Supplemental Digital Content is available in the text
Keywords: cardio-cerebral vascular disease, CYP2C19, genotype, nephropathy
Abstract
In recent years, the genetic factor has become one of the important predisposing factors of nephropathy susceptibility. There is a high incidence of nephropathy in CCVd. The CYP2C19 enzyme metabolizes most the drugs, including proton pump inhibitors commonly used medicines to treat CCVd, CYP2C19 genetic polymorphisms is association with multi-pathogenesis factors of nephropathy. The purpose of the study is to reveal the association between CYP2C19 genotype and the susceptibility of nephropathy in the CCVd patients. The study is composed of 623 samples from CCVd treated by anticoagulation. The patients were studied, including CCVd with hyperuricemia, coronary heart disease, diabetes, and other complication. Biochemical tests and CYP2C19 variants measurements were performed by the gene chip method. The association among CYP2C19 variants, complications, and nephropathy was analyzed in the CCVd. There is no correlation between nephropathy and complications in CCVd. In hyperuricemia, coronary heart disease and diabetes groups, the differences of renal function tests were significant between CYP2C19 mutant (P < 0.05). The nephropathy risk of wild genotype is 3.288 times higher than of mutation genotype in hyperuricemic group, 1.928 times higher than mutation genotype in coronary heart disease group, and 5.248 times higher than CYP2C19 mutation genotype in the diabetic group. There was significant correlation between the CYP2C19 wild type and the nephropathy susceptibility in CCVd patients. The CYP2C19 gene plays a potential maker to evaluate nephropathy in CCVd patients. We deduced that identification of CYP2C19 gene type may benefit for reducing and avoiding nephropathy caused by abnormal metabolism function in CCVd patients.
1. Introduction
With high incidence, lethal rate, and more complications, cardiovascular and cerebrovascular diseases are a serious threat to mankind's health and life. In all complications, nephropathy is concerned as result of its own high incidence rate and population, 10% of the population worldwide is affected by chronic kidney disease (CKD), half of people aged 75 or more have some degree of CKD, 1 in 5 men and 1 in 4 women between the ages of 65 and 74 have CKD. Nowadays, 26 million American adults have CKD and millions of others are at increased risk. Recent studies have shown that some complications in CCVd, including hyperuricemia, coronary heart disease, and diabetes are top 3 risk factors for nephropathy.[1] Nephrosis, noninflammatory nephropathy, often has no symptoms, and it can go undetected until very advanced. Therefore, the determination of susceptible population of CKD is necessary to effectively prevent and treat this disease.[2]
The occurrence of nephropathy is related to genetic factors, eating habits, environment, and so on.[3] Fundamentally, genetic factors including receptors and key enzymes have been a hot research topic in recent years. The cytochrome P450 gene super-family is involved in the synthesis of steroids and the metabolism of cholesterol. CYP2C19 plays an important role in drug metabolizing enzyme in CCVd treated by anticoagulation, which involves in proton pump inhibitor, platelet aggregation inhibitor, antidepressants, and so on.[4] However, the most basic function is to participate in the biosynthesis and metabolism of glucocorticoids, sugar, fat, and protein.[5]
The CYP2C19 gene consists of 9 exons spanning ∼90 kb and encodes a protein of 490 amino acids (NM_000769.1). Approximately 30 variant alleles of CYP2C19 have been reported so far. Many researchers have investigated the molecular mechanism of CYP2C19 enzyme polymorphisms, which include the wild-type CYP2C19∗1, ∗2, ∗3, ∗4, ∗5, ∗6, ∗7 and ∗8. CYP2C19∗2 and ∗3 are the main variants among all of CYP2C19.[6] So we carried out this study to illustrate the association between the CYP2C19 genotype and nephropathy in CCVd patients.
2. Methods
2.1. Study design and sample collection
This study was conducted between 2012 and 2016, in the population covered by the Chengdu Military General Hospital, constituted by 623 samples of patients with cardio-cerebral vascular disease (CCVd). All of the patients rule out infectious diseases, pregnancy, and congenital nephrotic syndrome. This study consisted on a basic information collection and physical examination. A blood sample for further biochemical analysis was also collected. Participants were selected using a simple random sampling scheme. Diagnosis of CCVd, diabetes, and nephropathy is according to the WHO diagnostic criteria, excluding renal injury due to improper drug treatment. The research was approved by the ethics committee.
2.2. Measurements and blood sample collection
The CYP2C19 genotype and biochemical measurements, including total cholesterol (TC), triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), cystatin C, blood urea nitrogen (BUN), creatinine serum (CREA), creatinine clearance rate (CCR), uric acid (UA), retinol binding protein (RBP), fasting blood-glucose (FBG), were performed in accordance with the recommendations proposed by national center for clinical laboratories.[7] Genomic DNA was isolated from whole blood using a commercially available DNA isolation kit (TaKaRa, China) according to the manufacturer's instruction. CYP2C19 variants measurements were performed based on the gene chip method.[8]
2.3. Statistical analysis
The mean values of total TC, TG, HDL-C, LDL-C, cystatin C, BUN, CREA, CCR, UA, RBP, FBG were collected in 3 months (once every month). Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables were reported as counts and percentages. Analyses of t tests and chi-square tests were used to test for differences between groups for continuous and categorical variables, respectively. Multivariate logistic regression analysis was used to identify independent predictors of nephropathy. Analyses were performed using SPSS version 19.0 statistical software. A value of P < 0.05 (2-sided) was considered statistically significant.[9] The P value is accurate to 3 decimal places when we calculated using SPSS.[10]
3. Presentation of Results
Overall, 623 patients with CCVd were enrolled in this study, including 497 cases of male, 126 cases of female. In all the cases, there were 141 CCVd patients with hyperuricemia, 358 patients with coronary heart disease, and 124 patients with diabetes (Supplemental Table 1).
3.1. Significant difference of renal function between CYP2C19 wild-type and mutation genotype in CCVd patients with hyperuricemia
As compared with CCVd patients with normal uric acid, hyperuricemic carriers with CYP2C19 wild type tended to have higher risk to get nephropathy (OR: 2.365, 95%CI: 1.357–4.122, P = 0.002). Therefore, we compared the CYP2C19 wild type with mutation genotype in CCVd patients with hyperuricemia. The proportions of coronary heart disease, sex and age were no significant difference between in the CYP2C19 wild-type group and the mutation group. As compared with mutation genotype group, the levels of cystatin C, BUN and CREA were significantly higher in the wild-type group. And the levels of CCR were significantly lower in the wild-type group than in the mutation group. In the levels of TC, TG, HDL-C, LDL-C, UA, RBP, and FBG, there were also no significant associations between 2 groups in the levels of TC, TG, HDL-C, LDL-C, UA, RBP, and FBG (Supplemental Table 2).
To identify CYP2C19 wild type associated with nephropathy in CCVd patients with hyperuricemia, further analysis of renal function test result showed in Fig. 1. CCVd patients with hyperuricemia were divided into 5 age groups. The levels of cystatin C, BUN, CCR, and CREA were no significant difference under age 60 between the wild-type group and the mutation group. However, there was significant difference in the 4 renal function test result between the 2 groups. Obviously, the renal function situation in patients with CYP2C19 wild type was much worse than with CYP2C19 mutation. Especially shown in cystatin C, the renal function gets worse and worse as the age grows. Despite the strong correlation between serum creatinine and cystatin C, cystatin C is less affected by weight and muscle mass and might represent a better alternative for the assessment of renal function.
Figure 1.
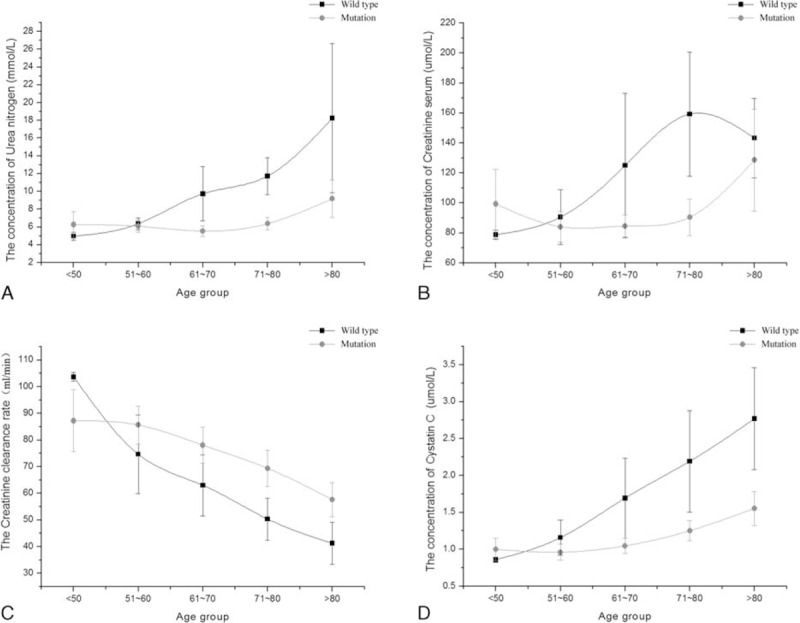
The relative index of renal function between CYP2C19 wild type and mutation in CCVd patients with hyperuricemia. Results represented means ± standard deviation: (A) the concentration trend chart of blood urine nitrogen in different age groups; (B) the concentration trend chart of serum creatinine in different age groups; (C) the trend chart of creatinine clearance rate in different age groups; (D) the concentration trend chart of serum cystatin C in different age groups. CCVd = cardio-cerebral vascular disease.
3.2. CYP2C19 wild type is high risk factor for nephropathy in CCVd patients with hyperuricemia
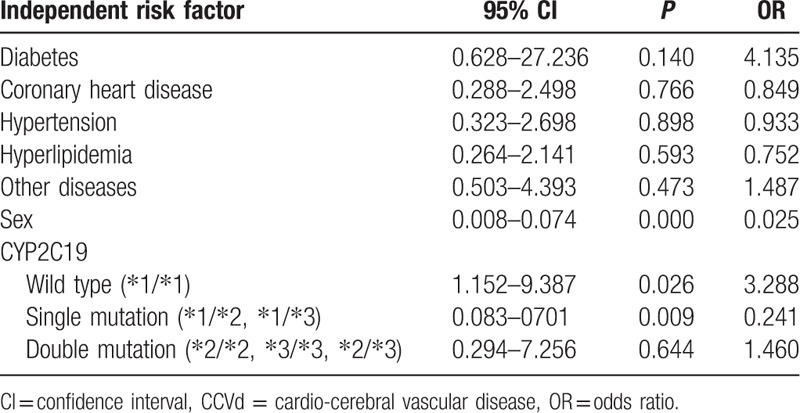
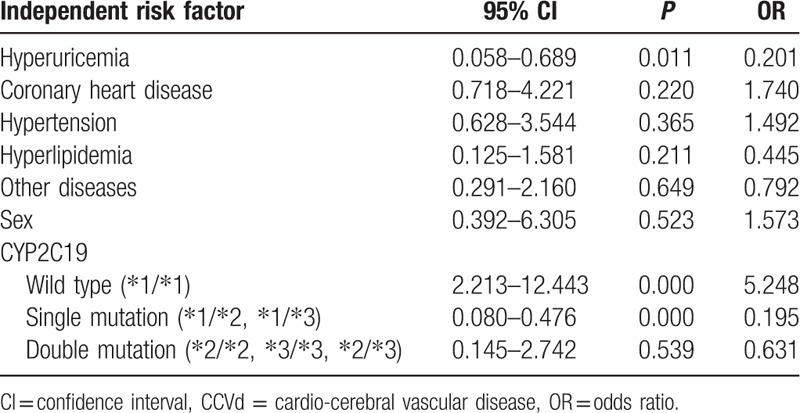
As shown in Table 1, CYP2C19 wild type (∗1/∗1) was an independent risk factor for nephropathy in CCVd patients with hyperuricemia (odds ratio [OR] 3.288, 95% confidence interval [CI] 1.152–9.387; P = 0.026) by multivariate logistic regression analysis. No significant associations between any of the other factors (diabetes, coronary heart disease, sex, single mutation, double mutation genotype) and nephropathy were observed in CCVd patients with hyperuricemia.
Table 1.
Multivariate logistic regression analysis of independent risk factor for nephropathy in CCVd patients with hyperuricemia.
3.3. Significant difference of renal function between CYP2C19 wild type and mutation genotype in CCVd patients with coronary heart disease
The statistical analysis was performed by t-tests and chi-square tests. A 2-sided P value was used to test for significance (threshold, P < 0.05). The levels of cystatin C, BUN, CREA, and UA were higher in the wild-type group than in the mutation group (P < 0.05). And the levels of CCR were significantly lower in the wild-type group than in the mutation group (P < 0.01). The difference of Uric acid level can be observed, and we deduced that the Uric acid level of CYP2C19 wild type generally higher than of mutation genotype in CCVd patients with coronary heart disease. No significant differences in sex and age factors between the CYP2C19 wild-type group and the mutation group were observed, nor did the biochemical test results of TC, TG, HDL-C, LDL-C, RBP, and FBG (Supplemental Table 3).
To further illustrate renal function difference in different age groups, the trend chart was plotted between the CYP2C19 wild-type group and the mutation group in Fig. 2. We analyzed individuals’ renal function difference by every 10 years. The levels of cystatin C, BUN, CCR, and CREA were no significant difference under age 60 between the wild-type group and the mutation group. There were some differences in the 4 renal function test results over aged 60 between the 2 groups. Obviously, the renal function situation in patients with CYP2C19 wild type was worse than with CYP2C19 mutation, especially in cystatin C.
Figure 2.
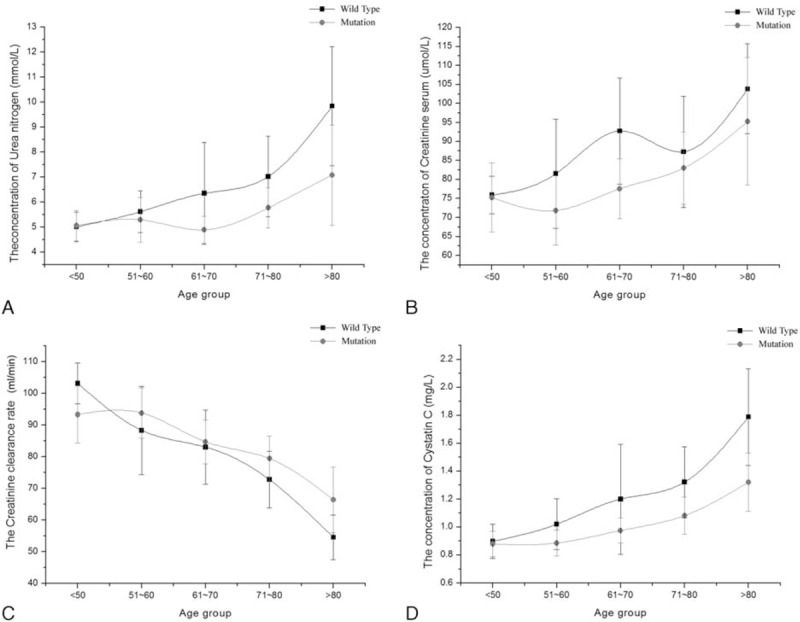
The relative index of renal function between CYP2C19 wild type and mutation in CCVd patients with coronary heart disease. Results represented means ± standard deviation: (A) the concentration trend chart of blood urine nitrogen in different age groups; (B) the concentration trend chart of serum creatinine in different age groups; (C) the trend chart of creatinine clearance rate in different age groups; (D) the concentration trend chart of serum cystatin C in different age groups. CCVd = cardio-cerebral vascular disease.
3.4. CYP2C19 wild type is high risk factor for nephropathy in CCVd patients with coronary heart disease
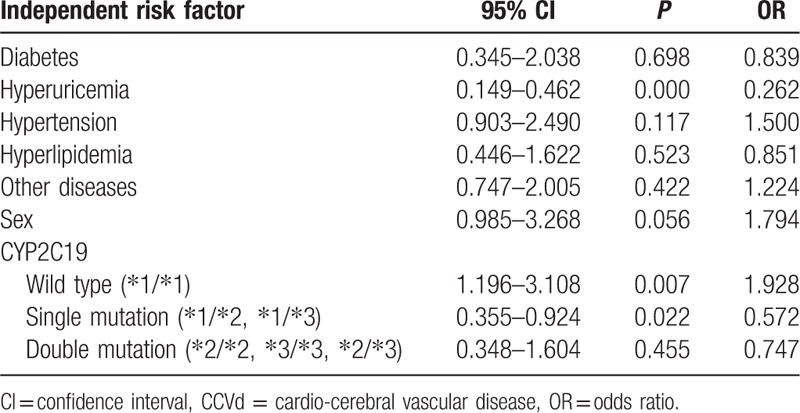
We calculated ORs for risk of nephropathy by multivariate logistic regression analysis, progressively adjusted for diabetes, hyperuricemia, male, CYP2C19 wild type, single mutation, and double mutation (Table 2). Analysis showed that the CYP2C19 wild type was an independent risk factor for nephropathy (OR 1.928, 95% CI: 1.196–3.108; P = 0.007).
Table 2.
Multivariate logistic regression analysis of independent risk factor for nephropathy in coronary heart disease.
3.5. Significant difference of renal function between CYP2C19 wild type and mutation genotype in CCVd patients with diabetes
Baseline clinical test results of the CYP2C19 wild-type group and the CYP2C19 mutation group are analyzed. The proportions of coronary heart disease, sex, and age were no significant differences between in the wild-type group and the CYP2C19 mutation group. The levels of cystatin C, BUN, and CREA were significantly higher in the wild-type group than in the mutation group. And the levels of CCR were significantly lower in the wild-type group than in the mutation group. There were also no significant differences in the levels of TC, TG, HDL-C, LDL-C, UA, RBP, and FBG (Supplemental Table 4).
In Fig. 3, the trend chart was plotted between the CYP2C19 wild-type group and the mutation group in 5 age groups. We analyzed individuals’ renal function difference by every 10 years. The levels of cystatin C, BUN, CCR, and CREA were no significant difference under age 70 between the wild-type group and the mutation group. There were some differences in the 4 renal function test results over aged 70 between the 2 groups. Obviously, the renal function situation in patients with CYP2C19 wild type was worse than with CYP2C19 mutation.
Figure 3.
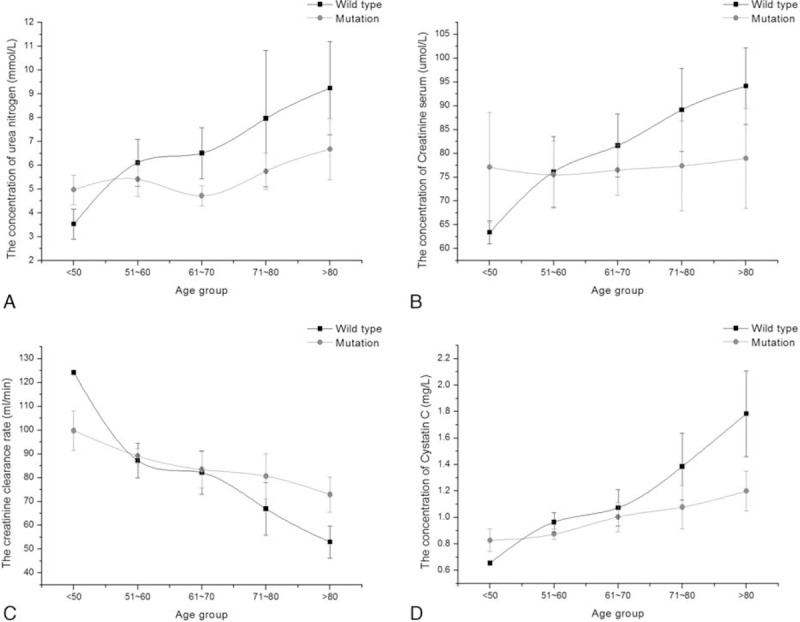
The relative index of renal function between CYP2C19 wild type and mutation in CCVd patients with diabetes. Results represented means ± standard deviation: (A) the concentration trend chart of blood urine nitrogen in different age groups; (B) the concentration trend chart of serum creatinine in different age groups; (C) the trend chart of creatinine clearance rate in different age groups; (D) the concentration trend chart of serum cystatin C in different age groups. CCVd = cardio-cerebral vascular disease.
3.6. CYP2C19 wild type is high risk factor for nephropathy in CCVd patients with diabetes
We calculated ORs for risk of nephropathy by multivariate logistic regression analysis, progressively adjusted for coronary heart disease, hyperuricemia, male, CYP2C19 wild type, single mutation, and double mutation (Table 3). Analysis showed that the CYP2C19 wild type was an independent risk factor for nephropathy (OR 5.248, 95% CI: 2.213–12.443; P = 0.000).
Table 3.
Multivariate logistic regression analysis of independent risk factor for nephropathy in diabetes.
4. Discussion
Both CYP2C19 and CYP2C9 play important role in drug metabolizing enzyme in CCVd treated by anticoagulation. There are 35 alleles found in the Chinese populations (∗2, ∗3, ∗8, ∗11, ∗13, ∗14, ∗16, ∗19, ∗23, ∗27, ∗29, ∗31, ∗33, ∗34, ∗36–∗56). ∗2 and ∗3 are the main variants among all of CYP2C9. However, the mutation frequency of ∗2 and ∗3 is very low (about 2%) in Asian.[11] So CYP2C19 become a focus in this study.
CYP2C19 is located within a cluster of cytochrome P450 genes on chromosome 10q24, which contains 9 exons and 8 introns.[12] The gene encodes a 490-aa long protein of approximately 56kDa, which is a member of the cytochrome P450 superfamily of enzymes. CYP2C19 is a clinically relevant drug-metabolizing enzyme for which genotyping and phenotyping information has the potential to improve drug safety and efficacy.[13] At least 27 variant alleles for CYP2C19 have been identified, with the most extensively described being CYP2C19∗2, CYP2C19∗3.[14] Since CYP2C19∗2 and ∗3 covering >90% poor metabolism population the mutation genotype contained ∗1/∗2, ∗1/∗3, ∗2/∗2, ∗2/∗3, and ∗3/∗3.
Stage 5 CKD is often called end-stage renal disease (ESRD), which is the terminal state of the kidney.[15] Both genetic and environmental factors effect on its development and progression, and that lead to the emergence of a variety of complications.[16] In order to avoid having that happen, prevention and treatment of early nephropathy is particularly important. Recently, various studies indicated that genetic component is the key point for the susceptibility of ESRD.[17] Research has shown that protein and mRNA expression of hepatic cytochrome P450 enzymes including CYP3A1, CYP3A2, and CYP2C11 were reduced in experimental models of CKD.[18] However, it has been unthorough about the effects of genetic factors on the susceptibility for nephropathy. This study aims to reveal the association between CYP2C19 genotype and nephropathy.
Firstly, this study showed that the renal function differences between CYP2C19 wild type and mutation genotype in CCVd patients with hyperuricemia. Independent risk factor analysis showed that CYP2C19 wild type is the only positively related risk factor for nephropathy. Sex is also related to nephropathy in CCVd patients with hyperuricemia, but this phenomenon can be caused by men being prone to get CCVd.[19] What is more, we analyzed biochemical results between CYP2C19 wild type and mutation genotype in CCVd patients with coronary heart disease and with diabetes. We found the similar conclusions that CYP2C19 wild type was an independent risk factor for nephropathy in CVVd patients.
Hypertension is the most important risk factor for cardio-cerebral vascular disease, while the kidney is the main target organ of hypertensive damage.[20] We cannot underestimate the risk of hypertension leading to nephropathy. But in this study, hypertension is not an independent risk factor which causes nephropathy in CVVd patients. Thus, the detection of CYP2C19 genotype has more important significance for the assessment of the susceptibility of nephropathy, at least in CVVd patients.
In order to achieve the purpose of diagnosis and treatment, variety of drugs were used to patient body in the era of modern medicine.[21] Unfortunately, some of these drugs lead to adverse side effect and systemic toxicity, including impaired renal function. Many classes of drugs, including prescription drug and blame prescription drug, caused drug-induced renal impairment.[22] Patients undergoing clopidogrel treatment are often required to take concomitant medication. Therefore, the mechanism of resulting in kidney toxicity is unclear. No clear evidence was found that any of the anticoagulants separately cause renal damage but it is possible that the other constituents of the compound tablets and possibly other anticoagulants are necessary to cause nephropathy.[23]
For most drugs, the faster drug metabolized in the liver, the more dosage was needed. Most of the drug can interfere with normal transport mechanisms in the kidney, leading to a variety of impairment of renal function.[24] Cytochrome P450 (CYP) is a superfamily of drug-metabolizing enzymes that is involved in the metabolism of most of the clinically used drugs. CYP2C19 wild type is the fastest metabolism type in all of CYP2C19 genotype.[25] The ultrafast drug metabolism forces to increase dosages, which result in kidney toxicity and nephropathy. Hence, the use of the prodrug and tailored drug to protect against nephropathy is necessary.
5. Conclusion
To conclude, the association between CYP2C19 wild type and nephropathy is extremely significant, which suggests that CYP2C19 wild type is related to the nephropathy susceptibility in CCVd patients. CYP2C19 wild type is an independent risk factor for nephropathy, and the risk of nephropathy is 3.288 times higher than CYP2C19 mutation genotype in hyperuricemic CCVd patients, 1.928 times higher than CYP2C19 mutation genotype in CCVd patients with coronary heart disease, and 5.248 times higher than CYP2C19 mutation genotype in diabetic CCVd patients. The CYP2C19 gene plays a potential maker to evaluate nephropathy in CCVd patients. Furthermore, the deteriorative degree of nephropathy is more and more obvious as age grows when the age is >60 years. On the basis of our current results, we recommend optimizing drug choice and dose for a more effective therapy, avoid serious adverse effects, and decrease medical costs. Because most of the drug can interfere with normal transport mechanisms in the kidney, the use of prodrug and tailored drug can contribute to the decrease of drug toxicity and nephropathy in CCVd patients with CYP2C19 wild type. Since the limited sample size, further studies in a large population are needed to confirm these findings.
Supplementary Material
Footnotes
Abbreviations: BUN = blood urea nitrogen, CCR = creatinine clearance rate, CI = Confidence interval, CKD = chronic kidney disease, CREA = creatinine serum, CVVd = cardio-cerebral vascular disease, CYP = cytochrome P450, ESRD = end-stage renal disease, FBG = fasting blood-glucose, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, OR = odds ratio, RBP = retinol binding protein, SD = Standard deviation, TC = total cholesterol, TG = triglyceride, UA = uric acid.
KC and ZJ contributed equally to the work.
Funding: This work was supported by an applied basic research project of the Science and Technology Department in Sichuan (2013JY0173).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Tabata N, Hokimoto S, Akasaka T, et al. Chronic kidney disease status modifies the association of cyp2c19 polymorphism in predicting clinical outcomes following coronary stent implantation. Thromb Res 2014; 134:939–944. [DOI] [PubMed] [Google Scholar]
- 2.Toda A, Ishizaka Y, Tani M, et al. Hyperuricemia is a significant risk factor for the onset of chronic kidney disease. Nephron Clin Pract 2014; 126:33–38. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez AM, Maceira BM, Perez E, et al. Genetics, environment, and diabetes-related end-stage renal disease in the Canary Islands. Genet Test Mol Biomarkers 2012; 16:859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uno Y, Matsushita A, Shukuya M, et al. CYP2C19 polymorphisms account for inter-individual variability of drug metabolism in cynomolgus macaques. Biochem Pharmacol 2014; 91:242–248. [DOI] [PubMed] [Google Scholar]
- 5.Niwa T, Murayama N, Imagawa Y, et al. Regioselective hydroxylation of steroid hormones by human cytochromes P450. Drug Metab Rev 2015; 47:89–110. [DOI] [PubMed] [Google Scholar]
- 6.Oestreich JH, Best LG, Dobesh PP. Prevalence of CYP2C19 variant alleles and pharmacodynamic variability of aspirin and clopidogrel in Native Americans. Am Heart J 2014; 167:413–418. [DOI] [PubMed] [Google Scholar]
- 7.Sottas PE, Kapke GF, Leroux JM. Adaptive Bayesian analysis of serum creatinine as a marker for drug-induced renal impairment in an early-phase clinical trial. Clinic Chem 2012; 58:1592–1596. [DOI] [PubMed] [Google Scholar]
- 8.Dodgen TM, Hochfeld WE, Fickl H, et al. Introduction of the AmpliChip CYP450 Test to a South African cohort: a platform comparative prospective cohort study. BMC Med Genet 2013; 14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang K, Qiu F, Chen M, et al. Engineering the MEP pathway enhanced ajmalicine biosynthesis. Biotechnol Appl Biochem 2014; 61:249–255. [DOI] [PubMed] [Google Scholar]
- 10.Ariyoshi K, Okuya S, Kunitsugu I, et al. Ultrasound analysis of gray-scale median value of carotid plaques is a useful reference index for cerebro-cardiovascular events in patients with type 2 diabetes. J Diabetes Investig 2015; 6:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YH, Pan PP, Dai DP, et al. Effect of 36 CYP2C9 variants found in the Chinese population on losartan metabolism in vitro. Xenobiotica 2013; 44:270–275. [DOI] [PubMed] [Google Scholar]
- 12.Lamoureux F, Duflot T, Woillard JB, et al. Impact of CYP2C19 genetic polymorphisms on voriconazole dosing and exposure in adult patients with invasive fungal infections. Int J Antimicrob Agents 2016; 47:124–131. [DOI] [PubMed] [Google Scholar]
- 13.Tabata N, Hokimoto S, Akasaka T, et al. Patients with both CYP2C19 loss-of-function allele and peripheral endothelial dysfunction are significantly correlated with adverse cardiovascular events following coronary stent implantation. J Cardiol 2016; 67:104–109. [DOI] [PubMed] [Google Scholar]
- 14.Hu LM, Dai DP, Hu GX, et al. Genetic polymorphisms and novel allelic variants of CYP2C19 in the Chinese Han population. Pharmacogenomics 1012; 13:1571–1581. [DOI] [PubMed] [Google Scholar]
- 15.Molinaro M, Ameri P, Marone G, et al. Recent advances on pathophysiology, diagnostic and therapeutic insights in cardiac dysfunction induced by antineoplastic drugs. Biomed Res Int 2015; 2:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reznichenko A, Böger CA, Snieder H, et al. Umod, as a susceptibility gene for end-stage renal disease. BMC Med Genet 2012; 13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agrawal S, Agarwal S, Naik S. Genetic contribution and associated pathophysiology in end-stage renal disease. Appl Clin Genet 2010; 3:65–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ladda MA, Goralski KB. The effects of CKD on cytochrome P450-mediated drug metabolism. Adv Chronic Kidney Dis 2016; 23:67–75. [DOI] [PubMed] [Google Scholar]
- 19.Babiker FA, Lips DJ, Delvaux E, et al. Oestrogen modulates cardiac ischaemic remodelling through oestrogen receptor-specific mechanisms. Acta Physiologica 2007; 189:23–31. [DOI] [PubMed] [Google Scholar]
- 20.Joyner MJ, Limberg JK. Blood pressure: return of the sympathetics? Curr Hypertension Rep 2016; 18:1–6. [DOI] [PubMed] [Google Scholar]
- 21.Serebruany VL, Tomek A, Pokov AN, et al. Clopidogrel, prasugrel, ticagrelor or vorapaxar in patients with renal impairment: do we have a winner. Expert Rev Cardiovasc Ther 2015; 13:1–12. [DOI] [PubMed] [Google Scholar]
- 22.Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis 2014; 7:457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang XL, Samant S, Lesko LJ, et al. Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clinic Pharmacokinet 2015; 54:147–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kóbori L, Kõhalmy K, Porrogi P, et al. Drug-induced liver graft toxicity caused by cytochrome P450 poor metabolism. Brit J Clin Pharmacol 2008; 65:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Gong Y, Zeng DL, et al. Inhibitory effect of apigenin on losartan metabolism and CYP2C9 activity in vitro. Pharmacology 2016; 98:183–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


