Abstract
Background
Asthma is common among children, adolescents, and adults. However, management of asthma often fails to follow evidence-based guidelines. Control assessments have been developed, validated against expert opinion, and disseminated. However, in primary care, assessment of control is only one step in asthma management. To facilitate integration of the evidence-based guidelines into practice, tools should also guide the next steps in care. The Asthma APGAR tools do just that, incorporating a control assessment as well as assessment of the most common reasons for inadequate and poor control. The Asthma APGAR tool is also linked to a care algorithm based on the 2007 National Heart, Lung, and Blood Institute asthma guidelines. The objective of this study is to assess the impact of implementation of the Asthma APGAR on patient asthma outcomes in primary care practices.
Methods
A total of 1400 patients aged 5–60 years with physician-diagnosed asthma are enrolled in 20 practice-based research network (PBRN) practices randomized to intervention or usual care. The primary outcomes are changes in patient self-reported asthma control, asthma-related quality of life, and rates of exacerbations documented in medical records over the 18–24 months of enrollment. Process measures related to implementation of the Asthma APGAR system into daily care will also be assessed using review of medical records. Qualitative assessments will be used to explore barriers to and facilitators for integrating the Asthma APGAR tools into daily practice in primary care.
Discussion
Data from this pivotal pragmatic study are intended to demonstrate the importance of linking assessment of asthma and management tools to improve asthma-related patient outcomes. The study is an effectiveness trial done in real-world PBRN practices using patient-oriented outcome measures, making it generalizable to the largest possible group of asthma care providers and primary care clinics.
Keywords: asthma, asthma control, asthma management, primary care, pragmatic research, practice-based research, protocol
Introduction
Asthma affects as many as 18% of US children by the age of 18 years and 5% of adults, with another 5% of children and adults reporting exercise-induced asthma.1–7 Asthma is the 15th most common condition seen by family physicians,8 and the majority of the 11.9 million annual asthma-related medical visits are made to primary care physicians.2,3,9–11 Asthma is associated with significant morbidity and mortality,3,12–16 much of which could be prevented17–19 by broader implementation of the four major tenets of the 2007 National Heart, Lung, and Blood Institute (NHLBI) asthma guidelines.20–28 These tenets include accurate diagnosis, appropriate treatment, ongoing monitoring and assessment, and developing partnerships between health professionals and families.29–34 Simple tools have been developed to monitor asthma control.35–45 However, none of the control scores recommended in the US guidelines are linked to the next steps of asthma care.7,21,43,46–49
Asthma is a chronic inflammatory disease with overlying acute episodes of increased inflammation and bronchoconstriction.18 Determining how to specify, address, and prevent the inflammation of asthma better is the major therapy question, while the translational question of most importance is how to facilitate, operationalize, systematize, and integrate guideline-directed care into everyday practice.7,43,46–48,50,51
Assessment of asthma “control” requires knowledge of the patient’s symptom burden, eg, daytime and night-time symptoms and need to modify activities. Primary care medical records consistently lack this information.7,43,46,47,50,52 Existing highly promoted control assessments collect the information to assess control.21,22,46,47,52 The control assessments leave the patient labeled as in or out of control and may predict future exacerbations, but provide no further guidance related to therapy. Primary care physicians need systems and tools to guide daily practice and not just to label or risk-stratify patients.
Therefore, asthma remains an important target for translational studies and testing of tools that facilitate all four of the NHLBI’s major tenets of asthma care.18 The Asthma APGAR system uses tools developed, validated, and demonstrated to work in primary care practices. This multicomponent system includes audit with feedback and patient-reported signs and symptoms, as well as information on adherence, triggers, and response to therapy in a system that allows flexibility and adaptability in implementation.54,55 This clinical trial assesses the effectiveness of the Asthma APGAR system54 in primary care, focusing on patient-oriented asthma outcomes. The trial is being done at community practice-based research network (PBRN) primary care sites to enhance the generalizability of the results while maintaining adequate internal validity.56–60
Materials and methods
Overview
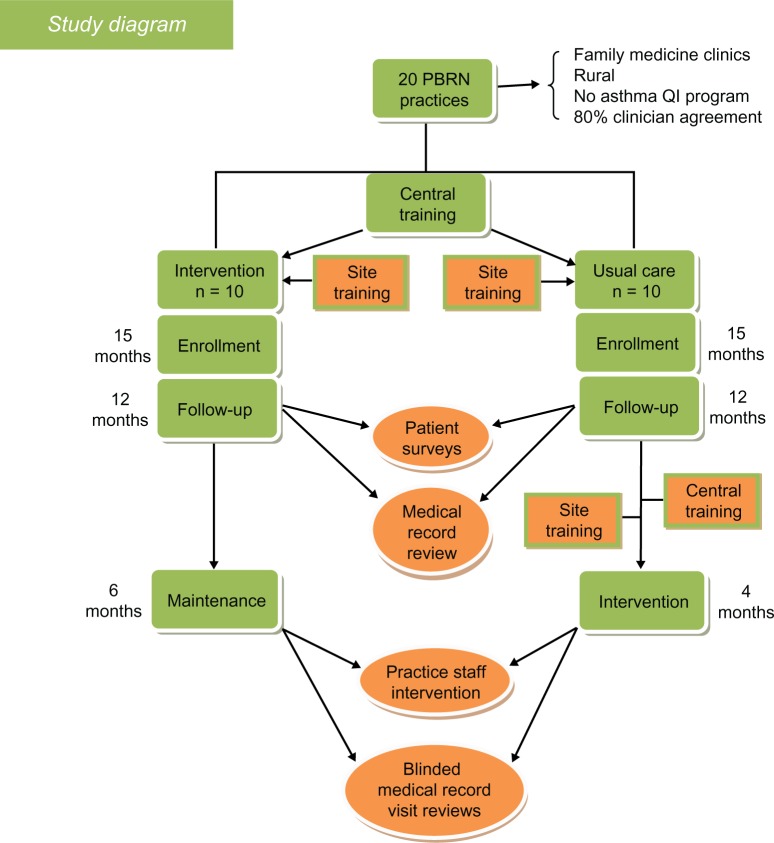
This is a pragmatic, randomized, controlled effectiveness trial (Figure 1) of a practice system change for asthma evaluation and management and is now presently underway in 20 family medicine and pediatric practices (all members of the PBRN). Randomization was 1:1 (intervention to usual care) and stratified by residency status (yes or no) and type of practice (pediatric or family medicine practice). A total of 1400 patients are to be enrolled. The primary outcomes will be changes in self-reported asthma control, self-reported quality of life, and rates of asthma exacerbations documented by medical records. Secondary outcomes are care process measures, including documentation of asthma control, education on or review of inhaler technique, and assessment of adherence during clinic visits. Exploratory outcomes will be assessed using qualitative methods (semistructured interviews) to explore factors associated with the feasibility of implementing Asthma APGAR tools in the intervention practices.
Figure 1.
Study design.
Abbreviations: PBRN, practice-based research network; QI, quality improvement.
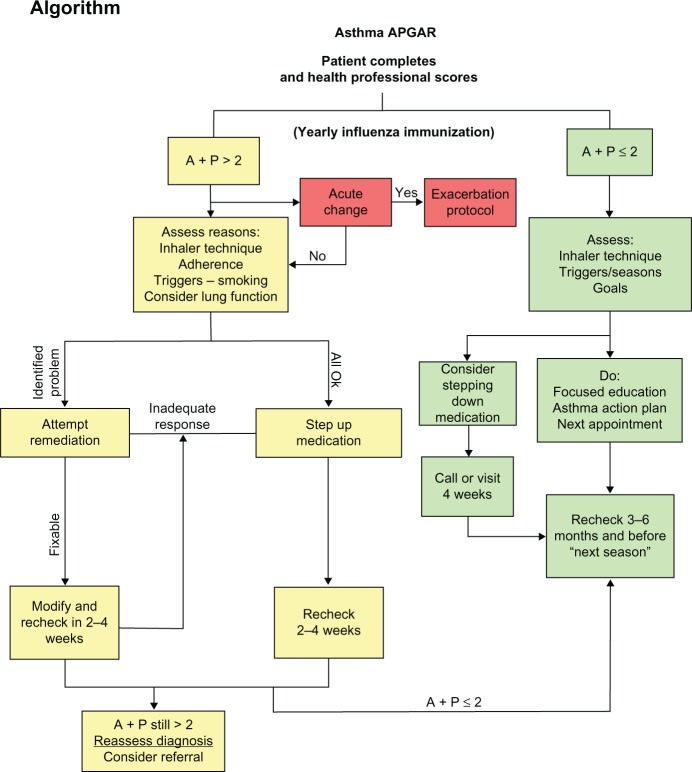
The intervention consists of a facilitated practice systems change to integrate the Asthma APGAR tools into daily management of asthma. The Asthma APGAR tools address five domains critical to the tracking, assessment, monitoring, and management of asthma (Table 1). The Asthma APGAR tools include: a five-question practice asthma care audit used to motivate, monitor, and report baseline asthma care processes (Figure 2); a patient-completed survey issued at all asthma visits to assess and track control as well as explore the most common reasons for lack of control (Figure 3A and B); and a care algorithm linked to the control, adherence, and trigger assessment using evidence from the 2007 NHLBI asthma guidelines (Figure 4). The algorithm incorporates both drug and nondrug management strategies, eg, stepped medication care, asthma education, and evaluation of inhaler technique. The intervention tools have been pretested and validated to change and sustain processes centered on the Asthma APGAR tools.54 Use of the tools has been shown to facilitate guideline-adherent asthma care and should thereby improve patient outcomes.
Table 1.
APGAR domains: essential elements
| A (Activity limitation) |
| P (Persistence of symptoms, day and night) |
| G (TriGger identification) |
| A (Adherence to medications) |
| R (Response to therapy) |
Figure 2.
Asthma APGAR audit.
Figure 3.
Asthma APGAR Plus. (A) Front and (B) back of questionnaire.
Figure 4.
Asthma APGAR algorithm.
Abbreviations: A, activities; P, persistence.
Practices
The practices enrolled are members of the American Academy of Family Physicians National Research Network (http://www.aafp.org/nrn) or the American Academy of Pediatrics Quality Improvement Innovation Network (http://www.aap.org/en-us/professional-resources/practice-support/quality-improvement/Quality-Improvement-Innovation-Networks/Pages/Quality-Improvement-Innovation-Networks-QuIIN.aspx).
Inclusion criteria
Practice located in a non-inner city or urban center with >250,000 population
Practice includes 2–12 primary care clinicians
Within the practice, 80% of all primary care physicians agree to participate in the project for three years
Practice has had at least 100 patients aged 5–60 years making asthma visits in past 12 months
Practice agrees to recruit at least 70 patients with asthma over the 15-month enrollment period of the study
Practice is willing to sign an individual investigator agreement with the American Academy of Family Physicians institutional review board or have an affiliation with a local institutional review board.
Exclusion criterion
Practice has been involved in any formal asthma care improvement program during the previous three years.
Each practice signed a practice agreement attesting to the support of the practice leadership and acknowledging the $1300 per year they would receive based on attaining specific enrollment goals and copying and mailing of medical records goals. Practices were not and could not be blinded to their randomization status. However, the patients who are enrolled will not know the randomization status of the practice they attend.
Patients
Patients are recruited in two ways, ie, as they are seen in the enrolled practices for a patient visit or by identification from an asthma registry and an invitation to come for an asthma checkup and enrollment in the study. The enrollment process is the same for patients in an intervention or a usual care practice.
Inclusion criteria
Patient is aged 5–60 years
Patient has a physician diagnosis of asthma and a current prescription for an asthma drug
Patient or parent agrees to complete the five study packets (baseline, and at 6, 12, 18, and 24 months after enrollment) and allow review of the enrollee’s medical record by the central study team members.
Exclusion criteria
Patient or parent is unable to read or speak English
Patient has a concomitant diagnosis of cystic fibrosis, chronic obstructive pulmonary disease (COPD), or other chronic lung disease.
Children younger than 5 years of age are excluded to avoid the problem of diagnostic uncertainty often found in preschool children.61,62 Adults over 60 years of age were also excluded because of concerns about COPD that has been misdiagnosed as asthma.63 Treatment for cystic fibrosis, COPD, active tuberculosis, or other chronic lung disease is significantly different from that recommended for asthma, and inclusion of these patients could confound our results. Spirometry is not required because it is unlikely to be available in most of the primary care practices enrolled and because most asthma seen in primary care is diagnosed on the basis of symptoms and response to therapy.64–66
Study procedures
Site training
Two members from each practice (a lead study physician and a lead nurse) were brought together at a central site to introduce the study procedures to all sites and the Asthma APGAR system to the intervention sites. Training on the study procedure lasted eight hours, beginning with a short overview of the 2007 NHLBI asthma guidelines. The remainder of the day focused on methods to identify asthma patients for potential enrollment, informed consent, tracking forms to assess enrollment and refusal rates, and methods (e-faxing and faxing) used to send study data to the central site. An opportunity to complete the required human subjects training was included.
The intervention site training continued with six hours of work on the next day. That time was used to introduce the Asthma APGAR tools and to discuss and demonstrate how they could be integrated into daily asthma care. Interaction methods included case presentations, discussion, and interactive brainstorming of ways to facilitate use of the Asthma APGAR. Because implementation of the intervention includes some flexibility, time was spent working with each practice team individually to discuss practice-specific implementation. Each team was provided with an arm-specific slide presentation designed to be used by the team leaders at an all-practice local study training session. The slides included a shortened version of the information presented during central training. The principal investigator for the study attended all of these local training sessions by telephone conference call to lend support and answer questions.
Implementation at intervention sites
Based on experience from previous work, introduction of the Asthma APGAR system in the intervention practices was accompanied by “motivational” work to improve practice engagement in the project. Each practice’s current asthma care was evaluated using the Asthma APGAR audit which assesses the presence or absence of documentation in selected medical records of the elements required to evaluate asthma control (Figure 2).
These audit data were summarized, graphed, and presented to the entire practice staff as part of the local study training led by the site lead physician and nurse as described above. One goal of the sessions was to help the practices assess the strengths and gaps in asthma care before initiating the study. Strengths were discussed first.
By including discussion of what the practice does well, discussion appeared to begin more readily than when focusing first on gaps. When discussing gaps, invariably one or two clinicians pointed out they had done much more than was documented. These comments facilitated discussion on the purpose of the information documented in the medical record and its use, not only at the current visit but also during future visits to assess changes in asthma status over time. Guided discussion also addressed the potential value of the Asthma APGAR data elements (Figure 3A and B) in identifying reasons for inadequate control, such as the role of triggers, adherence, and medication failures in asthma control.
Introducing a new tool into practice requires planning and often trial and error. All intervention practices were allowed a six-week period to adapt implementation of the Asthma APGAR into their practice before adding the additional burden of patient enrollment. No specific study visits are required in this pragmatic trial. When patients do visit the clinic for any reason, they receive the patient Asthma APGAR survey. The Asthma APGAR information is used and documented as the physician/clinician chooses during each visit.
A coordinator from the central study team is assigned to each practice as the practice liaison. The liaison interacts with the site nurse leader weekly during the early implementation phase and then biweekly or as needed throughout the rest of the study.
Usual care group
After returning home from central training, the usual care study leaders also provide a short education session for their practice members. The focus of the education session is “whole practice participation” in identifying patients eligible for enrollment and assuring that all practice staff are aware of the study. As in the intervention program, no visits or visit frequency is dictated by the study. All care decisions are at the discretion of the physician/clinician and patient. Usual care sites are also assigned a liaison from the central study team to work with the local sites on patient enrollment.
Patient-reported outcomes
Patient or parent subjects are asked to complete five survey packets at baseline and at 6, 12, 18, and 24 months after enrollment. Each packet includes the Patient Assessment of Chronic Illness Care (PACIC), the Asthma Quality of Life Questionnaire/Pediatric Asthma Quality of Life Questionnaire (AQLQ/PAQLQ), the Asthma Control Test (ACT), Asthma APGAR patient questions, and a group of health care utilization questions (Table 2). The initial packet also includes demographic data. To facilitate continued participation in the study, a central site coordinator calls each enrolled person (or parent) within 72 hours of signing the informed consent.
Table 2.
Variables, instruments, and links to study aims
| Aim and outcome | Instruments | Source of data |
|---|---|---|
| Patient outcomes | ||
| Asthma-related quality of life | AQLQ | Patient/parent |
| Asthma control | ACT | Patient/parent |
| Exacerbations | Number per year | Medical record and patient/parent |
| Chronic care approach | PACIC | Patient/parent |
| Maintenance | Use of APGAR Use of algorithm |
Medical records Medical records |
| Barriers | ||
| Number of new systems | PPC | Key staff |
| Types of new systems | PACIC | Key staff |
| Implementation issues | Surveys and interviews | Key informants |
| Fidelity measures | ||
| Reach of patient APGAR Reach of algorithm |
Both by medical record review | Both by medical records |
Abbreviations: PACIC, Patient Assessment of Chronic Illness Care; AQLQ, Asthma Quality of Life Questionnaire; ACT, Asthma Control Test; PPC, Practice Process Content.
Practice process data
Medical record data are required to measure practice asthma care processes and to assess asthma exacerbation rates. All data abstraction is done centrally. Inter-rater reliability testing will be accomplished within each site and across sites and must remain at 90% or greater. This will be done by abstraction of the same record by multiple abstractors across and within sites. If agreement is lower than 90% for at least five major items within the abstraction, additional training and testing will be undertaken.
Fidelity measures
Translational and effectiveness studies differ from efficacy and traditional randomized controlled trials in that no central study staff are present in the study practice sites. Therefore, it is important to have some measure of how well the intervention is actually implemented, ie, a study fidelity metric. In this study, uptake of the intervention is assessed using mixed methods, including semistructured interviews with the lead physician and nurse at the study site and medical record review at the end of the study period to assess documentation of use of the Asthma APGAR tools.
Exploratory data concerning barriers and facilitators
The central site staff will conduct interviews to collect information on use of the Asthma APGAR tools. Each interviewee will be queried regarding barriers and facilitators of the Asthma APGAR implementation.
Data analysis
The patient data will be summarized and presented in both graphic and tabular form, separately by site and pooled intervention versus control. The primary patient-oriented outcomes will be analyzed using linear mixed effects or generalized linear random effects models, with random mean terms for patient and practice, and fixed-effect terms for patient age and gender, and a fixed-effect term for the intervention. For these patient outcomes, the random effects for practices are likely to be important, given that there are likely to be fairly large differences in patient characteristics across different practices.
Whenever patient-reported outcomes are used, some level of nonresponse is expected. To minimize the number of records excluded from analysis, we will use multiple imputations to fit missing responses. Results of the multiple imputation analysis will be compared with an analysis of complete data; if results differ, both will be presented.
The ACT and AQLQ/PAQLQ scores are reasonably Gaussian, and we will fit linear models for them. For asthma exacerbations and number of missed work/school days, we will fit generalized mixed linear models with a random effect term to adjust for differences between practices. A significant coefficient for the intervention term will be interpreted as indicating an intervention effect. For assessment of exacerbations (requiring short bursts of oral steroids), data are only relevant for those visits dealing with exacerbations. Therefore, for this measure, analysis will be restricted to those visits where that treatment is appropriate.
Information from visits will be compared between intervention and control practices starting at three months, to allow a run-in period for the intervention to be implemented. Per-practice rates for each of these procedures will be computed both before (baseline data) and after the three-month run-in period (study period), and will be presented in both graphic and tabular form. The hypotheses for the five measures will be tested through logistic regression with random effects (generalized linear mixed models), with random mean terms for patient and practice, fixed-effect terms for patient age and gender, and a fixed-effect term for the intervention. Baseline data obtained from both the intervention and control practices will be included in the models, but the visit-specific value for the intervention term will be 0 (not intervention) for these records, because at that time the intervention would not yet have been implemented. Inclusion of baseline data will allow good estimation of practice random effects, and will adjust for differences not accounted for by randomization. A significant coefficient for the intervention term will be interpreted as evidence of an intervention effect.
The number of asthma-related practice systems in place for each practice will be assessed at time 0 and at 24 months using the PACIC modified for asthma care. In each of the six domains, three clinically important types of system will be identified, so the number of new systems could range from 0 to 18. The within-practice number of new systems will be computed by subtracting the number of systems in place at study completion from baseline, and will be compared across practices using the Wilcoxon rank-sum test. The PACIC is a patient visit-level measure that we are using to quantify the practice-level intervention effect.67 Its distribution is approximately Gaussian, and we will fit a linear random effects model, with random mean terms for patient and practice, fixed-effect terms for patient age and gender, and a fixed-effect term for the intervention. Inclusion of the baseline data will allow good estimation of the practice random effects, and will adjust for differences not accounted for by randomization. A significant coefficient for the intervention term will be interpreted as evidence of an intervention effect.
Analysis of the key personnel interviews will be descriptive and exploratory. We will attempt to identify any unanticipated barriers and facilitators to implementation. In addition, the interviews will provide information about underused or ineffective systems.
Sample size
We will enroll approximately 1400 patients, averaging 70 per practice. In our study of post-partum depression40 we were able to attain 12-month response rates better than 60%; this group of asthma patients is likely to be less mobile, and we believe that assuming a 60% complete response is conservative. Based on simulated data with 840 subjects, we estimate that we will have over 80% power to detect a change in exacerbation rate from 12% to 8%, and over 90% power to detect a difference between a mean of 5.0 versus a mean of 4.0 missed work/school days. For the AQLQ/PAQLQ, the overall score is approximately Gaussian with a standard deviation of about 0.8;68 we will have approximately 80% power to detect a mean difference of 0.15. A difference of 0.5 represents an important clinical change for an individual.69
Discussion
Effectiveness and PBRN translational studies are different from efficacy studies and clinical trials based at academic centers.58–60,70–75 Not only is the subject and goal usually different, but the study design must be built around the strengths and weaknesses inherent in doing research in a real-world practice. In national studies, it is rarely feasible to send research coordinators or facilitators to each of the study sites on a repeated basis.57,76 Therefore, the study must be designed in a manner that allows the study personnel to carry out the study with limited use of practice resources. This difference is highlighted in the sections on study and intervention implementation. The all-practice meetings led by the two practice leaders are supported heavily by the central team, and include development and dissemination of pre-prepared educational slide programs and handouts, the simple practice audit, and a proven format in which to present the audit data. Telephone support from the principal investigator and the central site lead study coordinator demonstrates the central team’s commitment to provide help at each stage of the study.77 The provision of a study liaison for continuity of contact has resulted in local sites identifying with their liaison and funneling their questions and concerns through a single individual. Making sure that all interactions with practices are at all times convenient for the practices not the principal investigator or central study team is necessary to engage the local sites. Unlike many large clinical trials, there can be no expectation that a PBRN study comes ahead of any clinical or practice issue. Familiarity with practice flow and challenges in sites similar to those enrolled in the study facilitates discussion and keeps expectations of the central site in line with those of the enrolled sites. Certain issues can be anticipated in all PBRN studies, and are discussed in the following sections.
Time pressures
Time pressures will be an issue for these practices. Meetings with the central study staff will be held by distance interaction (telephone or web-based) at a time that is convenient for the practice. Each practice will be paid $1300 in each of the study years. This will not cover their time or effort, but does serve to recognize the important contributions of the practice. We will limit staff time away from or interference with patient care as far as possible.
Practice politics
It can be difficult to engender open and honest discussion in some practices or suggest that baseline care is not of the highest quality. To avoid including practices with no hope of collaboration, we will query each practice about previous attempts at practice change and ask for assurance that at least 80% of their primary care physicians are committed to this study.
Reluctance to use asthma guidelines
The Asthma APGAR is designed to make it feasible to use the guidelines in practices that have previously been unable or unwilling to do so.
Lack of resources to make practice changes
We anticipate that our concepts and tools will minimize such difficulties and may even make the care of asthma easier in these practices.
Failure to institutionalize changes
Many previous quality improvement efforts have suffered from the mentality of “fix it and move on”, that often results in a decline in practice systems or process improvement and potentially in the quality of care. We will test the level of the Asthma APGAR’s sustainability and practice institutionalization by including a maintenance phase, which is similar to the intervention phase but without support or calls from the central site staff.
This study has limitations that must be recognized in its initial design and goals. The study will occur in practices that do not have the research personnel usually associated with a randomized controlled trial. This is an advantage for generalizability of the results, but might be viewed as a limitation by those only familiar with trials of efficacy conducted in carefully controlled environments. With careful monitoring, frequent contact, and collection of fidelity data, PBRNs have been shown to be capable of producing reliable and accurate effectiveness results.57 Asthma is defined clinically, and no pulmonary function data are required. Many of the enrolled practices do not have experience with spirometry testing. This is comparable with the 40%–60% of all primary care practices in the US that do not use spirometry on a regular basis. Other researchers, such as Juniper39 and Nathan et al,37 have found that assessment of asthma control and patient outcomes is possible without pulmonary function testing. This study will be generalizable to the defined asthma population of most primary care practices. Outcomes assessment will rely heavily on patient self-reporting of asthma control and asthma-related quality of life. Not all patients will return the surveys containing this information. However, with good follow-up, response rates should be in the range of 65%–75%. The rate of exacerbations will use medical records data which may not fully reflect patient-reported symptoms; however, it is a very good resource for identifying prescribed medications, ie, oral steroids, which is what we are using to define significant asthma exacerbations.
Summary
Asthma continues to be associated with preventable morbidity and mortality that could be lowered through more consistent delivery of guideline-compliant care in primary care practices. This project uses patient outcomes to test the effectiveness of the Asthma APGAR system and tools developed in collaboration with practicing family physicians and their staff members. The patient asthma APGAR guides collection of the important information required to assess asthma control and the results are linked to action items in the care algorithm. The project uses the available evidence about translating research into practice, including addressing motivation, the process of change, and development of a systemic care process tailored to rural practices. The intervention is simple, requires limited investment of time or money on the part of the practice, and is therefore likely to be feasible for broad dissemination in the future.
Acknowledgments
Funding for this study was provided by the Agency for HealthCare Research and Quality (R01-HS0118431).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hedman L, Bjerg A, Lundback B, Ronmark E. Conventional epidemiology underestimates the incidence of asthma and wheeze – a longitudinal population-based study among teenagers. Clin Transl Allergy. 2012;2(1):1. doi: 10.1186/2045-7022-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;94:1–8. [PubMed] [Google Scholar]
- 3.Bloom B, Cohen RA, Freeman G. Summary health statistics for US children: National Health Interview Survey, 2010. Vital Health Stat. 2011;250:1–80. [PubMed] [Google Scholar]
- 4.Mannino DM, Homa DM, Akiinbami LJ, et al. Surveillance for asthma – United States, 1980–1999. MMWR CDC Surveill Summ. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 5.Ginde AA, Espinola JA, Camargo CA. Improved overall trends but persistent racial disparities in emergency department visits for acute asthma, 1993–2005. J Allergy Clin Immunol. 2008;122(2):313–318. doi: 10.1016/j.jaci.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong GW, Chow CM. Childhood asthma epidemiology: insights from comparative studies of rural and urban populations. Pediatr Pulmonol. 2008;43(2):107–116. doi: 10.1002/ppul.20755. [DOI] [PubMed] [Google Scholar]
- 7.Yawn BP, Wollan P, Kurland MJ, Scanlon P. A longitudinal study of asthma prevalence in a community population of school age children. J Pediatr. 2002;140(5):576–581. doi: 10.1067/mpd.2002.123764. [DOI] [PubMed] [Google Scholar]
- 8.Yawn BP, Fryer GE, Phillips RL, Dovey SM, Lanier D, Green LA. Using the ecology model to describe the impact of asthma on patterns of health care. BMC Pulm Med. 2005;5:1–7. doi: 10.1186/1471-2466-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newacheck PW, McManus M, Fox HB, Hung YY, Halfon N. Access to health care for children with special health care needs. Pediatrics. 2000;105(4 Pt 1):760–766. doi: 10.1542/peds.105.4.760. [DOI] [PubMed] [Google Scholar]
- 10.Lozano P, Sullivan S, Smith D, Weiss K. The economic burden of asthma in US children: estimates from the National Medical Expenditure Survey. J Allergy Clin Immunol. 1999;104(5):957–963. doi: 10.1016/s0091-6749(99)70075-8. [DOI] [PubMed] [Google Scholar]
- 11.To T, Dell S, Dick P, Cicutto L. The burden of illness experienced by young children associated with asthma: a population-based cohort study. J Asthma. 2008;45(1):45–49. doi: 10.1080/02770900701815743. [DOI] [PubMed] [Google Scholar]
- 12.Rowe BH, Voaklander DC, Wang D, et al. Asthma presentations by adults to emergency departments in Alberta, Canada: a large population-based study. Chest. 2009;135(1):57–65. doi: 10.1378/chest.07-3041. [DOI] [PubMed] [Google Scholar]
- 13.Sears MR. Epidemiology of asthma exacerbations. J Allergy Clin Immunol. 2008;122(4):662–668. doi: 10.1016/j.jaci.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Henderson AJ. What have we learned from prospective cohort studies of asthma in children? Chron Respir Dis. 2008;5(4):225–231. doi: 10.1177/1479972308097327. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Mannino DM, Homa DM, et al. Self-reported asthma and health-related quality of life: findings from the behavioral and risk factor surveillance system. Chest. 2003;123(1):119–127. doi: 10.1378/chest.123.1.119. [DOI] [PubMed] [Google Scholar]
- 16.Savage-Brown A, Mannino DM, Redd SC. Lung disease and asthma severity in adults with asthma: data from the Third National Health and Nutrition Examination. J Asthma. 2005;42(6):519–523. doi: 10.1081/JAS-67605. [DOI] [PubMed] [Google Scholar]
- 17.Pappas G, Hadden WC, Kozak LJ, Fisher GF. Potentially avoidable hospitalizations: inequalities in rates between US socioeconomic groups. Am J Public Health. 1997;87(5):811–816. doi: 10.2105/ajph.87.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Heart, Lung, and Blood Institute . National Asthma Education and Prevention Expert Panel report 3: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health; 2007. [Accessed March 30, 2013]. Available from: http://www.nhlbi.nih.gov/guidelines/asthma/method.htm. [Google Scholar]
- 19.Lundback B, Ronmark E, Lindberg A, Jonsson AC, Larsson LG, James M. Asthma control over 3 years in a real-life study. Respir Med. 2009;103(3):348–355. doi: 10.1016/j.rmed.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Yawn BP. Applying the 2007 Expert Panel Report in caring for young children with asthma. J Clin Outcomes Manage. 2007;14(12):667–681. [Google Scholar]
- 21.Cabana MD, Rand CS, Becher OJ, Rubin HR. Reasons for pediatrician nonadherence to asthma guidelines. Arch Pediatr Adolesc Med. 2001;155(9):1057–1062. doi: 10.1001/archpedi.155.9.1057. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan SD, Lee TA, Blough DK, et al. A multi-site randomized trial for the effects of physician education and organizational change in chronic asthma care. Cost-effectiveness analysis of the Pediatric Asthma Care Patient Outcomes Research Team II (PAC-PORT II) Arch Pediatr Adolesc Med. 2005;159(5):428–434. doi: 10.1001/archpedi.159.5.428. [DOI] [PubMed] [Google Scholar]
- 23.Perry TT, Vargas PA, McCracken A, Jones SM. Underdiagnosed and uncontrolled asthma: findings in rural school children from the Delta region of Arkansas. Ann Allergy Asthma Immunol. 2008;101(4):375–381. doi: 10.1016/S1081-1206(10)60313-4. [DOI] [PubMed] [Google Scholar]
- 24.Yawn BP, Mainous AG, III, Love MM, Hueston W. Do rural and urban children have comparable asthma care utilization? J Rural Health. 2001;17(1):32–39. doi: 10.1111/j.1748-0361.2001.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 25.Hagmolen Of Ten Have W, van den Berg NJ, van der Palen J, van Aalderen WM, Bindels PJ. Limitations of questioning asthma to assess asthma control in general practice. Respir Med. 2008;102(8):1153–1158. doi: 10.1016/j.rmed.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Levy ML. Guideline-defined asthma control: a challenge for primary care. Eur Respir J. 2008;31(2):229–231. doi: 10.1183/09031936.00157507. [DOI] [PubMed] [Google Scholar]
- 27.Braido F, Baiardini I, Stagi E, Piroddi MG, Balestracci S, Canonica GW. Unsatisfactory asthma control: astonishing evidence from general practitioners and respiratory medicine specialists. J Investig Allergol Clin Immunol. 2010;20(1):9–12. [PubMed] [Google Scholar]
- 28.Liu AH, Gilsenan AW, Stanford RH, Lincourt W, Ziemiecki R, Ortega H. Status of asthma control in pediatric primary care: results from the pediatric Asthma Control Characteristics and Prevalence Survey Study (ACCESS) J Pediatr. 2010;157(2):276–281. doi: 10.1016/j.jpeds.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Norton SP, Pusic MV, Taha F, Heathcote S, Carleton BC. Effect of a clinical pathway on the hospitalization rates of children with asthma: a prospective study. Arch Dis Child. 2007;92(1):60–66. doi: 10.1136/adc.2006.097287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster JM, Hoskins G, Smith B, Lee AJ, Price D, Pinnock H. Practice development plans to acute asthma: randomized controlled trial. BMC Fam Pract. 2007;8:23. doi: 10.1186/1471-2296-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rastogi D, Shetty A, Neugebauer R, Harijith A. National Heart, Lung, and Blood Institute guidelines and asthma management practices among inner-city pediatric primary care providers. Chest. 2006;129(3):619–623. doi: 10.1378/chest.129.3.619. [DOI] [PubMed] [Google Scholar]
- 32.Doherty SR, Jones PD, Davis L, Ryan NJ, Treeve V. Evidence-based implementation of adult emergency department: a controlled trial. Emerg Med Australas. 2007;19(1):31–38. doi: 10.1111/j.1742-6723.2006.00910.x. [DOI] [PubMed] [Google Scholar]
- 33.Yawn B, Madison S, Bertram S, et al. Automated patient and medication payment method for clinical trials. Open Access J Clin Trials. 2012;4:1–9. [Google Scholar]
- 34.Doboszynska A, Swietlik E. Asthma management at primary care level: symptoms and treatment of 3305 patients with asthma diagnosed by a family physician. J Physiol Pharmacol. 2008;59(Suppl 6):231–241. [PubMed] [Google Scholar]
- 35.Vollmer W. Assessment of asthma control and severity. Ann Allergy Asthma Immunol. 2004;93(5):409–413. doi: 10.1016/S1081-1206(10)61406-8. [DOI] [PubMed] [Google Scholar]
- 36.Vollmer WM, Markson LE, O’Connor E, Frazier EA, Berger M, Buist AS. Association of asthma control with health care utilization: a prospective evaluation. Am J Respir Crit Care Med. 2002;165(2):195–199. doi: 10.1164/ajrccm.165.2.2102127. [DOI] [PubMed] [Google Scholar]
- 37.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the Asthma Control Test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Schatz M, Nakahiro R, Jones CH, Roth RM, Joshua A, Petitti D. Asthma population management: development and validation of a practical 3-level risk stratification scheme. Am J Manag Care. 2004;10(1):25–32. [PubMed] [Google Scholar]
- 39.Juniper EF. Assessing asthma control. Curr Allergy Asthma Rep. 2007;7(5):390–394. doi: 10.1007/s11882-007-0059-2. [DOI] [PubMed] [Google Scholar]
- 40.Schatz M, Zeiger RS, Vollmer WM, Mosen D, Cook EF. Determinants of future long-term asthma control. J Allergy Clin Immunol. 2006;118(5):1048–1053. doi: 10.1016/j.jaci.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 41.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Schatz M, Mosen DM, Kosinski M, et al. Validity of the Asthma Control Test completed at home. Am J Manag Care. 2007;13(12):661–667. [PubMed] [Google Scholar]
- 43.Yawn BP, Brenneman SK, Allen-Ramey FC, Cabana MD, Markson LE. Assessment of asthma severity and asthma control in children. Pediatrics. 2006;118(1):322–329. doi: 10.1542/peds.2005-2576. [DOI] [PubMed] [Google Scholar]
- 44.Skinner E, Diette G, Algatt-Bergstrom P, et al. The Asthma Therapy Assessment Questionnaire (ATAQ) for children and adolescents. Dis Manag. 2004;7(4):305–313. doi: 10.1089/dis.2004.7.305. [DOI] [PubMed] [Google Scholar]
- 45.Pinnock H, Juniper EF, Sheikh A. Concordance between supervised and postal administration of the Mini Asthma Quality of Life Questionnaire (MiniAQLQ) and Asthma Control Questionnaire (ACQ) was very high. J Clin Epidemiol. 2005;58:809–814. doi: 10.1016/j.jclinepi.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 46.Cabana M, Slish K, Nan B, Lin X, Clark N. Asking the correct questions to assess asthma symptoms. Clin Pediatr (Phila) 2005;44(4):319–325. doi: 10.1177/000992280504400406. [DOI] [PubMed] [Google Scholar]
- 47.Diette G, Patino C, Merriman B, et al. Patient factors that physician use to assign asthma treatment. Arch Intern Med. 2007;167(13):1360–1366. doi: 10.1001/archinte.167.13.1360. [DOI] [PubMed] [Google Scholar]
- 48.Herman EJ, Garbe PL, McGeehin MA. Assessing community-based approaches to asthma control: the Controlling Asthma in American Cities project. J Urban Health. 2011;88(Suppl 1):1–6. doi: 10.1007/s11524-010-9480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ragazzi H, Keller A, Ehrensberger R, Irani AM. Evaluation of a practice-based intervention to improve the management of pediatric asthma. J Urban Health. 2011;88(Suppl 1):38–48. doi: 10.1007/s11524-010-9471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yawn BP, Goodwin M, Zyzanski S, Stange K. Time use during acute and chronic illness visits to a family physician. Fam Pract. 2003;20(4):474–477. doi: 10.1093/fampra/cmg425. [DOI] [PubMed] [Google Scholar]
- 51.Lisspers K, Stallberg B, Hasselgren M, Johansson G, Svardsudd K. Primary health care centres with asthma clinics: effects on patients knowledge and asthma control. Prim Care Respir J. 2010;19(1):37–44. doi: 10.4104/pcrj.2009.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yawn B, Bertram S, Wollan P. Introduction of Asthma APGAR tools improve asthma management in primary care practices. [Accessed March 29, 2013];J Asthma Allergy. 2008 1:1–10. doi: 10.2147/jaa.s3595. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3121335/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juniper ER, Bousquet J, Abetz L, Bateman ED. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100(4):616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 54.Yawn BP. Factors accounting for asthma variability: achieving optimal symptom control for individual patients. Prim Care Respir J. 2008;17(3):138–147. doi: 10.3132/pcrj.2008.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mintz M, Gilsenan AW, Bui CL, et al. Assessment of asthma control in primary care. Curr Med Res Opin. 2009;25(10):2523–2531. doi: 10.1185/03007990903218655. [DOI] [PubMed] [Google Scholar]
- 56.Fried RA, Miller RS, Green LA, Sherrod P, Nutting PA. The use of objective measures of asthma severity in primary care: a report from ASPN. J Fam Pract. 1995;41(2):139–143. [PubMed] [Google Scholar]
- 57.Pace W, Fagnan L, West D. The Agency for Health Care Research and Quality (AHRQ). Practice-Based Research Network (PBRN) relationship: delivering on a opportunity, challenges, and future directions. J Am Board Fam Med. 2011;24(5):489–492. doi: 10.3122/jabfm.2011.05.110080. [DOI] [PubMed] [Google Scholar]
- 58.Westfall JM, Mold J, Fagnan L. Practice-based research – “Blue Highways” on the NIH roadmap. JAMA. 2007;297(4):403–436. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 59.Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299(2):211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]
- 60.Solberg LI, Brekke ML, Fazio CJ, et al. Lessons from experienced guideline implementers: attend to many factors and use multiple strategies. Jt Comm J Qual Improv. 2000;26(4):171–188. doi: 10.1016/s1070-3241(00)26013-6. [DOI] [PubMed] [Google Scholar]
- 61.Winklerprins V, Walsworth DT, Coffey JC. Clinical Inquiry. How best to diagnose asthma in infants and toddlers? J Fam Pract. 2011;60(3):152–154. [PubMed] [Google Scholar]
- 62.Rodriguez-Martinez CE, Sossa-Briceno MP, Castro-Rodriguez JA. Discriminative properties of two predictive indices for asthma diagnosis in a sample of preschoolers with recurrent wheezing. Pediatr Pulmonol. 2011;46(12):1175–1181. doi: 10.1002/ppul.21493. [DOI] [PubMed] [Google Scholar]
- 63.Miravitlles M, Andreu I, Romero Y, Sitjar S, Altes A, Anton E. Difficulties in differential diagnosis of COPD and asthma in primary care. Br J Gen Pract. 2012;62(595):e68–e75. doi: 10.3399/bjgp12X625111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D’Urzo AD. Must family physicians use spirometry in managing asthma patients?: NO. Can Fam Physician. 2010;56(2):127. [PMC free article] [PubMed] [Google Scholar]
- 65.Kaplan A, Stanbrook M. Must family physicians use spirometry in managing asthma patients?: YES. Can Fam Physician. 2010;56(2):126. [PMC free article] [PubMed] [Google Scholar]
- 66.Holton C, Crockett A, Nelson M, et al. Does spirometry training in general practice improve quality and outcomes of asthma care? Int J Qual Health Care. 2011;23(5):545–553. doi: 10.1093/intqhc/mzr039. [DOI] [PubMed] [Google Scholar]
- 67.Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and Validation of the Patient Assessment of Chronic Illness Care (PACIC) Med Care. 2005;43(5):436–444. doi: 10.1097/01.mlr.0000160375.47920.8c. [DOI] [PubMed] [Google Scholar]
- 68.Juniper EF. Effect of asthma on quality of life. Can Respir J. 1998;5(Suppl A):77A–84A. [PubMed] [Google Scholar]
- 69.Juniper EF, Norman GR, Cox RM, Roberts JN. Comparison of the standard gamble, rating scale, AQLQ and SF-36 for measuring quality of life in asthma. Eur Respir J. 2001;18(1):38–44. doi: 10.1183/09031936.01.00088301. [DOI] [PubMed] [Google Scholar]
- 70.Agency for Healthcare Research and Quality . Translating Research Into Practice (TRIP)-II. Fact sheet. AHRQ Publication No 01-P017. Rockville, MD: Agency for Healthcare Research and Quality; 2001. [Accessed March 30, 2013]. Available from: http://www.ahrq.gov/research/trip2fac.htm. [Google Scholar]
- 71.Farquhar CM, Stryer D, Slutsky J. Translating research into practice: the future ahead. Int J Qual Health Care. 2002;(1493):233–249. doi: 10.1093/oxfordjournals.intqhc.a002615. [DOI] [PubMed] [Google Scholar]
- 72.Van Bokhoven MA, Kok G, van der Weijden T. Designing a quality improvement intervention: a systematic approach. Qual Saf Health Care. 2003;12(3):215–220. doi: 10.1136/qhc.12.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26:13–24. doi: 10.1002/chp.47. [DOI] [PubMed] [Google Scholar]
- 74.Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii–iv. 1–72. doi: 10.3310/hta8060. [DOI] [PubMed] [Google Scholar]
- 75.Freemantle N, Harvey EL, Wolf F, Grimshaw JM, Grilli R, Bero LA. Printed educational materials: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2000;2:CD000172. doi: 10.1002/14651858.CD000172. [DOI] [PubMed] [Google Scholar]
- 76.Fagnan L, Dorr D, Davis M, et al. Turning on the care coordination switch in rural primary care: voices from the practices – clinician champions, clinician partners, administrators, and nurse care managers. J Ambul Care Manage. 2011;34(3):304–318. doi: 10.1097/JAC.0b013e31821c63ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ. 1998;317(7156):465–468. doi: 10.1136/bmj.317.7156.465. [DOI] [PMC free article] [PubMed] [Google Scholar]