Abstract
This study aims to investigate whether neutrophil to lymphocyte ratio (NLR) is an independent predictor in newly diagnosed diffuse large B-cell lymphoma (DLBCL) patients in the rituximab era. Data from newly diagnosed DLBCL patients at Nanjing Drum Tower Hospital from 2006 to 2015 were retrospectively reviewed. We used the receiver operating characteristic (ROC) curve analysis to generate the optimal cutoff value for NLR. Among those 156 patients enrolled, the NLR was < 3.0 in 46.8% (73/156) of the patients, and the remaining 53.2% (83/156) had an NLR ≥ 3.0. Patients with higher pretreatment NLR were found to correlate with poorer OS and PFS than these with lower NLR (hazard ratio [HR] = 2.66, 95% confidence interval [CI] = 1.43–4.97, P = 0.002 and HR = 1.79, 95% CI = 1.05–3.07, P = 0.034, respectively). The multivariate Cox proportional hazard model analysis further showed that high NLR was found independently predictive of poor OS (HR = 0.40; CI = 0.19–0.84, P = 0.015) and PFS (HR = 0.57; CI = 0.33–0.98, P = 0.042). Consequently, pretreatment NLR was an independent prognostic predictor in patients with DLBCL in the rituximab era.
Keywords: diffuse large B-cell lymphoma, neutrophil-lymphocyte ratio, prognosis, rituximab
1. Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin's lymphoma (NHL) subtype, accounting for 25% to 35% of all new NHL diagnoses worldwide each year.[1] The important advance in treatment of DLBCL was the addition of rituximab, to the combination chemotherapy of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). Researches have shown that the new combined method provides a greater survival benefit for patients with DLBCL.[2–4] However, still>30% of DLBCL patients will become refractory to initial therapy or will relapse as a result.[5]
The International Prognostic Index (IPI) was widely used to predict aggressive lymphoma patients’ outcome and to choose the best therapeutic treatment.[6,7] Nevertheless, many efforts have been made to improve the capacity of IPI, such as the elderly IPI (E-IPI),[8] the revised IPI (R-IPI),[9] the National Comprehensive Cancer Network IPI (NCCN-IPI),[10] and the biological marker-adjusted IPI (A-IPI).[11] Unfortunately, these models are still unsatisfactory in identifying patients who would not benefit from RCHOP therapy. There are evidence that some DLBCL patients achieved no significant survival benefit from RCHOP compared with CHOP.[12] Herein, new survival model's discrimination in the rituximab era are in urgent need. Several considerations of such a model have been proposed. For example, the C-reactive protein at diagnosis was reported to be associated with prognosis in R-CHOP-treated DLBCL patients.[13] Watanabe et al[14] suggested lymphocyte–monocyte ratio (LMR) should be a predictor too, consistent with the previous study by Belotti et al.[15] The prognostic value of an interim fluorine-18-fluorodeoxyglucose positron emission tomography (PET-CT)[16,17] and Beta-2 microglobulin[18] was also evaluated. However, the ideal marker predicting the DLBCL remains elusive.
Increasing evidence has shown the close link between immune factors such as chronic inflammation, immunodeficiencies, autoimmunity, and infections, and lymphoma.[19,20] The abnormal inflammatory reaction and immune status are close to the pathobiology of lymphoma, thereby affecting the outcome of patients with lymphoma. Absolute lymphocyte counts were found to predict clinical outcome in DLBCL.[21] It has also been found that neutrophils, a common marker of body inflammatory reaction, plays a prominent role in the first line of the innate immune defense against infectious diseases.[22] Furthermore, the neutrophil-lymphocyte ratio (NLR), an inexpensive and repeatable marker that comprehensively reflect the inflammatory and immune status in cancer patients has been proved to be a significant predictor of survival in patients with diverse types of cancer such as laryngeal carcinoma, esophageal cancer, renal cell carcinoma, and gallbladder carcinoma.[23–26] Whether NLR is associated with survival in hematological malignancy is under considerable research interest. In this study, we aimed to determine whether the pretreatment NLR acts as a predictor of survival in Chinese patients with DLBCL treated with RCHOP.
2. Material and method
2.1. Patients
A total of 180 patients with newly diagnosed DLBCL were followed up at the Nanjing Drum Tower Hospital from 2006 to 2015. Eighteen patients were excluded because pretreatment NLR and pretreatment bone marrow biopsy were not available. Six patients, who changed to CHOP chemotherapy after only 1 cycle of R-CHOP chemotherapy, were also excluded. At last, we retrospectively reviewed data from 156 newly diagnosed DLBCL patients. All data were extracted from patients’ medical records by a trained reviewer. This study was approved by the Ethics Committee of the Nanjing Drum Tower Hospital and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
2.2. Statistical analysis
NLR was calculated by dividing the neutrophil count with the lymphocyte count. We used the receiver operating characteristic (ROC) curve analysis to generate the optimal cutoff value for NLR. Clinical characteristics and NLR were compared by using the Pearson Chi-squared test or Fisher's exact test. PFS was defined as the duration from diagnosis until disease relapse or progression. The Kaplan–Meier method (log-rank test) and Cox proportional hazards model were used to assess the survival differences. All data were analyzed using SPSS 19.0 (IBM Corp., Armonk, NY). Differences were considered statistically significant when the P-value < 0.05.
3. Results
3.1. Clinical characteristics
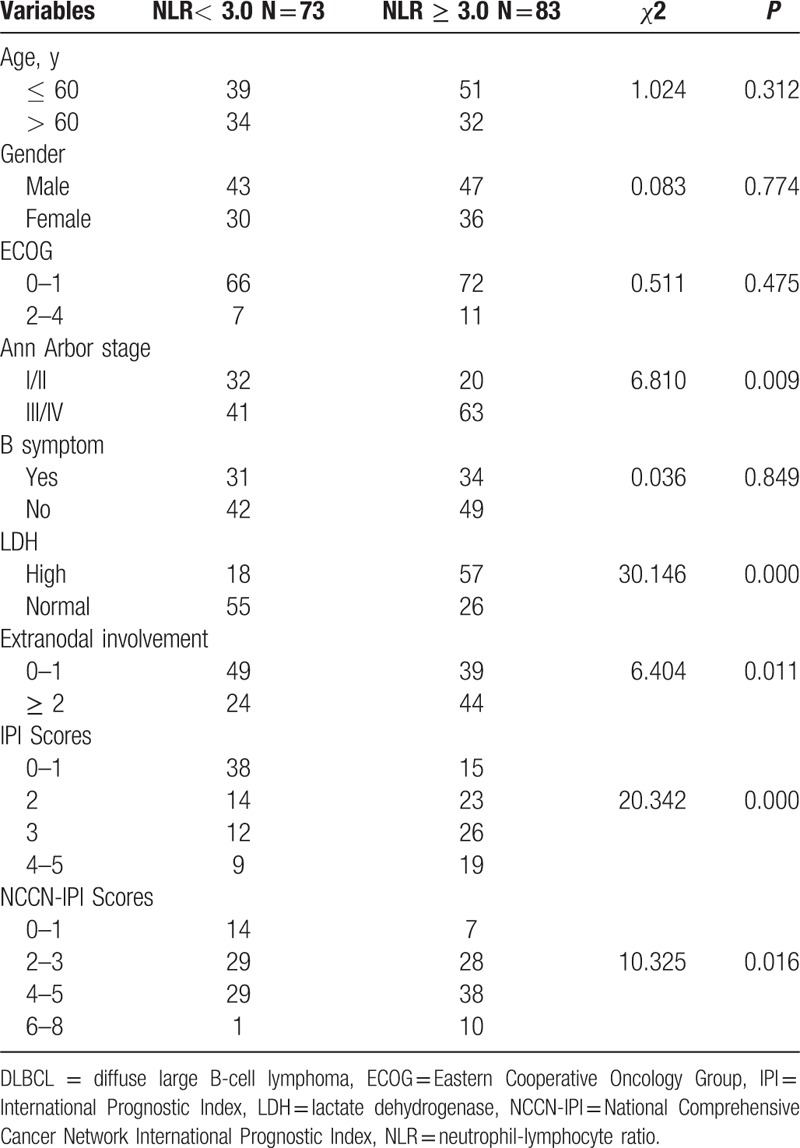
Among the 156 patients, 40 died of DLBCL, whereas 116 remain alive by November 1, 2015. The median follow-up was 29 months (range 2–122 months). The baseline characteristics of DLBCL patients, such as age, sex, Ann Arbor stage, LDH level, and NLR, were shown in Table 1.
Table 1.
Associations between pretreatment NLR and baseline characteristics of DLBCL patients.
3.2. Cut-off value in the NLR group
We determined by ROC curve analysis a cutoff value of 3.0 for the NLR to be the optimal cutoff point to discriminate between patients’ survival and death in our study (Fig. 1).
Figure 1.
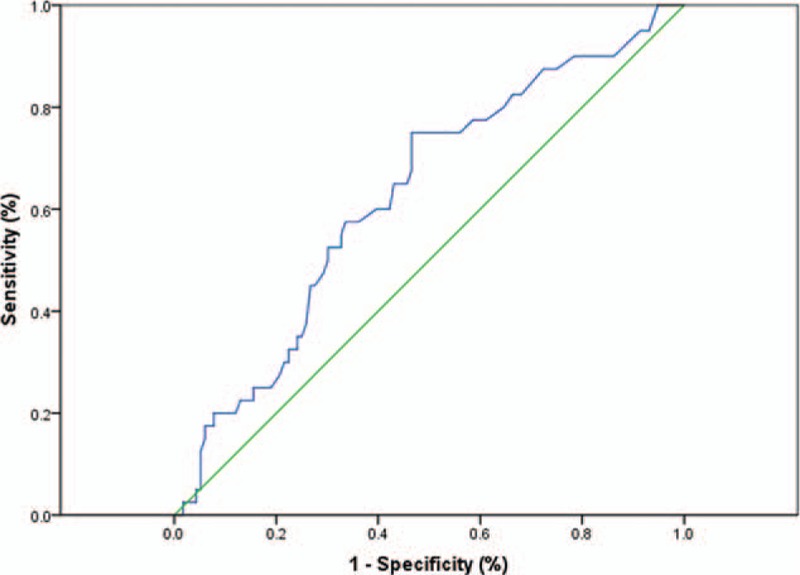
Receiver operating characteristic curve (ROC) and area under the curve (AUC) for NLR at diagnosis (AUC = 0.627, P = 0.017; 75.0% sensitivity and 53.4% specificity).AUC = area under the curve, NLR = neutrophil-lymphocyte ratio, ROC = receiver operating characteristic curve.
3.3. Associations of NLR with clinical characteristics
A total of 83 (53.2%) patients had high NLR, while the other 73 (46.8%) had low NLR. As shown in Table 1, there was no significant difference in age, gender, ECOG performance status, and B symptoms between the 2 groups (P > 0.05). In contrast, a higher NLR was significantly correlated with Ann Arbor stage (P = 0.009), LDH level (P = 0.000), and extranodal disease (P = 0.011). Patients in the high NLR group was significantly correlated with high IPI and NCCN-IPI (P = 0.000, P = 0.016, respectively).
3.4. Survival analysis
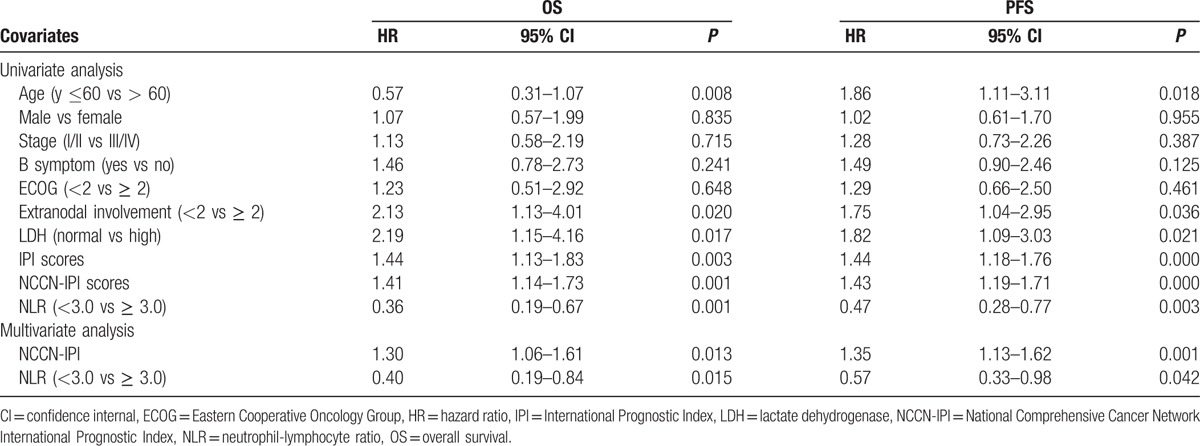
The univariate survival analysis demonstrates that patients in the high NLR group had significantly poorer OS and PFS than those in the low NLR group (P = 0.001 and P = 0.003, respectively) (Fig. 2A and B). The 5-year OS and PFS in the high NLR group and low NLR group were 57.5% versus 82.5% and 30.0% versus 64.5%, respectively. The multivariate survival analysis is shown in Table 2. On univariate analysis, age > 60 years (P = 0.008, P = 0.018), extranodal involvement ≥ 2 (P = 0.020, P = 0.036), elevated LDH level (P = 0.017, P = 0.021), high IPI scores (P = 0.003, P = 0.000), high NCCN-IPI scores (P = 0.001, P = 0.000), and high NLR (P = 0.001, P = 0.003) were significantly associated with poorer OS and PFS. However, multivariate analysis showed that, only pretreatment NLR (P = 0.015, P = 0.042, respectively) and NCCN-IPI (P = 0.013, P = 0.001, respectively) remained as independent prognostic factors.
Figure 2.
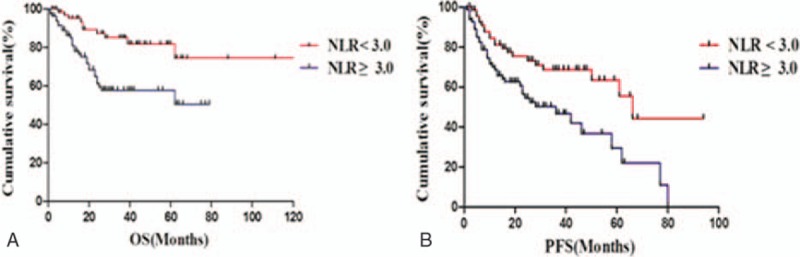
Survival (log-rank test). (A) Overall survival (P = 0.001). (B) Progression-free survival (P = 0.003).
Table 2.
Univariate and multivariate analysis for OS and PFS outcomes.
4. Discussion
Although it has been suggested that pretreatment NLR is associated with survival in patients with many kinds of solid tumors, only a few studies investigated the prognostic role of pretreatment NLR in DLBCL patients treated with RCHOP.
In accordance with previous studies, the results of the present study showed that the patients in the high NLR group have advanced disease stages (stages III and IV), evaluated LDH level, high IPI and NCCN-IPI, and more extranodal involvement.[27–28] These factors, associated with inflammation and tumor burden, were also revealed to be significantly related to survival in the present study. Based on the multivariable analysis, NLR was a predictor of survival in DLBCL patients treated with RCHOP, as well as NCCN-IPI. A similar study, conducted by Ho et al[29] in Taiwan, indicated for the first time that NLR pretreatment is associated with poor outcome; however, no statistical significance was found in the subsequent multivariate analysis. Recently, a retrospective study by Keam et al[27] concluded that NLR ≥ 3 at diagnosis was independently associated with poor OS (HR = 2.89, P < 0.001) and PFS (HR = 2.19, P < 0.001) in DLBCL patients in the rituximab era, which was consistent with our present study.
NLR at diagnosis was reported to affect the survival of the patients with DLBCL in the rituximab era. The potential mechanism remains unclear. The present study provides preliminary explanations for the association of high NLR between and poor survival in DLBCL patients. First, the emerging role of pro-inflammatory cytokines was observed in patients with high NLR, and as a result, these inflammatory cytokines may establish and perpetuate a tumor microenvironment for aggressive tumor behavior, thus leading to a poor outcome.[30] As concluded in previous studies, the elevated circulating level of interleukin-17 (IL-17) and IL-8 were detected in patients with high NLR.[31] Second, high NLR reflects relatively elevated neutrophils, which were regarded as a reservoir of vascular endothelial growth factor (VEGF), which has been found to play a vital role in tumor development and angiogenesis.[31] As expected, we found that high NLR with elevated circulating concentrations of VEGF was associated with heavy tumor burden and poor prognosis. Third, high NLR often leads to relatively depleted lymphocytes, which may manifest as a decreased host immune response to malignancy.[21] However, in the current study, no statistical significance was observed between adaptive immune cells and NLR.[27] Further investigations are necessary to provide more robust evidence. Furthermore, antibody-dependent cell cytotoxicity is the principal mechanism of action of rituximab, which may be impacted by relatively depleted lymphocytes too.
The usage of prognostic factors at diagnosis, such as NLR, to identify patients not recommended for RCHOP therapy offers the chance to improve the outcome in DLBCL patients by selecting the more efficient therapy option. In recent years, many molecular signatures have been shown to have prognostic value in DLBCL, such as TIM-3, PD-1, and E2F1 levels.[32,33] However, molecular markers are often costly and inefficient, making them unsuitable for clinical laboratories. By contrast, blood cell count is an inexpensive and easily available measure in daily clinical practice. We assure the observation duration and the number of patients should be extended in the future.
In conclusion, we found that NLR at diagnosis was an independent predictor of DLBCL in the rituximab era and may provide additional prognostic information in conjunction with the NCCN-IPI. Further studies with a larger number of participants are necessary to validate the prognostic role of NLR in DLBCL patients in the rituximab era.
Footnotes
Abbreviations: AUC = area under the curve, DLBCL = diffuse large B-cell lymphoma, ECOG = Eastern Cooperative Oncology Group, HR = hazard ratio, IPI = International Prognostic Index, LDH = lactate dehydrogenase, LMR = lymphocyte-monocyte ratio, NCCN = National Comprehensive Cancer Network, NLR = neutrophil-lymphocyte ratio, OS = overall survival, PFS = progression-free survival, ROC = receiver operating characteristic.
JW, MZ, and JX contributed equally to this work and should be considered as co-first authors.
Authorship: All authors contributed toward data analysis, drafting and revising the paper, and agree to be accountable for all aspects of the work.
Funding: This work was supported by Medical Science and Technology Key Project of Nanjing (ZKX14015), Medical Foundation of Nanjing Health and Family Planning Commission (YKK15068) and Peak of Six Talent in Jiangsu Province ( 2014-WSN-049).
The authors have no conflicts of interest to disclose.
References
- 1.Cultrera JL, Dalia SM. Diffuse large B-cell lymphoma: current strategies and future directions. Cancer Control 2012; 19:204–213. [DOI] [PubMed] [Google Scholar]
- 2.Jurczak W, Ochrem B, Giza A, et al. Role of rituximab in the first-line therapy of high-risk diffuse large B-cell lymphoma: a retrospective analysis by the Polish Lymphoma Research Group. Pol Arch Med Wewn 2015; 125:741–748. [DOI] [PubMed] [Google Scholar]
- 3.Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006; 7:379–391. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Liu Z, Cao J, et al. Rituximab in combination with CHOP chemotherapy for the treatment of diffuse large B cell lymphoma in China: a 10-year retrospective follow-up analysis of 437 cases from Shanghai Lymphoma Research Group. Ann Hematol 2012; 91:837–845. [DOI] [PubMed] [Google Scholar]
- 5.Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26 suppl 5:v116–125. [DOI] [PubMed] [Google Scholar]
- 6.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993; 329:987–994. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Wang J, Sui X, et al. Prognostic and clinicopathological value of survivin in diffuse large B-cell lymphoma: a meta-analysis. Medicine (Baltimore) 2015; 94:e1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Advani RH, Chen H, Habermann TM, et al. Comparison of conventional prognostic indices in patients older than 60 years with diffuse large B-cell lymphoma treated with R-CHOP in the US Intergroup Study (ECOG 4494, CALGB 9793): consideration of age greater than 70 years in an elderly prognostic index (E-IPI). Br J Haematol 2010; 151:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007; 109:1857–1861. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014; 123:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Zhou M, Xu JY, et al. MYC and BCL-2 adjusted-International Prognostic Index (A-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Histol Histopathol 2016; 31:285–292. [DOI] [PubMed] [Google Scholar]
- 12.Jia B, Shi Y, Kang S, et al. Addition of rituximab is not associated with survival benefit compared with CHOP alone for patients with stage I diffuse large B-cell lymphoma. Chin J Cancer Res 2015; 27:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Zhou M, Wang X, et al. Pretreatment C-reactive protein was an independent prognostic factor for patients with diffuse large B-cell lymphoma treated with RCHOP. Clin Chim Acta 2016; 459:150–154. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe R, Tomita N, Itabashi M, et al. Peripheral blood absolute lymphocyte/monocyte ratio as a useful prognostic factor in diffuse large B-cell lymphoma in the rituximab era. Eur J Haematol 2014; 92:204–210. [DOI] [PubMed] [Google Scholar]
- 15.Belotti A, Doni E, Bolis S, et al. Peripheral blood lymphocyte/monocyte ratio predicts outcome in follicular lymphoma and in diffuse large B-cell lymphoma patients in the rituximab era. Clin Lymphoma Myeloma Leuk 2015; 15:208–213. [DOI] [PubMed] [Google Scholar]
- 16.Ceriani L, Martelli M, Zinzani PL, et al. Utility of baseline 18FDG-PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B-cell lymphoma. Blood 2015; 126:950–956. [DOI] [PubMed] [Google Scholar]
- 17.Mamot C, Klingbiel D, Hitz F, et al. Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large B-cell lymphoma treated with R-CHOP-14 (SAKK 38/07). J Clin Oncol 2015; 33:2523–2529. [DOI] [PubMed] [Google Scholar]
- 18.Miyashita K, Tomita N, Taguri M, et al. Beta-2 microglobulin is a strong prognostic factor in patients with DLBCL receiving R-CHOP therapy. Leuk Res 2015; pii: S0145-2126(15)30368-4. [DOI] [PubMed] [Google Scholar]
- 19.Bizjak M, Selmi C, Praprotnik S, et al. Silicone implants and lymphoma: the role of inflammation. J Autoimmun 2015; 65:64–73. [DOI] [PubMed] [Google Scholar]
- 20.Baecklund E, Smedby KE, Sutton LA, et al. Lymphoma development in patients with autoimmune and inflammatory disorders—what are the driving forces? Semin Cancer Biol 2014; 24:61–70. [DOI] [PubMed] [Google Scholar]
- 21.Kim DH, Baek JH, Chae YS, et al. Absolute lymphocyte counts predicts response to chemotherapy and survival in diffuse large B-cell lymphoma. Leukemia 2007; 21:2227–2230. [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann SH. Immunology's foundation: the 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat Immunol 2008; 9:705–712. [DOI] [PubMed] [Google Scholar]
- 23.Zeng YC, Chi F, Xing R, et al. Pretreatment neutrophil-to-lymphocyte ratio predicts prognosis in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy. Jpn J Clin Oncol 2016; 46:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yutong H, Xiaoli X, Shumei L, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with esophageal cancer in a high incidence area in China. Arch Med Res 2015; 46:557–563. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Dahl DM, Dong L, et al. Preoperative neutrophil-to-lymphocyte ratio and neutrophilia are independent predictors of recurrence in patients with localized papillary renal cell carcinoma. Biomed Res Int 2015; 2015:891045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Jiang C, Li J, et al. Prognostic significance of preoperative neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in patients with gallbladder carcinoma. Clin Transl Oncol 2015; 17:810–818. [DOI] [PubMed] [Google Scholar]
- 27.Keam B, Ha H, Kim TM, et al. Neutrophil to lymphocyte ratio improves prognostic prediction of International Prognostic Index for patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma 2015; 56:2032–2038. [DOI] [PubMed] [Google Scholar]
- 28.Porrata LF, Ristow K, Habermann T, et al. Predicting survival for diffuse large B-cell lymphoma patients using baseline neutrophil/lymphocyte ratio. Am J Hematol 2010; 85:896–899. [DOI] [PubMed] [Google Scholar]
- 29.Ho CL, Lu CS, Chen JH, et al. Neutrophil/lymphocyte ratio, lymphocyte/monocyte ratio, and absolute lymphocyte count/absolute monocyte count prognostic score in diffuse large B-cell lymphoma: useful prognostic tools in the rituximab era. Medicine (Baltimore) 2015; 94:e993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013; 88:218–230. [DOI] [PubMed] [Google Scholar]
- 31.Motomura T, Shirabe K, Mano Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol 2013; 58:58–64. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Du H, Xiao TW, et al. Prognostic value of PD-1 and TIM-3 on CD3+ T cells from diffuse large B-cell lymphoma. Biomed Pharmacother 2015; 75:83–87. [DOI] [PubMed] [Google Scholar]
- 33.Samaka RM, Aiad HA, Kandil MA, et al. The Prognostic Role and Relationship between E2F1 and SV40 in Diffuse Large B-Cell Lymphoma of Egyptian Patients. Anal Cell Pathol (Amsterdam) 2015; 2015:919834. [DOI] [PMC free article] [PubMed] [Google Scholar]


