Abstract
Cellular migration assays are useful tools to investigate physiologic events on the bench top. Furthermore, this migration assay can be utilized to investigate wound healing therapeutics (those that encourage or accelerate wound closure) as well as deleterious agents (ones that mitigate or slow wound closure). The current study used an in vitro scratch assay to measure the effects of platelet-rich plasma (PRP) and depleted uranium (DU) in the form of uranyl acetate on cellular migration of human neonatal dermal fibroblasts in an in vitro simulation of wound healing. Data analyses included percent wound closure measured as the distance between cell margins, and rates of wound closure versus untreated controls. The highest doses of PRP (0.063, 0.125%) resulted in 50–65% wound closure after 4–8 hours relative to 38–44% in controls and the low-dose treatment group (0.031%). The high-dose treatments of PRP (0.125, 0.063%) reached 100% wound closure at 12 hours postwound versus 16 hours for controls and the low-dose treatment group (0.031%). Conversely, the higher doses of DU treatments (50 and 100 μM) resulted in <80% closure versus 100% closure in controls after 16 hours, with full closure observed at 20 hours. The highest dose of DU (1,000 μM) resulted in <20% closure versus 100% closure in controls after 16 hours. The use of the described scratch assay serves as a translatable bench-top model that has the potential to predict in vivo outcomes, and in many early studies can help to demonstrate proof-of-concept before moving into complex biological systems.
Keywords: : depleted uranium, in vitro, platelet-rich plasma, scratch assay, wound healing
Introduction
The scratch assay has been demonstrated to be an effective model to evaluate cellular migration using an in vitro system.1 This assay is designed to provide early study data that can be analyzed and repeated in an economical, high-throughput, and timely manner before moving into more complicated physiological systems, models, or entire organisms. The assay begins with scratching a confluent monolayer of cells with a sterile pipet tip, capturing high-resolution images at specified time intervals, and subsequent image analysis of the migration margin of the cells into the wound site.1 In the current studies, the scratch assay was utilized as a bench-top wound healing model to evaluate the effect of both a therapeutic (blood product) and a cytotoxic agent (depleted uranium, DU) on cellular migration as an indicator of wound closure. Human neonatal dermal fibroblasts (hDFn) were used for the scratch assays, as these cells are found in the integument (skin) in abundance and play a large role in the normal wound healing process.2,3 They work simultaneously with matrix metalloproteinases and other cells to degrade and lay down new extracellular matrix, composed primarily of collagen, for newly synthesized cells to adhere, proliferate, and take residence.4 Moreover, these fibroblasts that play a key role in wounding events in vivo make for an accurate in vitro skin model for multiwell scratches with both cytotoxic and therapeutic additives, such as platelet-rich plasma (PRP).
Platelets play a key role in the normal wound-healing process.5 The initial clotting effect of platelets prevents excess blood loss of damaged tissues and surrounding blood vessels. Additionally, growth factors such as vascular endothelial growth factor, fibroblast growth factor, and various other cytokines are released from platelet granules, thus initiating migration and chemotaxis of inflammatory cells to the wound site, commencing the healing process.4 Platelets are currently being evaluated in many ways to help promote an expedited wound healing response to injuries.6 Vascular chronic wounds are an example of injuries that have a lack of blood flow and cellular migration to the wound site, inhibiting wound resolution or repair, thus needing a stimulatory additive.6 Autologous PRP is a form of blood that is now commonly used in the clinical field to treat wounds and can be readily created and utilized as an expedited wound healing supplement.7 Through a double-centrifugation process, whole blood is spun down and separated into a dense erythrocyte layer, a buffy coat layer, and a plasma layer. The buffy layer, which is concentrated with platelets, is extracted and re-spun to concentrate the platelets and ultimately create a platelet-rich solution.8,9 This solution can then be applied to wounds to encourage an increased healing response and subsequent expedited wound closure.10
In addition to the applied therapeutics (PRP), the effect of a cytotoxic agent, DU, was assessed in the current study. DU is a byproduct of the uranium enrichment process and has been used by the U.S. Department of Defense in the manufacturing of armor-piercing military rounds and as shielding for military vehicles.11 Furthermore, current military personnel and veteran exposure to uranium is inevitable whether it is from shrapnel, coated bullets, or other sources.12 What has yet to be determined is if the skin could be a potential target organ for DU that would delay or possibly inhibit the wound healing response. Recent studies have shown that veterans with embedded shrapnel with unknown amounts of DU in their skin produce measurable levels of uranium excretion in their urine.11,12 The solubilization and dispersion could lead to implications in wound healing. DU contamination of battlefield wounds and burns may present immediate clinical challenges to the soldier as well as long-term concerns if the DU is not neutralized or rendered biologically ineffective.13 Preliminary data in the current study suggest that DU exposure to dermal fibroblast cultures in a scratch assay negatively affects wound closure. This indicates a potential pathway for uranium to become chemically toxic in DU-contaminated skin wounds.
Materials and Methods
Scratch assays
Human neonatal dermal fibroblasts (hDFn, 3rd passage; Life Technologies, Carlsbad, CA) were grown as a monolayer into a tissue-culture-treated T75 culture flask (Corning Inc., Corning, NY) at a density of 5,000 cells/cm2. The cells were grown in Dulbecco's modified Eagle's medium (DMEM; Life Technologies) supplemented with 10% fetal bovine serum (FBS; Life Technologies). Cells were incubated at 37°C, 5% CO2. Once the cells reached optimal density (>50% and <70% confluence) they were subcultured into a tissue-culture-treated 12-well plate (Corning Inc.) and incubated for 2 days or until the cells reached 100% confluence. Upon reaching confluence, the cells were scratched using a P200 pipet tip (Gilson Inc., Middleton, WI) across the monolayer with the initial scratch width measuring 1.5 ± 0.5 mm. Next, the media was removed, the cells were rinsed with 1× Hanks balanced salt solution (HBSS; Life Technologies), and the respective treatments were added (Fig. 1A–F).
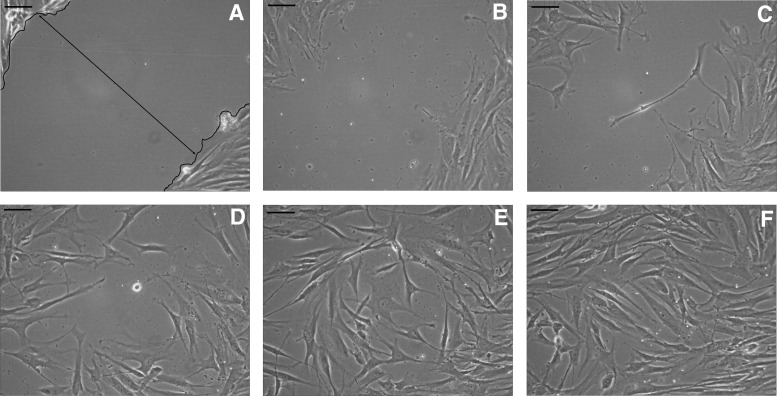
FIG. 1.
(A–F) Inverted microscopic images of the scratch “wound” assay at 100× magnification. The black line outlines the wound margin with cells located on the edges and the “mock” wound devoid of cells. (A) Captured at 0 hours, immediately after the wound was created. (B) Captured at 4 hours postwound; the cells have begun to migrate to cover the wound site. (C) Captured at 8 hours postwound; the cells continue to migrate to cover the wound site. (D) Captured at 12 hours postwound; the cells continue to migrate to cover the wound site. (E) Captured at 16 hours postwound; the cells have completely invaded the wound site, indicating 100% wound closure. (F) Captured at 20 hours postwound. All scale bars = 200 μm.
Platelet-rich plasma
Porcine whole-blood anticoagulated with acid citrate dextrose was purchased from Lampire Biological Laboratories (Pipersville, PA.) The whole-blood samples were centrifuged at 200 × g for 20 minutes with the acceleration and deceleration of the centrifuge set to 5 × g. Upon completion of the initial centrifugation, the buffy coat was transferred to a 15 mL conical tube. The sample underwent a second centrifugation at 400 × g for 15 minutes with the acceleration and deceleration of the centrifuge set to 5 × g. Concentrations of PRP at 0.031%, 0.063%, and 0.125% were mixed in a 10% FBS/DMEM and used as a 1 mL working volume in each well. These specific concentrations were used in order to effectively create an environment where PRP would interact with the hDFn cells while also preventing the mixed solution from forming a clot, which can prevent accurate and interpretable image capture of the wound margins. This solution was applied to the scratched monolayer of hDFn cells and observed over defined time points. Images were collected at 0, 4, 8, 12, and 16 hours after application of each PRP concentration. An ANOVA test with a Tukey post-hoc adjustment was used to identify statistical significance between data sets (p < 0.05).
Depleted uranium
DU concentrations of 25, 50, 100, and 1,000 μM were prepared via sterile filtration in DMEM with 10% FBS medium from a 4 mM uranyl acetate (Spectrum Chemical MFG. Corp., Gardena, CA) stock solution and used as a 1 mL working volume in each well. These concentrations were selected based on published ranges of exposure seen in the environment (25, 50, and 100 μM)14,15 and to test a maximal dose that has also been reported in the literature in contaminated wells in Wyoming, a 1,000 μM dose was also evaluated in the current study (Osiensky et al., 1984).16 Treatments were applied to the cells in their respective concentrations. Images were collected at 0, 4, 8, 12, 16, and 20 hours after application of each DU concentration. The Kruskal–Wallis test with a Bonferroni post-hoc adjustment was used to identify statistical significance between data sets (p < 0.05).
Image analysis
Images were captured using OptixCam image software (OCView V7.3.1.8; The Microscope Store, LLC, Roanoke, VA) on an Olympus CK2 inverted microscope (Olympus Corporation, Waltham, MA). Photographs were analyzed using ImageJ (V1.48) analysis software (National Institute of Health, Bethesda, MD). Wound widths in each well were measured at 10 unique locations along the length of the scratch, and an average value was reported.
Statistical analysis
All statistics were evaluated using the R-Program for Statistical Computation V3.2.2 GUI (2014).
Results
Platelet-rich plasma
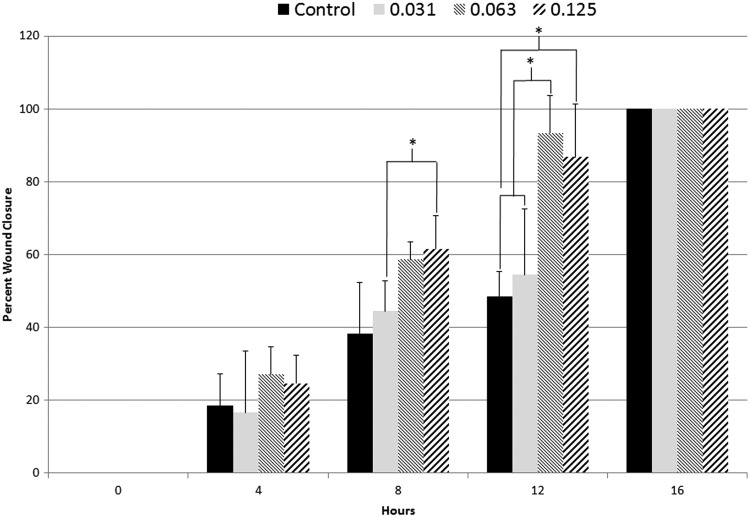
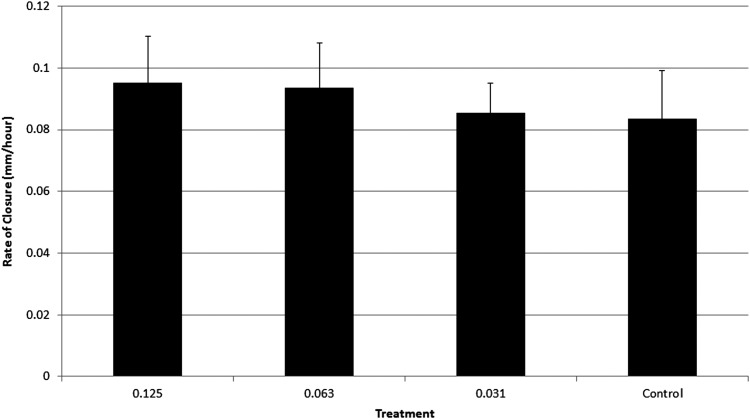
Sample sizes (n) for all PRP treatment experiments were n = 6. Scratch widths at 0 hours postwound measured 1.5 ± 0.5 mm. PRP was added to the 10% FBS medium, and no clotting was observed. Four hours (4 hours) postwound, all treatment groups, including control, had closed 16–27% relative to the 1.5 ± 0.5 mm average wound width. During the 4–8-hour time interval, the higher doses (0.125, 0.063%) of PRP stimulated the fastest cellular migration and resulted in 58–61% wound closure. In comparison, the low dose of PRP (0.031%) along with the control resulted in 38–44% wound closure at the same time point. During the 8–12-hour time interval, the higher doses of PRP (0.125, 0.063%) again were associated with the fastest wound closure and resulted in 86–93% wound closure at these time points. In contrast, the low dose of PRP (0.031%) along with the control resulted in 48–54% wound closure. At the 16-hour timepoint, all treatment groups were 100% closed (Fig. 2). Rate of closure was also calculated and reported for the scratch assay to determine the movement of cells in millimeters/hour. The 0.125% PRP treatment resulted in the fastest rate of closure of 0.095 mm/hr. The 0.063% PRP treatment had a rate of closure of 0.094 mm/hr. The lower dose treatment along with the control had the lowest rates of closure at 0.086 mm/hr for the 0.031% treatment and 0.084 mm/hr for the control (Fig. 3).
FIG. 2.
Percent wound closure analysis of the PRP-treated scratch wounds (n = 6). Wound closure occurred statistically faster in the 0.125% and 0.063% treatment groups compared with control and the 0.031% treatment group (p < 0.05). PRP, platelet-rich plasma.
FIG. 3.
Rate of closure analysis of the PRP-treated scratch wounds (n = 6). No statistical differences were seen in the rate of closure analysis. A dose–effect trend was observed in the rate of closure analysis; as the PRP dose increased, healing occurred at a faster rate. Additionally, as the dose decreased, healing rate also decreased and the benefit of expedited wound closure was diminished.
Depleted uranium
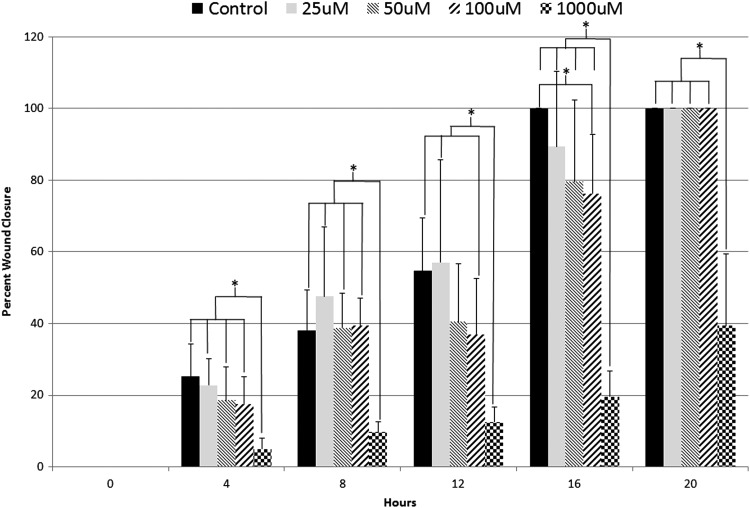
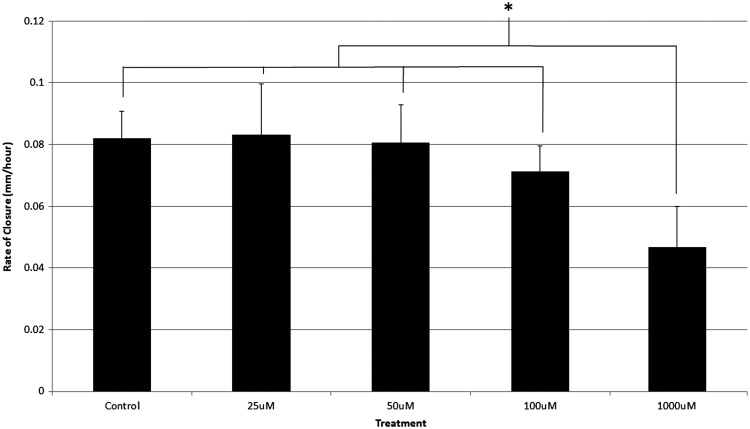
Sample sizes (n) for the 25, 50, and 100 μM DU treatment experiments were n = 9. Sample size for the 1,000 μM DU treatment experiment was n = 6. Scratch widths at 0 hours postwound measured 1.5 ± 0.5 mm. Trends were observed in which the control group and the lowest DU dose (25 μM) demonstrated the highest percent closure throughout the duration of the experiment. At 16 hours postwound the control was completely closed, whereas the 25, 50, 100, and 1,000 μM treatments remained open. Additionally, a dose-dependent response was seen at the 16-hour time point, with the 100 and 1,000 μM treatment groups demonstrating significantly lower percent wound closure compared with controls (p < 0.05). At 20 hours postwound, all remaining treatments were completely closed with the exception of the 1,000 μM treatment, which remained open. The experiment concluded at 24 hours postwound with the 1,000 μM treatment approximately 40% healed (Fig. 4). Rate of closure was also calculated and reported for the scratch assay to determine the movement of cells in millimeters/hour. The control and 25 μM treatment resulted in the fastest rate of closure measuring 0.082 and 0.083 mm/hr. The 50 μM treatment had a rate of closure measuring 0.081 mm/hr. The 100 μM treatment had a rate of closure measuring 0.071 mm/hr and the 1,000 μM treatment resulted in the slowest rate of closure measuring 0.047 mm/hr (Fig. 5).
FIG. 4.
Percent wound closure analysis of the DU-treated wounds (n = 9 for control, 25, 50, and 100 μM; n = 6 for 1,000 μM). Wound closure occurred statistically faster in control wounds compared with the 1,000 μM throughout the 24-hour study. Control was also statistically different when compared with the 100 μM treatment at 16 hours postwound. *Indicates statistical significance between treatment groups. DU, depleted uranium.
FIG. 5.
Rate of closure analysis of the DU-treated wounds (n = 9 for control, 25, 50, and 100 μM; n = 6 for 1,000 μM). The 1,000 μM treatment group closed at a statistically slower rate compared with control and all other treatment groups throughout the 24-hour study. *Indicates statistical significance between treatment groups.
Discussion
The optimization of the scratch assay as a bench-top wound model has been successfully adopted from previously published work to evaluate therapeutic agents as well as deleterious compounds.17,18 The simplistic, high-throughput, and timely evaluation made it possible to explore a therapeutic blood product and a cytotoxic agent, both of which demonstrated promising results to be evaluated in further in vivo studies. Wound closure was mitigated with the addition of DU, whereas an increased rate of wound healing was observed with PRP supplementation. In this way, the scratch assay as a wound closure model was demonstrated to be useful in evaluating compounds that stimulate or interfere with wound closure, thus demonstrating that a wide range of treatments can be investigated with this bench-top model. In many studies, first understanding the effects of various treatments using in vitro models can be critically important to the investigation of mechanistic pathways that may not be best studied in living organisms in which the complexity of the entire organism's physiology may complicate the analysis. For example, in the current study, the scratch assay could be used to evaluate various soluble or insoluble factors released by the cellular monolayer as a result of therapeutic applications. Additionally, mRNA expression levels of various key wound healing indicators such as collagen production or inflammatory markers can be easily targeted in a closed, controlled in vitro model. In these ways, first exploring in vitro models can be a critical initial step before moving into further research using in vivo models.19 By using a scratch model assay as an early wound healing model, new mechanistic pathways can be explored of various therapeutics before moving into a living biological system. This allows for higher throughput screening, cost efficiencies, and the collection of preliminary data to potentially allow for more successful in vivo experimentation.19,20
The lure and tendency to quickly move into in vivo experimentation is common among researchers. Many investigators suggest that physiologic events, such as wound healing, can be appropriately evaluated only within the living system. In many cases this holds true; however, the cost of in vivo biological experimentation can exceed budgets while utilizing a great deal of time and resources.19 In contrast, the in vitro scratch assay is cost effective, uses small amounts of reagents and consumables, and yields clear and concise results in a rapid manner.
The accuracy and repeatability of the scratch assay was validated through the successful re-creation of the scratch that resulted in similar wounds throughout all of the experiments. Both sets of control groups for the DU and PRP study closed within the 16-hour time point. This control result was demonstrated multiple times through repeat experimentation. The same-size (P200) pipet tip was utilized to ensure that a scratch margin was carefully created at 1.5 ± 0.5 mm. The wound width was measured by the leading edge of the wound margin. The percent wound closure was calculated for each scratch using the starting wound width for that specific well, resulting in relative percent wound closure data to help account for slight variations in scratches between treatment groups. Rate of closure was also calculated using a similar strategy that utilized the starting and ending width for each scratch.
In the current study, the addition of PRP in the presence of a “mock” in vitro wound demonstrated expedited wound closure compared with control wounds. All treatment groups showed an increased rate of closure as well as an increased percent wound closure. The 0.125% PRP treatment demonstrated the fastest rate of closure of all groups. A dose effect trend was observed in the rate of closure analysis; as the PRP dose increased, healing occurred at a faster rate. Additionally, as the dose decreased, healing rate also decreased and the benefit of expedited wound closure was lost. Similar results were shown in percent wound closure analysis. The high-dose treatments of PRP (0.125, 0.063%) reached >87% wound closure at 12 hours postwound, whereas the 0.031% treatment and control reached >49% wound closure during the same time. These data are consistent with numerous in vitro proliferation assays as well as human and animal in vivo studies,21,22 and further confirm that PRP behaves as a promoter of wound healing in an in vitro model of wound healing.
Conversely, in separate experiments, DU was added to the scratch assay to determine if deleterious effects of wound closure could be observed using this in vitro assay. Results from the current study demonstrate that DU as a contaminant in the scratch assay negatively affected wound closure compared with control wounds. When environmentally relevant doses of DU at 25, 50, 100, and 1000 μM were evaluated,23 percent wound closure decreased compared with controls. Control results from the PRP experiment were similar to control results from the DU experiment, where control wounds had an average closure time of 16 hours. In comparison, the four doses of DU treatments resulted in longer closure times, >20 hours for complete wound closure. DU results also showed a dose effect as seen in the PRP experiments, however, it was an inverse relationship. As the DU dose increased, healing occurred at a slower rate. As the dose decreased, healing rate increased.
Future in vitro work using the scratch assay can be performed to evaluate methods or strategies to neutralize the DU in a contaminated wound. In this way, the scratch assay may continue to be helpful in preliminary efforts to screen various DU-neutralizing compounds before comprehensive testing in in vivo models.
Conclusions
The bench-top scratch assay wound model described in the current study demonstrated success in being able to evaluate agents that positively and negatively influence wound closure. The scratch assay allows for early screening of soluble factors' influence on wound closure and allows for mechanistic studies to be performed. This assay is cost effective, is reproducible, and generates reliable data in a high-throughput manner for efficient data analysis. Many study questions can be answered using the scratch assay, which can help to shape the study design for future in vivo experiments.
Acknowledgments
1. Northern Arizona University Initiative for Maximizing Student Diversity in the Biomedical Sciences, National Institute of Health (NIH #R25GM056931-14).
2. Technology and Research Initiative Fund (TRIF). Northern Arizona University Health Research Initiative Grant. Title: “The Effects of Depleted Uranium Contamination on Wound Healing.”
Author Disclosure Statement
No competing financial interests exist in this study.
References
- 1.Liang C, Park A, Guan J. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007:2;329–333 [DOI] [PubMed] [Google Scholar]
- 2.Moulin V, Auger F, Garrel D, et al. Role of wound healing myofibroblasts on re-epithelialization of human skin. Burns 2000:26;3–12 [DOI] [PubMed] [Google Scholar]
- 3.Calderon M, Lawrence W, Banes A. Increased proliferation in keloid fibroblasts wounded in vitro. J Surg Res 1996:61;343–347 [DOI] [PubMed] [Google Scholar]
- 4.Kakudo N, Minakata T, Mitsui T, et al. Proliferation-promoting effect of platelet-rich plasma on human adipose–derived stem cells and human dermal fibroblasts. Plast Reconstr Surg 2008:122;1352–1360 [DOI] [PubMed] [Google Scholar]
- 5.Moon H, Yong H, Lee A. Optimum scratch assay condition to evaluate connective tissue growth factor expression for anti-scar therapy. Arch Pharm Res 2011:35;383–388 [DOI] [PubMed] [Google Scholar]
- 6.Sadoghi P, Lohberger B, Aigner B, et al. Effect of platelet-rich plasma on the biologic activity of the human rotator-cuff fibroblasts: A controlled in vitro study. J Orthop Res 2013:31;1249–1253 [DOI] [PubMed] [Google Scholar]
- 7.Rozman P, Bolta Z. Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol Alp Panonica Adriat 2007:16:156–165 [PubMed] [Google Scholar]
- 8.Nagata M, Messora M, Flavia F, et al. Effectiveness of two methods for preparation of autologous platelet-rich plasma: An experimental study in rabbits. Eur J Dent 2010:4;395–402 [PMC free article] [PubMed] [Google Scholar]
- 9.Kushida S, Kakudo N, Morimoto N, et al. Platelet and growth factor concentrations in activated platelet-rich plasma: A comparison of seven commercial separation systems. J Artif Org 2014:17;186–192 [DOI] [PubMed] [Google Scholar]
- 10.Kim D, Je Y, Kim C, et al. Can platelet-rich plasma be used for skin rejuvenation? Evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol 2011:23;424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiu J, Gaitens J, Squibb K, et al. Significance of dermatologic findings in a cohort of depleted uranium-exposed veterans of Iraqi conflicts. Am Contact Dermat Soc 2015:26;142–147 [DOI] [PubMed] [Google Scholar]
- 12.McClain D, Benson K, Dalton , et al. Biological effects of embedded depleted uranium (DU): Summary of Armed Forces Radiobiology Research Institute research. Sci Total Environ 2001:274;115–118 [DOI] [PubMed] [Google Scholar]
- 13.McClain D, Benson K, Dalton T, et al. Health effects of depleted uranium. Mil Med 2002:167;117. [PubMed] [Google Scholar]
- 14.Abdelouas A, Lutze W, Gong W, et al. Biological reduction of uranium in groundwater and subsurface soil. Sci Total Environ 2000:250;21–35 [DOI] [PubMed] [Google Scholar]
- 15.Brooks S. Waste characteristics of the former s-3 ponds and outline of uranium chemistry relevant to the NABIR field research center studies. NABIR Field Res Center Stud 2001:1–21 [Google Scholar]
- 16.Osiensky JL, Winter GV, Williams RE. Monitoring and mathematical modeling contaminated ground-water plumes in fluvial environments. Ground Water 1984:22;298–306 [Google Scholar]
- 17.Moulin V, Castilloux G, Jean A, et al. In vitro models to study wound healing fibroblasts. Burns 1996:22;359–362 [DOI] [PubMed] [Google Scholar]
- 18.Yarrow J, Perlman Z, Westwood N, et al. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol 2004:4;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walum E, Hedander J, Garberg P. Research perspectives for pre-screening alternatives to animal experimentation: On the relevance of cytotoxicity measurements, barrier passage determinations and high throughput screening in vitro to select potentially hazardous compounds in large sets of chemicals. Toxicol Appl Pharmacol 2005:207;393–397 [DOI] [PubMed] [Google Scholar]
- 20.Doke S, Dhawale S. Alternatives to animal testing: A review. Saudi Pharm Co 2015:23;223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranzato E, Mazzucco L, Patrone M, et al. Platelet lysate promotes in vitro wound scratch closure of human dermal fibroblasts: Different roles of cell calcium, P38, ERK and PI3K/AKT. J Cell Mol Med 2008:13;2030–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cáceres M, Martínez C, Martínez J, et al. Effects of platelet-rich and -poor plasma on the reparative response of gingival fibroblasts. Clin Oral Implants Res 2012:23;1104–1111 [DOI] [PubMed] [Google Scholar]
- 23.Bleise A, Danesi P, Burkart W. Properties, use and health effects of depleted uranium: A general overview. J Environ Radioact 2003:64;93–112 [DOI] [PubMed] [Google Scholar]