Abstract
Background:
The concomitant incidence of cancer and pregnancy has increased in recent years because of the increase in maternal age at the time of the 1st pregnancy. The diagnosis of cancer in a pregnant woman causes ethical and therapeutic problems for both the patient and the physician. The main aim of this paper is to describe the available evidence concerning the short- and long-term neonatal impact of chemotherapy given to pregnant women.
Methods:
The relevant publications in English were identified by a systematic review of MEDLINE and PubMed for the last 15 years. The search strategy included “cancer[Title/Abstract] OR tumor[Title/Abstract] AND pregnancy[Title/Abstract] OR pregnant[Title/Abstract] AND embryo[Title/Abstract] or fetus[Title/Abstract] or neonate[Title/Abstract] or newborn[Title/Abstract] or pediatric[Title/Abstract] or child[Title/Abstract] AND English[lang].”
Results:
An analysis of the literature showed that only the administration of chemotherapy during the embryonic stage of conceptus is dangerous and can lead to the termination of the pregnancy. When the disease is diagnosed in the 2nd or 3rd trimester of gestation or when it is possible to delay the initiation of chemotherapy beyond the 14th week, the risk of severe problems for the fetus are low, and pregnancy termination is not required.
Conclusion:
Data regarding the final outcome of children who have received in utero chemotherapy seem reassuring. Only the administration in the embryonal stage of conceptus is dangerous and can lead to the termination of pregnancy. When the disease is diagnosed in the 2nd or 3rd trimester of gestation or when it is possible to delay the initiation of chemotherapy beyond the 14th week, the risk of severe problems for the fetus are low and pregnancy termination is not needed. Increased knowledge of how to minimize the risks of chemotherapy can reduce improper management including unnecessary termination of pregnancy, delayed maternal treatment, and iatrogenic preterm delivery.
Keywords: cancer, chemotherapy, children, embryo, fetus, neonate, pregnancy
1. Introduction
The concomitant incidence of cancer and pregnancy is a rare event and is estimated to account for only 1 to 2 cases per 1000 pregnancies. However, the numbers have increased in recent years because of the increase in maternal age at the time of the 1st pregnancy.[1–3] The most common tumors diagnosed during pregnancy are the same that are common in females of childbearing age and include breast cancer, cervical cancer, leukemia, lymphoma, and lung cancer (Table 1).[4] Breast cancer is the most common form of cancer diagnosed during pregnancy and occurs in 1 to 3 cases per 10,000 pregnancies. Diagnosis is frequently delayed during pregnancy because some symptoms, including breast enlargement and changes in breast texture, are initially considered physiological manifestations of pregnancy, which makes diagnosis of cancer-related breast changes difficult to detect.[5] Rarely, routine procedures performed during pregnancy can uncover cancer. A Pap test, when performed as part of standard pregnancy care, can also detect cervical cancer, and an ultrasound, when performed to monitor fetal development, can also detect ovarian cancer.
Table 1.
Most common malignancies diagnosed in pregnant women and chemotherapy usually prescribed.
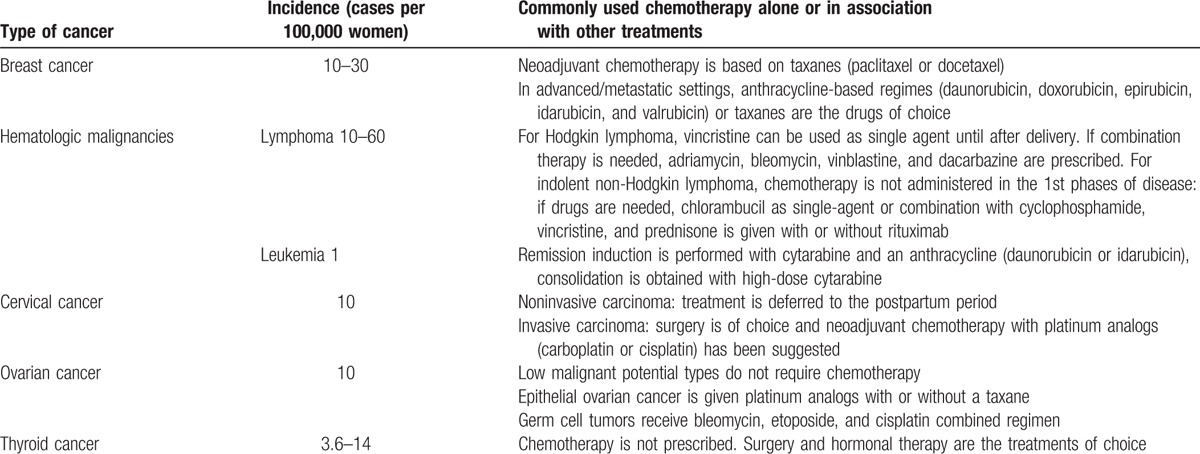
The diagnosis of cancer in pregnant women causes ethical and therapeutic problems for both the patient and the physician. Most of the problems arise from the treatment options and in particular, from chemotherapy. Pregnant women with cancer tend to abstain from treatment for the fear of fetal damage. Conversely, physicians have to balance embryo and fetal well-being with maternal prognosis, taking in account that, even if rarely, vertical transmission of cancer can occur and the child can suffer of the same disease of the mother.[6] To reduce the risk of damage, it is important to understand the optimum use of cytotoxic drugs in pregnant women.[7] Unfortunately, this knowledge is lacking in the field, and the time course for therapy initiation, the appropriate drug choice, and the total daily dose and fractioning have not been clearly established. It is well known that physiological changes in pregnancy can significantly affect drug disposition, especially in the 2nd and 3rd trimester of pregnancy. Moreover, the chemosensitivity of cancer cells in pregnant women has only been defined for some anticancer drugs but not for other drugs that are effective in certain types of tumors.[8] The decision of whether and how to administer chemotherapy could be improved by data regarding the final outcome of children born to mothers with cancer who were adequately treated during pregnancy; however, data are limited and are mainly derived from retrospective case series or reports. Therefore, the main aim of this paper is to describe the available evidence concerning the short- and long-term impact of chemotherapy given to pregnant women with cancer on the neonate.
2. Methods
The relevant publications in English were identified by a systematic review of MEDLINE and PubMed for the last 15 years. The search strategy included “cancer[Title/Abstract] OR tumor[Title/Abstract] AND pregnancy[Title/Abstract] OR pregnant[Title/Abstract] AND embryo[Title/Abstract] or fetus[Title/Abstract] or neonate[Title/Abstract] or newborn[Title/Abstract] or pediatric[Title/Abstract] or child[Title/Abstract] AND English[lang].” Randomized controlled trials, prospective/retrospective studies, and case reports in the English language were included. If the article was not published in English, we relied on the abstract as it appeared in the search engine. No authors declared conflict of interest. Ethics Committee approval was not requested because it is not needed for systematic reviews of the literature according to the Israeli and Italian laws.
3. Results
3.1. The impact of chemotherapy on the embryo and fetus
The effects of chemotherapy on the conceptus depends on several factors, including the duration and timing of the exposure, the dose of the drugs that reaches the embryo or the fetus, and the modalities with which they interfere with cell metabolism.[9] Older-generation alkylators (i.e., procarbazine, busulfan, chlorambucil, and nitrogen mustard) and the antimetabolites aminopterin and methotrexate have high teratogenic and abortive potential; conversely, anthracyclines and vinca alkaloids (i.e., vinblastine and vincristine) have lower fetotoxic potential.[10] The use of cytotoxic drugs during fertilization or implantation (i.e., during the 1st 10 days after conception) can lead to one of 2 opposite phenomena: the death of the embryo or the proper development of the embryo. The 1st trimester is the period during which the majority of organogenesis occurs and chemotherapy can exert a significant teratogenic effect, particularly on the heart, limbs, palate, neural tube, eyes, and ears.[11] Therefore, the administration of cytotoxic drugs during the 1st trimester is particularly dangerous because it is frequently associated with the development of malformations, embryo death, and spontaneous abortion. The risk of malformations is approximately 7% to 17% when a single agent treatment is used and increases to 25% in cases of combination therapy.[12] The strict association between chemotherapy during the 1st 12 weeks of gestation and the risk of malformations explains why experts agree with the recommendation of delaying the initiation of chemotherapy in pregnant women with cancer until after the end of the 1st trimester of gestation.[13] Outside this period, the influence of chemotherapy in fetal and child outcomes is significantly lower, and the risk of malformations has not been explicitly demonstrated. Doll et al[14] reported major malformations in 1.3% of children born to mothers with cancer who had been treated with chemotherapy after the 1st trimester, which is a risk value similar to the general population without chemotherapy treatment. Moreover, although it has been reported that the eyes, genitalia, central nervous system, and hematopoietic systems remain vulnerable to continued exposure to cytotoxic drugs during the last months of gestation, and intrauterine growth retardation is a possible complication of that exposure,[15,16] several studies have shown that chemotherapy administered during the 2nd or 3rd trimester of pregnancy has little effect on the long-term outcome of the child, which favors its use to control cancer in pregnant women.[17–19]
The relatively good tolerance of the fetus to maternal chemotherapy is, at least in part, explained by the limited exposure of the conceptus to the cytotoxic drugs compared to the pregnant mother. Placental passage of a drug is a function of multiple factors including protein binding, lipid solubility, and ionization constant; however, fetal exposure to drugs depends on maternal pharmacokinetics including the volume of distribution, the rate of metabolism and excretion by the placenta, the pH difference between maternal and fetal fluids, and the effect of hemodynamic changes in the mother during pregnancy.[20] Many drugs used to treat cancer have characteristics that favor passive diffusion through the placenta because they have a low molecular weight and are lipid-soluble and nonionized. Despite this, these drugs typically reach in the fetal circulatory system at concentrations that are significantly lower than those present in the mother.[21] Several placental transporters (i.e., multidrug-resistant proteins, P-glycoprotein, and breast cancer resistance proteins) regulate the uptake and efflux of drugs used in pregnancy to reduce the concentrations in fetal blood.[22] Moreover, the absorption of drugs from the gastrointestinal tract during pregnancy is modified because of changes in gastric secretion and motility. Furthermore, maternal drug metabolism may be altered due to the elevation of endogenous hormones such as progesterone.[23,24] Finally, therapeutic concentrations of the active drug may also be affected by hemodynamic changes that take place throughout pregnancy. For example, blood volume increases by 40% and total body water content increases by 5% to 8%, due to the expansion of the extracellular fluid space and the growth of new tissue.[25] Body water also accumulates in the fetus, placenta, and amniotic fluid. This contributes to an increase in volume of distribution and may lower the concentration of drugs by increasing their elimination half-life. Realistically, all the drugs used to treat cancer reach the fetus in a relatively low concentration, although there are significant differences among the individual drugs. Observations in primates show the passage can be marginal (paclitaxel, 0%–1%), hardly valuable (anthracyclines, 5%–7%), or relatively high (carboplatin, 60%) depending on the drug assessed; however, the concentrations are always significantly lower compared to the mother.[26,27]
3.2. The impact of chemotherapy on pregnancy
Chemotherapy has been associated with an increased risk of stillbirth and fetal growth restriction.[28,29] Van Calsteren et al[29] detected a demonstrable increase in preterm delivery in pregnant women exposed to cytotoxic therapy in comparison to healthy unexposed women (4% vs 11.8%, P = 0.01). However, a definitive conclusion cannot be made because in some studies no differences were detected in both the gestational age and birth weight between children born to mothers with cancer receiving chemotherapy and those born to healthy women.[30–34] Conversely, the occurrence of prematurity can cause relevant problems; however, it is unclear whether these problems depend on the drug administration or is linked to other factors related to the disease itself.
3.3. The perinatal and long-term effects on children from chemotherapy administered during pregnancy
3.3.1. Perinatal effects
At birth and in the 1st few weeks of life, a number of children born to mothers treated with chemotherapy present with transient myelosuppression, which involve leukopenia (white blood cell count <5000/mm3) and/or neutropenia (absolute neutrophil count <1500/mm3) with anemia and/or thrombocytopenia (platelet count <15,000/mm3).[35,36] Moreover, in children born to mothers treated with rituximab, a monoclonal antibody against the protein CD20 used to treat B-cell non-Hodgkin lymphoma, selective B-cell depletion was observed.[35]
Generally, transient myelosuppression is maximally evident in the 1st days of life and is resolved within 2 to 10 weeks. Its frequency varies from 43% and 33% in the studies by Aviles and Neri[34] and Reynoso et al,[28] respectively, to 4% observed by Cardonick and Iacobucci[9] in a review of 321 published case reports. Data from an international cancer and pregnancy registry collected from 1995 to 2008 showed that among 157 neonates exposed to various chemotherapy drugs during pregnancy, only 2 infants suffered from transient myelosuppression or anemia at birth, and neither suffered from opportunistic infections.[35] These differences may have several explanations, including the different therapeutic regimens. However, the most important reason may be the timing of therapy suspension with respect to the timing of childbirth; specifically, when treatment is maintained until the end of pregnancy, the risk of transient myelosuppression is significantly higher whereas the risk is greatly reduced the more extensive time period between the suspension of chemotherapy and childbirth. For this reason, it is recommended that delivery is avoided during the maternal nadir period, and chemotherapy should not be administered after the 35th week of gestation in order to allow the fetus to eliminate the cytotoxic drugs.[36] Theoretically, transient myelosuppression might lead to severe infections, and systematic monitoring of at-risk neonates at birth is recommended. Additionally, supportive care including thrombocyte transfusions, erythrocyte transfusions, erythropoietin, and recombinant granulocyte colony-stimulating factor administration are given when needed. However, in general, transient myelosuppression is mild and does not cause clinical problems.[37] The same is true for transient myelosuppression-related immune depression. If transient myelosuppression is resolved within 10 weeks, even when maternal treatment is continued until delivery, it is reasonable to expect that in children that are not exposed to chemotherapy in the last week of gestation, immune depression would also last for a limited period of time and should not induce an immune response to inactivated vaccines given according to the recommended pediatrician schedule. Consistent with this conclusion, data collected in children born to mothers who received rituximab to treat hematologic tumors show that the administration of rituximab is associated with a selective inhibitory effect on the development of newborn B-cells.[38] However, the condition is reversible, and B-cell levels return to normal by 3 to 6 months of age. In the short-term follow-up, no significant infections were identified, and subsequent immunological controls revealed an adequate response to standard vaccinations.[39,40] However, because no substantial data to this regard is currently available, further studies on the immune response of children receiving chemotherapy in utero are needed to exclude the possibility that vaccines (mainly the live attenuated ones) administered in the 1st days of life may be dangerous. For example, severe neonatal neutropenia and fatal dissemination of Bacillus Calmette–Guérin has been reported in children born to mothers treated with biologicals.[41]
3.3.2. Long-term effects
Several studies have evaluated the long-term impact of cytotoxic drugs administered during pregnancy on children. Together with growth and general development, the main target of most of these studies was the neurological and psychological development because the central nervous system is extremely sensitive to these drugs. Mennes et al,[42] found that children with acute lymphoblastic leukemia, treated exclusively with chemotherapy, processed information slower compared to control children, especially when more information has to be processed or when attention has to be focused precisely. However, contrary to what was feared, the 1st evaluation of the impact of in utero chemotherapy did not find any neurological problems, even in subjects whose mothers were treated during the 1st trimester of pregnancy.[34] Subjects were evaluated during high-school or college and showed normal neurological and psychological development. Furthermore, the educational performance of these children corresponded with the economic and social status of their families, and no learning disabilities were observed. Similar results were obtained with other studies including those published by Hahn et al[43] and Amant et al.[44] Average neuropsychological development was demonstrated in the great majority of chemotherapy-exposed children. In addition, when neuropsychological issues were present, they could be ascribed to prematurity or maternal stress. Despite these reassuring findings, the conclusions were not considered definitive due to the small sample size of the enrolled children and the limited follow-up for a number of the cases. Recently studies have concluded that prenatal exposure to maternal cancer with or without treatment does not impair the cognitive development of children. Cardonick et al[18] examined 57 children of mothers diagnosed with cancer while pregnant, 35 of whom had been exposed to chemotherapy in utero. The children underwent developmental testing and ranged in age from 18 months to 10.4 years. Based on age, the Bayley Scales of Infant Development–Third Edition, the Wechsler Preschool and Primary Scale of Intelligence-Revised, the Wechsler Intelligence Scale for Children, Third Edition, or the Wechsler Individual Achievement Test were administered. All parents or primary caregivers completed the Child Behavior Checklist, which is a parent questionnaire to assess behavioral and emotional issues. No significant differences were noted in cognitive ability, school performance, or behavioral competencies between the chemotherapy-exposed group and the unexposed children. Cognitive assessments were within the normal limits in 95% of the cases. In addition, 71% and 79% of children demonstrated at or above age equivalency in mathematics and reading scores, respectively, and 79% of children scored within the normal limits on measures of behavior.[18] However, older children had significantly higher rates of internalizing behavioral problems. Amant et al[19] performed a similar study where 96 children (median age, 22 months; range, 12–42 months) were prospectively assessed (by means of a neurologic examination and the Bayley Scales of Infant Development) at 18 months, 36 months, or both and compared with a control group. No significant between-group differences were observed in cognitive development on the basis of the Bayley score or in subgroup analyses. Prematurity was correlated with a worse cognitive outcome, but this effect was independent of cancer treatment.
A 2nd largely studied problem is the potential toxicity of some drugs, especially anthracyclines and trastuzumab, on fetal heart development. Interest in the effects of these 2 drugs is based on the results from several studies. Anthracyclines are well-known for their cardiotoxic effects that depend on multiple mechanisms, including oxidative damage, changes in calcium metabolism, and activation of apoptotic pathways, leading to progressive deterioration in cardiac function.[45,46] Moreover, anthracycline toxicity has been shown to be enhanced by the concomitant use of trastuzumab, a monoclonal antibody that inhibits proliferation of cells overexpressing human epidermal growth factor receptor tyrosine kinase, which is used in breast cancers where these cells are present.[47] Several case reports have described the development of fetal heart toxicity after the administration of anthracycline to pregnant women and have shown these affects are mostly reversible.[48] Finally, data from pediatric cancer survivors have shown that anthracycline exposure at a young age can result in progressive left ventricular dysfunction and clinical heart failure 10 to 20 years after stopping chemotherapy.[49]
Transplacental passage of anthracyclines has been studied in vitro and in animals but available data seem to suggest that delivery to the fetus is relatively poor and umbilical artery sample concentrations were no higher than 65% of the levels detected in the mother.[50] However, because the fetal myocardium significantly differs from the adult myocardium, it is theoretically possible that lower drug concentrations may induce toxic effects in the fetus. Fortunately, no evidence of cardiac disease was found in the studies in which the heart function of children exposed to chemotherapy in utero was evaluated. In the most recent study, cardiac function was evaluated in 50 children, aged 36 months, who had been exposed to chemotherapy in utero and 26 of the cases involved exposure to anthracyclines.[19] A 12-lead electrocardiography and a detailed echocardiographic examination were performed. In addition, standard views and measurements were carried out according to the guidelines of the American Society of Cardiology. The results were compared with 47 healthy children matched for age and sex. No structural abnormalities were detected. All measures of cardiac-chamber dimension and cardiac wall thickness were within normal ranges, and there were no between-group differences in ejection fraction, fractional shortening, or values for global longitudinal strain and circumferential strain.[19] In particular, there were no differences in the subjects exposed to anthracyclines compared to the subjects exposed to other chemotherapy drugs.
Finally, no differences in growth and general development have been found between exposed children and healthy controls.[19]
4. Conclusions
Although studies are limited and results have been obtained on limited number of patients with no possibility to define the level of evidence with a scale, data regarding the final outcome of children who have received in utero chemotherapy seem reassuring. Table 2 summarizes the main effects of chemotherapy on embryo and fetus development. Only the administration in the embryonal stage of conceptus is dangerous and can lead to the termination of pregnancy.[13] When the disease is diagnosed in the 2nd or 3rd trimester of gestation or when it is possible to delay the initiation of chemotherapy beyond the 14th week, the risk of severe problems for the fetus are low and pregnancy termination is not needed.
Table 2.
Main effects of chemotherapy during pregnancy on embryo and fetus development.
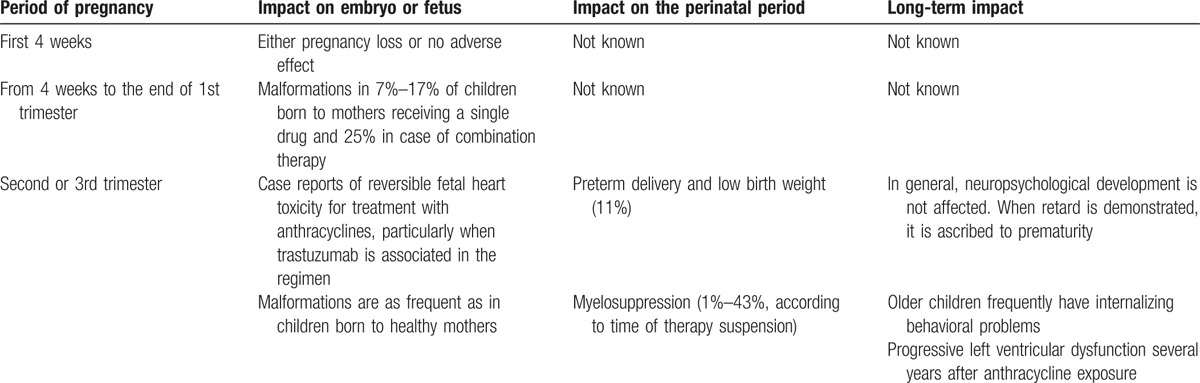
However, these conclusions should be interpreted with caution because several aspects of chemotherapy in pregnant woman have not been fully documented. First, many of the drugs given to pregnant women at the usual dosages show lower blood concentrations in pregnant women compared to nonpregnant subjects suggesting that an increased dose is needed. This increase could expose the mother and the fetus to subsequent problems. Second, severe neutropenia can occur in pregnant women with cancer treated with chemotherapy. Usually this condition is treated with granulocyte colony-stimulating factor but the effectiveness and safety of this compound during pregnancy has not definitively been established. Third, any new drugs have to be adequately evaluated to establish the pharmacokinetic and pharmacodynamic characteristics in pregnant women during the different periods of gestation to measure the amount of drug that reaches the fetus. Finally, studies on immunogenicity, safety, and tolerability of routine vaccines administered in the 1st few months of life are necessary in order to understand whether children born to mothers with cancer who were treated with chemotherapy should follow the routine schedule or postpone/anticipate the administration of some vaccines. Increased knowledge of how to minimize the risks of chemotherapy can reduce improper management including unnecessary termination of pregnancy, delayed maternal treatment, and iatrogenic preterm delivery, which are frequently applied in the treatment of pregnant cancer patients.[51]
Footnotes
Funding/support: This review was partially supported by a grant from the Italian Ministry of Health (Progetto Ricerca Corrente 2016 850/01 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico).
The authors have no conflicts of interest to disclose.
References
- 1.Williams TJ, Turnbull KE. Carcinoma in situ and pregnancy. Obstet Gynecol 1964; 24:857–864. [PubMed] [Google Scholar]
- 2.Pavlidis NA. Coexistence of pregnancy and malignancy. Oncologist 2002; 7:279–287. [PubMed] [Google Scholar]
- 3.Smith LH, Danielsen B, Allen ME, et al. Cancer associated with obstetric delivery: result of linkage with the California cancer registry. Am J Obstet Gynecol 2003; 189:1128–1135. [DOI] [PubMed] [Google Scholar]
- 4.Salani R, Billingsley C, Crafton S. Cancer and pregnancy: an overview for obstetricians and gynaecologist. Am J Obstet Gynecol 2014; 211:7–14. [DOI] [PubMed] [Google Scholar]
- 5.Amant F, Loibl S, Neven P, et al. Breast cancer in pregnancy. Lancet 2012; 379:570–579. [DOI] [PubMed] [Google Scholar]
- 6.Tolar J, Neglia JP. Transplacental and other routes of cancer transmission between individuals. J Pediatr Hematol Oncol 2003; 25:430–434. [DOI] [PubMed] [Google Scholar]
- 7.Mir O, Berveiller P. Increased evidence for use of chemotherapy in pregnancy. Lancet Oncol 2012; 13:852–854. [DOI] [PubMed] [Google Scholar]
- 8.Berveiller P, Mir O. Taxanes during pregnancy: probably safe, but still to be optimized. Oncology 2012; 83:239–240. [DOI] [PubMed] [Google Scholar]
- 9.Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol 2004; 5:283–291. [DOI] [PubMed] [Google Scholar]
- 10.Azim HA, Jr, Pavlidis N, Peccatori FA. Treatment of the pregnant mother with cancer: a systematic review on the use of cytotoxic, endocrine, targeted agents and immunotherapy during pregnancy. Part II: hematological tumors. Cancer Treat Rev 2010; 36:110–121. [DOI] [PubMed] [Google Scholar]
- 11.Walton JR, Prasad MR. Obstetric and neonatal outcomes of cancer treated during pregnancy. Clin Obstet Gynecol 2011; 54:567–573. [DOI] [PubMed] [Google Scholar]
- 12.Ebert U, Loffler H, Kirck W. Cytotoxic therapy and pregnancy. Pharmacol Ther 1997; 74:207–220. [DOI] [PubMed] [Google Scholar]
- 13.Lishner M, Avivi I, Apperley JF, et al. Hematologic malignancies in pregnancy: management guidelines from an International Consensus Meeting. J Clin Oncol 2016; 34:501–508. [DOI] [PubMed] [Google Scholar]
- 14.Doll DC, Ringenberg QS, Yarbro JW. Antineoplastic agents and pregnancy. Semin Oncol 1989; 16:337–346. [PubMed] [Google Scholar]
- 15.Kalter H, Warkany J. Medical progress. Congenital malformations: etiologic factors and their role in prevention (first of two parts). N Engl J Med 1983; 308:424–431. [DOI] [PubMed] [Google Scholar]
- 16.Vandenbroucke T, Verheecke M, Van Calsteren K, et al. Fetal outcome after prenatal exposure to chemotherapy and mechanisms of teratogenicity compared to alcohol and smoking. Expert Opin Drug Saf 2014; 13:1653–1665. [DOI] [PubMed] [Google Scholar]
- 17.Cardonick E, Gilmandyar D, Somer RA. Maternal and neonatal outcomes of dose-dense chemotherapy for breast cancer in pregnancy. Obstet Gynecol 2012; 120:1267–1272. [DOI] [PubMed] [Google Scholar]
- 18.Cardonick EH, Gringlas MB, Hunter K, et al. Development of children born to mothers with cancer during pregnancy: comparing in utero chemotherapy-exposed children with nonexposed controls. Am J Obstet Gynecol 2015; 212:658.e1-8. [DOI] [PubMed] [Google Scholar]
- 19.Amant F, Vandenbroucke T, Verheecke M, et al. Pediatric outcome after maternal cancer diagnosed during pregnancy. N Engl J Med 2015; 373:1824–1834. [DOI] [PubMed] [Google Scholar]
- 20.Gedeon C, Koren G. Designing pregnancy centered medications: drugs which do not cross the human placenta. Placenta 2006; 27:861–868. [DOI] [PubMed] [Google Scholar]
- 21.Eshkoli T, Sheiner E, Ben-Zvi Z, et al. Drug transport across the placenta. Curr Pharm Biotechnol 2011; 12:707–714. [DOI] [PubMed] [Google Scholar]
- 22.Staud F, Ceckova M. Regulation of drug transporter expression and function in the placenta. Expert Opin Drug Metab Toxicol 2015; 11:533–555. [DOI] [PubMed] [Google Scholar]
- 23.Langer O, Conway D, Berkus M, et al. A comparison of glyburide in insulin in women with gestational diabetes mellitus. N Engl J Med 2000; 343:1134–1138. [DOI] [PubMed] [Google Scholar]
- 24.Lobstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. In. Maternal Fetal Toxicology: A Clinician Guide 3rd ed.Toronto, Ontario, Canada: Marcel Dekker Inc; 2001. 1–21. [Google Scholar]
- 25.Bournissen G, Feig D, Koren G. Maternal–fetal transport of hypoglycemic drugs. Clin Pharmacokinet 2003; 42:303–313. [DOI] [PubMed] [Google Scholar]
- 26.Van Calsteren K, Verbesselt R, Devlieger R, et al. Transplacental transfer of paclitaxel, docetaxel, carboplatin, and trastuzumab in a baboon model. Int J Gynecol Cancer 2010; 20:1456–1464. [DOI] [PubMed] [Google Scholar]
- 27.Van Calsteren K, Verbesselt R, Beijnen J, et al. Transplacental transfer of anthracyclines, vinblastine, and 4-hydroxycyclophosphamide in a baboon model. Gynecol Oncol 2010; 119:594–600. [DOI] [PubMed] [Google Scholar]
- 28.Reynoso EE, Shepherd FA, Messner HA, et al. Acute leukemia during pregnancy: the Toronto leukemia study group experience with long-term follow-up of children exposed in utero to chemotherapeutic agents. J Clin Oncol 1987; 5:1098–1106. [DOI] [PubMed] [Google Scholar]
- 29.Val Calsteren K, Heyns L, De Smet F, et al. Cancer during pregnancy: an analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J Clin Oncol 2010; 28:683–689. [DOI] [PubMed] [Google Scholar]
- 30.Hahn KM, Johnson PH, Gordon N, et al. Treatment of pregnant breast cancer patients and outcomes of children exposed to chemotherapy in utero. Cancer 2006; 107:1219–1226. [DOI] [PubMed] [Google Scholar]
- 31.Zemlickis D, Lishner M, Degendorfer P, et al. Maternal and fetal outcome after breast cancer in pregnancy. Am J Gynecol 1992; 166:781–787. [DOI] [PubMed] [Google Scholar]
- 32.Giacalone PL, Laffargue F, Benos P. Chemotherapy for breast carcinoma during pregnancy: a French national survey. Cancer 1999; 86:2266–2272. [PubMed] [Google Scholar]
- 33.Hoffman MA, Wiernik PH, Kleiner GJ. Acute promyelocytic leukemia and pregnancy. Cancer 1995; 76:2237–2241. [DOI] [PubMed] [Google Scholar]
- 34.Aviles A, Neri N. Hematologic malignancies and pregnancy: a final report of 84 children who received chemotherapy in utero. Clin Lymphoma 2001; 2:173–177. [DOI] [PubMed] [Google Scholar]
- 35.Cardonick E, Usmani A, Ghaffar S. Perinatal outcomes of a pregnancy complicated by cancer, including neonatal follow-up after in utero exposure to chemotherapy: results of an international registry. Am J Clin Oncol 2010; 33:221–228. [DOI] [PubMed] [Google Scholar]
- 36.Buekers TE, Lallas TA. Chemotherapy in pregnancy. Obstet Gynecol Clin North Am 1998; 25:323–329. [DOI] [PubMed] [Google Scholar]
- 37.Udink ten Cate FE, ten Hove CH, Nix WM, et al. Transient neonatal myelosuppression after fetal exposure to maternal chemotherapy. Case report and review of the literature. Neonatology 2009; 95:80–85. [DOI] [PubMed] [Google Scholar]
- 38.Klink DT, van Elburg RM, Schreurs MW, et al. Rituximab administration in third trimester of pregnancy suppresses neonatal B-cell development. Clin Dev Immunol 2008; 2008:271363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Decker M, Rothermundt C, Hollander G, et al. Rituximab plus CHOP for treatment of diffuse large B-cell lymphoma during second trimester of pregnancy. Lancet Oncol 2006; 7:693–694. [DOI] [PubMed] [Google Scholar]
- 40.Friedrichs B, Tiemann M, Salwender H, et al. The effect of rituximab treatment during pregnancy on a neonate. Haematologica 2006; 91:1426–1427. [PubMed] [Google Scholar]
- 41.Ling J, Koren G. Challenges in vaccinating infants born to mothers taking immunoglobulin biologicals during pregnancy. Expert Rev Vaccines 2016; 15:239–256. [DOI] [PubMed] [Google Scholar]
- 42.Mennes M, Stiers P, Vandenbussche E, et al. Attention and information processing in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. Pediatr Blood Cancer 2005; 44:478–486. [DOI] [PubMed] [Google Scholar]
- 43.Hahn KM, Johnson PH, Gordon N, et al. Treatment of pregnant breast cancer patients and outcomes of children exposed to chemotherapy in utero. Cancer 2006; 107:1219–1226. [DOI] [PubMed] [Google Scholar]
- 44.Amant F, Van Calsteren K, Halaska MJ, et al. Long-term cognitive and cardiac outcomes after prenatal exposure to chemotherapy in children aged 18 months or older: an observational study. Lancet Oncol 2012; 13:256–264. [DOI] [PubMed] [Google Scholar]
- 45.Sawyer DB, Peng X, Chen B, et al. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis 2010; 53:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen B, Peng X, Pentassuglia L, et al. Molecular and cellular mechanisms of anthracycline cardiotoxicity. Cardiovasc Toxicol 2007; 7:114–121. [DOI] [PubMed] [Google Scholar]
- 47.Telli ML, Hunt SA, Carlson RW, et al. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol 2007; 25:3525–3533. [DOI] [PubMed] [Google Scholar]
- 48.Gziri MM, Amant F, Debiève F, et al. Effects of chemotherapy during pregnancy on the maternal and fetal heart. Prenat Diagn 2012; 32:614–619. [DOI] [PubMed] [Google Scholar]
- 49.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009; 339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marnitz S, Kohler C, Oppelt P, et al. Cisplatin application in pregnancy: first in vivo analysis of 7 patients. Oncology 2010; 79:72–77. [DOI] [PubMed] [Google Scholar]
- 51.Han SN, Kesic VI, Van Calsteren K, et al. Cancer in pregnancy: a survey of current clinical practice. Eur J Obstet Gynecol Reprod Biol 2013; 167:18–23. [DOI] [PubMed] [Google Scholar]


