in rats. 3. Kinetics of the response. J Exp Med. 1968 Nov 1;128(5):1157–1181. doi: 10.1084/jem.128.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Kisielow P. Self-nonself discrimination by T cells. Science. 1990 Jun 15;248(4961):1369–1373. doi: 10.1126/science.1972594. [DOI] [PubMed] [Google Scholar]



