Abstract
Cardiovascular diseases are prevalent worldwide and are the most frequent causes of death in the United States. Although spending in drug discovery/development has increased, the amount of drug approvals has seen a progressive decline. Particularly, adverse side effects to the heart and general vasculature have become common causes for preclinical project closures, and preclinical models do not fully recapitulate human in vivo dynamics. Recently, organs-on-a-chip technologies have been proposed to mimic the dynamic conditions of the cardiovascular system—in particular, heart and general vasculature. These systems pay particular attention to mimicking structural organization, shear stress, transmural pressure, mechanical stretching, and electrical stimulation. Heart- and vasculature-on-a-chip platforms have been successfully generated to study a variety of physiological phenomena, model diseases, and probe the effects of drugs. Here, we review and discuss recent breakthroughs in the development of cardiovascular organs-on-a-chip platforms, and their current and future applications in the area of drug discovery and development.
Key words: : cardiovascular, drug discovery, heart-on-chip, organ-on-a-chip, vasculature-on-a-chip
Introduction
Cardiovascular diseases (CVDs) are the most frequent causes of death in the United States.1 In 2011, 800,000 people were killed by CVDs; approximately 60% of those deaths were attributable to coronary heart disease (such as myocardial infarction [MI]) and hypertensive heart disease.1 CVDs also include diseases affecting the general vasculature, such as atherosclerosis, hypertension, and other vascular cell dysfunctions that can result in occlusion and thrombosis.2,3 Over the years, these cardiovascular complications have been treated with numerous interventional4 and pharmacological agents.5–7 Several drugs have been developed against heart and vasculature diseases, but the increasing number of patients affected by them motivates a strong need for new and improved treatments. However, drug discovery and development has seen a decline each year in approved cardiovascular drugs.8
The cost of developing a new drug varies approximately between $0.8 and $1.2 billion U.S. dollars.9 After an intensive and costly research period, new drug candidates are subjected to clinical trials to assess safety and efficacy, before reaching regulatory decision by the U.S. Food and Drug Administration (FDA). However, despite extensive preclinical laboratory and animal testing, high rates of potential drug candidates still fail in clinical trials, especially during later stages of development.8,10 For instance, in the year of 2000, clinical safety (such as toxicity) and efficacy issues accounted for approximately 30% of drug project failures.11 Between 1991 and 2000, the rate of clinical trial success in cardiovascular drug development was as low as 20%.11 Moreover, over the past five decades, adverse side effects have been a major reason for cardiovascular drugs being rejected or retracted from the market.12–14 This can be partially attributed to the lack of effective and predictive preclinical models. Although conventional two-dimensional (2D) cultures and animal (disease) models have successfully contributed to the development of a wide range of therapeutics, many of these models do not adequately mimic human in vivo conditions. For example, there are essential genetic and metabolic differences between human and animals that can lead to significant pharmacokinetic mismatch.15 These differences motivate the development of better and more predictive human-based in vitro models to assess the safety and efficacy of potential new drugs. Ideally, these models should consist of platforms in which biochemical, physical, and electrochemical factors can be integrated and controlled in order to recapitulate the dynamic microenvironment of tissues in vivo.
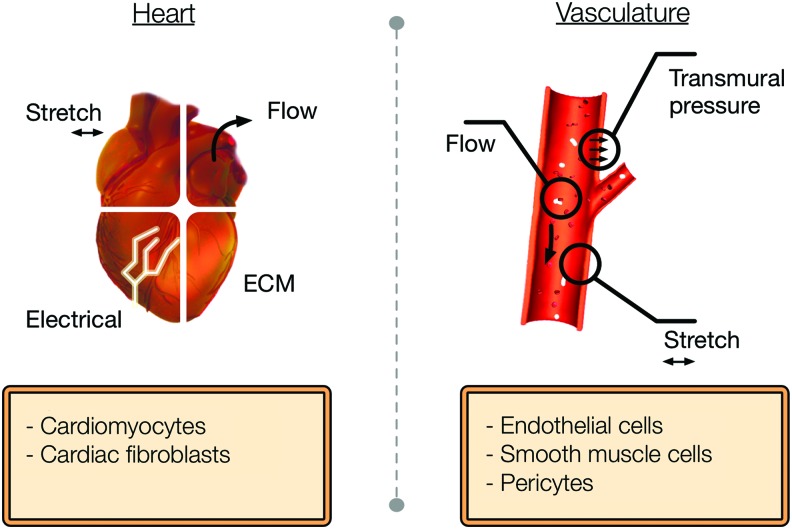
Several studies have been able to microengineer biologically relevant heart and general vasculature tissue models in vitro.16,17 The development of these three-dimensional (3D) engineered microtissues can potentially be used for in vitro drug testing, disease modeling, and biological mechanistic studies.18–20 Moreover, these models exhibit the physiological characteristics of myocardium and blood vessels because of the presence of tissue-like properties such as multiple cell interaction (e.g., paracrine signaling and cell–cell signaling), cell–extracellular matrix (ECM) interactions, and mechanical stimulation (Fig. 1). Importantly, in the past few years, novel microfluidic platforms have been introduced and have led to the creation of biomimetic cardiovascular tissues in vitro.21–25 These microfluidic organs-on-a-chip models can recapitulate important organ-level functions, multicellular microarchitecture, and environment dynamics (Fig. 1), thus providing a technological platform capable of accelerating cardiovascular drug development. Therefore, these engineered and novel heart- and vasculature-on-a-chip systems could contribute to the development of suitable high-throughput platforms for drug development and disease modeling of major CVDs such as MI, hypertension, heart failure, and atherosclerosis.
FIG. 1.
Key design parameters required to mimic a heart and general vasculature-on-a-chip model. Color images available online at www.liebertpub.com/aivt
Here, we discuss the challenges for the validation of cardiovascular organs-on-a-chip platforms and their potential applications on the pharmaceutical industry drug development pipeline. Furthermore, we describe and discuss the advances of the past decade in the development of heart- and vasculature-on-a-chip platforms to study heart and general vasculature physiology and disease, pinpointing their successes and shortcomings. Additionally, we identify opportunities for in vitro disease modeling and drug development.
Challenges and Translational Opportunities
Need for cardiovascular organ-on-a-chip models
CVD is the leading cause of death in the United States.1,26 Approximately 85.6 million people suffer from a CVD, resulting in 2,150 deaths per day in the United States alone and costing annually $320 billion and predicted to continue to rise.1 As a result, there is a need for development of novel drugs either for prevention27 or for treatment of CVD.28 Despite such a demand, the number of new pharmaceutical compounds approved per amount of dollars spent on research and development (R&D) has halved approximately every 9 years since 1950.29 Although most of the scientific and technological fields have seen exponential advances, the past 60 years of drug development has not followed that trend.29 However, spending continues to increase, with a reported $40 billion expenditure in 2014 in pharmaceutical R&D.30 Together, this suggests that there is a need for a disruptive technology that allows for a more predictive and efficient drug discovery/development process.8,31,32 Such technology would have the potential to lead to the discovery of new and improved drugs to treat CVD, while reducing the current patient burden.
Some of the key challenges that current drug discovery models face have been previously summarized by Pound et al.33 Most of these drawbacks fall into one main problem—positive/negative findings in animals do not necessarily link to positive/negative findings in humans.34,35 Below, we have listed some of the shortcomings associated with the current paradigm of drug development, as appeared in the literature:
1. CVD animal models are poor predictors of human responses.36
2. Adverse effects caused by systemic toxicity or toxicity of metabolites are organism dependent.37
3. Some drugs can benefit a certain group of ethnicity, sex, age, and/or genotype. Such phenomenon is not necessarily captured in a limited clinical trial study. For example, it is known that the average volunteer for clinical trials is a midaged female.38,39 This does not recapitulate the multitude of possible users of a particular drug.
4. The current paradigm is lengthy and costly.40
For all of the reasons mentioned above and others discussed in the literature, the process of drug development in the cardiovascular arena, obtaining regulatory approval, and achieving market entry is becoming less efficient and more costly. In fact, industry faces an increasing FDA regulatory scrutiny,9 while less drugs are being allowed to the market. However, this is not unfounded: regulatory agencies are requiring more evidence of safety to avoid postmarket issues, because clinical trials do not offer a complete measure of protection.41,42 Together, the above-described reasons highlight the role of new models to better predict safety in humans.
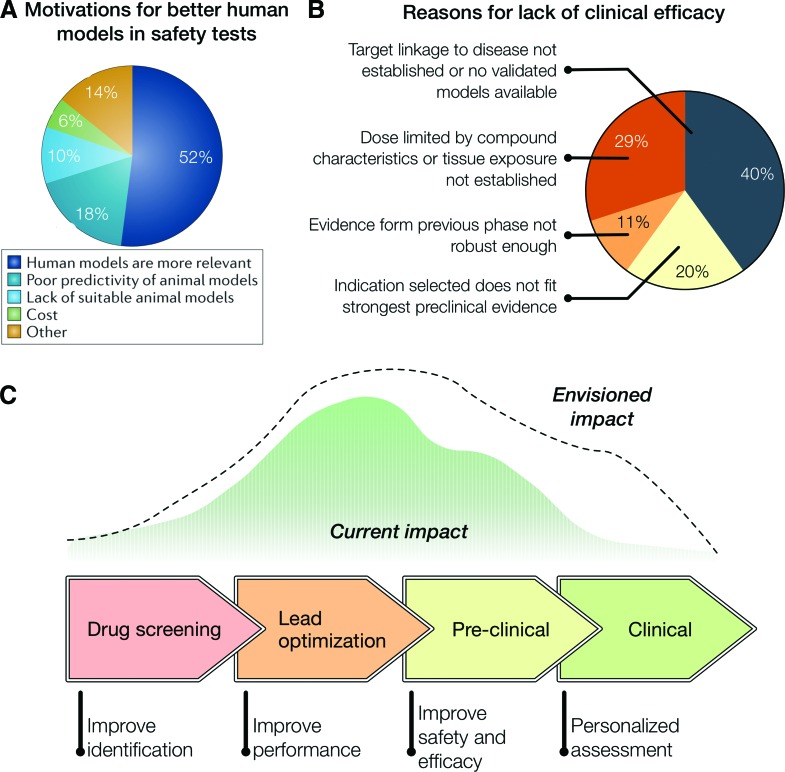
Some researchers underscore that inefficiency in the drug discovery pipeline could perhaps be attributed to current cardiovascular models. In fact, these models are not entirely capable of mimicking human conditions and dynamics. For example, a survey showed that industries were interested in adopting human tissue-based approaches for drug testing, despite the fact that they were not widely used (Fig. 2A).31 Interestingly, costs were not seen as a major issue toward adopting such models, while 50% reasoned that human models were more relevant.31 A study analyzing AstraZeneca's drug pipeline from 2005 to 2010 showed that 82% of drug project closures in the preclinical phase was because of safety issues.8 Interestingly, among the organ systems involved in the safety failures, the cardiovascular one accounted for as much as 17% failures, the highest of all the organ systems analyzed.8 The solution could be the introduction of a paradigm shift using a disruptive technology that presents a physiologically relevant in vitro model of the human heart and general vasculature system.43 In contrast to preclinical stages, the project closures in clinical phase are mainly because of efficacy issues. Remarkably, in 40% of the cases of lack of clinical efficacy (Fig. 2B), the reason is no target linkage to disease or no validated models available, whereas other aspects are related to results not fitting preclinical evidence.8
FIG. 2.
(A) Survey on the motivations behind adopting human tissue-based approaches for safety pharmacology studies.31 (B) AstraZeneca's small-molecule projects from 2005 to 2010. the terminated projects were analyzed to understand the causes for failure and identify potential predictors of success (total of 28 projects).8 (C) Impact of organs-on-a-chip technologies on the drug development pipeline. [(A) adapted from Holmes et al.31; (B) adapted from Cook et al.8]. Color images available online at www.liebertpub.com/aivt
Organs-on-a-chip platforms have gained wide scientific attention because of their potential in achieving a physiological resemblance in vitro, with a hope to potentially change the way scientists and industry can test the effect of drugs on human cells and organs. Indeed, pioneering work has focused on mimicking cardiovascular tissue in vitro.44–46 These organs-on-a-chip platforms have thus far been able to make interesting additional in vitro models. However, to date, it is still difficult to claim that the associated studies have created examples that are adopted by industry, as means toward cost-effective development of new drugs that would not have been possible otherwise. Even though such models have produced exciting scientific studies, there has not been a major reception for them by the industry. Furthermore, focusing on scalability and ease of handling of the technology would accelerate the adoption of such platforms.
The needs of the industry can broadly be divided into a need for better in vitro models predicting the behavior of animal and human organs (i.e., heart), and human organ systems (i.e., heart–vascular interactions). While actual proof of clinical predictability is necessary to make an impact and engage with regulatory agencies, scientific studies to date have demonstrated that their platforms could be potentially capable of performing a high-throughput screen.47,48 In the path toward achieving broad application by the pharmaceutical industry are two key challenges: guaranteeing clinical relevancy and reproducibility.
Translating cardiovascular organ-on-a-chip models
Once the advantages over current technologies/models are proven, the following step is to have a commercialization strategy. Aside from the academic examples mentioned above, it is also worthwhile considering some of the ventures established toward commercializing and implementing the associated technologies. There are a number of enterprises aiming at commercializing products that encompass single and multiple organs-on-a-chip.49 These companies have mainly developed products for research laboratories, but ongoing efforts are being made to apply such technologies at a broader scale in the pharmaceutical industry.
The market need from pharmaceutical industry is expected to be high, mainly because of the ever-decreasing number of new drug approvals and the challenges faced with preclinical models. However, these in vitro platforms would require regulatory approval for cardiovascular applications, either for heart- and vasculature-on-a-chip systems alone, or for a potential combination of both. An opportunity in this arena is to show comparable results to animal tests, followed by a strong demonstration of reproducibility and clinical significance. Another path for application of cardiovascular organs-on-a-chip could be direct regulatory endorsement. The current platforms required by FDA for such assessments have remained limited to petri dish, animal studies, and limited clinical trials—models still lacking the level of accuracy and predictability required. Here, the dialog with regulatory agencies such as FDA would be a starting point, in order to establish standards expected for such platforms from the regulatory point-of-view.
Some of the open challenges that are being addressed by research teams and companies are related to standardization, optimization, and scaling up for manufacturing (discussed in detail by Vladisavljevic et al.50). While the scientific community is geared toward innovating and creating new platforms, the growth of companies in this area could lead to their optimization for standardization, reproducibility, and scaling-up. These key challenges, once tackled, will allow the widespread usage of cardiovascular organ-on-a-chip for drug discovery, while securing regulatory endorsement. The impact of such technologies for drug discovery and development is expected to be vast (Fig. 2C). Current opportunities exist for improving performance at later stages of lead optimization, and safety and efficacy at early stages of preclinical studies. Furthermore, we envision that such technologies would be able to provide significant impact on preclinical stages in the near future, and personalized medicine approaches at a later stage.
Heart-on-a-Chip Platforms in Drug Discovery
Mimicking cardiac environment
Cardiac tissue contractile function is mediated through cardiomyocytes (CMs) that are organized in parallel arrays of myofibril bundles. The contractility of CMs is mediated through chemical, mechanical, and electrical stimuli.51,52 In addition to CMs, cardiac tissue is composed of fibroblasts and microvessels, which consist of endothelial cells (ECs) and vascular smooth muscle cells. Cellular composition and anisotropic organization, together with continuous exposure to multiple dynamic stimuli, plays a critical role in maintaining cardiac physiological function.53–55 Microfluidic in vitro heart-on-a-chip platforms can provide precise individual control over each these essential factors, allowing for novel opportunities to study cardiac physiology, pathology, and pharmacology. In particular, these platforms may hold great promise for the development of high-throughput assays that could be valuable in drug screening and toxicity studies.21,22,24,25,56
Recent advances have enabled the engineering of physiologically relevant microscale myocardial tissues by using microfluidic organ-on-a-chip systems. Such systems are often fabricated using poly(dimethyl siloxane) (PDMS) because of its biocompatibility, nontoxicity, and low cost.57,58 In addition, PDMS is an elastomeric and transparent material that can be rapidly prototyped and customized into a microfluidic system by using soft and photolithography techniques.57 However, the hydrophobic nature of PDMS limits the attachment and spreading of cells.58 This challenge can be addressed by treating the PDMS surface with proteins (e.g., fibronectin) that facilitate cellular attachment.58 Recently, Annabi et al.59 developed a technique to overcome this challenge by coating microfluidic channels with hydrogels (Fig. 3A), which provided a suitable environment for on-chip cardiac cell culture. Specifically, methacrylated tropoelastin (MeTro) and gelatin methacryloyl (GelMA) facilitated cellular adhesion inside the microfluidic channels,59 which was also reported in other cell types.60,61 The seeded CMs revealed not only an increased attachment, but also proliferation and beating rate on MeTro hydrogels compared to GelMA (Fig. 3B).62 This was mainly attributable to the presence of cell-interactive amino acid sequences and the more elastic nature of tropoelastin compared to GelMA. In addition, continuous perfusion of medium through the heart-on-a-chip platform provided the possibility to mimic blood flow-induced shear stress on myocardial tissue. Therefore, this platform could potentially be useful to study biomimetic cues on cardiac function. The results of this study suggested that tropoelastin-based hydrogels might offer a suitable microenvironment for proper functioning of CMs in vitro. Important properties of tropoelastin that support this are the tunable elasticity and biocompatibility. Moreover, this microfluidic system could be used to study safety and efficacy of drugs under physiologic-like conditions.62 However, this simplified ECM approach might not recapitulate the complexity of cardiac ECM composition. Further challenges include identifying the minimal design criteria for ECM composition and organization that are required to properly mimic the heart, which might, among others, require the screening of different ECM compositions in a high-throughput manner.
FIG. 3.
(A) Schematic of a PDMS-based microfluidic heart-on-a-chip model developed to culture cardiomyocytes. These microfluidic channels were coated by gelatin- and tropoelastin-based hydrogels to induce cellular attachment. (B) The effect of 10% (w/v) tropoelastin and 10% (w/v) gelatin-based hydrogels on the beating pattern (left) and beating frequency (right) of cardiomyocytes (CMs) inside microfluidic microchannels. (C) The schematic of a heart-on-a-chip microdevice designed for pharmacological testing. (D) The effect of isoproterenol on the contractility of CM-seeded muscular thin films, showing an increase in beating rate compared to control. Adapted with permission from Annabi et al.59 and Agarwal et al.69 Color images available online at www.liebertpub.com/aivt
Although native myocardial cells, scaffold elasticity, and (natural) ECM proteins are critical to engineering biomimetic cardiac tissues in vitro, additional components of native heart tissue are required to recapitulate proper cardiac tissue function. Here, we will highlight some of these factors and discuss their use and application in the fabrication of heart-on-a-chip devices.
One of the factors important for the fabrication of heart-on-a-chip devices is electrical stimulation and CM anisotropy. In vivo, myocardial pumping arises from the electrical stimulation of CMs by automated pacemaker cells in the sinoatrial node. These electrical signals propagate through the left and right atria to go to the atrioventricular node and eventually reach the right and left ventricles. As a result, the anisotropic and elongated CMs are depolarized, which results in regular and synchronized cardiac muscle contraction. Consequently, external electrical stimulation and an anisotropic organization of CMs may contribute to the development of more physiologic in vitro heart tissue. Anisotropic alignment of CMs and ECM significantly affects electrical and mechanical functioning of cardiac tissue.63,64 Integrating structural and functional anisotropy used in cardiac tissue engineering65 into heart-on-a-chip devices could result in better contractile force generation and better electrical propagation of action potentials.65 Grosberg et al.66 used a muscular thin film (MTF) technique to design a heart-on-a-chip with anisotropically organized CMs. MTF platforms were created by microcontact printing fibronectin patterns on a thin, deformable PDMS film, later seeded with primary neonatal rat ventricular CMs. Additionally, the self-organized CMs on the functionalized surface were electrically stimulated by platinum electrodes. By electrically stimulating CMs on the chip, the in vivo generation of electrical signals by pacing cells could be recapitulated. Moreover, electrical stimulation of cells increased the cellular alignment, differentiation, and functionality of engineered cardiac tissue.67 This study showed that contractile behavior and electrophysiological properties such as the action potential morphology of MTFs could be measured successfully. Consequently, these measurements could be used in the evaluation of pharmacological intervention on the contractile function of multiple cardiac microtissues. Using a dose-range experiment, the effect of epinephrine concentrations on the frequency of contractions was calculated based on the MTF contractility stress profiles. This demonstrated that MTFs in a microfluidic chip can be used to assess contractility in the presence of drugs or physiologic-like cues such as hydrodynamic, mechanical, and electrical stimuli. Although this 2D monolayer of CMs provides an opportunity to analyze deformation of cardiac microtissue in a 3D manner, it is not able to recapitulate the 3D microenvironment of myocardial tissue. A solution to this challenge could be found by combining cellular encapsulation and microengineering strategies to form aligned 3D microtissues.68
A similar MTF approach was used to create an aluminum-based microdevice for high-throughput pharmacological testing of isoproterenol (Fig. 3C).69 This platform provided the advantage of a semiautomated fabrication method that could be scaled up and automated for higher throughput screening of drugs. Moreover, a metallic heating element, electrodes, and a transparent top were incorporated in the device for simultaneous temperature control, electrical field stimulation, and optical contraction analysis, respectively. The microfluidic device's high-throughput capacity was leveraged to test the effect of isoproterenol on the contractile stress of MTFs (Fig. 3D). In this experiment, a series of increasing drug concentrations were tested within the same microdevice. The MTFs showed an increase in contractility stress when compared to control. Furthermore, by using this system, the same level of isoproterenol potency in vitro was comparable to the reported values for rat hearts.69 This microdevice enabled the incorporation of an MTF-based heart-on-a-chip and allowed automatic fluidic control, wash-in and washout after each drug dosage. In addition, this system was shown to be suitable for simultaneous analysis of cardiac tissue contractility during drug compound testing, thus demonstrating its ability to perform rapid and accurate drug analysis. Consequently, this microdevice could potentially improve pharmacological in vitro studies on a higher throughput manner that is not feasible with in vivo studies.
A cell culture chip to form in vitro cardiac microtissues through the application of electrical stimulation and topographical cues was created by Au and colleagues.70 Here, neonatal rat CMs were seeded and cultured onto polystyrene cell culture chips with integrated topographical microgrooves. CMs showed attachment and alignment along the grooves. In addition, electrical stimulation of the CMs increased the cellular maturation in terms of sarcomeric protein (such as sarcomeric alpha-actinin) and gap junction protein expression. This work highlights the importance of combining several different factors toward achieving an in vivo-like cellular response.
Another factor important for the fabrication of heart-on-a-chip device is cardiac tissue perfusion. The complex architecture of myocardial tissue, consisting of cardiac bundles and associated microvessels, can be more accurately recreated by fabricating in vitro systems that can recapitulate cardiac tissue perfusion. Xiao et al.71 designed a more physiologically relevant heart-on-a-chip by creating a perfusable cardiac microtissue with a poly(tetrafluoroethylene) microtubing to induce CMs to align and elongate along the tubing template. This system demonstrated the feasibility of mimicking cardiac tissue by showing spontaneous beating, expression of sarcomeric troponin-T, and gap junction markers (connexin-43). In addition, the functionality of this system as a platform for pharmacological studies was examined by testing the system with nitric oxide (NO). After exposure to NO for 24 hours, the beating rate of the CMs was significantly decreased in comparison with the untreated control. These results indicated that this platform could serve as suitable biomimetic in vitro system for both drug development and drug-induced changes in beating behavior.
Cardiac disease models
Many stem cell-based, tissue-engineered, and organ-on-a-chip systems for in vitro modeling of cardiac disease have been developed. Cardiac diseases such as rhythm disorders72,73 and dilated cardiomyopathy74 have been studied by using induced pluripotent stem cells (iPSCs) in monolayer disease models. In addition, cardiac conditions, including MI and re-entry arrhythmia, have been successfully re-created in 2D tissue-engineered heart models.34 Although these models were able to represent specific phenotypic disease characteristics, they still lack the incorporation of proper environmental factors such as 3D cell–ECM and cell–cell interactions. To overcome this hurdle, several biomaterials have been mixed with different cell sources (e.g., iPSCs, rat neonatal ventricular cells) toward the development of 3D structures of cardiac disease models.35,75–77 Recent advances in the field of microfluidics have allowed researchers to construct novel heart-on-a-chip systems for disease modeling and drug testing. Next, we will summarize some of the recent findings on the development of these microfluidic systems.
Several chronic CVDs, such as hypertension and aortic valve stenosis, can cause pressure overload in the left ventricle and lead to cardiac hypertrophy and fibrosis.78 Increased mechanical stress during disease is a critical factor that drives the initiation of hypertrophy, fibrotic remodeling, and heart failure.79,80 These dynamic changes are partly attributable to mechanotransduction, a process in which external mechanical forces are translated into internal chemical and electrical cellular responses.81 Therefore, it is important to develop an in vitro heart-on-a-chip model that can provide insight into the pathophysiology of this phenomenon. McCain et al.48 successfully applied an MTF platform toward the creation of a failing myocardium-on-a-chip.82 Mechanical cyclic stretch of the MTFs resulted in pathological tissue remodeling, with several genetic indicators of pathological remodeling being upregulated. Furthermore, by the application of transverse stretch, structural organization of micropatterned CMs was disrupted, resulting in disorganized CMs similar to hypertrophic cardiomyopathy (Fig. 4A).48,82 Further development of similar heart failure on-a-chip models could potentially aid in a better understanding of cardiac remodeling, while providing valuable tools for pharmaceutical industries.
FIG. 4.
(A) The effect of transverse mechanical stretch showing a disorganized architecture of cardiomyocytes cultured on muscular thin films (scale bar = 50 μm).48 (B) Schematic illustration of a microfluidic heart-on-a-chip model that ressembled the native interface of myocardial cells and microcapillaries. Reprinted with permission from Ren et al.83 (copyright 2013 American Chemical Society). (C) Representation of fluorescence images of cytoskeletal changes in cardiomyocytes under hypoxic conditions, which were induced by the introduction of an oxygen consumption blocker in one of the microfluidic side channels. Reprinted with permission from Ren at al.83 (copyright 2013 American Chemical Society). (D) Schematic representation of a bi-compartmental co-culture of valvular endothelial (VEC) and valvular interstitial cells (VIC) (top panel). VECs were embedded on a thin and porous membrane on top of a VIC-loaded gelatin methacryloyl (GelMA) (bottom channel).86 Color images available online at www.liebertpub.com/aivt
Ren et al.83 fabricated a heart-on-a-chip to study hypoxia-induced myocardial injury. By designing a PDMS-based microfluidic device with four functional units, the interface of myocardial tissue and microcapillaries was mimicked in vitro. The four units consisted of a central cell culture chamber and two lateral channels that were separated from each other by micropillar arrays, representing the interface between the blood vessel and cardiac tissue (Fig. 4B). To mimic hypoxia-induced conditions, a specific oxygen consumption blocking reagent, FCCP (cyanide p-trifluoromethoxyphenylhydrazone), was introduced into one of the side channels (Fig. 4C).83 CMs presented normal attachment, spreading, and proliferation under normoxic conditions, but underwent morphological changes such as shrinkage and disassembling of intracellular actin bundles after induction of hypoxia. During myocardial ischemia (or MI) there is an increased CM apoptosis, a feature that was accurately recapitulated within this device.
Various (stem) cell transplantation therapies for cardiac repair after MI have gained considerable attention in the past several years.84 Many groups have explored the potential of numerous cells types such as embryonic stem cells, cardiac progenitor cells, skeletal myoblasts, and iPSCs.84 To study the interactions as well as the potential role of skeletal myoblasts in the repair of hypoxia-injured CMs, a comparable microfluidic device was created in a follow-up study by He et al.85 In this system, skeletal myoblasts and rat CMs were co-cultured in separate chambers that were connected through permeable microvalves. The results of this study demonstrated that skeletal myoblasts were capable of repairing the hypoxia-injured CMs through cell–cell interactions.
Hydrogels and microfluidic systems that are developed to mimic myocardial tissue could also be leveraged to mimic valvular tissue and disease.86 Chen et al.86 used a multicompartmental approach to design a valvular and vascular compartment in a 3D microenvironment, allowing cells to communicate through paracrine signaling (Fig. 4D). A thin porous membrane separated the cells and enabled the paracrine communication between valvular endothelial cells (VECs) and valvular interstitial cells (VICs). Fibroblast-like VICs were encapsulated in GelMA hydrogels and VECs seeded on fibronectin-coated PDMS microchannels. In addition, VECs could be stimulated physiologically by the introduction of flow-induced shear stress. Activation of quiescent VICs into alpha smooth muscle actin (α-SMA)-expressing myofibroblasts are thought to be one of the hallmarks of valvular pathological remodeling, because of their increase in ECM synthesis and contractile features.87,88 Myofibroblasts have the ability to increase calcium deposition by differentiating into osteoblast-like cells.89 Consequently, myofibroblasts play an important role in calcific aortic disease and valvular fibrosis.90,91 A study by Chen et al. showed that VECs are able to inhibit VIC activation under both static and dynamic fluid flow, which is in line with in vivo conditions.86 This biomimetic microfluidic system could be used to study biological and pathological VEC-VIC interaction, as well as drug-associated toxicity. In addition, this platform may be an appropriate testing model for potential drug candidates in treatment of aortic valve disease.
Recent developments in stem cell biology have further enabled the use of patient-specific iPSC-derived CMs for generating in vitro models of cardiac disease.92 These advances may lead to the creation of physiologically and genetically relevant models of cardiac disease for drug discovery and drug toxicity.93 By using iPSCs, MTF, and microfluidic technologies, Wang et al.94 successfully engineered the cardiomyopathy of Barth syndrome (BTHS) in an in vitro disease model.94 iPSCs were generated from patients with BTHS and subsequently differentiated into patient-specific iPSC-derived CMs. In this study, the pathophysiology of BTHS cardiomyopathy was demonstrated by seeding iPSC-derived CMs on MTFs. The BTHS-derived cardiac microtissues were shown to have impaired sarcomeric structures as compared to control. Additionally, MTFs from BTHS iPSC-CM revealed a significantly lower contractile performance compared to controls under the same conditions. Moreover, this “BTHS cardiomyopathy on-a-chip’’ was used to test potential therapeutical options (e.g., pharmacological and genetic modifications), indicating its suitability for identifying new therapeutical targets.
All together, these studies point toward a better understanding of CM physiology under a range of dynamic conditions. The new in vitro models of heart-on-a-chip present a strong step toward testing cardiotoxicity in human-relevant models, but models of disease are still an area filled with challenges and opportunities. In particular, we propose that heart disease-on-a-chip models represent a high-value area for new drug screening platforms.
Vasculature-on-a-Chip Platforms in Drug Discovery
Mimicking vascular environment
It is challenging to mimic the vascular environment of the human body in vitro. Arteries, arterioles, veins, venules, and capillaries are all part of the vascular system, but differ in the structural and cellular compositions. In addition, blood vessels are subject to a range of biophysical stimuli because of the pulsatile nature of blood flow. ECs lining the lumen of vessels experience flow-induced pulsatile wall shear stress and transmural pressure. ECs and vascular smooth muscle cells (VSMCs) both experience cyclic mechanical stretching, which causes the vessels to increase in diameter in response to blood flow. Hemodynamic parameters contribute to the maintenance of homeostasis in the vessel wall, with several microfluidic models studying the effects of hemodynamics in vitro.95–98 Because the vascular system is constantly under these biophysical stimuli (Fig. 1), microfluidic technologies are an advantageous way to recapitulate these forces. Recently, several microfluidic-based platforms have been described for distinct vascular applications,97 including the formation of tissue-engineered vascular networks, angiogenesis, and vascular disease models. The end goal of such models is to gain mechanistic and contextual insight on the vascular biology processes, while providing a superior platform for drug discovery and development.
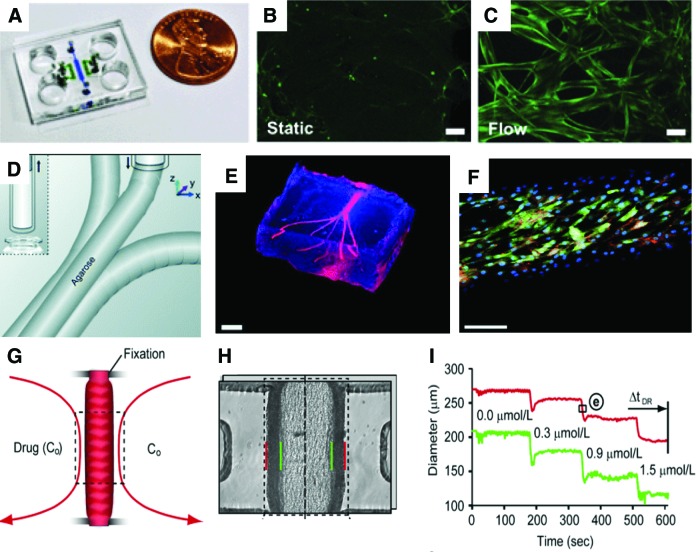
The use of hydrogels in microfluidics is a simple platform to provide relevant 3D matrixes to vascular cells, while maintaining the ability to apply shear stress. Kim et al. used the flexibility of PDMS to generate a microfluidic device (Fig. 5A) capable of containing fibrin matrices.99 The multichannel device enabled the study of vasculogenesis and angiogenesis, and the fibrin matrix was able to support cell growth. Kim and colleagues were able to show the formation of a perfusable vascular network, and the maintenance of barrier function. Additionally, the vascular networks under flow exhibited an increased nitric oxide production compared with static conditions99 (Fig. 5B, C). Similar methodologies have been used with different hydrogels100–103 with the end goal of creating vascular networks in vitro to study different aspects of vascular biology. Morgan et al.102 used type I collagen gels with embedded cells and hollow channels that were later seeded with ECs. This simple system allows for the creation and design of defined microvascular endothelialized geometries that are useful for the study of permeability and blood–vasculature interactions in a tissue-engineered construct.102 In another example, Wang et al.103 utilized sodium alginate as artificial templates to cast a hydrogel mixture containing gelatin, agarose, and collagen. The resulting matrices were seeded with HUVECs, and displayed good barrier properties and response to flow. Other fibrin-based approaches have been pursued in combination with other cell types.101,104 Hasenberg et al.101 aimed to incorporate capillary networks in fibrin gels for multi organ-chip constructs; these vascular networks were stable in serum-free media, which is an important aspect in designing such systems for drug development. Schimek et al.105 designed a similar microfluidic chip, which focused on designing an on-chip peristaltic micropump to provide the vascular network with pulsatile shear stress.
FIG. 5.
In vitro microfluidic models to study vascular networks and vascular functions. (A) Microfluidic chip for forming vascular networks and to study angiogenesis. (B, C) Nitric oxide production in static and flow conditions inside the device A (scale bar = 50 μm). (D) Bioprinting methodology to produce vascular networks with agarose sacrificial layers. (E) Bioprinted network (scale bar = 3 mm). (F) Bioprinted vascular network with ECs (green, GFP; blue, DAPI; red, CD31; scale bar = 250 μm). (G) Artery-on-a-chip model where a small mouse artery segment is held in place and perfused through the lumen and outside walls. (H) Microphotograph of artery-on-a-chip model (red, outer side wall; green, inner side wall). (I) Response of the artery-on-a-chip to phenylephrine. [(A–C) adapted with permission from Kim et al.99; (D–F) adapted with permission from Bertassoni et al.100; (G–I) adapted with permission from Gunther et al.47.] Color images available online at www.liebertpub.com/aivt
Others have used different fabrication techniques such as bioprinting to produce vascular networks.100 In one example, Bertassoni et al. utilized bioprinting of template sacrificial layers to generate vascular networks in hydrogels (Fig. 5D). Combined with UV-crosslinkable GelMA hydrogels, this bioprinting methodology allows an on-demand precise control of microarchitectures, yielding potentially complex structures that can mimic different vascular regions (Fig. 5E, F) observed in the human body.100 This bioprinting approach can generate 3D constructs in a fast, controllable, and inexpensive way, which can be useful for testing drug compounds in a wide range of vascular flow settings and on a large scale. These technologies106 aim to create 3D vascular beds utilizing a wide range of hydrogels. This allows the fine-tuning of mechanical properties and ECM cues to target the specific regions of interest in the vascular system. These systems can have the potential to provide meaningful impact on drug discovery by mimicking in vitro vascular tissue microenvironments.
Conversely, some studies have used microfluidics in combination with ex vivo vascular segments.47,107 Gunther et al.47 generated a small but complex microfluidic device that captured a mouse mesenteric artery segment and held it in place. Once this segment was fixed, it could be imaged and exposed to drugs through the lumen or external wall (Fig. 5G, H). The contraction or dilation of the artery segment was then imaged upon drug exposure. The exposure to phenylephrine in this system resulted in a segment contraction in a dose-dependent way (Fig. 5I). Although this represents a major step forward in microfluidics for vascular drug discovery and development, the usage of animal vessels limits its application and translational relevance to humans.
Vascular disease models
Rosano et al.108 mapped a mouse cremaster muscle and used it to build a PDMS mold of the vascular network. This biomimetic vascular model can be analogous to human vascular structures, possessing different geometries and regions with different shear stresses. Such models can comprise complex bifurcation and geometric intricacies inherent to vascular networks that change the way cells interact with drugs. Importantly, they can potentially be applied to generate individualized vascular networks and simulate scenarios of thrombus or atherosclerosis formations.
Besides recreating vascular structures in vitro, researchers have also pursued models to study angiogenesis.109,110 Galie et al.110 highlighted the influence of shear stress in angiogenic sprouting. Both wall shear stress and transmural flow in the endothelium above a certain threshold (>10 dyn/cm2) induce endothelial sprouting. Local narrowing of blood vessels induces high shear stress regions, which can trigger further sprouting. Such information can be valuable in the context of pharmacological interventions that promote or inhibit angiogenesis. In a cancer setting, vascular microfluidic tools can help identify molecules inhibiting tumor angiogenesis and help elucidate the relationship of tumor extravasation in the vasculature. In order to better understand the early phase of metastatic processes, some microfluidic models have been designed to study the process of tumor extravasation.111–113 Buchanan et al.111 used co-cultures of MDA-MB-231 and ECs to study tumor angiogenesis. Interestingly, high wall shear stress had protective effects, downregulating several genes important for tumor angiogenesis such as MMP9, HIF1, and VEGFA. Jeon et al.112 used a microfluidic device to develop a 3D model of metastatic breast cancer extravasation in different environments. Using a bone- and muscle-mimicking environment, extravasation rates were found significantly increased in the bone-mimicking models. This microfluidic model elucidated the effects of different microenvironments and their effect in cancer cell extravasation. Additionally, the blockage of A3 adenosine receptors in cancer cells resulted in increased extravasation, enabling a deeper mechanistic insight into cancer extravasation, while also providing a valuable platform to test new pharmaceutical agents.
Several microfluidic platforms have been used for in vitro drug screening114 and for the development of drug delivery systems.24 Microfluidic models can be applied to study thrombosis,115,116 occlusion,117 and stenotic regions.118 Li et al.115 designed a microfluidic system (Fig. 6A) to study stenotic regions and thrombus formation under different shear rates. Low shear rates lead to longer occlusion times. However, the administration of increasing concentrations of the antiplatelet eptifibatide (Fig. 6B) in high shear stress did not reduce the occlusion times compared to no drug. This highlights the need of studying drug effects, in vitro, together with relevant biophysical stimuli such as shear rates. Another system that mimics a stenotic region (Fig. 6C, D) was used to design a “smart” drug.118 Blood vessels are often narrowed in thrombotic regions. At this site, wall shear stress can rapidly increase by two orders of magnitude. Korin et al.118 engineered microparticles that were responsive to shear stress that would thus breakup into smaller nanoparticles when exposed to the shear stresses observed in stenotic regions. By incorporating tissue plasminogen activator (tPA) in the nanoparticles, Korin et al. were able to show local delivery and rapid thrombolysis (Fig. 6E–G). This innovative platform illustrates how microfluidic biomimetic vascular systems can be used in the context of drug development. In this field, researchers have used the physical properties of the stenotic regions to target drugs, thus avoiding the need for systemic delivery of tPA and the resultant adverse side effects. Other vascular models have been used to study drug carriers and physical characteristics.24,119,120 Thrombotic and stenotic models are closely related; Muthard et al.116 engineered a microfluidic system (Fig. 6I) to probe the effects of wall shear stress and trans-thrombus pressure gradients in the thrombogenesis. In this system, a side flow region was filled with collagen gel with the pressure across it controlled by computer. The collagen area was exposed to the main fluidic channel and visualized directly on the device. As expected, the perfusion of whole blood induced thrombus formation at the collagen site (Fig. 6J, K). Interestingly, by varying the pressure gradient in the trans-thrombus collagen area, the authors observed a decrease in thrombin with higher pressure-gradients. This device can act as a useful tool to assess thrombotic areas and the hemodynamics of pressure gradients in the vessel wall.
FIG. 6.
Vascular models to study stenosis and thrombosis in vitro. (A) Microfluidic model of stenosis to study the effect of drugs on occlusion. (B) Effect of drug epitifibatide concentration on dissolving clots over time. (C) Microfluidic stenosis model used to test shear-activated microparticle formulation. (D) Photograph of the microfluidic device. (E) Exposure of clots to free or encapsulated t-PA (shear-activated microparticles). (F–H) Time-dependent thrombolysis of clots upon exposure to encapsulated t-PA. (I) Microfluidic device to study thrombosis in vitro containing a flow region (Q1) and a collagen region where pressure gradient is varied (ΔP). (J, K) Thrombus formation on collagen and collagen/TF hydrogels (white arrow indicates flow direction). [(A, B) adapted with permission from Li et al.115; (C–H) adapted with permission from Korin et al.118; (I–K) adapted with permission from Muthard et al.116.] Color images available online at www.liebertpub.com/aivt
Vascular permeability and dynamic condition models
Studies have also been conducted to evaluate the involvement of ECs in wound healing.121 In order to better understand the vascular wound healing processes, Franco et al.121 focused on the combined effect of flow and topographical cues on endothelial migration. A device was fabricated combining 1-μm-grated surfaces and a fluid flow parallel to these gratings. Interestingly, ECs cultured in such surfaces exhibited a much faster migration velocity compared to flat surfaces under the same flow rate. The research highlights the role of surface modifications and topographical microarchitectures, particularly ones mimicking the basal matrix, in wound healing. Therefore, in certain settings, wound healing might benefit from approaches that change topography, in addition to pharmacological interventions.
In a recent study by Arends et al.122 the authors developed a microfluidic device to probe the diffusive transport of analytes through basal lamina interfaces. By using ECM gels made from Engelbreth–Holm–Swarm sarcoma of mice, the authors were able to study permeability and the accumulation of different molecules at the interface. The accumulation of molecular at the ECM gel interface was charge-selective, and the results were corroborated in vivo. The system provided a platform to screen drugs and evaluated how changes in properties could affect interactions with the vascular basal lamina. Several other systems have been developed to study the permeability changes in the vasculature.123–127 Microfluidic collagen gels were fabricated123,125 and seeded with ECs to study barrier functions and permeability. Chrobak et al.123 showed that cells formed a strong barrier in these gels and responded to inflammatory stimuli by rapidly decreasing barrier permeability and increasing leucocyte adhesion. Price et al.125 demonstrated that low flows were related to higher permeability, whereas higher flows promoted a stronger barrier function, while increasing the lifespan of the endothelial constructs in vitro. This demonstrates the effects of biophysical parameters such as shear and transmural pressure in mediating barrier functions. Additionally, Lee et al.124 designed a simple microfluidic device to generate a tubular, perfusable microvessel network. Similarly, Chrobak et al.123 found that microvascular networks were responsive to inflammatory stimuli such as histamine and TNFα.124 Furthermore, U87MG cancer cells were used to mimic high-permeability conditions seen in neoplastic settings. A drug administered in vivo—bevacizumab—was able to reinstate permeability and improve barrier functions in agreement with other in vivo data.124 A more complex system envisioned by Sato et al.126 focused on barrier function and the vascular permeability of ECs and lymphatic ECs (LECs). The microfluidic chip (Fig. 7A) consisted of two channels separated by a porous membrane (Fig. 7B). LECs (Fig. 7C) and ECs (Fig. 5D) were cultured and maintained in different channels. With flows mimicking that of blood and lymphatic vessels, transport across and from EC channel to LEC channel was observed. In accordance to the aforementioned reports, histamine stimulated also the increase in permeability, and the authors further tested the system with habu snake venom (Fig. 7E), showing its effect on the barrier function.126 With the development of vascular permeability assays, it is essential to track changes in permeability and other parameters in real time. Young et al.127 developed a microfluidic system and technique to measure real-time endothelial permeability by using laser-induced fluorescence. Additional work developed by Li et al.128 generated a vascular lumen integrated with a nanowire array that was capable of measuring nitric oxide produced by ECs. Such systems could pave the way to the application of different permeability and barrier function assays within ECs.
FIG. 7.
Microfluidic model to study vascular permeability. (A) Photograph of microfluidic dual-channel model to study permeability under shear stress; (B) Schematic of the microfluidic device with the upper channel containing blood endothelial cells (BECs) and the lower channel containing lymphatic endothelial cells (LECs), separated by a porous membrane. (C) Immunostaining for LECs marker podoplanin (green) and DAPI (blue). (D) Immunostaining with endothelial-specific marker claudin-5. (E) Changes in permeability induced by exposure to habu snake venom. Adapted with permission from Sato et al.126 Color images available online at www.liebertpub.com/aivt
In addition to a considerable amount of research on vascular networks and fluid flow, some studies have incorporated additional biophysical hemodynamic parameters.129,130 Shao et al.129 generated a microfluidic chip that applied pulsatile and oscillatory shear stress to ECs, and Zheng et al.130 focused on the combined effects of shear and stretch. Together, these works highlight the need to incorporate complex dynamic conditions in order to fully mimic the vascular microenvironment.
There has been an increasing amount of work developed to recreate vascular networks in vivo. By combining biomaterials, microfluidics, and microarchitectures, we expect to be able to mimic the vascular wall in different settings, modeling disease, and ultimately generate better in vitro models for drug discovery and development.
Conclusions
Mimicking the cardiovascular system is challenging because of its high-level dynamics such as blood flow, stretching, and electrical stimulation. Several approaches have produced a wide range of microfluidic-based organs-on-a-chip to study different aspects of the cardiovascular system. Some of these have been used, in an academic context, to test and design different drugs and formulations. Although they present a tremendous leap forward for scientific knowledge, further work is required to translate and apply such technologies in drug discovery and development in industry.
Toward the translational pathway, a few startup companies have emerged, having raised significant amounts of funds and starting collaborations with industry partners. Here, we highlighted also the importance of regulatory agencies in the process of identifying new models and addressing concerns of reproducibility and standardization across the industry.
Although promising, cardiovascular organs-on-a-chip have not revolutionized the drug discovery process as of yet, and key challenges still lay ahead. Partnering industry with multidisciplinary academic teams can leverage the translational potential and help accelerate the development of technology. In short, the current progress and opportunities discussed highlight that cardiovascular organs-on-a-chip models still hold great promise for streamlining the drug discovery process.
Acknowledgments
The authors acknowledge funding from the National Science Foundation (EFRI-1240443), IMMODGEL (602694), and the NIH (EB012597, AR057837, DE021468, HL099073, AI105024, and AR063745). J.R. acknowledges the support from the Portuguese Foundation for Science and Technology (SFRH/BD/51679/2011). L.F. acknowledges the funds of FEDER through the program COMPETE and Portuguese funds through FCT in context of the project “CARDIOSTEM—Engineered Cardiac Tissues and Stem Cell-Based Therapies for Cardiovascular Applications” (Ref.: MITP-TB/ECE/0013/2013). J.L. acknowledges financial support from Innovative Research Incentives Scheme Veni #14328 of the Netherlands Organization for Scientific Research (NWO).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2015 Update: A report from the American Heart Association. Circulation 2014:131;e29–e322 [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature 2002:420;868–874 [DOI] [PubMed] [Google Scholar]
- 3.Lusis AJ. Atherosclerosis. Nature 2000:407;233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolini F, Gherli T. Alternatives to transplantation in the surgical therapy for heart failure. Eur J Cardiothorac Surg 2009:35;214–228 [DOI] [PubMed] [Google Scholar]
- 5.The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987:316;1429–1435 [DOI] [PubMed] [Google Scholar]
- 6.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991:325;293–302 [DOI] [PubMed] [Google Scholar]
- 7.Lechat P, Packer M, Chalon S, et al. Clinical effects of adrenergic blockade in chronic heart failure: A meta-analysis of double-blind, placebo-controlled, randomized trials. Circulation 1998:98;1184–1191 [DOI] [PubMed] [Google Scholar]
- 8.Cook D, Brown D, Alexander R, et al. Lessons learned from the fate of AstraZeneca's drug pipeline: A five-dimensional framework. Nat Rev Drug Discov 2014:13;419–431 [DOI] [PubMed] [Google Scholar]
- 9.Ciociola AA, Cohen LB, Kulkarni P. How drugs are developed and approved by the FDA: Current process and future directions. Am J Gastroenterol 2014:109;620–623 [DOI] [PubMed] [Google Scholar]
- 10.Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: The pharmaceutical industry's grand challenge. Nat Rev Drug Discov 2010:9;203–214 [DOI] [PubMed] [Google Scholar]
- 11.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 2004:3;711–715 [DOI] [PubMed] [Google Scholar]
- 12.Menna P, Salvatorelli E, Minotti G. Cardiotoxicity of antitumor drugs. Chem Res Toxicol 2008:21;978–989 [DOI] [PubMed] [Google Scholar]
- 13.Piccini JP, Whellan DJ, Berridge BR, et al. Current challenges in the evaluation of cardiac safety during drug development: Translational medicine meets the Critical Path Initiative. Am Heart J 2009:158;317–326 [DOI] [PubMed] [Google Scholar]
- 14.Shah RR. Can pharmacogenetics help rescue drugs withdrawn from the market? Pharmacogenomics 2006:7;889–908 [DOI] [PubMed] [Google Scholar]
- 15.Chu X, Bleasby K, Evers R. Species differences in drug transporters and implications for translating preclinical findings to humans. Expert Opin Drug Metab Toxicol 2013:9;237–252 [DOI] [PubMed] [Google Scholar]
- 16.Hirt MN, Hansen A, Eschenhagen T. Cardiac tissue engineering: State of the art. Circ Res 2014:114;354–367 [DOI] [PubMed] [Google Scholar]
- 17.Zhang WJ, Liu W, Cui L, et al. Tissue engineering of blood vessel. J Cell Mol Med 2007:11;945–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benam KH, Dauth S, Hassell B, et al. Engineered in vitro disease models. Annu Rev Pathol 2015:10;195–262 [DOI] [PubMed] [Google Scholar]
- 19.Li S, Sengupta D, Chien S. Vascular tissue engineering: From in vitro to in situ. WIREs Syst Biol Med 2014:6;61–76 [DOI] [PubMed] [Google Scholar]
- 20.Mathur A, Ma Z, Loskill P, et al. In vitro cardiac tissue models: Current status and future prospects. Adv Drug Deliv Rev 2016;96:203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 2014:32;760–772 [DOI] [PubMed] [Google Scholar]
- 22.Polini A, Prodanov L, Bhise NS, et al. Organs-on-a-chip: A new tool for drug discovery. Exp Opin Drug Discov 2014:9;335–352 [DOI] [PubMed] [Google Scholar]
- 23.Zhang YS, Aleman J, Arneri A, et al. From cardiac tissue engineering to heart-on-a-chip: Beating challenges. Biomed Mater 2015:10;034006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhise NS, Ribas J, Manoharan V, et al. Organ-on-a-chip platforms for studying drug delivery systems. J Control Release 2014:190;82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghaemmaghami AM, Hancock MJ, Harrington H, et al. Biomimetic tissues on a chip for drug discovery. Drug Discov Today 2012:17;173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics. Underlying Cause of Death 1999–2013. Center for Disease Control and Prevention; 2015 [PubMed] [Google Scholar]
- 27.World Health Organization. Prevention of Cardiovascular Disease: Guidelines for Assessment and Management of Cardiovascular Risk. Geneva: WHO; 2007 [Google Scholar]
- 28.Fuster V. Top 10 cardiovascular therapies and interventions for the next decade. Nat Rev Cardiol 2014:11;671–683 [DOI] [PubMed] [Google Scholar]
- 29.Scannell JW, Blanckley A, Boldon H, et al. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov 2012:11;191–200 [DOI] [PubMed] [Google Scholar]
- 30.Bloomberg Business. Drug Testing, Now Without the Chimp. Bloomberg Business; 2015 [Google Scholar]
- 31.Holmes A, Bonner F, Jones D. Assessing drug safety in human tissues—what are the barriers? Nat Rev Drug Discov 2015;14:585–587 [DOI] [PubMed] [Google Scholar]
- 32.Dahlin JL, Inglese J, Walters MA. Mitigating risk in academic preclinical drug discovery. Nat Rev Drug Discov 2015:14;279–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pound P, Ebrahim S, Sandercock P, et al. Where is the evidence that animal research benefits humans? BMJ 2004:328;514–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang MG, Zhang Y, Chang CY, et al. Spiral waves and reentry dynamics in an in vitro model of the healed infarct border zone. Circ Res 2009:105;1062–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katare RG, Ando M, Kakinuma Y, et al. Engineered heart tissue: A novel tool to study the ischemic changes of the heart in vitro. PloS One 2010:5;e9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bracken MB. Why animal studies are often poor predictors of human reactions to exposure. J R Soc Med 2009:102;120–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnard ND, Kaufman SR. Animal research is wasteful and misleading. Sci Am 1997:276;80–82 [DOI] [PubMed] [Google Scholar]
- 38.Cato A, Sutton L, Cato A., III Clinical Drug Trials and Tribulations, Revised and Expanded. New York: Marcel Dekker, Inc.; 2002 [Google Scholar]
- 39.Mattison DR. Improving pediatric drug development: Challenges, opportunities and lessons learned. Front Pharmacol 2010:1;1–221607058 [Google Scholar]
- 40.Institute of Medicine (U.S.) Committee on Accelerating Rare Diseases Research and Orphan Product Development. Rare Diseases and Orphan Products: Accelerating Research and Development (2011). Washington, DC: National Academies Press; 2010 [Google Scholar]
- 41.Junod SW. FDA and Clinical Drug Trials: A Short History. U.S. Food and Drug Administration; 2014 [Google Scholar]
- 42.Ralph Landau BA, Scriabine A. Pharmaceutical Innovation: Revolutionizing Human Health. New York: Chemical Heritage Foundation; 1999 [Google Scholar]
- 43.Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov 2010:9;203–214 [DOI] [PubMed] [Google Scholar]
- 44.Alcendor DJ, Block FE, III, Cliffel DE, et al. Neurovascular unit on a chip: Implications for translational applications. Stem Cell Res Ther 2013:4;1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathur A, Loskill P, Shao K, et al. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep 2015:5;8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang G, McCain ML, Yang L, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with iPSC and heart-on-chip technologies. Nat Methods 2014:20;616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gunther A, Yasotharan S, Vagaon A, et al. A microfluidic platform for probing small artery structure and function. Lab Chip 2010:10;2341–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCain ML, Sheehy SP, Grosberg A, et al. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc Natl Acad Sci U S A 2013:110;9770–9775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reardon S. ‘Organs-on-chips’ go mainstream. Nature 2015:523;266. [DOI] [PubMed] [Google Scholar]
- 50.Vladisavljevic GT, Khalid N, Neves MA, et al. Industrial lab-on-a-chip: Design, applications and scale-up for drug discovery and delivery. Adv Drug Deliv Rev 2013:65;1626–1663 [DOI] [PubMed] [Google Scholar]
- 51.Gregorio CC, Antin PB. To the heart of myofibril assembly. Trends Cell Biol 2000:10;355–362 [DOI] [PubMed] [Google Scholar]
- 52.Holt E, Lunde PK, Sejersted OM, et al. Electrical stimulation of adult rat cardiomyocytes in culture improves contractile properties and is associated with altered calcium handling. Basic Res Cardiol 1997:92;289–298 [DOI] [PubMed] [Google Scholar]
- 53.Brutsaert DL. Cardiac endothelial-myocardial signaling: Its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev 2003:83;59–115 [DOI] [PubMed] [Google Scholar]
- 54.Roberts DE, Scher AM. Effect of tissue anisotropy on extracellular potential fields in canine myocardium in situ. Circ Res 1982:50;342–351 [DOI] [PubMed] [Google Scholar]
- 55.Souders CA, Bowers SLK, Baudino TA. Cardiac fibroblast: The renaissance cell. Circ Res 2009:105;1164–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neuži P, Giselbrecht S, Länge K, et al. Revisiting lab-on-a-chip technology for drug discovery. Nat Rev Drug Discov 2012:11;620–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duffy DC, McDonald JC, Schueller OJ, et al. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem 1998:70;4974–4984 [DOI] [PubMed] [Google Scholar]
- 58.Halldorsson S, Lucumi E, Gómez-Sjöberg R, et al. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron 2015:63;218–231 [DOI] [PubMed] [Google Scholar]
- 59.Annabi N, Selimović Š, Acevedo Cox JP, et al. Hydrogel-coated microfluidic channels for cardiomyocyte culture. Lab Chip 2013:13;3569–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nichol JW, Koshy ST, Bae H, et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010:31;5536–5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wise SG, Mithieux SM, Weiss AS. Engineered tropoelastin and elastin-based biomaterials. Adv Protein Chem Struct Biol 2009:78;1–24 [DOI] [PubMed] [Google Scholar]
- 62.Annabi N, Shin SR, Tamayol A, et al. Highly elastic and conductive human-based protein hybrid hydrogels. Adv Mater 2016;28:40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saffitz JE, Kanter HL, Green KG, et al. Tissue-specific determinants of anisotropic conduction velocity in canine atrial and ventricular myocardium. Circ Res 1994:74;1065–1070 [DOI] [PubMed] [Google Scholar]
- 64.Spach MS, Heidlage JF, Dolber PC, et al. Changes in anisotropic conduction caused by remodeling cell size and the cellular distribution of gap junctions and Na(+) channels. J Electrocardiol 2001:34;69–76 [DOI] [PubMed] [Google Scholar]
- 65.Bian W, Jackman CP, Bursac N. Controlling the structural and functional anisotropy of engineered cardiac tissues. Biofabrication 2014:6;024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grosberg A, Alford PW, McCain ML, et al. Ensembles of engineered cardiac tissues for physiological and pharmacological study: Heart on a chip. Lab Chip 2011:11;4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tandon N, Cannizzaro C, Chao PH, et al. Electrical stimulation systems for cardiac tissue engineering. Nat Protoc 2009:4;155–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aubin H, Nichol JW, Hutson CB, et al. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials 2010:31;6941–6951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agarwal A, Goss JA, Cho A, et al. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 2013:13;3599–3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Au HT, Cui B, Chu ZE, et al. Cell culture chips for simultaneous application of topographical and electrical cues enhance phenotype of cardiomyocytes. Lab Chip 2009:91;564–575 [DOI] [PubMed] [Google Scholar]
- 71.Xiao Y, Zhang B, Liu H, et al. Microfabricated perfusable cardiac biowire: A platform that mimics native cardiac bundle. Lab Chip 2014:14;869–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davis RP, Casini S, van den Berg CW, et al. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation 2012:125;3079–3091 [DOI] [PubMed] [Google Scholar]
- 73.Itzhaki I, Maizels L, Huber I, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 2011:471;225–229 [DOI] [PubMed] [Google Scholar]
- 74.Sun N, Yazawa M, Liu J, et al. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med 2012:4;130ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mosadegh B, Dabiri BE, Lockett MR, et al. Three-dimensional paper-based model for cardiac ischemia. Adv Healthc Mater 2014:3;1036–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song H, Zandstra PW, Radisic M. Engineered heart tissue model of diabetic myocardium. Tissue Eng Part A 2011:17;1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stöhr A, Friedrich FW, Flenner F, et al. Contractile abnormalities and altered drug response in engineered heart tissue from Mybpc3-targeted knock-in mice. J Mol Cell Cardiol 2013:63;189–198 [DOI] [PubMed] [Google Scholar]
- 78.Li AH, Liu PP, Villarreal FJ, et al. Dynamic changes in myocardial matrix and relevance to disease: Translational perspectives. Circ Res 2014:114;916–927 [DOI] [PubMed] [Google Scholar]
- 79.Ieda M, Tsuchihashi T, Ivey KN, et al. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell 2009:16;233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schroer AK, Merryman WD. Mechanobiology of myofibroblast adhesion in fibrotic cardiac disease. J Cell Sci 2015:128;1865–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCain ML, Parker KK. Mechanotransduction: The role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch 2011:462;89–104 [DOI] [PubMed] [Google Scholar]
- 82.Ho CY. Hypertrophic cardiomyopathy: Preclinical and early phenotype. J Cardiovasc Transl Res 2009:2;462–470 [DOI] [PubMed] [Google Scholar]
- 83.Ren L, Liu W, Wang Y, et al. Investigation of hypoxia-induced myocardial injury dynamics in a tissue interface mimicking microfluidic device. Anal Chem 2013:85;235–244 [DOI] [PubMed] [Google Scholar]
- 84.Garbern JC, Lee RT. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell 2013:12;689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He J, Ma C, Liu W, et al. On-chip monitoring of skeletal myoblast transplantation for the treatment of hypoxia-induced myocardial injury. Analyst 2014:1391;4482–4490 [DOI] [PubMed] [Google Scholar]
- 86.Chen MB, Srigunapalan S, Wheeler AR, et al. A 3D microfluidic platform incorporating methacrylated gelatin hydrogels to study physiological cardiovascular cell-cell interactions. Lab Chip 2013:13;2591–2598 [DOI] [PubMed] [Google Scholar]
- 87.Taylor PM, Batten P, Brand NJ, et al. The cardiac valve interstitial cell. Int J Biochem Cell Biol 2003:35;113–118 [DOI] [PubMed] [Google Scholar]
- 88.Wipff PJ, Rifkin DB, Meister JJ, et al. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 2007:179;1311–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Merryman WD, Schoen FJ. Mechanisms of calcification in aortic valve disease: Role of mechanokinetics and mechanodynamics. Curr Cardiol Rep 2013:15;355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rajamannan NM, Evans FJ, Aikawa E, et al. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation 2011:124;1783–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Simmons CA, Grant GR, Manduchi E, et al. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res 2005:96;792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martins AM, Vunjak-Novakovic G, Reis RL. The current status of iPS cells in cardiac research and their potential for tissue engineering and regenerative medicine. Stem Cell Rev 2014:10;177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mercola M, Colas A, Willems E. Induced pluripotent stem cells in cardiovascular drug discovery. Circ Res 2013:112;534–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang G, McCain ML, Yang L, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 2014:20;616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bianchi E, Molteni R, Pardi R, et al. Microfluidics for in vitro biomimetic shear stress-dependent leukocyte adhesion assays. J Biomech 2013:46;276–283 [DOI] [PubMed] [Google Scholar]
- 96.Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng 2007:9;229–256 [DOI] [PubMed] [Google Scholar]
- 97.Wong KH, Chan JM, Kamm RD, et al. Microfluidic models of vascular functions. Annu Rev Biomed Eng 2012:14;205–230 [DOI] [PubMed] [Google Scholar]
- 98.Zhang W, Zhang YS, Bakht M, et al. Elastomeric free-form blood vessels for interconnecting organs on chip systems. Lab Chip 2016. DOI: 10.1039/C6LC00001K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim S, Lee H, Chung M, et al. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 2013:13;1489–1500 [DOI] [PubMed] [Google Scholar]
- 100.Bertassoni LE, Cardoso JC, Manoharan V, et al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 2014:6;024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hasenberg T, Muhleder S, Dotzler A, et al. Emulating human microcapillaries in a multi-organ-chip platform. J Biotechnol 2015:216;1–10 [DOI] [PubMed] [Google Scholar]
- 102.Morgan JP, Delnero PF, Zheng Y, et al. Formation of microvascular networks in vitro. Nat Protoc 2013:8;1820–1836 [DOI] [PubMed] [Google Scholar]
- 103.Wang XY, Jin ZH, Gan BW, et al. Engineering interconnected 3D vascular networks in hydrogels using molded sodium alginate lattice as the sacrificial template. Lab Chip 2014:14;2709–2716 [DOI] [PubMed] [Google Scholar]
- 104.Yeon JH, Ryu HR, Chung M, et al. In vitro formation and characterization of a perfusable three-dimensional tubular capillary network in microfluidic devices. Lab Chip 2012:12;2815–2822 [DOI] [PubMed] [Google Scholar]
- 105.Schimek K, Busek M, Brincker S, et al. Integrating biological vasculature into a multi-organ-chip microsystem. Lab Chip 2013:13;3588–3598 [DOI] [PubMed] [Google Scholar]
- 106.Bogorad MI, DeStefano J, Karlsson J, et al. Review: in vitro microvessel models. Lab Chip 2015:15;4242–4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yasotharan S, Pinto S, Sled JG, et al. Artery-on-a-chip platform for automated, multimodal assessment of cerebral blood vessel structure and function. Lab Chip 2015:15;2660–2669 [DOI] [PubMed] [Google Scholar]
- 108.Rosano JM, Tousi N, Scott RC, et al. A physiologically realistic in vitro model of microvascular networks. Biomed Microdevices 2009:11;1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bischel LL, Young EW, Mader BR, et al. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 2013:34;1471–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Galie PA, Nguyen DH, Choi CK, et al. Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci U S A 2014:111;7968–7973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Buchanan CF, Verbridge SS, Vlachos PP, et al. Flow shear stress regulates endothelial barrier function and expression of angiogenic factors in a 3D microfluidic tumor vascular model. Cell Adh Migr 2014:8;517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jeon JS, Bersini S, Gilardi M, et al. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci U S A 2015:112;214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang XY, Pei Y, Xie M, et al. An artificial blood vessel implanted three-dimensional microsystem for modeling transvascular migration of tumor cells. Lab Chip 2015:15;1178–1187 [DOI] [PubMed] [Google Scholar]
- 114.Tsui JH, Lee W, Pun SH, et al. Microfluidics-assisted in vitro drug screening and carrier production. Adv Drug Deliv Rev 2013:65;1575–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li M, Hotaling NA, Ku DN, et al. Microfluidic thrombosis under multiple shear rates and antiplatelet therapy doses. PLoS One 2014:9;e82493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Muthard RW, Diamond SL. Side view thrombosis microfluidic device with controllable wall shear rate and transthrombus pressure gradient. Lab Chip 2013:13;1883–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsai M, Kita A, Leach J, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest 2012:122;408–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Korin N, Kanapathipillai M, Matthews BD, et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science 2012:337;738–742 [DOI] [PubMed] [Google Scholar]
- 119.Doshi N, Prabhakarpandian B, Rea-Ramsey A, et al. Flow and adhesion of drug carriers in blood vessels depend on their shape: A study using model synthetic microvascular networks. J Control Release 2010:146;196–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu R, Li F, Lv J, et al. Microfluidic analysis of pressure drop and flow behavior in hypertensive micro vessels. Biomed Microdevices 2015:17;9959. [DOI] [PubMed] [Google Scholar]
- 121.Franco D, Milde F, Klingauf M, et al. Accelerated endothelial wound healing on microstructured substrates under flow. Biomaterials 2013:34;1488–1497 [DOI] [PubMed] [Google Scholar]
- 122.Arends F, Sellner S, Seifert P, et al. A microfluidics approach to study the accumulation of molecules at basal lamina interfaces. Lab Chip 2015:15;3326–3334 [DOI] [PubMed] [Google Scholar]
- 123.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res 2006:71;185–196 [DOI] [PubMed] [Google Scholar]
- 124.Lee H, Kim S, Chung M, et al. A bioengineered array of 3D microvessels for vascular permeability assay. Microvasc Res 2014:91;90–98 [DOI] [PubMed] [Google Scholar]
- 125.Price GM, Wong KH, Truslow JG, et al. Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 2010:31;6182–6189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sato M, Sasaki N, Ato M, et al. Microcirculation-on-a-chip: A microfluidic platform for assaying blood- and lymphatic-vessel permeability. PLoS One 2015:10;e0137301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Young EW, Watson MW, Srigunapalan S, et al. Technique for real-time measurements of endothelial permeability in a microfluidic membrane chip using laser-induced fluorescence detection. Anal Chem 2010:82;808–816 [DOI] [PubMed] [Google Scholar]
- 128.Li LM, Wang XY, Hu LS, et al. Vascular lumen simulation and highly-sensitive nitric oxide detection using three-dimensional gelatin chip coupled to TiC/C nanowire arrays microelectrode. Lab Chip 2012:12;4249–4256 [DOI] [PubMed] [Google Scholar]
- 129.Shao J, Wu L, Wu J, et al. Integrated microfluidic chip for endothelial cells culture and analysis exposed to a pulsatile and oscillatory shear stress. Lab Chip 2009:9;3118–125 [DOI] [PubMed] [Google Scholar]
- 130.Zheng W, Jiang B, Wang D, et al. A microfluidic flow-stretch chip for investigating blood vessel biomechanics. Lab Chip 2012:12;3441–3450 [DOI] [PubMed] [Google Scholar]