Abstract
This study aimed to evaluate the utility of the 2006 Sendai and 2012 Fukuoka guidelines for differentiating malignant intraductal papillary mucinous neoplasm (IPMN) of the pancreas from benign IPMN.
Between January 2000 and March 2015, a total of 138 patients underwent surgery and had a pathologically confirmed pancreatic IPMN. Clinicopathological parameters were reviewed, and all patients were classified according to both the 2006 Sendai and 2012 Fukuoka guidelines. Univariate and multivariate analyses were used for identifying significant factors associated with malignancy in IPMN.
There were 9 high-grade dysplasia (HGD) and 37 invasive cancers (ICs) in the 138 patients. The positive predictive value (PPV) and negative predictive value (NPV) of the Sendai and Fukuoka guidelines for HGD/IC was 35.1%, 43.3%, 100%, and 85.4%, respectively. Of the 36 patients with worrisome features using the Fukuoka guideline, 7 patients had HGD/IC in their IPMNs. According to the multivariate analysis, jaundice, tumors of ≥3 cm, presence of mural nodule on imaging, and aged <65 years were associated with HGD/IC in patients with IPMN.
The Sendai guideline had a better NPV, but the Fukuoka guideline had a better PPV. We suggest that patients with worrisome features based on the Fukuoka guideline be aggressively managed.
Keywords: cystic lesion of the pancreas, guideline, intraductal papillary mucinous neoplasm (IPMN), pancreatectomy
1. Introduction
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas represents a group of mucinous cystic lesions that have malignant potential in the pancreas. Some IPMNs have premalignant or malignant components and should be resected in surgically fit patients. IPMNs have been classified as main-duct type (MD-IPMN), branch-duct type (BD-IPMN), and mixed type (MT-IPMN) based on involvement of the pancreatic duct.[1] The management of pancreatic IPMNs is still a controversial topic because they have a wide range of malignant potential. The reported incidence of malignancy varies from 57% to 92% in MD-IPMN and from 6% to 46% in BD-IPMN.[2] It is generally thought that all MD-IPMN and MT-IPMN should be resected because of its high risk of malignancy, whereas BD-IPMN may be treated conservatively according to its clinical risk of malignancy. The 2006 Sendai consensus guideline suggested surgical resection of all MD-IPMNs and BD-IPMNs involving symptomatic tumors of ≥3 cm, lesions with a mural nodule or thickened wall, and the main pancreatic duct (MPD) of ≥6 mm.[3] This guidelines seemed highly sensitive to detect malignant or premalignant BD-IPMNs; under this guideline, the most suspicious BD-IPMNs (i.e., high-grade dysplasia [HGD] or invasive cancer [IC]) would be resected.[4–7] However, the low specificity of lesions with HGD or IC on final pathology reports indicate a limitation of the 2006 Sendai guideline.[4,6,7] Some patients had undergone unnecessary operations because of the Sendai guideline, including complicated pancreaticoduodenectomies. Thus, the revised 2012 Sendai consensus guideline (i.e., Fukuoka guideline) leans toward a relatively conservative approach for pancreatic IPMNs. The Fukuoka guideline proposed “worrisome features” and “high-risk stigmata” categories in an attempt for further stratify patients regarding risk of malignancy.[8] Patients with high-risk malignant stigmata (obstructive jaundice, enhancing solid component within cysts, and a MPD of ≥10 mm in size) were suggested to undergo surgical resection. Patients with worrisome features (pancreatitis, tumor of ≥3 cm, thickened/enhancing cyst wall, non-enhancing mural nodule, abrupt change in caliber of pancreatic duct with distal pancreatic atrophy, and a main duct sized 5–9 mm) were suggested for observation, rather than immediate surgical resection if there was no evidence of a definite mural nodule, main duct features suspicious of involvement, or cytology suspicious or positive for malignancy based on additional endoscopic ultrasonography (EUS) studies. The Fukuoka guideline de-emphasizes IPMNs with worrisome features and indicates that a more conservative approach could be applied for those with a relatively low potential for suspicious lesions. However, several studies challenged the safety of both the new and old guidelines.[5,9–13] Wong et al[9] reported a high incidence of malignancy and HGD in BD-IPMN of <3 cm, and Fritz et al[10] reported malignancies in 25% of Sendai-negative BD-IPMN. Both studies indicated a significant proportion of malignancy in BD-IPMN that were presumed to be benign and would have been observed without resection using both the Sendai and Fukuoka guidelines; thus, a more aggressive resection policy for BD-IPMN was recommended.
According to the 2012 Fukuoka guideline, preoperative diagnosis of MD-IPMN would be determined based on segmental or diffuse dilation of >5 mm of the MPD without any other cause of obstruction.[8] However, MD-IPMN with mild MPD dilatation (5–9 mm) could be managed in a manner similar to BD-IPMN, for example, without any high-risk stigmata that necessitates further evaluation, but no immediate resection.[8] In clinical practice, it is difficult to determine preoperatively the definitive diagnosis of IPMN type, such as main-duct, mixed, or branch-duct type, because of some discrepancies between histologic and radiologic criteria.[14,15] Identifying IPMN with HGD or IC is important and practical in management of this pancreatic neoplasm, because only minor part of patients with IPMN will have malignancy in the follow-ups after initial diagnosis with extremely low mortality rate, but major part of patients with IPMN will not have malignancy during their lifelong follow-ups.[16] In our hospital, after 2006, we applied the Sendai guideline to manage patients with all suspected IPMN types (main-duct type, mixed-type, or branch-duct type). In this study, we aimed to evaluate the utility of the 2006 Sendai and 2012 Fukuoka guidelines for the management of all IPMN types within our cohort. We also sought to analyze the impact of the 3-cm threshold and symptoms (i.e., pancreatitis) on the risk of malignancy.
2. Methods
2.1. Method
This study was approved by the institutional review board of our hospital. From January 2000 to March 2015, 138 patients who underwent surgery with a pathologically confirmed diagnosis of IPMN at National Taiwan University Hospital were included in this study. Chart records of all included patients were retrospectively reviewed to obtain clinical data including demographics, tumor size and location, histopathological report, perioperative data, and follow-up status. One pathologist reviewed all pathology for the surgical specimens, and diagnosis of MD-IPMN, MT-IPMN, and BD-IPMN was made based on current histological criteria[17] after review of the pathological findings. The decision of whether these patients should undergo surgery was made by the initial imaging or presentation at diagnosis. Before 2006, patients were recommended with surgery because of large tumor size and/or symptoms (pancreatitis, jaundice). After 2006, patients with “Sendai positive” feature were recommended with surgery. Seven of 138 patients with “Sendai negative” feature underwent surgery because of their own will. Eight of 138 patients had been initially observed but developed high-risk features during follow-up and underwent surgeries.
2.2. Sendai consensus guidelines 2006
Patients were classified as “Sendai positive” if the tumor size was ≥3 cm, was symptomatic, had mural nodules or a thickened wall, or was accompanied by a dilated MPD of ≥6 mm. Patients who did not meet these criteria were considered “Sendai negative.”
2.3. Revised Sendai consensus guidelines 2012 (Fukuoka guideline)
Patients were classified as “Fukuoka high risk” if any of the following were present: obstructive jaundice, enhancing solid component, or MPD of ≥10 mm. Patients were classified as “Fukuoka worrisome” if they presented with any worrisome features (pancreatitis, a tumor of ≥3 cm, a thickened/enhancing cyst wall, nonenhancing mural nodules, an abrupt change in caliber of the pancreatic duct with distal pancreatic atrophy, and an MPD of 5–9 mm). According to the Fukuoka guideline, patients with worrisome features were suggested to obtain additional EUS exams, and those who had the presence of a definite mural nodule, suspicious MPD involvement, or suspicious cytology during EUS exams were suggested to undergo resection. In this study, patients with worrisome features might have undergone surgical resection without additional EUS exams after discussing the risks and benefits with a surgeon. Patients who did not meet the abovementioned criteria for “Fukuoka high risk” or “Fukuoka worrisome” were considered “Fukuoka negative.”
2.4. Statistical analysis
Categorical variables were compared using the Fisher exact tests and Pearson χ2 tests; continuous variables were compared using the Student t test and Mann–Whitney U test, as appropriate. A multivariate analysis was performed based on the Cox proportional hazards regression model. A P value of <0.05 was considered significant. The statistical analyses were performed using SPSS 18 for Windows v. 18.0 (SPSS Inc, Chicago, IL).
3. Results
3.1. Demographic characteristics
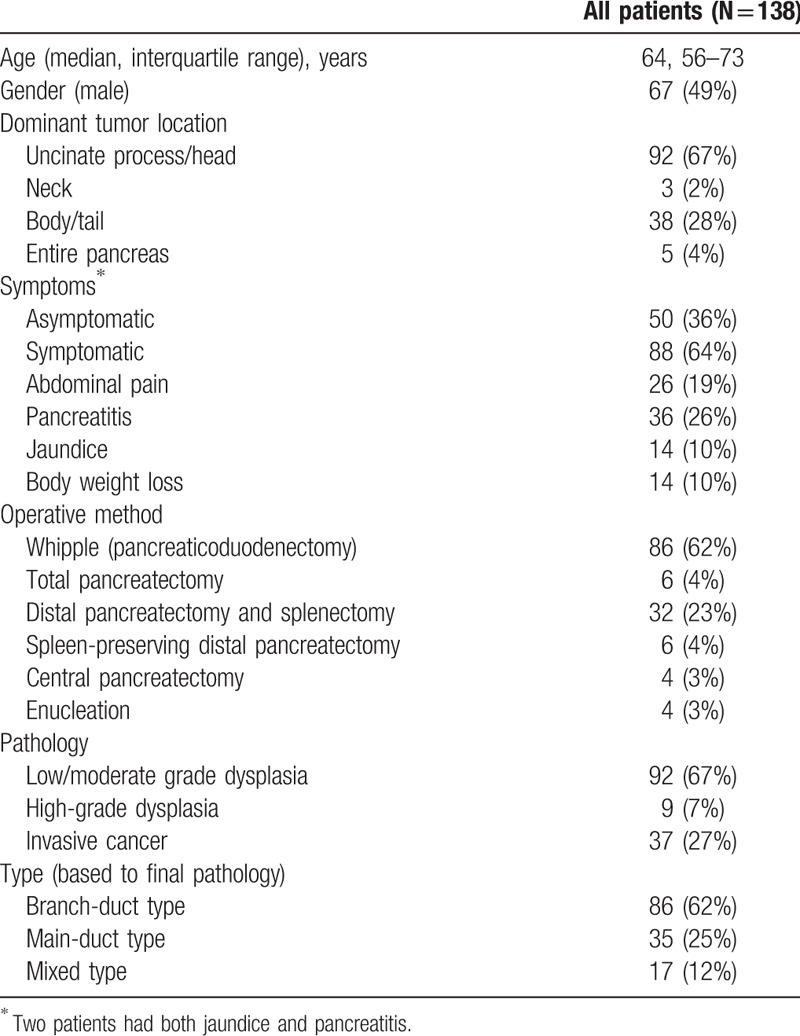
The demographics of the patients are summarized in Table 1. The 138 patients had a median age of 64 (interquartile range, 56–73) years, of which 71 (51.4%) were women. Eighty-eight (63.8%) patients were symptomatic, and 92 (66.7%) patients had lesions located at the uncinate process or pancreatic head. All patients underwent surgery including 6 (4.3%) total pancreatectomies, 86 (62.3%) pancreaticoduodenectomies, 38 (27.5%) distal pancreatectomies, 4 (2.9%) central pancreatectomies, and 4 (2.9%) enucleations. According to the Dindo–Clavien classification,[18] the overall complication rate was 34.8% (46 of 138 patients), most of them (42 patients) had grade I–II complication, 5 patients had grade III complication, and 1 patient had grade IV complication. There was no surgical-related mortality. Based on final histopathology, there were 86 (62.3%) BD-IPMNs, 35 (25.4%) MD-IPMNs, and 17 (12.3%) MT-IPMNs, of which 92 were low/moderate grade dysplasias, 9 were HGDs, and 37 were ICs.
Table 1.
Demographics, symptoms, and surgical and pathological outcomes of study patients.
3.2. Factors associated with pancreatic IPMN and high-grade dysplasia/invasive cancer
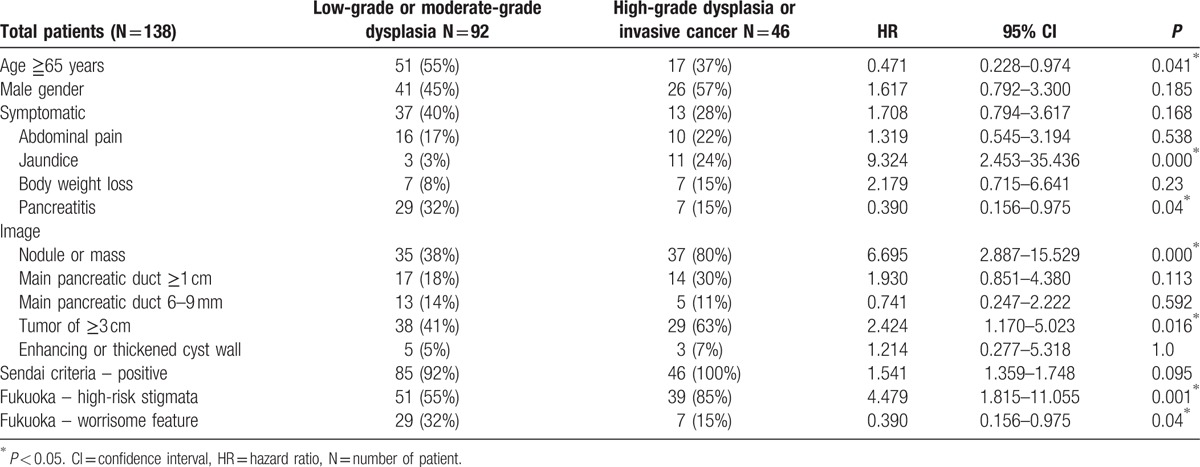
Forty-six (33.3%) of the 138 IPMNs had HGD or IC (Table 2). Factors associated with HGD/IC included aged <65 years, presence of jaundice, no pancreatitis, presence of a mural nodule in an image, or a tumor of ≥3 cm. On a multivariate analysis, presence of jaundice, tumor of ≥3 cm, presence of a mural nodule on imaging, or aged <65 years was associated with HGD/IC in IPMNs.
Table 2.
Univariate analysis of factors associated with high-grade dysplasia and invasive cancer.
3.3. Predictive value of the Sendai guideline for high-grade dysplasia or invasive cancer
Although applying the Sendai guideline, 131 (94.9%) patients in this study would have been recommended to undergo surgical resection (Fig. 1). Of these patients, 46 (35.1%) had HGD/IC. Seven patients who would have received a recommendation for observation, rather than resection, had pathologically confirmed an IPMN with low- or moderate-grade dysplasia after the operation. The sensitivity of the Sendai guideline for detecting HGD/invasive was 100%, and the specificity was 7.61%. The positive predictive value (PPV) and negative predictive value (NPV) for the Sendai guideline to detect HGD/invasive were 35.1% and 100%, respectively.
Figure 1.
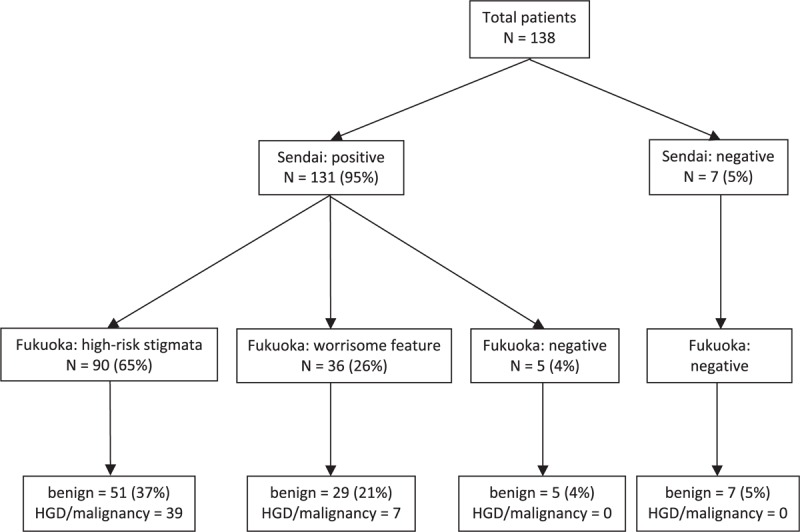
Applying the Sendai and Fukuoka Guidelines for evaluating the 138 patients. HGD = high grade dysplasia, N = number of patient.
3.4. Predictive value of the Fukuoka guideline for HGD or IC
Although applying the Fukuoka guideline, 90 (65.2%) patients in the study had high-risk stigmata and would have been recommended to undergo surgical resection (Fig. 1). Of these patients, 39 (43.3%) had HGD/IC. Thirty-six (26.1%) of the 138 patients had worrisome features and would have received a recommendation for close observation; however, 7 (19.4%) of these 36 patients had pathologically confirmed HGD/IC after the operation. Twelve (8.7%) patients in the “Fukuoka negative” group who would have received a recommendation for observation, rather than resection; all had a pathologically confirmed IPMN with low- or moderate-grade dysplasia after the operation. The sensitivity for high-risk stigmata in the Fukuoka guideline to detect HGD/invasive was 84.8%, and the specificity was 44.6%. The PPV and NPV for high-risk stigmata in the Fukuoka guideline to detect HGD/invasive was 43.3% and 85.4%, respectively.
4. Discussion
This study aimed to determine and compare the value of the 2006 Sendai and 2012 Fukuoka guidelines for the management of IPMN based on a retrospective review of 138 patients who underwent an operation. To our knowledge, studies comparing the Sendai and Fukuoka guidelines for the management of all IPMN types have not been published. Our results demonstrated a higher PPV but a lower NPV for the Fukuoka guideline than the Sendai guideline, and tumor size may still be an important factor for predicting HGD or malignancy. Pancreatic IPMN with worrisome features based on Fukuoka should receive more aggressive management because of a relative high risk of harboring HDG/IC in IPMN.
According to our findings, we recommend patients with clinical diagnosis of pancreatic IPMN with mural nodule or mass on image, jaundice, tumor ≥3 cm, or age younger than 65 years to undergo surgical resection by experienced surgeon in high volume center. Patients with those factors are associated with harboring malignancy, and surgery in high volume center is justified if taking into account of surgical risk and benefit, as well as residual life expectancy of the patients. Several studies validated the Sendai guideline for different cystic lesions of the pancreas (CLPs). For predicting malignancy, many studies reported a low PPV (11%–52%) but a high NPV (90%–100%) using the Sendai guideline for BD-IPMN, for mucinous CLPs, and even for all CLPs.[4,6,7,19,20] However, some studies reported a missed malignancy using the Sendai guidelines.[4,21,22] According to our study results, the PPV of the high-risk group under the Sendai and Fukuoka guidelines were 35.1% and 43.3%, respectively, indicating a slightly better predictive value for the “Fukuoka high-risk” group. However, the NPV of the high-risk group under the Sendai and Fukuoka guidelines were 100% and 85.42%, respectively. The Fukuoka guideline is more conservative than the Sendai guideline regarding the definition for high-risk patients, so many patients in the “Fukuoka worrisome” group would belong to the “Sendai high-risk” group. Without further evidence of high-risk features, patients in the “Fukuoka worrisome” group were recommended to obtain conservative treatment according to the Fukuoka guideline, but not surgical resection as suggested by the Sendai guideline. However, some patients (7/36, 19%) in the Fukuoka worrisome group in this study cohort had HDG/IC based on pathology. Although high-risk stigmata under the Fukuoka guidelines correlate with a malignant grade of pancreatic BD-IPMN, applying the high-risk stigmata of the Fukuoka guideline as a surgical indication may pose difficulties in identifying all HGD/IC for resection.[23] However, if we considered the worrisome group as a positive result, the NPV for the Fukuoka guideline was still 100%. In our study cohort, 7 and 12 patients would be classified as low risk based on the Sendai and Fukuoka guidelines, respectively. None of these 19 patients had HGD/IC, implying that patients in the low-risk group of both the Sendai and Fukuoka guidelines could safely be managed conservatively. In managing IPMN, applying the low-risk group of the Fukuoka guideline to select patients for conservative management seems better, or at least, not inferior to the Sendai guideline. In our study cohort, 5 more patients would have avoided unnecessary resection of their benign lesion by applying the Fukuoka guideline rather than the Sendai guideline.
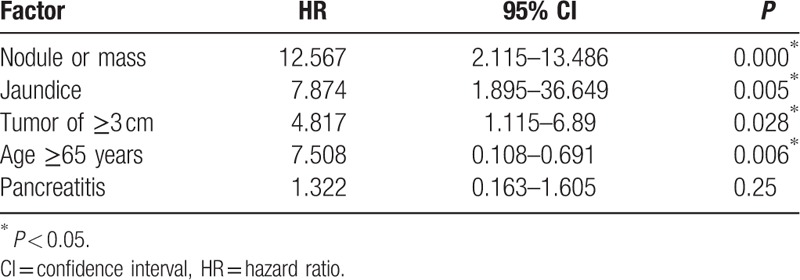
According to our results, several related factors could be associated with HGD/IC (Tables 2 and 3). There were significantly more patients aged ≥65 in the low/moderate group, implying that a younger age (<65 years old) may be a risk factor for harboring a malignancy in their IPMN. Young patients deserve more aggressive management as they may be more surgically fit, have a greater life expectancy, and harbor a higher risk of malignancy during long-term follow-up. Jaundice is one high-risk stigmata of the Fukuoka guideline; however, a nodule or mass lesion on imaging and a tumor size of ≥3 cm were 2 high-risk factors under the Sendai guideline, supporting the use of these predictors as an indication for surgery. Similar to our result, a recent study showed that the presence of a mural nodule, which had the highest hazard ratio for predicting factors in our result, was the most important predictor of malignancy for all IPMN types.[24] Pancreatitis is more prevalent in low/moderate dysplasia but not significant in the multivariate analysis, implying that pancreatitis may not be a factor related to HDG/IC. Many patients in this study had undergone surgery owing to symptoms of pancreatitis without HGD/IC in their IPMNs that also contributed to the result.
Table 3.
Multivariate analysis of factors associated with high-grade dysplasia and invasive cancer.
The current study agrees with the change in addressing “jaundice” as a predictive factor or surgical indication under the Fukuoka guideline rather than a nonspecific “symptom” under the Sendai guideline because jaundice is a factor associated with HDG/IC in this study, but pancreatitis is not. To manage asymptomatic IPMN, a 2015 guideline from the American Gastroenterological Association suggests that an EUS/fine-needle aspiration (FNA) exam for patients with ≥2 high-risk features, such as ≥3 cm, dilated MPD, or presence of solid components; these issues would lead to a recommendation for surgical resection for patients with both a solid component and dilated pancreatic duct, possibly accompanied by concerning features on EUS and FNA.[25] Although dilatation of the pancreatic duct is not a significant predictor of HGD/IC, it is worth close observation because it might be an early sign of jaundice in patients with asymptomatic IPMN. Although symptoms (except for jaundice) are not a good indication, surgery may still be justified in patients with repeat pancreatitis or if their symptoms are expected to be relieved by resection. Patients should be carefully evaluated to assess individual surgical risk and benefit.
Another important difference between the Fukuoka and Sendai guidelines is the 3-cm criteria. Although emphasizing the predictive value of the solid component and jaundice (or dilated pancreatic duct), the Fukuoka guideline (as well as the recent American Gastroenterological Association guideline) regards tumor size as a relatively weak predictor.[8,25] Tumors of ≥3 cm are an indication for resection under the Sendai guideline, but they have been de-emphasized in the Fukuoka guideline. According to the Fukuoka guideline, tumors of ≥3 cm indicate a worrisome feature rather than high-risk stigmata, and conservative treatment is still recommended unless these patients are proven to have high-risk features such as a definite mural nodule, suspicious main duct involvement, or suspicious cytology during EUS exams. Some studies reported that tumors of ≥3 cm in BD-IPMN is a risk factor of malignancy.[5,26,27] However, many studies reported that a tumor of ≤3 cm does not exclude malignancy, and EUS may have an advantage in identifying those patients with a malignancy.[9–13,21,22] Our data showed that a tumor of ≥3 cm is associated with HGD/IC, supporting the Sendai guideline that regards it as one high-risk feature and a surgical indication. Although tumors of ≥3 cm may be a risk factor for HGD/IC and highly suggestive of resection, tumors of <3 cm cannot be excluded from being HGD/IC and necessitate careful evaluation using an EUS exam.
Furthermore, EUS is a highly operator-dependent exam, and an inclusive result may be because of the poor sensitivity of the EUS findings and FNA cytology. It is difficult to expect that patients with inconclusive EUS results truly lack high-risk features. In our study cohort, nearly one-fifth of patients (7/36, 19%) in the Fukuoka worrisome feature group had HGD/IC. All of these patients were stratified to the Sendai positive group and would be recommended to undergo surgery, but they may receive conservative management if no high-risk features were detected in additional EUS exams according to the Fukuoka guideline. Using the Fukuoka guideline for the management of IPMN incurs a risk of missing HGD/IC, thus we suggest a more aggressive resection policy for those patients with Fukuoka worrisome features with inclusive EUS exams, especially those patients with tumors of ≥3 cm.
Our study had some limitations. This study was a single-center retrospective analysis. The study cohort comprised patients who underwent an operation and had pathologically confirmed IPMN; bias was inevitable and might have influenced the results. To evaluate only the operated patient could not know the exact sensitivity and specificity of both guidelines. Besides, the study cohort comprised patients who were observed over a long period; improvement in surgical expertise and advances in medication influenced management strategy, so the study cohort included heterogeneous patients who were managed with different surgical strategies.
In conclusion, both the Sendai and Fukuoka guidelines have utility for the management of IPMN. The Sendai guideline had a better NPV, but the Fukuoka guideline had a better PPV. Although managing IPMN, we suggest surgical resection for patients who have Fukuoka worrisome features with tumors of ≥3 cm, along with an inconclusive EUS exam. A more aggressive management policy towards patients with Fukuoka worrisome features may be important. Tumors of ≥3 cm are associated with malignancy in IPMN, but pancreatitis is not.
Footnotes
Abbreviations: BD-IPMN = branch-duct type intraductal papillary mucinous neoplasm, CLP = cystic lesion of the pancreas, EUS = endoscopic ultrasonography, FNA = fine-needle aspiration, HGD = high-grade dysplasia, IC = invasive cancer, IPMN = intraductal papillary mucinous neoplasm, MD-IPMN = main-duct type intraductal papillary mucinous neoplasm, MPD = main pancreatic duct, MT-IPMN = mixed type intraductal papillary mucinous neoplasm, NPV = negative predictive value, PPV = positive predictive value.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg 1999; 134:1131. [DOI] [PubMed] [Google Scholar]
- 2.Machado NO, Al Qadhi H, Al Wahibi K. Intraductal papillary mucinous neoplasm of pancreas. N Am J Med Sci 2015; 7:160–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006; 6:17–32.Review. [DOI] [PubMed] [Google Scholar]
- 4.Goh BK, Tan DM, Ho MM, et al. Utility of the sendai consensus guidelines for branch-duct intraductal papillary mucinous neoplasms: a systematic review. J Gastrointest Surg 2014; 18:1350–1357. [DOI] [PubMed] [Google Scholar]
- 5.Sahora K, Mino-Kenudson M, Brugge W, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg 2013; 258:466–475. [DOI] [PubMed] [Google Scholar]
- 6.Pelaez-Luna M, Chari ST, Smyrk TC, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol 2007; 102:1759–1764. [DOI] [PubMed] [Google Scholar]
- 7.Tang RS, Weinberg B, Dawson DW, et al. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol 2008; 6:815–819. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012; 12:183–197. [DOI] [PubMed] [Google Scholar]
- 9.Wong J, Weber J, Centeno BA, et al. High-grade dysplasia and adenocarcinoma are frequent in side-branch intraductal papillary mucinous neoplasm measuring less than 3 cm on endoscopic ultrasound. J Gastrointest Surg 2013; 17:78–84.discussion p.84-5. [DOI] [PubMed] [Google Scholar]
- 10.Fritz S, Klauss M, Bergmann F, et al. Small (Sendai negative) branch-duct IPMNs: not harmless. Ann Surg 2012; 256:313–320. [DOI] [PubMed] [Google Scholar]
- 11.Fritz S, Klauss M, Bergmann F, et al. Pancreatic main-duct involvement in branch-duct IPMNs: an underestimated risk. Ann Surg 2014; 260:848–855.discussion 855-6. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen AH, Toste PA, Farrell JJ, et al. Current recommendations for surveillance and surgery of intraductal papillary mucinous neoplasms may overlook some patients with cancer. J Gastrointest Surg 2015; 19:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KH, Lee SJ, Lee JK, et al. Prediction of malignancy with endoscopic ultrasonography in patients with branch duct-type intraductal papillary mucinous neoplasm. Pancreas 2014; 43:1306–1311. [DOI] [PubMed] [Google Scholar]
- 14.Waters JA, Schmidt CM, Pinchot JW, et al. CT vs MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg 2008; 12:101–109. [DOI] [PubMed] [Google Scholar]
- 15.Baiocchi GL, Portolani N, Missale G, et al. Intraductal papillary mucinous neoplasm of the pancreas (IPMN): clinico-pathological correlations and surgical indications. World J Surg Oncol 2010; 8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baiocchi GL, Portolani N, Grazioli L, et al. Management of pancreatic intraductal papillary mucinous neoplasm in an academic hospital (2005-2010): what follow-up for unoperated patients? Pancreas 2013; 42:696–700. [DOI] [PubMed] [Google Scholar]
- 17.Castellano-Megías VM, Andrés CI, López-Alonso G, et al. Pathological features and diagnosis of intraductal papillary mucinous neoplasm of the pancreas. World J Gastrointest Oncol 2014; 6:311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goh BK, Thng CH, Tan DM, et al. Evaluation of the Sendai and 2012 International Consensus Guidelines based on cross-sectional imaging findings performed for the initial triage of mucinous cystic lesions of the pancreas: a single institution experience with 114 surgically treated patients. Am J Surg 2014; 208:202–209. [DOI] [PubMed] [Google Scholar]
- 20.Goh BK, Tan DM, Thng CH, et al. Are the Sendai and Fukuoka consensus guidelines for cystic mucinous neoplasms of the pancreas useful in the initial triage of all suspected pancreatic cystic neoplasms? A single-institution experience with 317 surgically-treated patients. Ann Surg Oncol 2014; 21:1919–1926. [DOI] [PubMed] [Google Scholar]
- 21.Lee CJ, Scheiman J, Anderson MA, et al. Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg 2008; 12:234–242. [DOI] [PubMed] [Google Scholar]
- 22.Nagai K, Doi R, Ito T, et al. Single-institution validation of the international consensus guidelines for treatment of branch duct intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg 2009; 16:353–358. [DOI] [PubMed] [Google Scholar]
- 23.Aso T, Ohtsuka T, Matsunaga T, et al. High-risk stigmata” of the 2012 international consensus guidelines correlate with the malignant grade of branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2014; 43:1239–1243. [DOI] [PubMed] [Google Scholar]
- 24.Seo N, Byun JH, Kim JH, et al. Validation of the 2012 International Consensus Guidelines using computed tomography and magnetic resonance imaging: branch duct and main duct intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 2016; 263:557–564. [DOI] [PubMed] [Google Scholar]
- 25.Vege SS, Ziring B, Jain R, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015; 148:819–822. [DOI] [PubMed] [Google Scholar]
- 26.Kanno A, Satoh K, Hirota M, et al. Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. J Gastroenterol 2010; 45:952–959. [DOI] [PubMed] [Google Scholar]
- 27.Sugiyama M, Izumisato Y, Abe N, et al. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg 2003; 90:1244. [DOI] [PubMed] [Google Scholar]


