Abstract
Introduction
Intensity of exercise is believed to be a key determinant of response to chronic obstructive pulmonary disease (COPD) rehabilitation. We hypothesized that a higher intensity of exercise, in combination with physiotherapist-led instructions and education in management of breathlessness, would lead to better self-management, possibly delaying calls to the emergency service and preventing hospitalization.
Objective
We aimed to test this hypothesis in a subsequent randomized trial, and in order to test study processes and estimate hospitalization rates, we did a small preliminary prospective cohort study on severe COPD patients referred to outpatient rehabilitation.
Methods
In 2013, four rehabilitation courses were scheduled (spring, summer, autumn, and winter) each lasting 8 weeks and including eight to ten patients. This preliminary study was designed as a controlled cohort study. The bi-weekly exercise sessions in the spring and autumn courses included a high-intensity walking exercise at 95% of estimated VO2 max for as long as possible. The other two rehabilitation courses included the usual walking exercise intensity (85% of estimated VO2 max). Hospitalization rates were assessed from the participants’ medical records in an 18-month period.
Results
We were able to enroll 31 patients in total (15 in the high-intensity exercise group and 16 in regular intensity). There were no group differences in the hospitalization rates. However, during review of the medical records, we observed a striking mortality rate among participants who had attended the high-intensity rehabilitation courses (five deaths) compared to the standard rehabilitation (zero deaths). Four of the five deaths were COPD exacerbations. Fisher’s exact test was statistically significant (P=0.046), as was a log-rank test (P=0.019) of the Kaplan–Meier estimated survival rates.
Conclusion
These results from this small preliminary cohort study are alarming and raise concerns about the possible serious risks associated with high-intensity exercise rehabilitation of severe COPD patients.
Keywords: Chronic obstructive pulmonary disease, exercise, rehabilitation, mortality
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the world’s leading causes of death.1 A majority of COPD patients experience exacerbations that require hospitalization and drive up the cost and morbidity of COPD. Normally, priority is given to interventions that seek to delay the progression of the disease.
Rehabilitation is considered a cornerstone in treating and managing COPD, both in the stable phase and following exacerbations.2–4 At our outpatient lung clinic, four specialized 8-week pulmonary rehabilitation courses are run annually, each including eight to ten patients. Patients are eligible for this rehabilitation if they have moderate to very severe COPD (spirometric classification). The rehabilitation program includes physical exercise twice weekly and once weekly educational session. Physiotherapists supervise the exercise sessions, which include general warm-up exercises where patients are seated to control dyspnea. The general warm-up consisted of exercises for the shoulder, chest, and legs in combination with breathing exercises. Furthermore, patients received muscle endurance and strengthening, consisting of exercises in leg press for the lower extemities, and pull-down and elastic band exercises for the upper extremities. Finally, cardiovascular training was done on bicycles, step machines, and rowing ergometers, and by fast walking at 85% of predicted VO2 max estimated from the incremental shuttle walk test5,6 for as long as possible. The general exercise program and the walking intensity in our pulmonary rehabilitation courses are based on recommendation from the Danish Society of Respiratory Medicine.
Intensity of exercise is believed to be a key determinant of training response, but the aim of this preliminary study was to teach patients self-management of dyspnea by introducing high-intensity exercises that induced dyspnea. We hypothesized that a higher intensity of walking speed in combination with physiotherapist-led instructions and education in how to handle breathlessness would lead to better self-management of dyspnea, possibly delaying calls to the emergency service, and thereby prevent unnecessary hospitalizations. We aimed to test this hypothesis in a randomized trial, and in order to test study processes and estimate hospitalization rates (proposed primary outcome), we designed this small preliminary cohort study with a primary objective of assessing any effects of high-intensity exercises on hospitalization for COPD exacerbation.
Methods
In 2013, four rehabilitation courses were scheduled, and this preliminary study was designed as a controlled cohort study in which the bi-weekly exercise sessions in every other rehabilitation course (spring and autumn; a total of 15 patients) included a high-intensity walking exercise defined as walking at 95% predicted VO2 max (estimated as described earlier) for as long as possible and the general muscle endurance, strengthening, and cardiovascular training on bicycles, rowing ergometers, and step machines as described earlier. The other two rehabilitation courses (summer and winter; a total of 16 patients) included the usual walking exercise intensity (85% predicted VO2 max) and exercise program as described earlier. The order of courses with regular vs high exercise intensity (summer and winter vs spring and autumn) was based on randomization (coin flip) as we had no a priori assumptions regarding seasonal effects on the outcome. After the 8-week pulmonary rehabilitation program, the patients were encouraged to maintain exercise habits and instructed in a maintenance program, which was given to them either as individualized exercises, as a training digital video disc (DVD), or as information leaflet of training in community facilities, depending on patient preferences. As the two exercise programs were introduced as routine care (ie, provided to all referred patients) and regarded as a quality development project evaluating procedures, research ethical approval from the Regional Health Research Ethics Committee was not required according to Danish legislation (Act on Research Ethics Review of Health Research Projects), and therefore informed consent was not collected from the participants.
As part of the clinical examinations prior to rehabilitation, the patients underwent medical examination by a pulmonary physician and nurse where clinical data were recorded (Table 1). Patients were offered pulmonary rehabilitation if they were in the stable phase of their COPD and there were no records of mixed respiratory pathology. During the rehabilitation courses, three patients in each rehabilitation course experienced an exacerbation. To assess hospitalization rates (and causes), we reviewed the participants’ medical records in an 18-month period after the patients began the rehabilitation course.
Table 1.
Characteristics of participants in each group, and of participants who died during the observation period
| Variable | Regular-intensity rehabilitation (n=15) |
High-intensity rehabilitation
|
Group difference (regular vs high-intensity) mean (95% CI); P-value* |
|
|---|---|---|---|---|
| All participants (n=16) |
Of which died (n=5) |
|||
| Female sex, n (%) | 10 (67) | 9 (56) | 3 (60) | P=0.55** |
| Age, years | 69.7 (8.7) | 68.6 (8.7) | 73.6 (7.3) | 1.1 (−5.4 to 7.7); P=0.73 |
| Height, cm | 170.4 (8.3) | 168.3 (9.6) | 163.8 (6.7) | 2.2 (−4.4 to 8.8); P=0.51 |
| Body mass, kg | 67.3 (15.5) | 67.5 (19.6) | 55.0 (12.7) | 0.2 (−13.2 to 12.8); P=0.97 |
| BMI, kg/m2 | 23.2 (5.1) | 23.8 (6.3) | 20.5 (4.6) | −0.6 (−4.8 to 3.6); P=0.78 |
| Smoking status | P=0.55** | |||
| Current smoker (%) | 6 (40) | 6 (38) | 1 (20) | |
| Former smoker (%) | 8 (53) | 10 (62) | 4 (80) | |
| Never smoked (%) | 1 (7) | 0 (0) | 0 (0) | |
| Cigarette package years | 38.7 (17.9) | 63.4 (39.7) | 54.2 (28.8) | −24.6 (−47.4 to −1.9); P=0.035 |
| FEV1 | 0.88 (0.30) | 0.94 (0.40) | 0.80 (0.29) | −0.06 (−0.31 to 0.19); P=0.63 |
| FEV1 % predicted | 35.4 (11.4) | 36.2 (9.2) | 34.6 (8.8) | −0.74 (−8.37 to 6.90); P=0.84 |
| Estimated VO2 max, mL/min/kg | 8.78 (3.06) | 8.95 (3.45) | 7.62 (1.51) | −0.17 (−2.65 to 2.31); P=0.89 |
| Oxygen sat, at rest | 94.6 (1.8) | 94.5 (2.1) | 94.3 (2.1) | 0.10 (−1.40 to 1.60); P=0.89 |
| Oxygen supplement, yes (%) | 0 (0) | 2 (13) | 1 (20) | P=0.16** |
| CAT | 19.8 (7.7) | 20.6 (6.0) | 19.0 (4.8) | −0.8 (−6.1 to 4.4); P=0.74 |
| Number of previous exacerbations, median (first; third quartile) | 2 (0; 3) | 1 (0.25; 4) | 1 (0; 7.5) | P=0.14** |
| MRC | P=0.82** | |||
| 3, n (%) | 5 (33) | 5 (31) | 1 (20) | |
| 4, n (%) | 4 (27) | 3 (19) | 0 (0) | |
| 5, n (%) | 6 (40) | 8 (50) | 4 (80) | |
| COPD stage (GOLD) | P=0.87** | |||
| 2, n (%) | 1 (7) | 1 (6) | 0 (0) | |
| 3, n (%) | 8 (53) | 10 (63) | 3 (60) | |
| 4, n (%) | 6 (40) | 5 (31) | 2 (40) | |
| Comorbid cardiovascular disease, n (%) | 3 (20) | 8 (50) | 3 (60) | P=0.11** |
| Comorbid diabetes, n (%) | 2 (13) | 3 (19) | 1 (20) | P=0.68** |
| Number of hospitalizations from rehabilitation end +18 months | 1.20 (1.37) | 1.62 (2.75) | 2.20 (3.90) | −0.4 (−2.0 to 1.2); P=0.59 |
Notes: Data are mean (SD) unless otherwise stated.
Based on t-test.
Based on chi-square.
Abbreviations: BMI, body mass index; CAT, COPD assessment test; CI, confidence interval; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; MRC, Medical Research Council; SD, standard deviation.
Results
During this review, we discovered a striking mortality rate among participants who had attended the high-intensity rehabilitation courses (five deaths during observation; Figure 1) compared to the standard rehabilitation courses (zero deaths). Four of the five deaths were COPD exacerbations (Table 2), but none of these experienced an exacerbation during the 8-week rehabilitation. Fisher’s exact test was applied (due to small sample size), revealing that mortality between groups was statistically significant (P=0.046), as also illustrated from a log-rank test (P=0.019) of the Kaplan–Meier estimated survival rates (Figure 1). Causes of death were COPD exacerbation (n=4) and aortic aneurysm (n=1), and were distributed between 1 and 461 days after rehabilitation. There were no group differences in the hospitalization rates.
Figure 1.
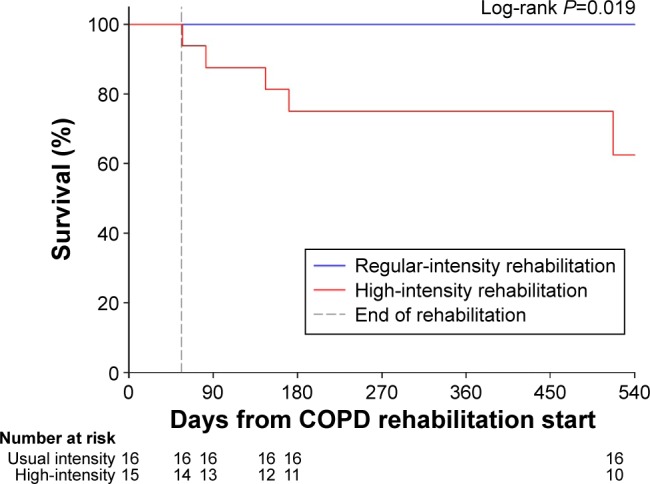
Kaplan–Meier curve for survival.
Table 2.
Individual participant data on the participants who died during the 18-month observation period
| Variable | Participant 1 | Participant 2 | Participant 3 | Participant 4 | Participant 5 |
|---|---|---|---|---|---|
| Sex | Female | Male | Female | Female | Male |
| Age, years | 73 | 71 | 64 | 84 | 76 |
| Height, cm | 159 | 167 | 173 | 156 | 164 |
| Body mass, kg | 59 | 75.6 | 49.0 | 45.1 | 46.3 |
| BMI, kg/m2 | 23.3 | 27.1 | 16.4 | 18.5 | 17.2 |
| Smoking status | Former | Former | Former | Former | Current |
| Cigarette package years | 37 | 100 | 49 | 25 | 60 |
| FEV1 | 0.51 | 1.20 | 0.64 | 0.64 | 1.00 |
| FEV1 % predicted | 25.7 | 44.5 | 25.1 | 37.3 | 40.4 |
| Oxygen saturation at rest | 92 | 93 | 96 | n/a | 96 |
| Oxygen supplement | No | Yes | No | No | No |
| CAT, 0–40 | 12 | 17 | 19 | 23 | 24 |
| Number of previous | 1 | 5 | 0 | 10 | 0 |
| exacerbations | |||||
| MRC score, 1–5 | 5 | 5 | 5 | 5 | 3 |
| COPD stage (GOLD classification, 1–4) | 4 | 3 | 4 | 3 | 3 |
| Comorbid cardiovascular disease | No | Yes, valve stenosis | No | Ischemic heart disease | Hypertension |
| Comorbid diabetes | No | Yes | No | No | No |
| Time to death from rehab stop, days | 1 | 26 | 90 | 115 | 461 |
| Cause of death | COPD exacerbation. Respiratory acidosis | COPD exacerbation. Respiratory arrest with cardiac arrest | COPD exacerbation. Respiratory acidosis | COPD exacerbation | Ruptured abdominal aortic aneurysm |
| Number of hospitalizations from rehabilitation end +18 months | 0 | 0 | 0 | 9 | 2 |
Abbreviations: BMI, body mass index; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; MRC, Medical Research Council; n/a, not/available.
We scrutinized available clinical and demographic data in search of possible explanations (only variables with complete data for all participants were analyzed), although the study size precludes firm statistical analyses. We found no obvious or statistically significant group differences, except for cigarette package years (mean difference 24.6 [95% confidence interval 1.9–47.4; P=0.035]). However, the five participants who died in the high-intensity group were in the lower range of package years (Table 2). Univariable Cox regression did not yield any statistical signals for package years (hazard ratio =1.00 [95% confidence interval 0.98–1.03]) and judged by the precision of the hazard ratio estimate, package years seem unlikely to be a confounder. We found no statistically significant hazard ratios for any of the recorded baseline variables (Table 3).
Table 3.
Hazard ratios from univariable Cox regression analyses of baseline characteristics predicting death
| Variable | Hazard ratio | 95% CI lower limit | 95% CI upper limit | P-value |
|---|---|---|---|---|
| Male sex | 1.14 | 0.19 | 6.88 | 0.89 |
| Age, years | 1.06 | 0.96 | 1.17 | 0.26 |
| Body mass, kg | 0.94 | 0.88 | 1.01 | 0.11 |
| BMI, kg/m2 | 0.89 | 0.73 | 1.08 | 0.23 |
| Smoking status | 0.53 | 0.10 | 2.75 | 0.45 |
| Cigarette package years | 1.00 | 0.98 | 1.03 | 0.85 |
| FEV1 | 0.19 | 0.00 | 11.64 | 0.43 |
| FEV1 % predicted | 0.98 | 0.90 | 1.08 | 0.71 |
| Estimated VO2 max, mL/min/kg | 0.85 | 0.57 | 1.28 | 0.45 |
| Oxygen sat, at rest | 0.93 | 0.55 | 1.56 | 0.78 |
| Oxygen supplement, yes | 2.53 | 0.81 | 7.95 | 0.11 |
| CAT | 0.97 | 0.84 | 1.11 | 0.64 |
| Number of previous exacerbations | 1.20 | 0.89 | 1.62 | 0.24 |
| MRC | 2.59 | 0.67 | 10.00 | 0.17 |
| COPD stage (GOLD) | 1.44 | 0.30 | 6.99 | 0.66 |
| Comorbid cardiovascular disease | 2.72 | 0.45 | 16.32 | 0.27 |
| Comorbid diabetes | 1.13 | 0.38 | 3.38 | 0.83 |
| Number of hospitalizations from rehabilitation end +18 months | 1.17 | 0.85 | 1.62 | 0.34 |
Abbreviations: BMI, body mass index; CAT, COPD assessment test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; MRC, Medical Research Council.
Discussion
These results are alarming and the first to indicate that even subtle increases in rehabilitation intensity are associated with unacceptable risks. It is possible that regular exercise intensity is challenging the participants to their maximum, and that even subtle increases in intensity may tip the load undesirably.
It is surprising that a minor adjustment in a subset of the rehabilitation may lead to such dramatic adverse effects, and these results suggest that COPD rehabilitation programs should be designed and executed very carefully.
Besides the small study size, this preliminary cohort study has several limitations. We did not record the muscle strength status of the patients at the start of the rehabilitation programs. Neither did we record any progression in exercise during the rehabilitation courses. Such information could have helped identify high-risk patients, such as very weak or fragile individuals, and aided in interpretation of the mortality data. Also, we did not record attendance or training volume, precluding assessment of adaptation to the exercise training or possible overtraining effects, which could have impacted immunity and health potentially explaining mortality rates. Furthermore, we did not collect data on the patients’ level of physical activity prior and post-participation in the pulmonary rehabilitation courses, which also could have aided in identification of inappropriate behavior associated with the rehabilitation. While such data could have informed this preliminary study, the study was designed to assess study-related procedures and estimate differences in hospitalization rates as a proposed primary outcome of a subsequent trial. For that reason, we did not plan an elaborate and detailed data collection of factors related to the exercise programs and change in physical activity habits.
We propose that rehabilitation programs should be individualized, although patient-specific information to be used for COPD rehabilitation remains elusive. The model of symptom/risk evaluation of COPD7 and medical history could be used as guidance in individual rehabilitation intensity and design. Also, day-to-day modifications according to COPD symptoms could be a means to ensure safety of rehabilitation.
Although our data are indicative at best, increased risk of mortality associated with high-intensity rehabilitation represents a potential red flag for clinical practice – a safety signal that should not be ignored without further scrutiny. Confirmation requires a large randomized trial; however, we anticipate that the present results prohibit such endeavors.
Conclusion
The results of this small preliminary cohort study are alarming and raise concerns about the possible serious risks associated with high-intensity exercise rehabilitation of severe COPD patients.
Acknowledgments
This study did not receive any specific funding. The Parker Institute operates under an unrestricted grant from the Oak Foundation. The Oak Foundation had no role in the planning, design, and execution of the study, or in the analysis of the results and the decision to submit for publication.
Footnotes
Author contributions
LS and MH conceived the study. LS, MH, and RC designed the study. LS collected data and drafted the manuscript. LEK and MH did the data analyses. All authors interpreted the data and critically reviewed successive drafts of the manuscript and approved the final version. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lopez-Campos JL, Ruiz-Ramos M, Soriano JB. Mortality trends in chronic obstructive pulmonary disease in Europe, 1994–2010: a joinpoint regression analysis. Lancet Respir Med. 2014;2(1):54–62. doi: 10.1016/S2213-2600(13)70232-7. [DOI] [PubMed] [Google Scholar]
- 2.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 3.Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(10):CD005305. doi: 10.1002/14651858.CD005305.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(4):CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Singh SJ, Morgan MD, Hardman AE, Rowe C, Bardsley PA. Comparison of oxygen uptake during a conventional treadmill test and the shuttle walking test in chronic airflow limitation. Eur Respir J. 1994;7(11):2016–2020. [PubMed] [Google Scholar]
- 6.Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47(12):1019–1024. doi: 10.1136/thx.47.12.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. [Accessed November 5, 2015]. Available from: http://goldcopd.org/


