Abstract
The molecular chaperone heat shock protein 90 (Hsp90) is a pivotal cellular regulator involved in the folding, activation and assembly of a wide range of proteins. Hsp90 has multiple roles in the retina and the use of different Hsp90 inhibitors has been shown to prevent retinal degeneration in models of retinitis pigmentosa and age-related macular degeneration. Hsp90 is also a potential target in uveal melanoma. Mechanistically, Hsp90 inhibition can evoke a dual response in the retina; stimulating a stress response with molecular chaperone expression. Thereby leading to an improvement in visual function and photoreceptor survival; however, prolonged inhibition can also potentially deleteriously affect vision. Here, we review the multiple roles of Hsp90 in the retina and the therapeutic potential of Hsp90 as a target.
22.1. Introduction
Hsp90 is an abundant molecular chaperone involved in many cellular processes. It plays a role in the folding, stability, maturation, intracellular transport, maintenance, and degradation of a number of client proteins. These clients include proteins involved in signal transduction, protein trafficking, and innate and adaptive immunity. Hsp90 is one of the most conserved heat shock proteins and is an essential component of the protective heat shock response, therefore playing a role in regulating cell physiology under normal and stressed conditions (McClellan et al. 2007). Hsp90 is expressed in the cytosol and the nucleus and contains an N-terminal ATP-binding domain that is essential for most of its cellular functions. Hsp90 has been shown to suppress the aggregation of a wide range of client proteins and hence acts as a general protective chaperone. Certain Hsp90 inhibitors (e.g. geldanamycin, 17-AAG or HSP990) bind with a high affinity to the ATP-binding pocket and block the chaperone ATPase cycle leading to the degradation of client proteins that can no longer be folded (Li and Buchner 2013). In addition, under resting conditions Hsp90 binds the stress responsive transcription factor, heat shock factor 1 (HSF-1), to silence the transcription factor activity and forms an auto-regulatory feedback loop that couples molecular chaperone levels to the need for chaperones to bind misfolded proteins (Neueder et al. 2014). Inhibition of Hsp90 leads to the release of HSF-1 and the activation of the stress response and an increase in molecular chaperones. Therefore, Hsp90 inhibition can either lead to the proteasome-mediated degradation of Hsp90 client proteins or upregulation of molecular chaperones, such as Hsp70 and Hsp40, which results in an enhanced protective effect against protein aggregation and reduced protein toxicity (Labbadia et al. 2011).
The retina is a complex tissue with a high metabolic demand, constantly exposed to stress (Athanasiou et al. 2013). To maintain cell homeostasis and prevent damage, the retina contains high levels of heat shock proteins under normal conditions (Urbak and Vorum 2010). Hsp90 is widely distributed in all retinal layers, from the retinal ganglion cells (RGC) to the inner segment (IS), the tips of the outer segment (OS) and retinal pigment epithelium (RPE) cells (Dean and Tytell 2001). Hsp90 plays an indispensable role in homeostasis of the retina as prolonged Hsp90 inhibition leads to photoreceptor cell death (Kanamaru et al. 2014).
22.2. Manipulation of Hsp90 as a potential therapy for retinal degeneration
Pharmacological intervention with compounds that target Hsp90 function could potentially be therapeutic against several different forms of retinal degeneration and pathology.
22.2.1. Retinitis pigmentosa (RP)
RP is the most common form of inherited photoreceptor degeneration and mutations in the rhodopsin gene are the most common cause of autosomal dominant RP. It has been previously shown that the Hsp90 inhibitor 17-N-allylamino-17-demethoxygeldanamycin (17-AAG) can protect against rhodopsin aggregation and toxicity in a cell model of a class II misfolding mutation in rhodopsin, P23H, which is the most common rhodopsin mutation in the USA (Mendes and Cheetham 2008). This protection appears to be dependent on HSF-1, as mouse embryonic fibroblasts from HSF-1 knock-out mice were not protected against P23H rhodopsin aggregation by 17-AAG, suggesting that the protective effect is dependent on induction of the stress response (Aguila et al. 2014). Systemic administration of the blood brain barrier permeable Hsp90 inhibitor, HSP990, can activate HSF-1 and stimulate molecular chaperone expression in vivo in the retina (Aguila et al. 2014). In a P23H rhodopsin transgenic rat model with progressive retinal degeneration, a single low dose of HSP990 was sufficient to mediate an improvement in visual function and photoreceptor survival several weeks later. Importantly, this treatment did not affect any phototransduction component, but did induce molecular chaperones and reduced rhodopsin aggregation, showing the ability of Hsp90 inhibition to stimulate the proteostasis machinery that protects against misfolded proteins (Aguila et al. 2014). Other examples of how imbalances in photoreceptor proteostasis can be targeted with Hsp90 inhibition are IMPDH misfolding mutations associated with RP10. In this instance, claudin 5 RNAi was used to transiently permeabilize the blood retinal barrier and allow 17-AAG to stimulate a protective response in photoreceptors expressing R224P mutant IMPDH, with a concomitant reduction in mutant IMPDH aggregation and protection of ONL structure (Tam et al. 2010).
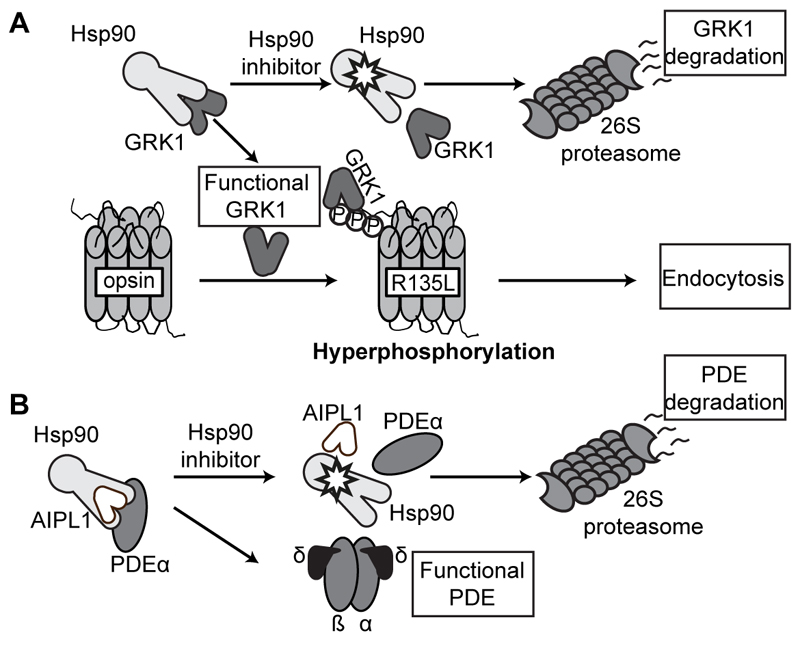
Interestingly, in a disease model for a different class of rhodopsin mutation (R135L) inhibition of Hsp90 was also protective, but this was independent of HSF-1. The R135L mutation causes rhodopsin hyperphosphorylation, arrestin binding and aberrant rhodopsin endocytosis (Fig. 22.1A), which deleteriously affects vesicular traffic (Chuang et al. 2004). Hsp90 inhibition blocked the recruitment of arrestin to R135L mutant rhodopsin and thereby alleviated aberrant endocytosis (Aguila et al. 2014). This effect was still maintained in HSF-1 null cells, showing that it was independent of HSF-1. Further investigation revealed that, like many kinases, rhodopsin kinase (GRK1) is an obligate Hsp90 client protein and the effect of Hsp90 inhibition on R135L rhodopsin arrestin binding was mediated by an upstream reduction in phosphorylation of R135L because of lack of an appropriate kinase (Aguila et al. 2014). This mechanism related to the reduction of a specific client protein that is mediating an adverse effect of a genetic mutation is distinct from the enhanced production of protective factors through the activation of the stress response to combat a mutational consequence. Overall, these data suggest that Hsp90 has multiple roles in the retina and that the use of Hsp90 inhibitors can be potentially protective against different types of RP through different mechanisms.
Figure 22.1. Hsp90 is required for GRK1 and PDE function.
(A) GRK1 requires Hsp90 for maturation. R135L rhodopsin mutant is hyperphosphorylated by functional GRK1 leading to arrestin binding and endocytosis. Hsp90 inhibitors prevent Hsp90 mediated GRK1 folding, leading to GRK1 degradation and loss of R135L hyperphosphrylation. (B) PDE needs Hsp90 and its co-chaperone AIPL1 for maturation. Hsp90 inhibition blocks the Hsp90-AIPL1 interaction, resulting in PDE degradation.
22.2.2. Age-related macular degeneration (AMD) and RPE biology
AMD is a complex multifactorial disease involving genetic, environmental, metabolic, and functional factors. Functional abnormalities and cell death in the RPE cells contribute to the development of AMD, and are associated with increased oxidative stress (Jarrett and Boulton 2012). Hsp90 is expressed in RPE cells and its expression increases significantly during the progression of AMD (Decanini et al. 2007). It has been suggested that Hsp90 expressed from necrotic RPE cells may function as a trigger for inflammatory responses in adjacent healthy RPE (Qin et al. 2011). Inflammatory responses in RPE cells can be blocked by Hsp90 inhibition (Wang et al. 2010). Moreover, the Hsp90 inhibitor geldanamycin inhibits VEGF expression induced by hypoxia in RPE cells (Wu et al. 2007), suggesting that Hsp90 inhibitors may be effective in blocking both inflammation and neovascularization.
22.2.3. Ocular oncology: Uveal melanoma
Hsp90 is a major target in oncology as several aspects of tumor cell viability are reliant on Hsp90 function. Uveal melanoma (UM) is the most common primary intraocular malignancy in adults (Egan et al. 1988) and Hsp90 is emerging as a potentially important target in UM. Focal adhesion kinase (FAK) is a cytoplasmic tyrosine kinase that plays a central role in several cellular processes including mediation of extracellular matrix-integrin signaling, cell migration, invasion and metastasis in several cancers, including UM (Hess et al. 2005). Hsp90 is crucial for the stability and functional conformation of FAK, as inhibition of Hsp90 interferes with its phosphorylation and stimulates its proteasome-mediated degradation (Faingold et al. 2008). Hsp90 inhibition resulted in a reduction of migration and invasion of cancer cells through FAK-mediated pathways (Faingold et al. 2008). Furthermore, the protein kinase Akt also requires Hsp90 for its activity and stability (Basso et al. 2002) and high levels of phosphorylated Akt (p-Akt) have been shown to be associated with a higher risk of metastatic disease in patients with UM (Saraiva et al. 2005). Treatment of human UM cell lines with 17-AAG resulted in a decrease of Akt and activated p-Akt, possibly contributing to cell growth arrest and induction of cell death. In addition, 17-AAG and 17-DMAG inhibited cell proliferation in WTB-Raf UM cell lines by downregulating the WTB-Raf protein. This downregulation led to the inactivation of the MEK/ERK module and the decrease in cyclin D1, which is necessary for the proliferation of UM cell lines (Babchia et al. 2008). Overall, these data suggest that Hsp90 inhibition could be a possible therapy against this type of cancer.
22.2.4. Therapeutic considerations
Recent reports from oncology clinical trials have suggested that some Hsp90 inhibitors, such as 17-DMAG and AUY922, might lead to visual disturbances (Sessa et al. 2013). In a recent clinical trial for advanced solid tumors using AUY922, 43% of the patients reported grades 1–3 visual symptoms, including night blindness, photopsia, blurred vision and visual impairment (Rajan et al. 2011). Fortunately, all the visual symptoms were reversible when drug use was discontinued. It is therefore important to identify the molecular mechanism by which Hsp90 inhibitors affect vision. As predicted by the studies on R135L rhodopsin, prolonged systemic Hsp90 inhibition led to a reduction of GRK1 levels in the retina, confirming that Hsp90 is required for GRK1 biosynthesis (Aguila et al. 2014). Furthermore, phosphodiesterase (PDE) levels were also specifically reduced in the retina following Hsp90 inhibition (Aguila et al. 2014). The Leber congential amaurosis (LCA) gene product AIPL1 is a cochaperone for Hsp90 and is essential for PDE biosynthesis (Hidalgo-de-Quintana et al. 2008), suggesting that Hsp90 and AIPL1 co-operate in PDE biosynthesis (Fig. 22.1B). Reduction in GRK1 and PDE could cause some of the most common visual side-effects of Hsp90 inhibitors observed in oncology patients. Therefore, the effects of Hsp90 inhibition on visual function are likely to relate to essential Hsp90 client proteins in the phototransduction pathway in the retina and potentially elsewhere in the eye.
22.3. Conclusions
A range of Hsp90 inhibitors have now been developed with different affinities and bioavailability. Importantly, several Hsp90 inhibitors have been studied in oncology clinical trials and their pharmacokinetic profile and side effects have been identified. Therefore, they could potentially be applied to RP and other neurodegenerative disease with prior knowledge of the risks and benefits. Collectively, the data show that Hsp90 has multiple roles in the retina and that the use of Hsp90 inhibitors can be potentially protective against retinal degeneration and ocular oncology, but their possible adverse effects on visual function also need to be considered.
Acknowledgments
This work is supported by the Wellcome Trust and RP Fighting Blindness.
References
- Aguila M, Bevilacqua D, McCulley C, et al. Hsp90 inhibition protects against inherited retinal degeneration. Hum Mol Genet. 2014;23:2164–2175. doi: 10.1093/hmg/ddt613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou D, Aguila M, Bevilacqua D, et al. The cell stress machinery and retinal degeneration. FEBS Lett. 2013;587:2008–17. doi: 10.1016/j.febslet.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babchia N, Calipel A, Mouriaux F, et al. 17-AAG and 17-DMAG-induced inhibition of cell proliferation through B-Raf downregulation in WT B-Raf-expressing uveal melanoma cell lines. Invest Ophthalmol Vis Sci. 2008;49:2348–2356. doi: 10.1167/iovs.07-1305. [DOI] [PubMed] [Google Scholar]
- Basso AD, Solit DB, Chiosis G, et al. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277:39858–39866. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- Chuang JZ, Vega C, Jun W, et al. Structural and functional impairment of endocytic pathways by retinitis pigmentosa mutant rhodopsin-arrestin complexes. J Clin Invest. 2004;114:131–140. doi: 10.1172/JCI21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DO, Tytell M. Hsp25 and -90 immunoreactivity in the normal rat eye. Invest Ophthalmol Vis Sci. 2001;42:3031–3040. [PubMed] [Google Scholar]
- Decanini A, Nordgaard CL, Feng X, et al. Changes in select redox proteins of the retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol. 2007;143:607–615. doi: 10.1016/j.ajo.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan KM, Seddon JM, Glynn RJ, et al. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol. 1988;32:239–251. doi: 10.1016/0039-6257(88)90173-7. [DOI] [PubMed] [Google Scholar]
- Faingold D, Marshall JC, Antecka E, et al. Immune expression and inhibition of heat shock protein 90 in uveal melanoma. Clin Cancer Res. 2008;14:847–855. doi: 10.1158/1078-0432.CCR-07-0926. [DOI] [PubMed] [Google Scholar]
- Hess AR, Postovit LM, Margaryan NV, et al. Focal adhesion kinase promotes the aggressive melanoma phenotype. Cancer Res. 2005;65:9851–9860. doi: 10.1158/0008-5472.CAN-05-2172. [DOI] [PubMed] [Google Scholar]
- Hidalgo-de-Quintana J, Evans RJ, Cheetham ME, et al. The Leber congenital amaurosis protein AIPL1 functions as part of a chaperone heterocomplex. Invest Ophthalmol Vis Sci. 2008;49:2878–2887. doi: 10.1167/iovs.07-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med. 2012;33:399–417. doi: 10.1016/j.mam.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamaru C, Yamada Y, Hayashi S, et al. Retinal toxicity induced by small-molecule Hsp90 inhibitors in beagle dogs. J Toxicol Sci. 2014;39:59–69. doi: 10.2131/jts.39.59. [DOI] [PubMed] [Google Scholar]
- Labbadia J, Cunliffe H, Weiss A, et al. Altered chromatin architecture underlies progressive impairment of the heat shock response in mouse models of Huntington disease. J Clin Invest. 2011;121:3306–3319. doi: 10.1172/JCI57413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Buchner J. Structure, function and regulation of the hsp90 machinery. Biomedical journal. 2013;36:106–117. doi: 10.4103/2319-4170.113230. [DOI] [PubMed] [Google Scholar]
- McClellan AJ, Xia Y, Deutschbauer AM, et al. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell. 2007;131:121–135. doi: 10.1016/j.cell.2007.07.036. [DOI] [PubMed] [Google Scholar]
- Mendes HF, Cheetham ME. Pharmacological manipulation of gain-of-function and dominant-negative mechanisms in rhodopsin retinitis pigmentosa. Hum Mol Genet. 2008;17:3043–3054. doi: 10.1093/hmg/ddn202. [DOI] [PubMed] [Google Scholar]
- Neueder A, Achilli F, Moussaoui S, et al. Novel Isoforms of Heat Shock Transcription Factor 1, HSF1gammaalpha and HSF1gammabeta, Regulate Chaperone Protein Gene Transcription. J Biol Chem. 2014;289:19894–19906. doi: 10.1074/jbc.M114.570739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Ni M, Wang X, et al. Inhibition of RPE cell sterile inflammatory responses and endotoxin-induced uveitis by a cell-impermeable HSP90 inhibitor. Exp Eye Res. 2011;93:889–897. doi: 10.1016/j.exer.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Rajan A, Kelly RJ, Trepel JB, et al. A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. Clin Cancer Res. 2011;17:6831–6839. doi: 10.1158/1078-0432.CCR-11-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva VS, Caissie AL, Segal L, et al. Immunohistochemical expression of phospho-Akt in uveal melanoma. Melanoma Res. 2005;15:245–250. doi: 10.1097/00008390-200508000-00003. [DOI] [PubMed] [Google Scholar]
- Sessa C, Shapiro GI, Bhalla KN, et al. First-in-human phase I dose-escalation study of the HSP90 inhibitor AUY922 in patients with advanced solid tumors. Clin Cancer Res. 2013;19:3671–3680. doi: 10.1158/1078-0432.CCR-12-3404. [DOI] [PubMed] [Google Scholar]
- Tam LC, Kiang AS, Campbell M, et al. Prevention of autosomal dominant retinitis pigmentosa by systemic drug therapy targeting heat shock protein 90 (Hsp90) Hum Mol Genet. 2010;19:4421–4436. doi: 10.1093/hmg/ddq369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbak L, Vorum H. Heat shock proteins in the human eye. International journal of proteomics. 2010;2010:479571. doi: 10.1155/2010/479571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YQ, Zhang XM, Wang XD, et al. 17-AAG, a Hsp90 inhibitor, attenuates the hypoxia-induced expression of SDF-1alpha and ILK in mouse RPE cells. Mol Biol Rep. 2010;37:1203–1209. doi: 10.1007/s11033-009-9490-x. [DOI] [PubMed] [Google Scholar]
- Wu WC, Kao YH, Hu PS, et al. Geldanamycin, a HSP90 inhibitor, attenuates the hypoxia-induced vascular endothelial growth factor expression in retinal pigment epithelium cells in vitro. Exp Eye Res. 2007;85:721–731. doi: 10.1016/j.exer.2007.08.005. [DOI] [PubMed] [Google Scholar]