Supplemental Digital Content is available in the text
Keywords: anesthetics, child, delirium, desflurane, inhalation, methyl ethers, sevoflurane
Abstract
Background:
The differences in the incidence and severity of emergence agitation (EA) and emergence times between desflurane and sevoflurane anesthesia have not been as clearly elucidated in children as in adults.
Methods:
The design of the study is a systematic review with meta-analysis of randomized controlled trials. The study methodology is based on the Cochrane Review Methods. A comprehensive literature search was conducted to identify clinical trials comparing the incidence or severity of EA and emergence times in children anesthetized with desflurane or sevoflurane. Two reviewers independently assessed each study according to predefined inclusion criteria and extracted data from each study using a prespecified data extraction form. The data from each study were combined using a fixed effect or random effect model to calculate the pooled risk ratio (RR) or standardized mean difference (SMD) and 95% confidence interval (CI). Funnel plots were used to assess publication bias. Subgroup and sensitivity analyses were performed.
Results:
Fourteen studies met the inclusion criteria. Among the 1196 patients in these 14 studies, 588 received desflurane anesthesia and 608 received sevoflurane anesthesia. The incidence of EA was comparable between the 2 groups (pooled RR = 1.21; 95% CI: 0.96–1.53; I2 = 26%), and so was the severity of EA (EA score) between the 2 groups (SMD = 0.12; 95% CI: −0.02 to 0.27; I2 = 0%). Extubation and awakening times were shorter in the desflurane group than in the sevoflurane group; the weighted mean differences were −2.21 (95% CI: −3.62 to −0.81; I2 = 93%) and −2.74 (95% CI: −3.80 to −1.69; I2 = 85%), respectively. No publication bias was found in the funnel plot. The subgroup analysis based on the type of EA scale showed a higher incidence of EA in the desflurane group than in the sevoflurane group in studies using 3-, 4-, or 5-point EA scales; the pooled RR was 1.38 (95% CI: 1.10–1.73; I2 = 37%).
Conclusion:
The incidence and severity of EA were comparable between desflurane and sevoflurane anesthesia in children; however, emergence times, including extubation and awakening times, were shorter in desflurane anesthesia.
1. Introduction
The use of inhalational anesthetics in children can often cause emergence agitation (EA) during recovery from general anesthesia. EA has also been referred to as emergence delirium or emergence excitement, and it may be associated with physical injury as well as negative postoperative behaviors in children.[1] The risk factors for EA include rapid emergence from anesthesia and use of short-acting volatile anesthetic agents like desflurane or sevoflurane. These factors may lead to a dissociative state with altered cognitive perception, excitation, and agitation during recovery from anesthesia in children,[2,3] which results in a higher incidence of EA. However, the differences in both EA incidence and emergence time between desflurane and sevoflurane anesthesia in children have not been clearly elucidated, although differences in emergence (recovery) times between the 2 agents in adults are well known.[4,5]
Therefore, we aimed to investigate the incidence of EA as a primary outcome and the severity of EA (as quantified by the EA score) and emergence times (extubation and awakening time) as secondary outcomes in desflurane and sevoflurane anesthesia. This was accomplished by performing a systematic review (SR) of randomized controlled trials (RCTs) that had compared EA occurrence after desflurane and sevoflurane anesthesia in children. We hypothesized that the incidence and severity of EA, as well as the emergence times, might be higher in desflurane than in sevoflurane anesthesia in children.
2. Methods
The SR and meta-analysis were performed to compare EA after sevoflurane and desflurane anesthesia in children. The study protocol is based on the Cochrane review methods.[6]
2.1. Data source and literature source
We searched MEDLINE (January 1, 1976–July 24, 2014), EMBASE (January 1, 1985–July 24, 2014), and the Cochrane Controlled Clinical Trials Register Database (January 1, 1987–July 24, 2014) using the Medical Subject Headings and free text terms without language restriction.
The following keywords were searched through Medline: sevoflurane, desflurane, methyl ethers, anesthetics, and inhalation. Search strategies were designed for each database (Supplementary table 1). Further, to identify unpublished or ongoing studies, we searched the WHO International Clinical Trials Registry Platform and the ClinicalTrials.gov database (http://clinicaltrials.gov/). After the initial electronic search, we checked the bibliographies of identified studies.
2.2. Study selection
Potentially eligible studies were screened and selected by 2 independent reviewers (HK and BGL) using the predefined inclusion criteria. The 2 reviewers independently determined which of the identified studies were suitable for inclusion. Final selection was based on a screening of the full texts. Discrepancies between reviewers were resolved by discussion.
Studies were included in our meta-analysis if they met the following criteria: they included only healthy pediatric patients, used sevoflurane as the control anesthesia (sevoflurane group) and desflurane as the intervention anesthesia (desflurane group), reported the incidence or severity of EA in the sevoflurane and desflurane group, were RCTs, and were performed in elective minor surgeries including ambulatory urologic, orthopedic, otorhinolaryngological, ophthalmologic, and plastic surgeries.
2.3. Data extraction
Two reviewers independently extracted data from each study using a prespecified data extraction form. The following variables were extracted from studies: mean and standard deviation of EA score (the severity of EA) and extubation or awakening times, and dichotomous data on the incidence of EA in the intervention and control groups; and demographic, clinical, and treatment characteristics (e.g., number of patients in the intervention and control groups). Disagreements between reviewers were resolved in consultation with a third reviewer (HK).
2.4. Assessment of methodological quality
The methodological quality of included studies was evaluated blindly by 2 reviewers (HK and BGL) using the Cochrane Collaboration's risk of bias tool, which includes selection, performance, attrition, detection, and reporting bias through assessment of the following: mentioned random sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessments; incomplete outcome reporting; selective outcome reporting; and other biases. We evaluated the possible existence and direction of the bias and whether it was likely to have an impact on the effect to be evaluated. Publication bias was assessed using the tests for funnel plot asymmetry and the Egger linear regression test.
2.5. Statistical analysis
The primary outcome of our review was the incidence of EA. It was defined as the number of participants with postoperative behavioral disturbances including moaning, restlessness, involuntary physical activity, and thrashing about, as measured by the authors of the included studies. Thus, EA scores were assessed by the 3-, 4-, 5-point, or pediatric anesthesia emergence delirium (PAED) scale; EA was defined as a PAED scale score ≥10 or 16, or >12, or a score of 3 on a 3-point scale, a score ≥3 on a 4-point scale, or a score ≥4 on a 5-point scale. The secondary outcomes were the severity of EA, EA score measured by the scales of the included studies, and emergence times, including extubation time and awakening time. Extubation and awakening times were defined as the time from the discontinuation of anesthetics (time = 0 minute) to tracheal extubation and spontaneous eye opening or response to verbal stimuli, respectively. The incidence of EA, defined in the study as a dichotomous variable, was analyzed using risk ratio (RR) with 95% confidence interval (CI). The severity of EA (EA score) was analyzed using the standardized mean difference (SMD) with 95% CI; analysis of extubation or awakening times—understood here as continuous variables—was performed using weighted mean difference (WMD) with 95% CI.
We examined the heterogeneity between studies by scrutinizing the forest plots and quantifying the impact of heterogeneity using the I2 statistic. If heterogeneity between studies was found (I2 statistic >50% or any clinical heterogeneity), a random effects model was utilized.
Initially, subgroup analyses were preplanned to compare different types of EA scales: PAED versus others; age groups (e.g., only preschool age vs other ages); types of surgery (e.g., head and neck surgery vs surgical procedure below the umbilicus), premedication (e.g., none vs midazolam), induction agents (e.g., inhalational vs intravenous), perioperative analgesic amount (e.g., sufficient vs insufficient), and sedation degree at extubation (i.e., standard vs deep), because sufficient numbers of studies were identified for each subgroup analysis. After we conducted the planned subgroup analyses, we found that—except for types of EA scales—the subgroup analyses did not show a specific significance in the primary and secondary outcomes. Therefore, we only presented the results of subgroup analysis according to different types of EA scales in the incidence of EA. We conducted a sensitivity analysis according to the methodological quality (risk of bias), for example, high versus others and type of language for example, English versus others. We used RevMan version 5.2 (The Cochrane Collaboration, Oxford, UK) and STATA version 13.0 (Stata Corporation, College Station, TX, USA) for performing these analyses.
3. Results
3.1. Identification of studies
Searches of the databases yielded 5275 articles (Fig. 1). Of these, 5248 publications were excluded, as it was clear from the title and abstract that they did not fulfill the selection criteria. For the remaining 27 articles, we obtained the full manuscripts; these were scrutinized to identify 14 potentially relevant studies. Thirteen publications were excluded because of the following reasons: they included adult patients or did not report the incidence or severity of EA. Therefore, the total number of studies included in the review was 14 (Fig. 1).
Figure 1.
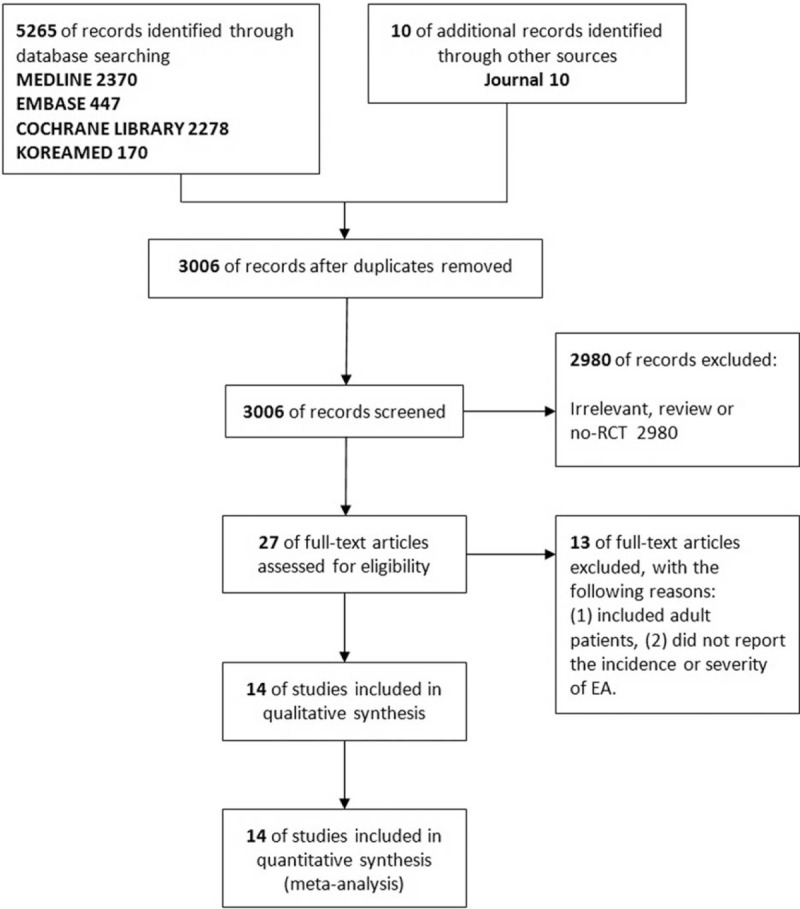
Meta-analysis flowchart. RCT = randomized controlled trial.
3.2. Study characteristics and patient populations
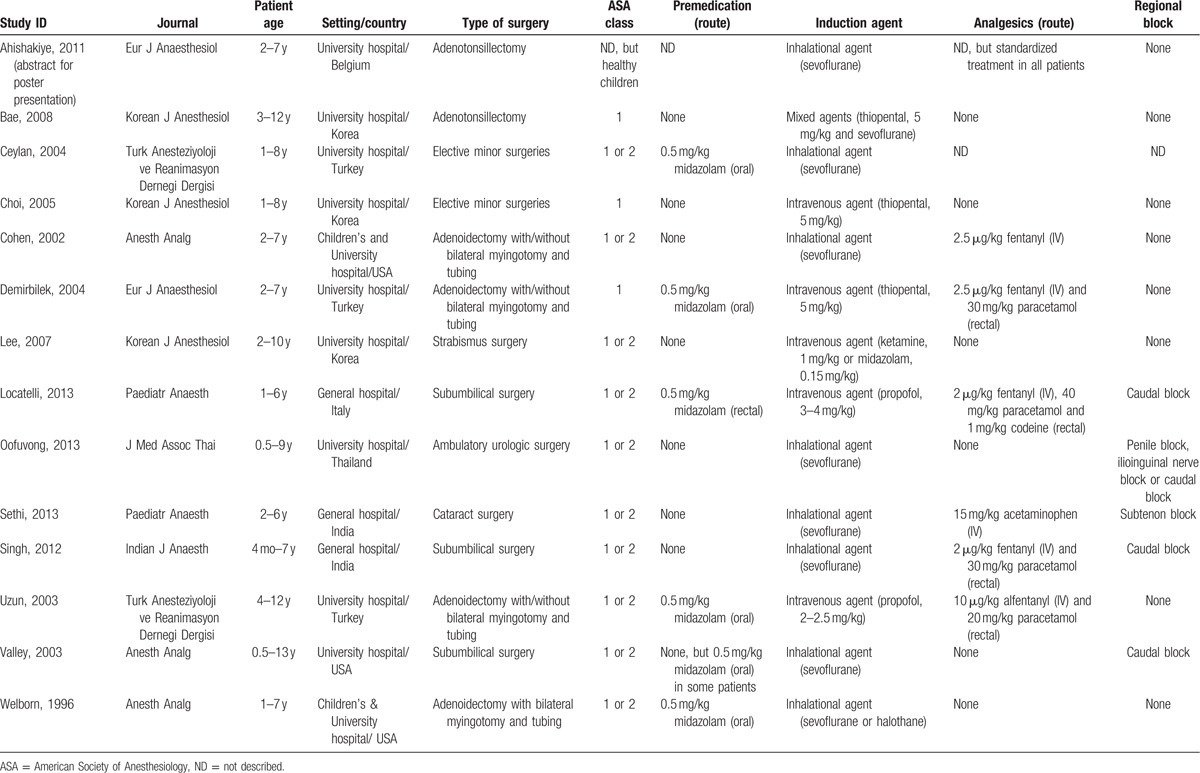
The details of the selected studies are summarized. As shown in Table 1, the included studies compared desflurane and sevoflurane anesthesia in elective minor surgeries including ambulatory urologic, orthopedic, ear–nose–throat, eye, and plastic surgeries in children. In 14 studies, a total of 1196 patients—588 patients anesthetized with desflurane and 608 patients anesthetized with sevoflurane—were evaluated for the incidence of EA. The included studies had a study design of RCTs, and they were conducted in Asian (43%) and European (36%) countries and in the United States (21%). All studies included preschool-aged children, and many studies (71%) limited inclusion to children 8 years of age or younger. Only 1 trial (7.1%) included children beyond the age of 12 years, and only 2 studies (14%) included infants younger than the age of 1 year. All studies excluded children with American Society of Anesthesiology status classification III or IV and those with a history of developmental delay or any neurological disease associated with agitation. Seven studies (50%) allowed no premedication, and 6 studies (43%) allowed oral or rectal midazolam. Eight studies (57%) were performed with inhalational anesthetics (sevoflurane) induction, and 5 studies (36%) were performed with intravenous anesthetics induction. All studies enrolled children undergoing elective minor surgeries including urologic, orthopedic, otorhinolaryngological, ophthalmologic, and plastic surgeries. Ten studies (71%) performed adequate and sufficient pre-/intraoperative analgesia using administration of analgesics including intravenous opioid or rectal paracetamol or similar analgesics, or regional analgesia including caudal analgesia or peripheral nerve block with local anesthetics; 4 studies involved children receiving potentially inadequate or no intraoperative analgesia. Postoperative behavioral disturbance during emergence from anesthesia was most commonly termed EA or agitation, although the term “emergence delirium” was also used. Several definitions of EA were based on different scales. In addition, different studies used various criteria or cutoffs to determine the presence or absence of EA. Nine studies (64%) used a 3-, 4-, or 5-point categorical scale, and 4 studies used the PAED scale. Most studies (86%) reported the incidence of EA, and this variable was the primary outcome for this review. Of the secondary outcomes, the severities of EA and extubation time were reported in 6 studies (43%), and awakening time was reported in 11 studies (79%).
Table 1.
Characteristics of included studies.
3.3. Quality of the included studies (risk of bias in included studies)
See Fig. 2.
Figure 2.
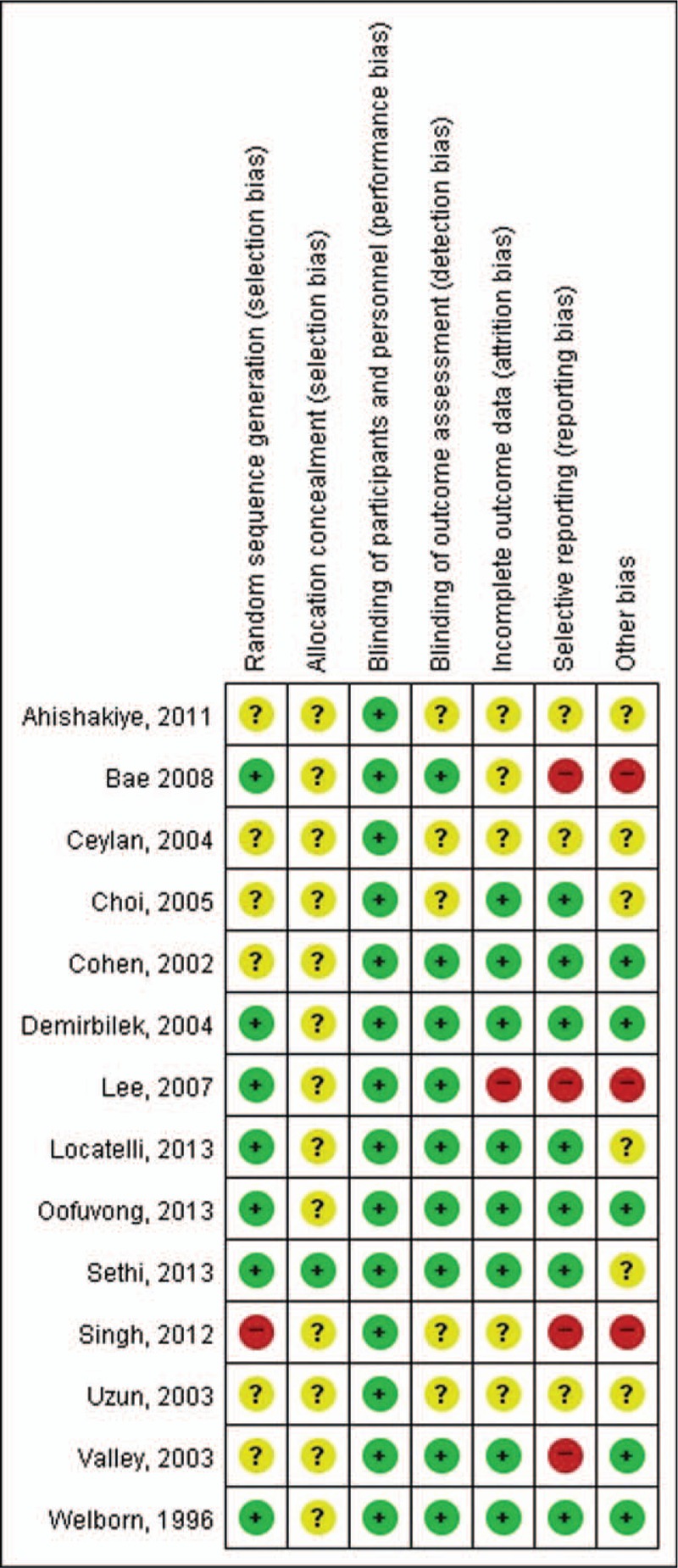
Risk of bias summary: review of authors’ judgments about each risk of bias item for each included study. Green circle: low risk of bias, yellow circle: unclear risk of bias, and red circle: high risk of bias.
3.4. Allocation
All of the 14 included studies reported that the study was randomized, but only 7 studies (50%) reported the method of random sequence generation applied. Allocation concealment was adequately reported in only 1 study (Sethi, 2013).
3.5. Blinding
Given that most participants were preschoolers, they were effectively blinded to the intervention of the studies. Nine studies (64%) that reported blinding of the outcome assessors were assessed as having low risk of bias, and other studies were considered to have unclear risk of bias.
3.6. Incomplete outcome data
Eight studies (57%) that reported the completeness of outcome data for each main outcome were assessed as having low risk of bias, and only 1 study (Lee, 2007)—which did not describe the attrition and exclusions from the analysis—was assessed as having high risk of bias.
3.7. Selective reporting
Seven studies (50%) were assessed as having low risk of bias; however, 4 studies (Bae, 2008; Lee, 2007; Singh, 2012; Valley, 2003) in which 1 or more reported primary outcomes were not prespecified or in which not all of the study's prespecified primary outcomes have been reported were assessed as having high risk of bias.
3.8. Other potential sources of bias
Three studies (Bae, 2008; Lee, 2007; Singh, 2012) that had a potential source of bias related to the specific study design used were assessed as having high risk of bias.
3.9. Result 1: the primary outcome
The incidence of EA was comparable between desflurane and sevoflurane group, the pooled RR of which was 1.21 (95% CI: 0.96–1.53; I2 = 26%) (Fig. 3A). This result showed low heterogeneity.
Figure 3.
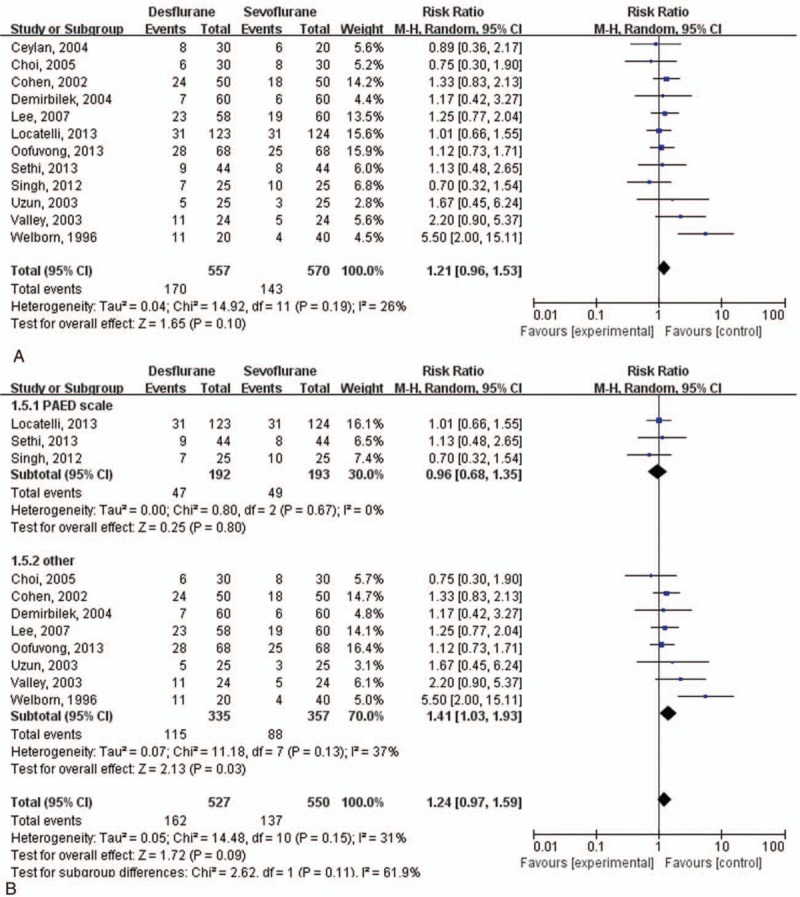
Incidence of emergence agitation (EA). (A) Incidence of EA in all study groups. (B) Incidence of EA in each subgroup as a result of use of different types of EA scales.
3.10. Result 2: the secondary outcomes
The severity of EA (EA score) was comparable between the 2 groups, the SMD of which was 0.12 (95% CI: −0.02 to 0.27; I2 = 0%) (Fig. 4). This result showed no heterogeneity. Extubation time and awakening time were shorter in the desflurane group than in sevoflurane group, the WMDs of which were −2.21 (95% CI: −3.62 to −0.81; I2 = 93%) and −2.74 (95% CI: −3.80 to −1.69; I2 = 85%), respectively (Fig. 5). These results showed high heterogeneity.
Figure 4.
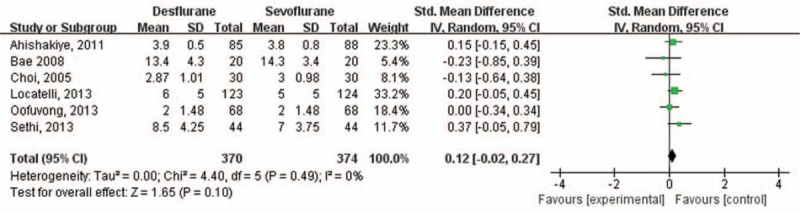
Severity of emergence agitation (EA) as determined by EA score.
Figure 5.
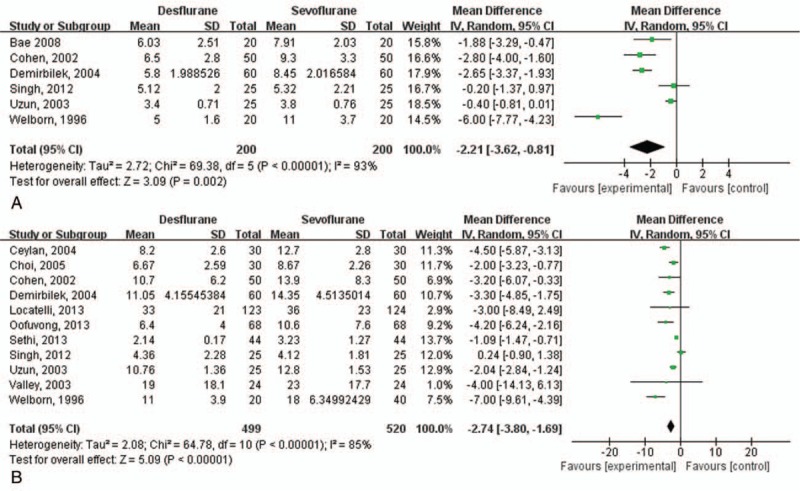
Extubation time and awakening time. (A) Extubation time, (B) awakening time.
3.11. Subgroup analysis
The subgroup analysis with different types of EA scale—PAED scale versus others—showed a different result in the incidence of EA (Fig. 3B). In the studies using 3-, 4-, or 5-point scales, the incidence of EA was higher in the desflurane group than in the sevoflurane group, the pooled RR of which was 1.38 (95% CI: 1.10–1.73; I2 = 37%). However, in the analyses using the PAED scale, EA incidence did not differ between desflurane and sevoflurane groups, the pooled RR of which was 0.96 (95% CI: 0.68–1.36; I2 = 0%) (Fig. 3B). Moreover, the heterogeneity index values (I2) of both subgroup analyses were significantly different (the subgroup analysis for studies using 3-, 4-, or 5-point scales showed higher heterogeneity than that for studies using the PAED scale) (P = 0.09, I2 = 61.9%).
3.12. Sensitivity analysis and publication bias
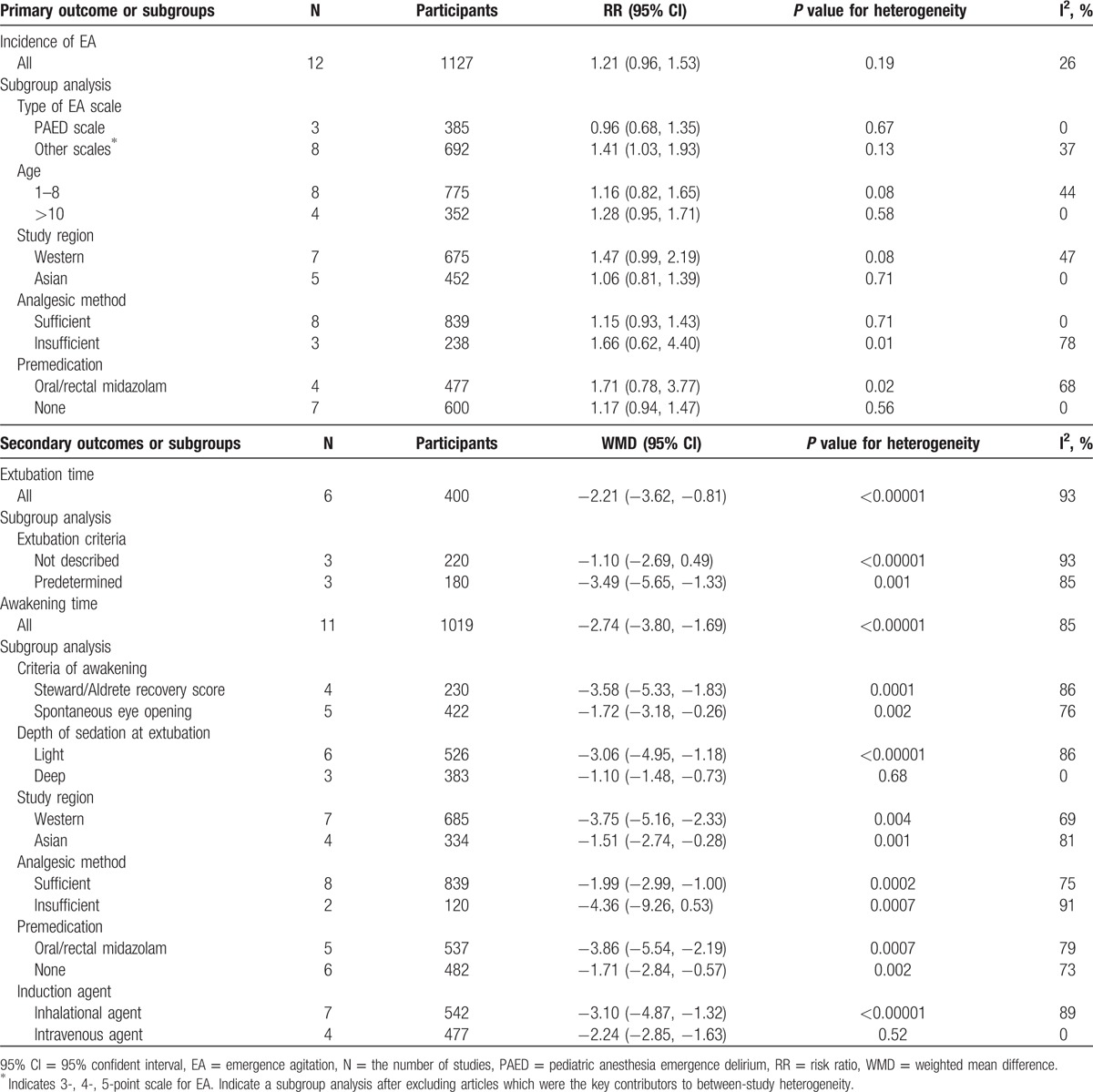
We found that there were no significant differences in heterogeneity according to the methodological quality (risk of bias; high vs others), study region (e.g., Western vs Asian), type of language (e.g., English vs others), or age group (e.g., preschool age vs others) in the primary outcome of incidence of EA (Table 2).
Table 2.
Summary of risk ratio or weighted mean difference for primary and secondary outcomes among subgroup.
Publication bias was not discovered on the results of the tests for funnel plot asymmetry (Fig. 6) or the Egger linear regression test (P = 0.36).
Figure 6.
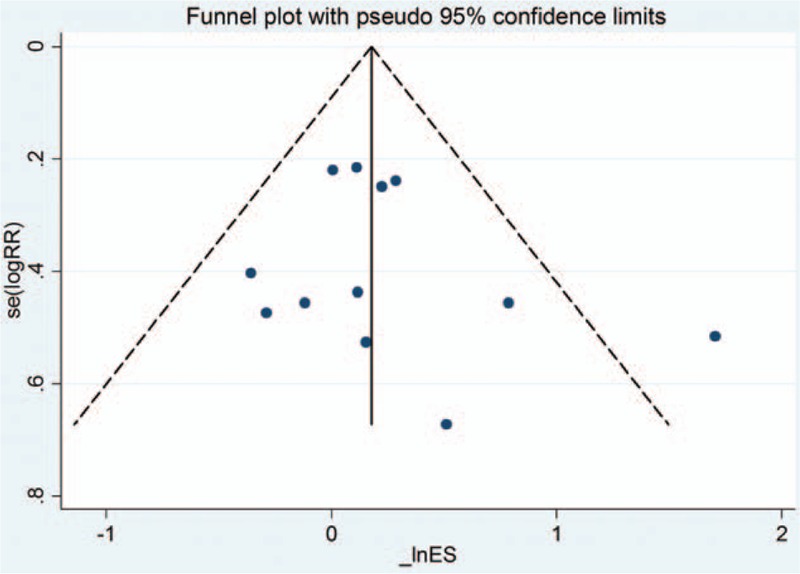
Funnel plot obtained using data from 14 trials comparing emergence agitation after sevoflurane and desflurane anesthesia in children. There was no publication bias.
4. Discussion
The SR of RCTs examined the incidence of EA after sevoflurane and desflurane anesthesia. Our main findings were that the incidence of EA after sevoflurane and desflurane is comparable and that the emergence time is shorter with desflurane. In terms of the overall completeness of this SR, the relatively large sample size of 14 RCTs with 1127 patients was able to show a significant difference between the 2 inhaled anesthetics. Furthermore, because of the comprehensive searching strategy, it is very unlikely that important studies were omitted. We found 14 RCTs related to EA due to sevoflurane and desflurane in pediatric patients, a robust finding that likely was a result of our complete comprehensive search strategies relative to other SRs.[7] We did not find any explicit publication bias (Fig. 6).
In the quality of evidence assessment, all 14 studies reported that the study was performed in a randomized trial design. However, only 7 studies reported the applied method of random sequence generation. Furthermore, it is unclear whether most of the studies were performed with adequate allocation concealment, which was found in only 1 study (Sethi, 2013). Ambiguous reporting could induce methodological weakness. It is possible that there are considerable confounding factors affecting the result of RCTs; such factors could include using different kinds of scales for EA, different cutoff values for EA within the same scale, and methods for reducing pain. It is well known that numerous factors are implicated in the development of EA, including age, type of surgery, premedication, and pain.[8–10] Conducting subgroup analysis, we confirmed in this SR that there are more potential EA-contributing factors with desflurane than sevoflurane anesthesia. The PAED scale is composed of 5 items: eye contact with caregiver, purposeful action, awareness of surrounding, restlessness, and inconsolability. Each item has 5 grades (0–4), and total PAED scores were calculated from the sum of the grades of each item. In contrast, scales using point systems (3-, 4-, and 5-point scales) simply assess the EA of patients as part of a rating scale from “not at all” to “extremely”. The precise scaling and threshold with the PAED scale provides relatively high sensitivity and specificity for predicting EA.[11,12] The discrepancy between the incidence of EA with desflurane using a scale such as PAED versus a 3-, 4-, and 5-point scale may result from the validity and reliability of the scale. An SR of the effects of desflurane and sevoflurane in pediatric anesthesia was recently published.[7] The authors analyzed many effects of desflurane and sevoflurane in pediatric patients, such as recovery time from anesthesia, extubation time, nausea, vomiting, pain, and agitation. They included 13 studies using sevoflurane and desflurane anesthesia in pediatric patients, and 5 studies were used for analyzing the incidence of agitation. The SR suggested that desflurane used in pediatric anesthesia had more favorable results than sevoflurane in terms of times to recovery, but agitation was found to be slightly higher in desflurane than in sevoflurane anesthesia. There are several possible reasons for the different results in our SR with regard to incidence of agitation: unlike our SR, which is focused on agitation, the study by He et al analyzed the overall effects of sevoflurane and desflurane in pediatric patients. Although there were 14 studies in our SR, 5 separate studies reported on agitation in the recent SR by He et al. The different number of included studies and participants may have influenced the results. In our SR, there were 3 studies using the PAED scale. In contrast, there were 4 studies using a 3-point scale in the SR by He et al and 1 study did not reveal the results concerning agitation. Consistent with this, our SR also showed that the EA incidence was similar to that in the SR by He et al (i.e., desflurane showed a higher incidence of agitation) when a 3- or 4-point scale was applied.
It is believed that rapid recovery from inhalation anesthetics with a relatively low blood/gas partition coefficient causes a higher incidence of EA.[13] However, more rapid emergence and extubation time after desflurane had not been associated with an increased incidence of EA. Despite more rapid emergence from anesthetics and more rapid extubation time with desflurane, these results should be interpreted with caution, because some studies did not clearly define the emergence or the extubation criteria that were used to assess the patient. There are some limitations in our review: this SR included studies with degrees of variability in scales and threshold and each study used different protocols, such as premedication, type of surgery, age range, and method of pain management.
Our review suggests that desflurane has a comparable incidence and severity of EA with sevoflurane. These findings are consistent with a review of 6 studies that focused on the effect of sevoflurane versus other general anesthetics on EA (Cohen, 2002; Demirbilek, 2004; Singh, 2012; Uzun, 2003; Valley, 2003; Welborn, 1996).[14] We have found additional 8 RCTs along with the 6 studies previously included in a review.
In conclusion, this SR revealed that there is comparable incidence of EA in children administered sevoflurane and in those receiving desflurane anesthesia, despite desflurane having a more rapid awakening time than sevoflurane. In the subgroup analysis, the incidence of EA was higher in the desflurane group than in sevoflurane group with studies using 3-, 4-, or 5-point scales for analysis.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, EA = emergence agitation, PAED = pediatric anesthesia emergence delirium, RCT = randomized controlled trial, SMD = standardized mean difference, SR = systematic review, WMD = weighted mean difference.
Institutional Review Board approval is not applicable to this systematic review and meta-analysis.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Kain ZN, Caldwell-Andrews AA, Maranets I, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg 2004; 99:1648–1654. [DOI] [PubMed] [Google Scholar]
- 2.Veyckemans F. Excitation phenomena during sevoflurane anaesthesia in children. Curr Opin Anaesthesiol 2001; 14:339–343. [DOI] [PubMed] [Google Scholar]
- 3.Jöhr M. Postanaesthesia excitation. Paediatr Anaesth 2002; 12:293–295. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Stierer T, Zuckerman R, et al. Comparison of recovery profile after ambulatory anesthesia with propofol, isoflurane, sevoflurane and desflurane: a systematic review. Anesth Analg 2004; 98:632–641. [DOI] [PubMed] [Google Scholar]
- 5.Macario A, Dexter F, Lubarsky D. Meta-analysis of trials comparing postoperative recovery after anesthesia with sevoflurane or desflurane. Am J Health Syst Pharm 2005; 62:63–68. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Oxford: The Cochrane Collaboration; 2011. [Google Scholar]
- 7.He J, Zhang Y, Xue R, et al. Effect of desflurane versus sevoflurane in pediatric anesthesia: a meta-analysis. J Pharm Pharm Sci 2015; 18:199–206. [DOI] [PubMed] [Google Scholar]
- 8.Aono J, Ueda W, Mamiya K, et al. Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys. Anesthesiology 1997; 87:1298–1300. [DOI] [PubMed] [Google Scholar]
- 9.Aono J, Mamiya K, Manabe M. Preoperative anxiety is associated with a high incidence of problematic behavior on emergence after halothane anesthesia in boys. Acta Anaesthesiol Scand 1999; 43:542–544. [DOI] [PubMed] [Google Scholar]
- 10.Vlajkovic GP, Sindjelic RP. Emergence delirium in children: many questions, few answers. Anesth Analg 2007; 104:84–91. [DOI] [PubMed] [Google Scholar]
- 11.Locatelli BG, Ingelmo PM, Emre S, et al. Emergence delirium in children: a comparison of sevoflurane and desflurane anesthesia using the Paediatric Anesthesia Emergence Delirium scale. Paediatr Anaesth 2013; 23:301–308. [DOI] [PubMed] [Google Scholar]
- 12.Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology 2004; 100:1138–1145. [DOI] [PubMed] [Google Scholar]
- 13.Lapin SL, Auden SM, Goldsmith LJ, et al. Effects of sevoflurane anaesthesia on recovery in children: a comparison with halothane. Paediatr Anaesth 1999; 9:299–304. [DOI] [PubMed] [Google Scholar]
- 14.Costi D, Cyna AM, Ahmed S, et al. Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst Rev 2014; 9:Cd007084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


