Abstract
Background
Disease burden and associated costs are not well understood among patients with gastroesophageal reflux disease (GERD) who have persistent symptoms despite optimized proton pump inhibitor (PPI) therapy. The aim of this study was to investigate disease burden and costs of GERD in partial responders to PPI therapy.
Methods
The Partial Response to PPI treatment: the Cost to Society and the Burden to the Patient in the US (REMAIN US) study was a 12-month, multicenter, noninterventional, observational study of 552 partial PPI responders in the USA. Participating sites were comprised of family practice (n = 30), internal medicine (n = 8), and specialist (gastroenterologist) centers (n = 15). GERD symptoms, health-related quality of life (HRQL), and impact on productivity were evaluated from patient-reported outcome instruments. Resource utilization data were also collected.
Results
Patients had a high symptom burden, impaired HRQL, and reduced productivity while at work and in daily activities, despite optimized PPI therapy. Mean annual GERD-related costs were US$9944 per patient, comprising total direct costs and mean productivity loss costs of US$4068 and US$5876 per patient, respectively.
Conclusion
Patients with GERD and a partial response to PPI therapy have considerable direct and indirect costs, along with substantial impairments in HRQL and productivity.
Keywords: gastroesophageal reflux disease, GERD, proton pump inhibitors, partial response
Introduction
Although there are biomarkers for defining and characterizing gastroesophageal reflux disease (GERD), patient reports are fundamental to both diagnosis and monitoring treatment response. The Montreal consensus definition of GERD emphasizes the importance of patient report by defining GERD as “a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications.”1 For this reason, clinicians focus on symptom reporting when planning treatment strategies and monitoring patient outcomes in GERD.
Currently, acid suppression is the mainstay of treatment for GERD, and among the acid suppressive agents, proton pump inhibitors (PPIs) are the treatment of choice.2 However, the results of a recent systematic review show that up to 45% of patients receiving a prescription for daily PPI therapy for GERD continue to experience troublesome symptoms of heartburn and regurgitation.3 Also, around one-quarter of patients reportedly augment PPI treatment with over-the-counter (OTC) drugs, indicating a need to improve symptom control.4 That is, while PPI therapy has revolutionized the management of symptoms among patients with GERD, it remains commonplace to find patients with persistent symptoms despite what treating physicians consider to be optimized PPI therapy. Many patients report some, yet only partial, improvement in GERD symptoms. Despite the high prevalence of this patient population, the burden of disease and associated costs among partial responders to PPI treatment is not well understood. As the prevalence of GERD rises in parallel with the aging population and the increasing obesity epidemic, clinicians will continue to manage GERD patients with partial, yet incomplete, response to PPI therapy.
The objective of the Partial Response to PPI treatment: the Cost to Society and the Burden to the Patient in the US (REMAIN US) study, therefore, was to provide empirical evidence about this patient population, by investigating the cost of illness, treatment patterns, and burden of GERD in partial responders to PPI therapy in the USA.
Methods
Study design
The multicenter, noninterventional, observational REMAIN US study was conducted at 53 primary and secondary care sites in the USA. Participating sites were comprised of family practice (n = 30), internal medicine (n = 8), and specialist (gastroenterologist) centers (n = 15). The protocol was approved by a central Institutional Review Board on October 16, 2008 (Sterling Independent Services, Inc, Atlanta, GA). Eligible patients were recruited during regular physician visits; specific appointments were not set up for patients who were to be screened for enrollment. The first patient was enrolled on November 12, 2008, the last patient was enrolled on June 12, 2009, and the last patient survey was received on January 12, 2010. At study entry, data regarding patients’ medical history and prescribed medications over the 6-month period prior to enrollment were collected retrospectively by the study physician using a case-report form; symptoms, health-related quality of life (HRQL), and productivity over the previous week were recorded using patient-reported outcome (PRO) instruments. Thereafter, two follow-up surveys were mailed to the patients at 3 and 6 months post-enrollment, respectively (each patient received a diary that was intended to act as a memory aid when patients were about to report retrospectively at these time points). The total duration of the REMAIN US study was therefore 12 months. Participating patients and physicians received a small remuneration for their contribution to the study.
Patients
Eligible patients were adults (≥18 years) identified as being partial responders to optimized PPI treatment (at, or prior to, the enrollment visit) for GERD (≥6 months’ documented history of GERD symptoms) who had provided written informed consent. Optimized PPI therapy was defined pragmatically as treatment that, according to the physician’s judgment, could not be further improved by changing the type or dosing schedule of the PPI. Patients also had to report ≥ 3 days/week of a burning feeling behind the breastbone of at least moderate intensity, and/or ≥3 days/week of an unpleasant movement of material upwards from the stomach of at least moderate intensity; these symptoms were assessed using the validated Reflux Symptom Questionnaire 7-day recall (RESQ-7).5
Patients with no improvement in GERD symptoms during PPI treatment were not eligible for inclusion.
Patient-reported outcomes
Disease-specific symptoms were evaluated at the baseline visit and at 3 and 6 months’ follow-up using RESQ-7, a PRO instrument partly based on the Reflux Disease Questionnaire.6 The RESQ-7 is a 26-item, self-administered questionnaire designed to assess the intensity and frequency of GERD symptoms over the past 7 days. Symptom intensity and frequency are scored on a six-point Likert scale, with higher scores indicating more intense or frequent symptoms. The symptom items can be combined into an overall symptoms domain, as well as into four separate domains: heartburn; regurgitation; hoarseness, cough, and difficulty swallowing; and burping.
At the baseline visit, all patients completed the Hospital Anxiety and Depression Scale (HADS)7 to detect states of anxiety and depression. The HADS is a 14-item self-assessment in which each item is scored by the patient on a four-point (0–3) response category. The scale yields an anxiety score and a depression score (0–7, no disorder; 8–10, “possible” anxiety or depression; ≥ 11, “probable” anxiety or depression).
HRQL was evaluated at the baseline visit and at 6 months’ follow-up using two generic instruments: the 36-item, Short-Form Health Survey Version 2 acute (SF-36v2 acute) with a 7-day recall period,8 and the European Quality of Life-5 Dimensions (EQ-5D).9 The SF-36v2 acute is a generic quality of life instrument that measures physical functioning, bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy/fatigue, and general health problems. The instrument also comprises physical and mental component summary scores. Scores were based on norm-based scoring (0–100, with higher scores representing a more favorable health state), using the SF-36v2 Health Survey 1998 general population norms (a mean score of 50 with a standard deviation [SD] of 10).
The EQ-5D9 is a health outcomes measure that provides a descriptive profile and index value for health status; five questions comprise dimensions of health (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) across three levels (no problems, some/moderate problems, and extreme problems), in addition to one measure of health status on a vertical graduated (0–100) visual analog scale (VAS). Higher EQ-5D Index and VAS scores represent a more favorable health state.
Disease-specific productivity impairment was evaluated at the baseline visit and at 3 and 6 months’ follow-up using the Work Productivity and Activity Impairment questionnaire for patients with GERD (WPAI-GERD), a six-item instrument that measures work productivity and activity impairment over the past 7 days.10 The WPAI-GERD questionnaire yields a number of scores, such as: absenteeism (hours of work-time missed), presenteeism (impairment at work/reduced on-the-job effectiveness), and activity impairment because of GERD. Presenteeism and activity impairment outcomes are expressed as percentages, with higher percentages indicating greater impairment and less productivity. The number of work hours lost due to presenteeism is calculated by multiplying the percentage of reduced productivity while at work by the number of hours the responder actually worked.
Resource utilization and costs
As part of retrospective data collection, physicians noted which PPIs and other medications considered to be relevant for patient care were prescribed during the 6 months before baseline.
For the 6-month prospective (follow-up) phase of the study, patients completed resource utilization forms to self-report prescribed GERD medication coupled with an open-ended question regarding OTC medication (“Nonprescription medication you used for the treatment of your GERD symptoms”). Patients also provided data on treatment duration for each therapy.
Physicians reported the date of any previous medical visits and hospitalizations due to GERD-related conditions occurring during the 6 months prior to baseline. The patient resource utilization forms were used to collect self-reported information regarding hospital and physician visits during the 6-month follow-up period.
Resource utilization data from the study were multiplied with unit cost data for medications,11 charges for medical consultations, tests and procedures,12 and hospitalizations13 (see Table S1). A mean hourly labor cost of US$29.37 was used for productivity cost calculations (the mean hourly labor cost for all occupations and all civilian workers, including all compensation costs during the fourth quarter of 2009, according to the Bureau of Labor Statistics14).
Number of hours absent from work and number of hours lost due to reduced work productivity were calculated over the preceding 7 days using WPAI-GERD results. The number of hours collected at baseline was applied for the 6-month period prior to baseline. Similarly, the number of hours reported at 6 months was applied for the period between baseline and 6 months. Productivity loss was adjusted to reflect the total study population when calculating productivity costs per patient. This was made by applying the costs to the number of patients for whom data on productivity loss was available, divided by the total number of patients at baseline and 6 months, respectively. Calculations were made separately for each productivity variable (hours absent from work and work hours lost due to reduced productivity).
Although resource utilization data were available separately for the two 3-month periods after baseline, cost calculations are presented here for the whole 6-month period after baseline.
Statistical analyses
All statistical analyses were descriptive. The study protocol specified a target enrollment sample of 550 patients with GERD and with partial response to optimized PPI treatment. Assuming an attrition rate of 15% per quarter over the course of the 6-month data collection period, the final sample size would be approximately 400 patients for analysis. Due to the descriptive objectives of the study, there were no hypotheses to test with statistical methods to predetermine a required sample size. As such, the sample size was pragmatic, and a target of 550 enrolled patients was deemed sufficient to meet satisfactory rates of precision for study variables, based on a previous US database study conducted among a relevant patient group.15
While patients completed diaries in order to help with retrospective reporting at 3 and 6 months’ follow-up, data were neither collected nor analyzed.
Results
Patient characteristics
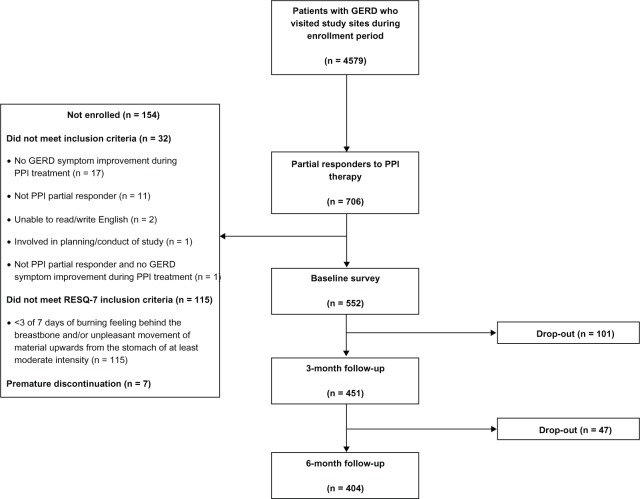
A total of 4579 patients with GERD visited the study sites during the enrollment period, of whom 706 were identified as potentially being partial responders to PPIs. Overall, 552 partial PPI responders met the eligibility criteria and completed the baseline survey; 164 patients (29.7%) were men and the mean age ± SD was 54.7 ± 14.4 (range 19–97) years. Clinical characteristics at baseline are presented in Table 1. A total of 404 patients completed the study until the 6-month follow-up period (Figure 1).
Table 1.
Summary of patients’ clinical characteristics at baseline
| Variable | Patientsa |
|---|---|
| Duration of GERD, years | 7.3 (6.5)b |
| Elapsed time from first observation of persistent symptoms of GERD, years | 2.6 (4.3)b |
| Body mass index, kg/m2 | 31.5 (7.7)b |
| Gastrointestinal history | |
| Dyspeptic symptoms | 304 (55.1) |
| History of hiatal hernia | 117 (32.1) |
| Diagnosis of irritable bowel syndrome | 92 (16.7) |
| Reflux esophagitis | 76 (13.8) |
| Endoscopic findingsc | |
| Hiatal hernia | 103 (40.6) |
| Esophagitis | 46 (18.1) |
| Barrett’s esophagus | 32 (12.6) |
| Gastric ulcer | 31 (12.2) |
| Esophageal stricture | 15 (5.9) |
| Duodenal ulcer | 4 (1.6) |
| Non-GERD medical historyd | |
| Hypertension | 206 (11.0) |
| Anxiety and/or depression | 156 (8.3) |
| Hyperlipidemia | 118 (6.3) |
| Diabetes mellitus | 69 (3.7) |
| Allergies | 65 (3.5) |
| Hypothyroidism | 63 (3.4) |
| Asthma/asthmatic bronchitis | 53 (2.8) |
| Osteoarthritis | 52 (2.8) |
| Back pain/chronic pain, low back pain/lower back pain | 41 (2.2) |
Notes:
All values are n (%), unless specified otherwise;
mean (standard deviation);
proportions of patients are based on 254 patients who had endoscopic data for the previous 6 months;
reported in ≥2% of patients.
Abbreviation: GERD, gastroesophageal reflux disease.
Figure 1.
Flow of patients through the REMAIN US study.
Abbreviations: REMAIN US, Partial Response to PPI treatment: the Cost to Society and the Burden to the Patient in the US; GERD, gastroesophageal reflux disease; PPI, proton pump inhibitor; RESQ-7, Reflux Symptom Questionnaire 7-day.
HADS scores at baseline classified 31.4% of patients as having probable anxiety disorders, and 15.4% of patients as having probable depression.
At baseline, 237 patients (42.9%) were receiving high-dose PPIs without adjunctive therapy, 202 patients (36.6%) were receiving standard-dose PPIs, and 89 patients (16.1%) were receiving PPIs in combination with other medications. The remaining patients (24 patients, 4.3%) were receiving other or no medication, consistent with the protocol that patients did not have to be receiving a PPI immediately prior to enrollment. Among the total of 512 (92.8%) patients who had been continuously treated with optimized PPI over the past 4 weeks, the most common reason for considering PPI therapy as being optimized (multiple responses possible) was “maximum dose” (90.2% of patients), followed by “tried different brands with no improvement” (72.3%), and “improvement by changing PPI unlikely due to symptom pattern” (48.2%).
Symptoms
According to the RESQ-7 questionnaire (overall symptoms domain), the patients included in this study experienced a high symptom burden at baseline, with 73% of patients reporting at least moderately severe symptoms, and 60.5% reporting daily symptoms despite PPI therapy. Both symptom intensity and frequency was lower at 6 months’ follow-up, with 46% of patients reporting at least moderately severe symptoms, and 43% of patients reporting daily symptoms based on the overall symptoms domain of RESQ-7. Similar findings were generally apparent across the four separate domains of RESQ-7 (data not shown).
Health-related quality of life
SF-36v2, EQ-5D Index, and VAS scores were consistent with impaired HRQL at both baseline and 6 months’ follow-up (Table 2). A relationship between greater intensity and frequency of the RESQ-7 heartburn domain symptoms and poorer HRQL, according to EQ-5D scores, was indicated (Figure 2).
Table 2.
Mean (± SD) SF-36v2, EQ-5D Index, and VAS scores at baseline and 6 months among evaluable patients (n = 550)
| Baseline | 6 months | |
|---|---|---|
| SF-36v2 domain | ||
| Physical functioning | 57.2 ± 30.2 | 55.2 ± 31.8 |
| Role – physical | 55.6 ± 30.7 | 55.6 ± 31.9 |
| Bodily pain | 43.4 ± 23.6 | 46.0 ± 27.1 |
| General health | 49.8 ± 23.2 | 46.0 ± 24.7 |
| Vitality | 43.3 ± 21.3 | 43.6 ± 22.8 |
| Social functioning | 59.7 ± 28.2 | 59.4 ± 30.9 |
| Role – emotional | 65.0 ± 31.9 | 64.2 ± 32.8 |
| Mental health | 61.4 ± 21.5 | 60.7 ± 23.6 |
| Physical component summary score | 38.9 ± 10.9 | 39.1 ± 11.8 |
| Mental component summary score | 42.2 ± 13.0 | 42.0 ± 14.0 |
| EQ-5D Index score | 0.69 ± 0.21 | 0.68 ± 0.23 |
| EQ-5D VAS scorea | 0.65 ± 0.20 | 0.65 ± 0.22 |
Note:
Normalized between 0 and 1 for purposes of comparison.
Abbreviations: EQ-5D, European Quality of Life-5 Dimensions; SD, standard deviation; SF-36v2, 36-item Short-Form Health Survey version 2 acute; VAS, visual analog scale.
Figure 2.
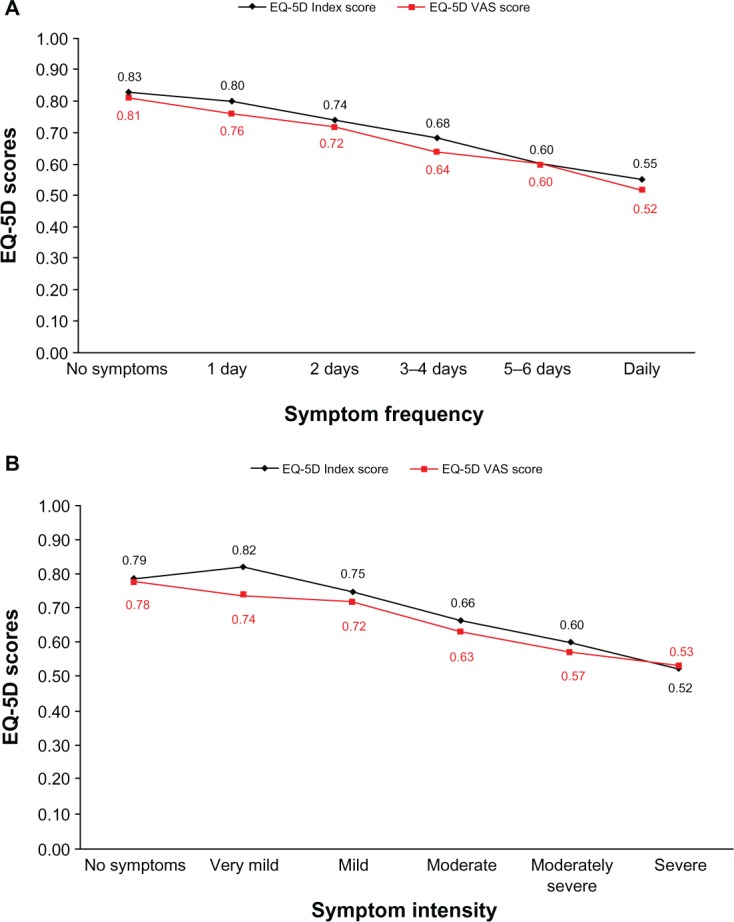
Mean EQ-5D Index and VAS scores by symptom frequency (A) and intensity (B) of RESQ-7 heartburn domain symptoms (using the symptom with the highest frequency) at 6 months’ follow-up.
Notes: For both EQ-5D scores, 0 represents a health state of being dead and 1 a health state of being at full health (the EQ-5D VAS score was normalized between 0 and 1 for comparative purposes). The relationship between symptoms and EQ-5D results is described at the 6-month assessment, since the number of patients in each symptom category was most evenly distributed at this time point.
Abbreviations: EQ-5D, European Quality of Life-5 Dimensions; VAS, visual analog scale; RESQ-7, Reflux Symptom Questionnaire 7-day.
Productivity impairment
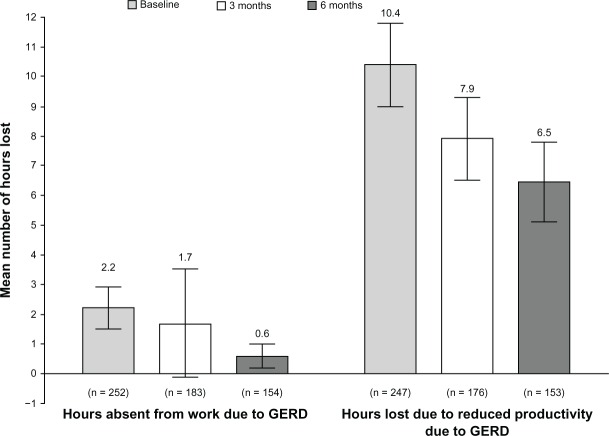
At baseline, the mean reduction in productivity while at work was 29.4% (95% confidence interval [CI] 25.9, 32.8; n = 254), the mean work time missed due to GERD was 8.0% (95% CI 5.3, 10.7; n = 235), and the mean reduced productivity while carrying out daily activities was 41.0% (95% CI 38.7, 43.4; n = 535). Subsequent results from the WPAI-GERD questionnaire revealed that the number of hours absent from work, as well as the number of hours lost due to reduced productivity while at work and during daily activities declined nominally over the course of the study (Figure 3).
Figure 3.
Results from the WPAI-GERD.
Note: Results are mean hours (95% confidence interval) for work absence due to GERD and reduced productivity due to GERD over the 7 days prior to assessment, at baseline, and at the 3- and 6-month follow-up visits.
Abbreviations: WPAI-GERD, Work Productivity and Activity Impairment questionnaire for patients with Gastroesophageal Reflux Disease; GERD, gastroesophageal reflux disease.
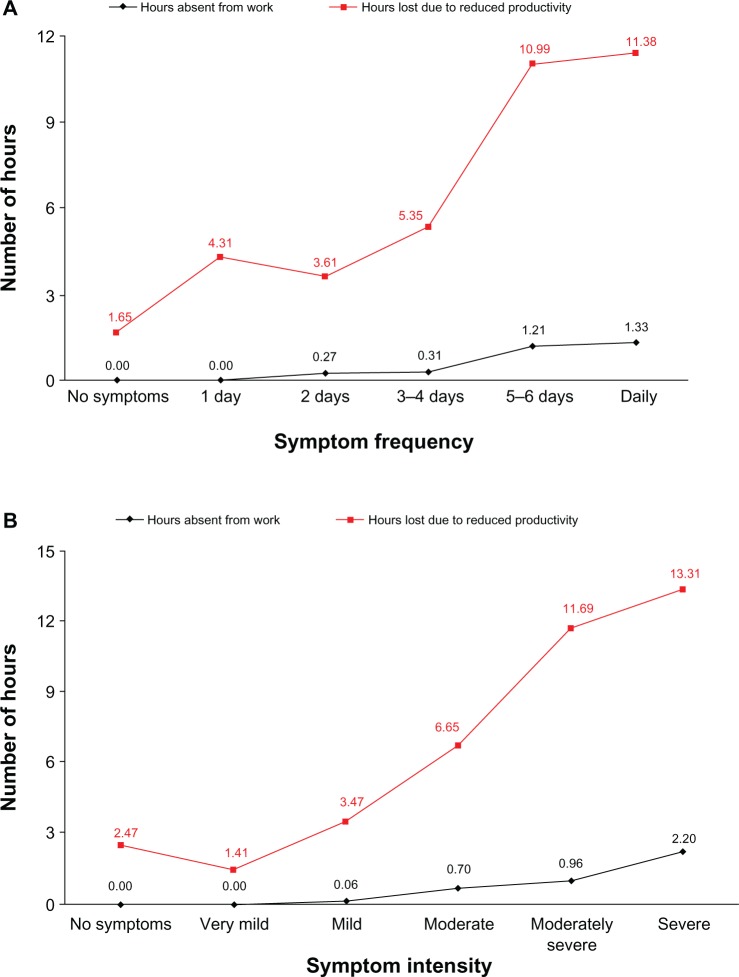
Reductions in productivity were greater with higher intensity and frequency of RESQ-7 heartburn domain symptoms (Figure 4).
Figure 4.
Mean number of hours absent from work and number of hours lost due to reduced work productivity by symptom frequency (A) and intensity (B) of RESQ-7 heartburn domain symptoms (using the symptom with the highest frequency) at 6 months’ follow-up.
Note: The relationship between symptoms and productivity results is described at the 6-month assessment, since the number of patients in each symptom category was most evenly distributed at this time point.
Abbreviation: RESQ-7, Reflux Symptom Questionnaire 7-day.
Resource utilization and costs
Over the 12-month study duration, the prescription of GERD medications remained relatively consistent. More than 90% of patients reported using OTC medications in addition to prescribed treatments.
The number of health care visits and hospitalizations over the study period is shown in Table 3.
Table 3.
Mean (± SD) number of health care visits (excluding screening visit) among all patients across the 12-month study period
| Health care visits per patient | |
|---|---|
| Visits between 6 months prior to baseline and baseline (n = 552), physician-reported; 6 months of retrospective data | |
| Primary care physician visits | 1.42 ± 2.14 |
| Specialist visits | 1.11 ± 1.92 |
| Emergency room visits | 0.07 ± 0.29 |
| Hospitalizations | 0.03 ± 0.17 |
| Visits between baseline and 3-month follow-up (n = 451), patient-reported; 3 months of prospective data | |
| Primary care physician visits | 0.95 ± 1.23 |
| Specialist visits | 0.62 ± 1.11 |
| Emergency room visits | 0.15 ± 0.62 |
| Outpatient hospital visits | 0.14 ± 0.57 |
| Inpatient hospitalizations | 0.07 ± 0.44 |
| Visits between 3- and 6-month follow-up (n = 404), patient-reported; 3 months of prospective data | |
| Primary care physician visits | 0.82 ± 1.36 |
| Specialist visits | 0.43 ± 0.97 |
| Emergency room visits | 0.11 ± 0.48 |
| Outpatient hospital visits | 0.14 ± 0.58 |
| Inpatient hospitalizations | 0.02 ± 0.17 |
Abbreviation: SD, standard deviation.
Over the course of the study, the mean annual GERD-related costs per patient totaled US$9944; the mean total direct cost per patient was US$4068, and the mean productivity loss cost per patient was US$5876. Reduced productivity at work accounted for around 80% of indirect costs (Table 4).
Table 4.
Cost analysis
| Type of cost | Mean ± SD cost per patient, US$
|
||
|---|---|---|---|
| 6 months prior to baseline, physician-reported (n = 552) | Months 1–6 after baseline, patient-reported (n = 404) | 6 months prior to baseline to 6-month follow-up (annual costs), physician- and patient-reported (n = 404)a | |
| Total costs | 5814 ± 8189 | 4934 ± 423 | 9944 ± 11,427 |
| Total direct costs | 1485 ± 1174 | 2564 ± 3111 | 4068 ± 3415 |
| Primary care physician visits | 82 ± 85 | 70 ± 90 | 155 ± 143 |
| Specialist visits | 65 ± 103 | 106 ± 176 | 171 ± 229 |
| Emergency room visits | 4 ± 18 | 84 ± 285 | 88 ± 289 |
| Outpatient visits | 25 ± 81 | ||
| Hospitalizations | 132 ± 807 | 354 ± 1481 | 523 ± 1780 |
| Prescribed medication (GERD-related) | 1074 ± 665 | 1606 ± 2170 | 2754 ± 2325 |
| OTC GERD medications | 67 ± 263 | ||
| Tests and procedures | 128 ± 270 | 253 ± 444 | 378 ± 549 |
| Upper GI endoscopy | 180 ± 302 | ||
| Dilation of stricture | 55 ± 181 | ||
| Acid perfusion | 5 ± 25 | ||
| Esophageal motility study | 14 ± 61 | ||
| Total work productivity loss cost | 4329 ± 7934 | 2370 ± 5528 | 5876 ± 10,787 |
| Cost of absence from work | 777 ± 3125 | 354 ± 3303 | 1114 ± 5060 |
| Cost of reduced productivity at work | 3552 ± 6932 | 2016 ± 4127 | 4762 ± 8783 |
Note:
Costs are derived based on the 404 patients with 12 complete months of data.
Abbreviations: GERD, gastroesophageal reflux disease; GI, gastrointestinal; OTC, over-the-counter; SD, standard deviation.
Discussion
Partial response to PPI therapy may be common in clinical practice, but little is known about the prevalence and associated burden in this important subpopulation of patients with GERD. This is the first noninterventional, observational study to evaluate the cost of illness and the burden of GERD in partial responders to PPI therapy in the US adult population.
There are several notable findings from this study. First, we found that patients with partial PPI response had a substantial impairment of HRQL, which appeared to be related to symptom burden. Such findings are in accordance with previous observations of patients with GERD.16 Second, few patients with partial PPI response changed treatment despite the fact that they continued to experience troublesome symptoms and diminished HRQL. This is an important point, as one explanation of the unmet need for different or more effective therapies for GERD is that patients with a partial response are continuing on the same therapy, and/or have been incorrectly diagnosed. However, 96% of enrolled patients had been previously diagnosed with GERD (according to medical records), and all were deemed to have been receiving optimized PPI therapy according to the physician’s judgment, which could not be further improved by changing the chosen PPI or dosing schedule. Third, a considerable direct and indirect cost of illness exists among such patients. Of the total cost associated with partial response to PPI therapy for GERD, for example, the cost of reduced productivity at work was the single largest contributor. This finding is consistent with the 2007 National Health and Wellness Survey data, which estimated that patients experienced a 28% reduction in productivity while at work due to disruptive GERD symptoms experienced among US respondents.17 Moreover, the level of productivity impairment is comparable to that of other diseases that are associated with substantial indirect costs such as irritable bowel syndrome (IBS),18 symptoms of which reduced total work productivity (absenteeism and presenteeism) by 21% according to one US study.19
An apparent decrease in symptom load over time was noted in the absence of marked changes in the medication prescribed. These findings were to be partly expected, given the fluctuating nature of GERD symptoms,20,21 and given the recruitment of patients with a high symptom burden. Other potential explanations include drop-outs (ie, the possibility that more symptomatic than nonsymptomatic patients dropped out from the study), and the different data collection settings at baseline (physician office) and follow-up (patient’s home).
Some authors have suggested that concomitant IBS in patients with GERD is associated with failure of PPI therapy;22 however, in the present subset of patients with GERD symptoms despite optimized PPI therapy, the proportion of patients whose gastrointestinal history included a diagnosis of IBS (17%) was equivalent to that reported previously for the general North American adult population.18,23 It should be noted that the absence of a previous IBS diagnosis does not rule out the experience of as-yet undiagnosed IBS symptoms.
The prevalence of probable anxiety, based on HADS classification, in the REMAIN US population (31%) was higher than among the general GERD population in Italy (in which 19% of patients were classified as having anxiety).24 This might be linked to undiagnosed anxiety and/or partial treatment response, and the belief that lack of symptom improvement might be attributable to a sinister cause. In the setting of antireflux surgery, psychological factors such as anxiety are also known to impact on treatment success.25
The relationship between, on the one hand WPAI-GERD and EQ-5D and, on the other hand, symptom load measured by RESQ-7 lends indirect support to the construct validity of the mentioned instruments in this study population, although it should be noted that supporting construct validity was not an objective of this study.
This study utilized patient-reported data to assess reduced productivity at work, the reliability of which may be in question due to the subjective nature of this assessment. However, comparisons with other GERD studies on productivity and cost indicate that the results presented here are largely consistent.26 Further, a study using objective measures of productivity lends support to the credibility of patient-reported productivity.27
Study strengths and limitations
The key strengths of this study include the large sample size and the use of established PRO instruments to evaluate HRQL and productivity. The limitations of the study include variation in data collection methods (eg, physician-versus patient-reported resource utilization), relatively long periods between patient recall for resource utilization (up to 3 months), and productivity data collected for relatively short periods (1 week each), which were then extrapolated. However, memory aids, in the form of diaries in which patients were encouraged to record use of medical care and medications for the treatment of GERD for the following 3 months, were sent to each patient together with the previous period’s survey, which may have aided in overcoming the limitations associated with memory recall.
While this study includes a convenient observational population, it is not a random sample of partial PPI responders and may not, therefore, be fully representative. It could also be argued that the identified problem of partial response to PPI therapy might be related to incorrect diagnosis. A diagnosis of GERD had been previously confirmed in 96% of enrolled patients, and this would explain why relatively few patients underwent objective tests of incompletely treated GERD during follow-up. However, the possibility of incorrect diagnosis cannot necessarily be overlooked. In a recent study, for example, Galindo et al28 described how a multimodal evaluation changed the diagnosis of GERD in around one-third of PPI treatment failures. Moreover, it was apparent that relatively few physicians in the present study explored adjunctive use of therapies such as prokinetics, for which there is some evidence of therapeutic benefit in patients with GERD who have not responded to PPIs.29–31 Physicians were not provided with clinical training on the management of GERD or related guidelines in this study, in order to obtain real-world data and not interfere with their routine management of patients with GERD. As such, the aforementioned findings of limited diagnostic follow-up and infrequent use of adjunctive therapies in PPI partial responders suggests that routine management of such patients could be improved. Finally, it should be noted that the REMAIN US study was not designed to assess changes in symptoms, HRQL, and productivity over time; thus, comparisons between baseline and follow-up assessments should be made with caution.
Conclusion
In summary, many US patients with GERD continue to experience persistent symptoms despite optimized PPI therapy, a symptom load that is associated with considerable direct and indirect costs, as well as substantial impairments in patients’ HRQL and productivity during work and daily activities. Addressing this significant unmet need remains a therapeutic challenge, and physicians should be encouraged to explore not only the possibility that PPI treatment is not optimized (even if they believe it to be), but also the likelihood of incorrect diagnosis and/or the potential benefits of adjunctive therapies.
Supplementary material
Table S1.
Unit cost data for medical consultations, hospitalizations, tests, procedures, and surgeries
| Description | Unit cost (US$) | Comment |
|---|---|---|
| Medical consultation charges | ||
| Primary care physician visits | 40.41 | 10-minute consultation for an established patient |
| Specialist visits | 102.10 | 30-minute consultation |
| Emergency room visits | 353.57 | Presenting with a problem of moderate severity |
| Outpatient visits | 91.64 | 30-minute consultation for an established patient |
| Charges for hospitalizations | ||
| Inpatient visits: general care | 2501.90 | Mean length of stay: 3.5 days |
| Inpatient visits: intermediate care | 6271.83 | Mean length of stay: 5.1 days |
| Charges for surgeries | ||
| Gastric ulcer surgery | 1443.20 | Stomach, esophageal, and duodenal procedures without CC/MCC |
| Charges for tests/procedures | ||
| Blood sample for laboratory tests | 3.39 | |
| Upper gastrointestinal endoscopy | 254.72 | Requires moderate sedation (with anesthesiologist cost is US$471.19) |
| Upper gastrointestinal endoscopy with biopsy | 309.56 | Requires moderate sedation (with anesthesiologist cost is US$562.11) |
| Radiologic examination | 99.58 | |
| Upper abdominal ultrasound | 231.27 | |
| 24-hour pH monitoring | 167.05 | |
| Esophageal motility study/esophageal manometry | 211.42 | |
| Bernstein test (acid perfusion test) | 122.67 | |
| Resting ECG | 12.27 | |
| Exercise ECG | 76.49 | |
| X-ray | 158.39 | |
| 24-hour Holter monitor (ambulatory ECG) | 80.10 | |
| Abdominal MRI | 289.72 | |
| Breath hydrogen | 62.42 | |
| Electrogastrogram | 26.70 | Professional component only |
| Gastric emptying scan | 248.95 | |
| HIDA scan | 294.77 | Hepatobiliary ductal system imaging, including gallbladder, with or without pharmacologic intervention, with or without quantitative measurement of gallbladder function |
| Pill cam | 611.54 | Capsule endoscopy |
| Pulmonary function test (spirometry) | 31.75 | |
| Sleep study: unattended | 195.91 | |
| Sleep study: attended | 406.61 | |
| Small bowel enteroscopy | 154.06 | Requires moderate sedation (with anesthesiologist cost is US$442.69) |
| Stretta procedure | 318.94 | Requires moderate sedation (with anesthesiologist cost is US$1198.55) |
| Echocardiogram | 101.02 | |
| Stool culture | 13.51 | Stool, aerobic, additional pathogens, isolation, and presumptive identification of isolates |
| Dilatation of stricture | 513.04 | Requires moderate sedation |
Abbreviations: CC/MCC, complications and comorbidities/major complications and comorbidities; ECG, electrocardiogram; HIDA, hepatobiliary iminodiacetic acid; MRI, magnetic resonance imaging.
Footnotes
Authors’ contributions
Study planning and interpretation of data: Brennan M Spiegel. Study planning, monitoring of conduct, and interpretation of data: Helena Granstedt Löfman, Peter Wahlqvist, and Nils-Olov Stålhammar. Monitoring of study conduct and interpretation of data: Maria Karlsson. Study planning and interpretation of data: Jørgen Næsdal. Study planning, conduct, and interpretation of data: M Todd Nelson and Nicolas Despiégel. All authors contributed to drafting and critical revision of the manuscript, and approved the final version for submission.
Disclosure
This study was supported by AstraZeneca R&D, Mölndal, Sweden. Medical writing services from Melanie Gatt and Steve Winter of inScience Communications, Springer Healthcare were funded by AstraZeneca. Brennan M Spiegel has received grant support from Shire-Movetis and Ironwood, and has received consultancy fees from Ironwood, Prometheus, and Shire-Movetis. Helena Granstedt Löfman, Maria Karlsson, Peter Wahlqvist, Jørgen Næsdal, and Nils-Olov Stålhammar are current, former, or retired employees of AstraZeneca. M Todd Nelson and Nicolas Despiégel are employees of OptumInsight, who conducted the study with funding from AstraZeneca. The authors report no other conflicts of interest in this work.
References
- 1.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus Group The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101(8):1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 2.Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135(4):1383–1391. doi: 10.1053/j.gastro.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther. 2010;32(6):720–737. doi: 10.1111/j.1365-2036.2010.04406.x. [DOI] [PubMed] [Google Scholar]
- 4.Jones R, Armstrong D, Malfertheiner P, Ducrotté P. Does the treatment of gastroesophageal reflux disease (GERD) meet patients’ needs? A survey-based study. Curr Med Res Opin. 2006;22(4):657–662. doi: 10.1185/030079906X100032. [DOI] [PubMed] [Google Scholar]
- 5.Vakil N, Björck K, Denison H, et al. Validation of the Reflux Symptom Questionnaire electronic diary in partial responders to proton pump inhibitor therapy. Clin Transl Gastroenterol. 2012;3:e7. doi: 10.1038/ctg.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw MJ, Talley NJ, Beebe TJ, et al. Initial validation of a diagnostic questionnaire for gastroesophageal reflux disease. Am J Gastroenterol. 2001;96(1):52–57. doi: 10.1111/j.1572-0241.2001.03451.x. [DOI] [PubMed] [Google Scholar]
- 7.Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 9.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 10.Wahlqvist P, Medin J, Karlsson M, et al. Responsiveness to change and construct validity of the Work Productivity and Activity Impairment questionnaire for gastroesophageal reflux disease (WPAI:GERD) in Swedish patients [abstract] Value Health. 2009;12:A60. [Google Scholar]
- 11.2009 Red Book: Pharmacy’s Fundamental Reference. Montvale, NJ: Thomson Reuters; 2009. [Google Scholar]
- 12.American Medical Association . 2009 National Physician Fee Schedule Relative Value File. Chicago, IL: American Medical Association; 2009. [Google Scholar]
- 13.Ingenix . DRG Expert 2009: A Comprehensive Guidebook to the DRG Classification System. 25th ed. Salt Lake City, UT: Ingenix, Inc; 2009. [Google Scholar]
- 14.Bureau of Labor Statistics . Employer costs for employee compensation [press release] Washington, DC: Bureau of Labor Statistics; Dec 8, 2010. [Google Scholar]
- 15.Toghanian S, Johnson DA, Stålhammar NO, Zerbib F. Burden of gastro-oesophageal reflux disease in patients with persistent and intense symptoms despite proton pump inhibitor therapy: a post hoc analysis of the 2007 national health and wellness survey. Clin Drug Investig. 2011;31(10):703–715. doi: 10.2165/11595480-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Wahlqvist P, Karlsson M, Johnson D, Carlsson J, Bolge SC, Wallander MA. Relationship between symptom load of gastro-oesophageal reflux disease and health-related quality of life, work productivity, resource utilization and concomitant diseases: survey of a US cohort. Aliment Pharmacol Ther. 2008;27(10):960–970. doi: 10.1111/j.1365-2036.2008.03671.x. [DOI] [PubMed] [Google Scholar]
- 17.Toghanian S, Wahlqvist P, Johnson DA, Bolge SC, Liljas B. The burden of disrupting gastro-oesophageal reflux disease: a database study in US and European cohorts. Clin Drug Investig. 2010;30(3):167–178. doi: 10.2165/11531670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel BM. The burden of IBS: looking at metrics. Curr Gastroenterol Rep. 2009;11(4):265–269. doi: 10.1007/s11894-009-0039-x. [DOI] [PubMed] [Google Scholar]
- 19.Dean BB, Aguilar D, Barghout V, et al. Impairment in work productivity and health-related quality of life in patients with IBS. Am J Manag Care. 2005;11(Suppl 1):S17–S26. [PubMed] [Google Scholar]
- 20.Isolauri J, Luostarinen M, Isolauri E, Reinikainen P, Viljakka M, Keyriläinen O. Natural course of gastroesophageal reflux disease: 17–22 year follow-up of 60 patients. Am J Gastroenterol. 1997;92(1):37–41. [PubMed] [Google Scholar]
- 21.Malfertheiner P, Nocon M, Vieth M, et al. Evolution of gastro-oesophageal reflux disease over 5 years under routine medical care – the ProGERD study. Aliment Pharmacol Ther. 2012;35(1):154–164. doi: 10.1111/j.1365-2036.2011.04901.x. [DOI] [PubMed] [Google Scholar]
- 22.Wu JC, Lai LH, Chow DK, Wong GL, Sung JJ, Chan FK. Concomitant irritable bowel syndrome is associated with failure of step-down on- demand proton pump inhibitor treatment in patients with gastro-esophageal reflux disease. Neurogastroenterol Motil. 2011;23(2):155–160. doi: 10.1111/j.1365-2982.2010.01627.x. [DOI] [PubMed] [Google Scholar]
- 23.Saito YA, Schoenfeld P, Locke GR., 3rd The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97(8):1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 24.Pacini F, Calabrese C, Cipolletta L, et al. Burden of illness in Italian patients with gastro-oesophageal reflux disease. Curr Med Res Opin. 2005;21(4):495–502. doi: 10.1185/030079905X38231. [DOI] [PubMed] [Google Scholar]
- 25.Biertho L, Sanjeev D, Sebajang H, Antony M, Anvari M. The influence of psychological factors on the outcomes of laparoscopic Nissen fundoplication. Ann Surg Innov Res. 2007;1:2. doi: 10.1186/1750-1164-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad M, Wahlqvist P, Shikiar R, Shih YC. A review of self-report instruments measuring health-related work productivity: a patient-reported outcomes perspective. Pharmacoeconomics. 2004;22(4):225–244. doi: 10.2165/00019053-200422040-00002. [DOI] [PubMed] [Google Scholar]
- 27.Wahlqvist P, Brook RA, Campbell SM, et al. Objective measurement of work absence and on-the-job productivity: a case-control study of US employees with and without gastroesophageal reflux disease. J Occup Environ Med. 2008;50(1):25–31. doi: 10.1097/JOM.0b013e31815dba5a. [DOI] [PubMed] [Google Scholar]
- 28.Galindo G, Vassalle J, Marcus SN, Triadafilopoulos G. Multimodality evaluation of patients with gastroesophageal reflux disease symptoms who have failed empiric proton pump inhibitor therapy. Dis Esophagus. 2012 doi: 10.1111/j.1442-2050.2012.01381.x. In press. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto M, Haruma K, Takeuchi K, Kuwabara M. Frequency scale for symptoms of gastroesophageal reflux disease predicts the need for addition of prokinetics to proton pump inhibitor therapy. J Gastroenterol Hepatol. 2008;23(5):746–751. doi: 10.1111/j.1440-1746.2007.05218.x. [DOI] [PubMed] [Google Scholar]
- 30.Futagami S, Iwakiri K, Shindo T, et al. The prokinetic effect of mosapride citrate combined with omeprazole therapy improves clinical symptoms and gastric emptying in PPI-resistant NERD patients with delayed gastric emptying. J Gastroenterol. 2010;45(4):413–421. doi: 10.1007/s00535-009-0173-0. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto M, Manabe N, Haruma K. Efficacy of the addition of prokinetics for proton pump inhibitor (PPI) resistant non-erosive reflux disease (NERD) patients: significance of frequency scale for the symptom of GERD (FSSG) on decision of treatment strategy. Intern Med. 2010;49(15):1469–1476. doi: 10.2169/internalmedicine.49.3615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Unit cost data for medical consultations, hospitalizations, tests, procedures, and surgeries
| Description | Unit cost (US$) | Comment |
|---|---|---|
| Medical consultation charges | ||
| Primary care physician visits | 40.41 | 10-minute consultation for an established patient |
| Specialist visits | 102.10 | 30-minute consultation |
| Emergency room visits | 353.57 | Presenting with a problem of moderate severity |
| Outpatient visits | 91.64 | 30-minute consultation for an established patient |
| Charges for hospitalizations | ||
| Inpatient visits: general care | 2501.90 | Mean length of stay: 3.5 days |
| Inpatient visits: intermediate care | 6271.83 | Mean length of stay: 5.1 days |
| Charges for surgeries | ||
| Gastric ulcer surgery | 1443.20 | Stomach, esophageal, and duodenal procedures without CC/MCC |
| Charges for tests/procedures | ||
| Blood sample for laboratory tests | 3.39 | |
| Upper gastrointestinal endoscopy | 254.72 | Requires moderate sedation (with anesthesiologist cost is US$471.19) |
| Upper gastrointestinal endoscopy with biopsy | 309.56 | Requires moderate sedation (with anesthesiologist cost is US$562.11) |
| Radiologic examination | 99.58 | |
| Upper abdominal ultrasound | 231.27 | |
| 24-hour pH monitoring | 167.05 | |
| Esophageal motility study/esophageal manometry | 211.42 | |
| Bernstein test (acid perfusion test) | 122.67 | |
| Resting ECG | 12.27 | |
| Exercise ECG | 76.49 | |
| X-ray | 158.39 | |
| 24-hour Holter monitor (ambulatory ECG) | 80.10 | |
| Abdominal MRI | 289.72 | |
| Breath hydrogen | 62.42 | |
| Electrogastrogram | 26.70 | Professional component only |
| Gastric emptying scan | 248.95 | |
| HIDA scan | 294.77 | Hepatobiliary ductal system imaging, including gallbladder, with or without pharmacologic intervention, with or without quantitative measurement of gallbladder function |
| Pill cam | 611.54 | Capsule endoscopy |
| Pulmonary function test (spirometry) | 31.75 | |
| Sleep study: unattended | 195.91 | |
| Sleep study: attended | 406.61 | |
| Small bowel enteroscopy | 154.06 | Requires moderate sedation (with anesthesiologist cost is US$442.69) |
| Stretta procedure | 318.94 | Requires moderate sedation (with anesthesiologist cost is US$1198.55) |
| Echocardiogram | 101.02 | |
| Stool culture | 13.51 | Stool, aerobic, additional pathogens, isolation, and presumptive identification of isolates |
| Dilatation of stricture | 513.04 | Requires moderate sedation |
Abbreviations: CC/MCC, complications and comorbidities/major complications and comorbidities; ECG, electrocardiogram; HIDA, hepatobiliary iminodiacetic acid; MRI, magnetic resonance imaging.