Abstract
Background
The ExtaviPro™ 30G autoinjector has been developed for self-administration of interferon beta-1b (Extavia®), which is used as a first-line, parenteral, disease-modifying therapy in multiple sclerosis (MS). The aim of this survey was to investigate patients’ perceptions of the importance of different general attributes of autoinjectors, and patient preferences when comparing the ExtaviPro™ 30G and Betacomfort® autoinjectors.
Method
The survey was conducted in France, Germany, Italy, and the USA in patients with relapsing-remitting MS who had been using an autoinjector for at least 1 year. Participants examined the ExtaviPro™ 30G and Betacomfort® devices, viewed fact sheets, and watched a video of these autoinjectors in use, then scored nine prespecified attributes of autoinjectors in terms of importance on a scale of 1–7 (1 = not at all important; 7 = extremely important). They then indicated which device they preferred, both overall and by individual attribute.
Results
Among the 201 participants who completed the survey, being reliable to use was considered the most important general attribute of autoinjectors, followed by attributes associated with convenience (ease of operation, one-handed injection, ease of reach of injection sites, ergonomic shape). For each of the nine attributes, a significantly higher proportion of participants (74%–94% by attribute; P < 0.05 for each) preferred ExtaviPro™ 30G to the Betacomfort® autoinjector, and 173 (86%) participants indicated that they preferred ExtaviPro™ 30G overall (P < 0.05).
Conclusion
The results of this survey suggest that patients with MS rate reliability and convenience as the most important general attributes of autoinjectors, and are more likely to prefer ExtaviPro™ 30G to the Betacomfort® autoinjector for routine self-administration of interferon beta-1b.
Keywords: autoinjector, subcutaneous injectors, interferon beta, multiple sclerosis, relapsing-remitting, patient preference, self-administration
Introduction
Data from randomized, controlled, phase 3 studies support the use of interferon beta-1b in patients with clinically isolated syndrome, relapsing-remitting multiple sclerosis (MS), and relapsing secondary progressive MS.1–6 It is generally accepted that treatment of MS with first-line, parenteral, disease-modifying therapies such as interferon beta-1b should be started as early as possible7,8 in order to control inflammatory lesion activity, prevent further relapses, and delay disability progression.3,5 Consistent with this, a large and clinically important survival benefit is associated with early treatment with interferon beta-1b.9 A follow-up study comparing long-term survival in patients randomized to receive interferon beta-1b with that in patients simultaneously randomized to receive placebo during their first 2 years of therapy found a 47% reduction in all-cause mortality in the former group at 21 years.9
Parenteral, disease-modifying therapies require regular administration by subcutaneous or intramuscular injection,10 and injection-site reactions (ISRs) are among the most common adverse events (AEs) reported.11 A systematic review of randomized trials and observational studies indicated that, following subcutaneous administration, ISRs were more common with interferon beta-1a and glatiramer acetate than with interferon beta-1b.11 Most ISRs are mild in severity and unlikely to lead to treatment discontinuation,12 but the use of autoinjectors can reduce the incidence and intensity of ISRs compared with manual administration,13 and their use has been shown to be a strong predictor of adherence.14
Assuming that an autoinjector functions reliably, there are several factors that may influence patient preference, including ease of use, convenience, and design. In a series of interviews reported by Kozubski that assessed 200 patients’ preferences when comparing two autoinjectors used to administer interferon beta-1b,10 71% of patients indicated that they would prefer to use ExtaviJect® 30G (Novartis Pharma AG, Basel, Switzerland) to Betaject® Comfort (Bayer Schering Pharma AG, Berlin, Germany). Importantly, the study revealed that attributes associated with convenience (ease of handling and ease of loading) were among the most common reasons for this preference.10 At the time of interview, patients had not used the autoinjectors to administer medication, so patient preferences were based on inspection of the devices as well as viewing instructional videos and fact sheets. In a separate clinical trial, improvements in patient satisfaction were demonstrated when patients used the ExtaviJect® 30G to administer interferon beta-1b during the 12-week non-interventional EXCELLENT study.15 These findings tend to support the hypothesis that the convenience perceived in the preference survey10 translated into actual convenience in the clinic.
The new ExtaviPro™ 30G autoinjector has been developed to replace the ExtaviJect® 30G device for self-administration of interferon beta-1b (Extavia®; Novartis Pharma AG). In order to address certain unmet clinical needs, the new device has been designed to be used one-handed, and has an ergonomic shape that is intended to sit well in the hand, which facilitates access to injection sites that are difficult to reach. The study reported here aimed to evaluate which general attributes of autoinjectors are considered most important by patients, and also recorded patient preferences when comparing the ExtaviPro™ 30G autoinjector (Novartis Pharma AG), pre-launch, with the marketed Betacomfort® autoinjector (the successor to the Betaject Comfort®; Bayer Schering Pharma AG), using an approach similar to that reported by Kozubski.10
Methods
Survey
This was a survey of adult patients aged 18–80 years, with relapsing-remitting MS conducted between April 23, 2013 and May 27, 2013 in France, Germany, Italy, and the USA, by an independent market research organization (MRO; Adelphi Research, Bollington, UK), in accordance with the codes of conduct stipulated by the British Healthcare Business Intelligence Association, the Market Research Society, ESOMAR (now the World Association of Opinion and Marketing Research Professionals), the European Pharmaceutical Marketing Research Association, and the Council of American Survey Research Organizations. The survey was conducted on an individual basis by the same moderator at a central location in each country.
At interview, as shown in Figure 1, the two autoinjectors (accompanied by fact sheets) were presented to each participant on a table; ExtaviPro™ 30G was labeled P3 and Betacomfort® was labeled Y6. For safety reasons, neither device contained any active substance or needle. Participants were encouraged to handle the devices and to read the fact sheets before being shown a short video demonstrating how to use each autoinjector. To control for possible presentation bias, the positions of the devices and fact sheets were alternated between interviews, so if device P3 and its fact sheet were placed to the left of the table for the first participant, they would be moved to the right before interviewing the second participant. In order to control for information bias, the sponsor prepared fact sheets in the same format for each autoinjector, using information from their package inserts that listed values for the same product characteristics for each device, but did not disclose the identity of the active substance to be delivered. The moderator did not read out the fact sheets or draw attention to any characteristic of either autoinjector.
Figure 1.

Layout of devices, fact sheets, and video reviewed by participants. The positions of the autoinjectors and their fact sheets were alternated between interviews. ExtaviPro™ 30G (Novartis Pharma AG, Basel, Switzerland) is on the left in this image.
Participants then answered the two-part questionnaire, administered by the moderator. Questionnaires were provided in the language appropriate to the interview location. In the first part (Table 1), the moderator asked participants to consider the importance of each prespecified attribute of autoinjectors in general, rather than specifically for device P3 or Y6. The full attribute descriptions provided to each participant and the related summary terms used here are given in Table 1. Participants assigned scores of importance to each attribute on a scale of 1 (not at all important) to 7 (extremely important). In part two of the questionnaire (Table 2) participants were asked to indicate for each attribute whether they preferred device P3 or device Y6. Finally, they were asked to indicate which device they preferred overall, and to state which prespecified attribute was the main reason for their choice. They were also allowed to provide a different reason from those that were prespecified (Table 2).
Table 1.
Survey questionnaire: the importance of general attributes of autoinjectors
| Summary term used here | Attribute description provided by the moderator |
| Ergonomic shape | “An ergonomic shape; by this I mean a design that is well adapted to your hand and therefore, is easy to handle” |
| Ability to read display | “The ability to read any information shown on the auto-injector for example injection window or the needle depth” |
| Reliable to use | “Reliable to use” |
| Intuitive to use | “Intuitive to use; by this I mean that you can understand how to use the device with little or no instruction” |
| Easy to operate | “Overall, easy to operate” |
| One-handed injection | “The ability to use the autoinjector with one hand for injection” |
| Reach | “The ability to reach all injection sites on your body” |
| Feel of autoinjector’s tip | “The feel of the tip of the autoinjector on your skin (ie, the nozzle)” |
| Attractive design | “An attractive design” |
Notes: Participants were asked to score each of the following nine general attributes of autoinjectors on a scale of 1–7, where 1 = not at all important and 7 = extremely important.
Table 2.
Survey questionnaire: autoinjector preference
| “Which of these two autoinjectors do you think has the best ergonomic shape – that is, the one with a design that is well adapted to your hand and, therefore, is easiest to handle?” |
| “Which do you think is easiest to read in terms of any information shown on the autoinjector (for example, the autoinjector window or the needle depth)?” |
| “Which do you think is the most reliable to use?” |
| “Which do you think is most intuitive to use – that is, the one you can understand how to use with little or no instruction?” |
| “Which do you think would be overall easiest to operate?” |
| “Which do you think would be easiest to use for injection with one hand?” |
| “Which do you think would offer the greatest ‘reach’ to all injection sites on your body?” |
| “Which autoinjector tip do you think has the best feel on your skin?” |
| “Which do you think is the most visually attractive to you?” |
| “Now, taking into account the information you received from the factsheets, the demonstration videos, and your own handling of the autoinjectors, overall which would you prefer to use for injections to treat your multiple sclerosis?” |
| “Autoinjector [device code] is your preferred choice. What is the main reason for your choice?” (The participant was shown a card summarizing the following options). |
| “An ergonomic shape; by this I mean a design that is well adapted to your hand and therefore, is easy to handle” |
| “The ability to read any information shown on the autoinjector for example injection window or the needle depth” |
| “Reliable to use” |
| “Intuitive to use; by this I mean that you can understand how to use the device with little or no instruction” |
| “Overall, easy to operate” |
| “The ability to use the autoinjector with one hand for injection” |
| “The ability to reach all injection sites on your body” |
| “The feel of the tip of the autoinjector on your skin (ie, the nozzle)” |
| “An attractive design” |
Notes: Having handled the autoinjectors, reviewed the fact sheets, and watched the demonstration video, participants were asked to indicate which device they preferred in response to the questions above. They were asked to consider only the devices, not the drugs they administer or their effects.
Eligibility criteria
Patients eligible for participation were those who had been receiving an injectable treatment for relapsing-remitting MS for at least 1 year and were using an autoinjector other than the Betacomfort® device. Eligible injectable treatments were interferon beta-1b (Betaseron® [Bayer HealthCare Pharmaceuticals] or Extavia® [Novartis Pharma AG]), interferon beta-1a (Rebif® [Merck Serono SA, Geneva, Switzerland]), and glatiramer acetate (Copaxone® [Teva Pharmaceutical Industries Ltd, Petach Tivka, Israel]). Reasons for exclusion were: not receiving a disease-modifying therapy; receiving interferon beta-1a (Avonex® [Biogen Idec, Weston, MA, USA], or Rebif® using the RebiSmart™ autoinjector [Merck Serono SA]); or fingolimod (Gilenya® [Novartis Pharma AG]) or natalizumab (Tysabri® [Biogen Idec]); mobility constraints that rendered the individual incapable of walking 25 feet in 20 seconds unaided; involvement in market research pertaining to MS in the preceding 3 months; and links to the pharmaceutical industry.
Patient recruitment and consent
To ensure that only those with a relevant diagnosis were interviewed, patients were recruited through their physicians, who provided them with the contact details of the MRO. It was the patient’s decision whether to contact the MRO, and on making contact they were screened to ensure eligibility criteria were met. Participants were required to provide informed consent before participating in the survey and were afforded anonymity and confidentiality in accordance with the codes of conduct described above.
Statistical analysis
The analysis population included all participants who completed the questionnaire. The minimum sample size required to provide a robust statistical test at the 5% level was 30 individuals per country. The significance of differences between subgroups of dichotomous data, such as autoinjector preference, was estimated using the z-test at the 5% level (P = 0.05). The significance of differences between subsets of continuous data, such as mean overall attribute scores, was estimated using the Student’s t-test at the 5% level (P = 0.05).
Results
Patient disposition
Recruitment of 200 patients in total from France, Germany, Italy, and the USA was planned, and 201 patients meeting the eligibility criteria provided informed consent and completed the questionnaire. Physicians discussed participation with a further 78 patients, of whom 36 never called to be screened, 26 were screened out, 8 declined to participate, and 8 agreed to participate but failed to attend the survey interview (Figure 2). Approximately 40% of participants who completed the questionnaire were from the USA and approximately 20% each were from France, Germany, and Italy (Table 3). Most participants (183 [91%]) were younger than 60 years of age, 153 (76%) were women and the distribution of disease duration among participants was relatively uniform. Of the eligible injectable disease-modifying therapies, glatiramer acetate was used most commonly (85 [42%]); there were 67 (33.3%) participants receiving interferon beta-1b, of whom 16 were receiving Extavia® (Table 3).
Figure 2.
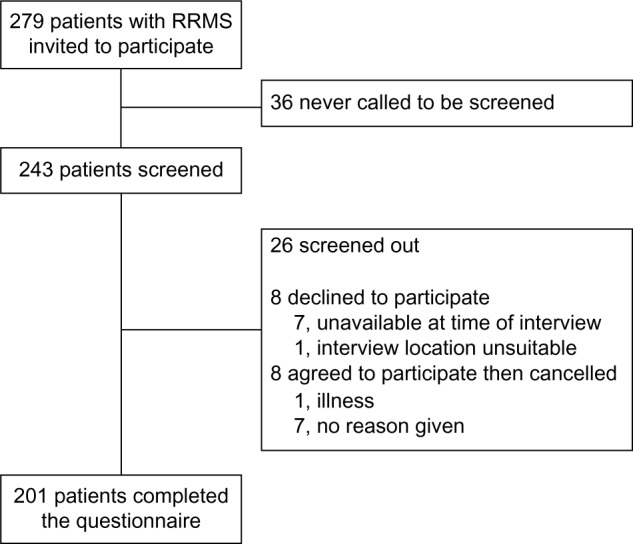
Patient flow.
Abbreviation: RRMS, relapsing-remitting multiple sclerosis.
Table 3.
Participant demographics (n = 201)
| Characteristic | n (%) | ||
|---|---|---|---|
| Sex, by country | Men | Women | Total |
| Total | 48 (23.9) | 153 (76.1) | 201 (100.0) |
| France | 8 (21.6) | 29 (78.4) | 37 (18.4) |
| Germany | 9 (22.5) | 31 (77.5) | 40 (19.9) |
| Italy | 23 (52.3) | 21 (47.7) | 44 (21.9) |
| USA | 8 (10.0) | 72 (90.0) | 80 (39.8) |
| Age group, years | |||
| 18–39 | 72 (35.8) | ||
| 40–59 | 111 (55.2) | ||
| 60–80 | 18 (9.0) | ||
| Time since diagnosis, years | |||
| 1–3 | 27 (13.4) | ||
| 4–6 | 44 (21.9) | ||
| 7–9 | 42 (20.9) | ||
| 10–12 | 39 (19.4) | ||
| 13–15 | 27 (13.4) | ||
| ≥16 | 22 (10.9) | ||
| Current injectable therapy | |||
| Copaxone® (glatiramer acetate)a | 85 (42.3) | ||
| Rebif® (interferon beta-1a)b | 49 (24.4) | ||
| Betaseron® (interferon beta-1b)c | 51 (25.4) | ||
| Extavia® (interferon beta-1b)d | 16 (8.0) |
Notes:
Teva Pharmaceutical Industries Ltd, Petach Tivka, Israel;
Merck Serono SA, Geneva, Switzerland;
Bayer HealthCare Pharmaceuticals, Berlin, Germany;
Novartis Pharma AG, Basel, Switzerland.
Prespecified autoinjector attributes
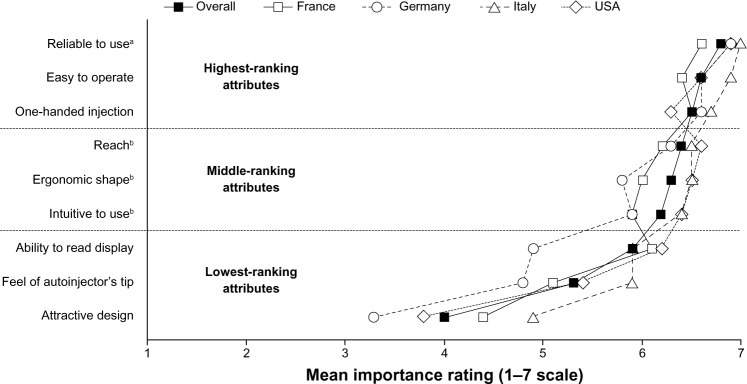
Among the three highest-ranking attributes (Figure 3), “reliable to use” was awarded the highest mean overall score (6.8; maximum possible score, 7.0), which was significantly higher than that awarded to any other attribute (P < 0.05). The difference in score between the next two highest-ranking attributes, “easy to operate” (6.6) and “one-handed injection” (6.5), was not significant. The three middle-ranking attributes were “reach” (6.4), “ergonomic shape” (6.3), and “intuitive to use” (6.2). These scores were all significantly higher (P < 0.05) than those of the three lowest-ranking attributes, “ability to read display” (5.9), “feel of autoinjector’s tip” (5.3), and “attractive design” (4.0). Considering the mean scores by attribute from each country, the range was narrower among the highest-ranked attributes than among the lowest-ranked ones. Compared to the overall mean scores, there was a tendency for higher mean scores to be awarded by participants from Italy and the USA, and for lower mean scores to be awarded by participants from France and Germany.
Figure 3.
Mean scores overall and by country for prespecified general attributes of autoinjectors. Data are presented as mean scores of importance, both overall and by country.
Notes: aP < 0.05, overall score compared to other attributes; bP < 0.05, overall scores compared to lowest-ranking attributes.
Autoinjector preference
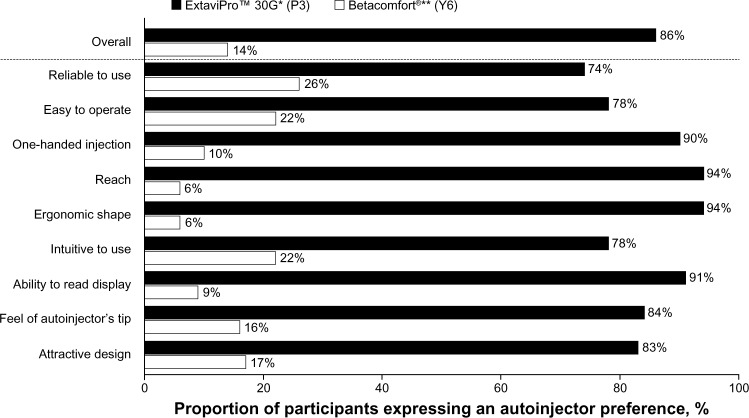
The majority of participants (86% [n = 173]) indicated that they preferred the ExtaviPro™ 30G autoinjector to the Betacomfort® device (P < 0.05); this result was also seen consistently across the participating countries (range, 81%–91%; P < 0.05 in each country). When participants were asked to indicate which device they preferred considering each attribute in turn, the ExtaviPro™ 30G autoinjector attracted a significantly higher proportion of preferences than the Betacomfort® device for all of the prespecified attributes (P < 0.05; Figure 4). Preference rates for the ExtaviPro™ 30G autoinjector by attribute ranged from 74% to 94%, with the highest rates recorded for “reach” (94%), “ergonomic shape” (94%), “ability to read display” (91%), and “one-handed injection” (90%). The highest preference rates recorded for the Betacomfort® autoinjector were for the attributes “reliable to use” (26%), “easy to operate” (22%), and “intuitive to use” (22%).
Figure 4.
Proportion of participants expressing an autoinjector preference, both overall and by attribute.
Notes: P < 0.05 for the difference between autoinjectors, both overall and by attribute. *Novartis Pharma AG, Basel, Switzerland; **Bayer Schering Pharma AG, Berlin, Germany.
Reasons for overall preference
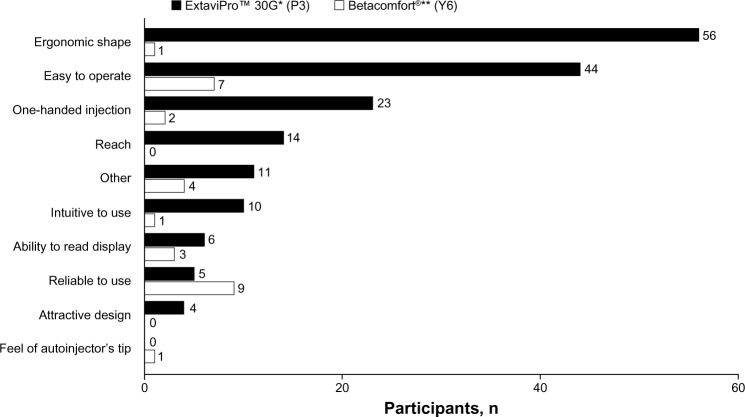
Among the 173 participants who indicated a preference for the ExtaviPro™ 30G autoinjector, the reason most commonly given for their preference was its “ergonomic shape” (n = 56), followed by “easy to operate” (n = 44) and “one-handed injection” (n = 23; Figure 5). Among the 28 participants who indicated a preference for the Betacomfort® device, the most common reasons given were “reliable to use” (n = 9) and “easy to operate” (n = 7).
Figure 5.
Number of participants citing each prespecified attribute as the main reason for their overall preference of autoinjector.
Notes: *Novartis Pharma AG, Basel, Switzerland; **Bayer Schering Pharma AG, Berlin, Germany.
Discussion
After reliability, general attributes of autoinjectors associated with convenience and ease of use were considered most important in this survey of patients with relapsing-remitting MS and at least 1 year’s experience of self-injection. For each of nine prespecified attributes, participants preferred the ExtaviPro™ 30G autoinjector to the Betacomfort® device, and 86% of participants indicated that they preferred ExtaviPro™ 30G overall.
With the exception of the attribute “reliable to use”, which was consistently awarded the highest importance score both in each country and overall, it was noticeable in our study that attributes associated with convenience of use were judged to be more important than other general attributes, such as the attributes associated with convenience, such as the attractiveness of the device or the feel of its tip on the skin. It is perhaps unsurprising that the reliability of an autoinjector is paramount in patients’ minds. Assessment of this is only truly possible in the context of normal use. However, it was included in the comparative part of the survey because any individual’s opinion of a device begins to form as soon as they handle it. Although small differences in absolute score were noted when comparing mean scores for each attribute by country, the range of mean scores was narrower among the higher-ranking attributes than among the lower-ranking ones, suggesting convergence of opinion across countries regarding which of these prespecified attributes are perceived as most important.
As well as indicating their overall preference for ExtaviPro™ 30G, participants indicated that they preferred it to the Betacomfort® device with respect to every attribute considered. These preferences were most marked among attributes associated with convenience, such as “reach”, “ergonomic shape”, “ability to read display”, and “one-handed injection”, for all of which ExtaviPro™ 30G achieved preference rates of at least 90%. “Ergonomic shape” was the most commonly given reason to explain patients’ preference for ExtaviPro™ 30G to the Betacomfort® device. It is hoped that a device that is designed to be easy to hold will facilitate self-administration for all patients, particularly for those individuals with impaired dexterity.
Convenience of use seemed to be an important driver of preference in an earlier study reported by Kozubski that compared the ExtaviJect® 30G autoinjector (currently used to administer Extavia®; interferon beta-1b) with the Betaject® Comfort device (which preceded the Betacomfort® autoinjector for administration of Betaseron; interferon beta-1b).10 In this study, 71% of participants said they would prefer to use the ExtaviJect® 30G device to the Betaject® Comfort, and approximately the same proportion indicated this preference for attributes associated with ease of use (67% thought the ExtaviJect® 30G would be easier to handle, and 73% thought it would be easier to load than the Betaject® Comfort).10
These perceptions of convenience reported by Kozubski also seemed to translate into improvements in convenience during clinical use of ExtaviJect® 30G. The EXCELLENT study, which was a single-cohort, non-interventional, 12-week follow-up of 582 patients with MS, used the Treatment Satisfaction Questionnaire for Medication (TSQM-9) assessment to measure patient satisfaction. While using the ExtaviJect® 30G autoinjector to administer interferon beta-1b, mean convenience scores within the TSQM-9 assessment improved significantly between week 6 and week 12 of the study (P < 0.001); additionally, adherence to therapy was high.15 As discussed, the perception of the convenience of the ExtaviJect® 30G autoinjector that was reported in a survey by patients who were yet to use it,10 was corroborated independently in the EXCELLENT study, in which another group of patients reported their experiences when using the device.15 Similarly, it is hoped that once ExtaviPro™ 30G becomes available, patients’ experience of it will reflect the perceptions of convenience and ease of use presented here.
A limitation of the current survey is that participants had no experience of using the devices either before or as part of the assessment. However, only participants with experience of using other autoinjectors for the treatment of relapsing-remitting MS were recruited, and would therefore be expected to have an awareness of any advantages or disadvantages stemming from design differences between the two devices. Participants were also provided with technical information that was presented without bias, devices devoid of visible branding to examine in parallel, and a video demonstration of the devices in use, to allow them to form their own opinions before the survey was conducted. Another limitation is that participants were only asked to compare two autoinjectors. Overall, Betacomfort® and ExtaviPro™ 30G are similar in their design and operation, so direct comparisons are relatively straightforward to make. More comprehensive conclusions may have been drawn had the survey included devices such as the RebiSmart™ autoinjector or the Avonex® pen. However, consideration of these devices, whose design and operation differ from ExtaviPro™ 30G, would have made direct comparisons of features among all of the devices very challenging within the scope of a short survey. Finally, this survey did not examine the important issue of patients’ psychological fear of injections in the context of autoinjector design. As the survey was based on participants’ opinions rather than actual experience of using the autoinjectors, and as the needles had been removed from the devices offered for scrutiny, comparison of this feature was deemed to be beyond the survey’s scope.
In conclusion, the importance of autoinjector attributes associated with convenience of use was emphasized in this international survey of 201 patients with relapsing-remitting MS. The survey also revealed patients’ preference for the ExtaviPro™ 30G autoinjector compared to the Betacomfort® device, both overall and for each of nine prespecified attributes. Further clinical studies may be warranted to evaluate whether the high level of preference for ExtaviPro™ 30G observed in this survey translates into high patient satisfaction and adherence during real-world use.
Acknowledgments
The authors would like to thank the patients who participated in this survey and the physicians who supported recruitment. The authors would also like to thank Kirstin Stricker (Novartis Pharma AG) for critical review of the manuscript.
Footnotes
Disclosure
The study was funded by Novartis Pharma AG. Kunal Thakur is an employee of Novartis Pharma AG; Laure Manuel is an employee of Adelphi Research Ltd, which was funded by Novartis Pharma AG to coordinate this survey; Mark Tomlinson receives funding as a consultant to Novartis Pharma AG. Writing and editorial support was provided by Jeremy Bright of Oxford PharmaGenesis™ Ltd and was funded by Novartis Pharma AG.
References
- 1.Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology. 1993;43(4):655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- 2.European Study Group on interferon beta-1b in secondary progressive MS Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet. 1998;352(9139):1491–1497. [PubMed] [Google Scholar]
- 3.Durelli L, Verdun E, Barbero P, et al. Independent Comparison of Interferon (INCOMIN) Trial Study Group Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN) Lancet. 2002;359(9316):1453–1460. doi: 10.1016/s0140-6736(02)08430-1. [DOI] [PubMed] [Google Scholar]
- 4.Panitch H, Miller A, Paty D, Weinshenker B, North American Study Group on Interferon beta-1b in Secondary Progressive MS Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology. 2004;63(10):1788–1795. doi: 10.1212/01.wnl.0000146958.77317.3e. [DOI] [PubMed] [Google Scholar]
- 5.Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006;67(7):1242–1249. doi: 10.1212/01.wnl.0000237641.33768.8d. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor P, Filippi M, Arnason B, et al. BEYOND Study Group 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol. 2009;8(10):889–897. doi: 10.1016/S1474-4422(09)70226-1. [DOI] [PubMed] [Google Scholar]
- 7.Hartung HP. Early treatment and dose optimisation BENEFIT and BEYOND. J Neurol. 2005;252(Suppl 3):iii44–iii50. doi: 10.1007/s00415-005-2017-z. [DOI] [PubMed] [Google Scholar]
- 8.Paolicelli D, Direnzo V, Trojano M. Review of interferon beta-1b in the treatment of early and relapsing multiple sclerosis. Biologics. 2009;3:369–376. [PMC free article] [PubMed] [Google Scholar]
- 9.Goodin DS, Reder AT, Ebers GC, et al. Survival in MS: a randomized cohort study 21 years after the start of the pivotal IFNβ-1b trial. Neurology. 2012;78(17):1315–1322. doi: 10.1212/WNL.0b013e3182535cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozubski W. Autoinjector improves injection-related tolerability issues in patients with multiple sclerosis – exploring the new ExtaviJect™ 30G system for the injection of interferon beta-1b. Eur Neurol Rev. 2010;5(2):77–81. [Google Scholar]
- 11.Giovannoni G, Southam E, Waubant E. Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherence. Mult Scler. 2012;18(7):932–946. doi: 10.1177/1352458511433302. [DOI] [PubMed] [Google Scholar]
- 12.Balak DM, Hengstman GJ, Çakmak A, Thio HB. Cutaneous adverse events associated with disease-modifying treatment in multiple sclerosis: a systematic review. Mult Scler. 2012;18(12):1705–1717. doi: 10.1177/1352458512438239. [DOI] [PubMed] [Google Scholar]
- 13.Brochet B, Lemaire G, Beddiaf A, et l’Epicure Study Group Reduction of injection site reactions in multiple sclerosis (MS) patients newly started on interferon beta 1b therapy with two different devices. Rev Neurol (Paris) 2006;162(6–7):735–740. doi: 10.1016/s0035-3787(06)75071-8. French. [DOI] [PubMed] [Google Scholar]
- 14.Pozzilli C, Schweikert B, Ecari U, Oentrich W, BetaPlus Study group Supportive strategies to improve adherence to IFN β-1b in multiple sclerosis – results of the βPlus observational cohort study. J Neurol Sci. 2011;307(1–2):120–126. doi: 10.1016/j.jns.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Boeru G, Milanov I, De Robertis F, et al. ExtaviJect® 30G device for subcutaneous self-injection of interferon beta-1b for multiple sclerosis: a prospective European study. Med Devices (Auckl) 2013 doi: 10.2147/MDER.S52590. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]