Abstract
Background
Abnormal Resting State electroencephalogram (RS-EEG) oscillations are reported in schizophrenia (SZ) and bipolar (BP) disorder, illnesses with overlapping symptoms and genetic risk. However, less evidence exists on whether similar EEG spectral abnormalities are present in both disorders, nor whether these abnormalities are present in their first degree relatives, possibly representing genetic predisposition for these disorders.
Methods
We examined 64-channel RS-EEG of 225 SZ probands, 201 of their first-degree relatives (SZR), 234 psychotic BP (PBP) probands, 231 of their first-degree relatives (PBPR) and 200 healthy controls (HC). Eight independent RS-EEG spectral components and associated spatial weights were derived using group independent component analysis. Analysis of covariance was conducted on spatial weights to evaluate group differences. Relative risk estimates and familiality was evaluated on spectral profiles abnormal in probands and relatives.
Results
Both SZ and PBP exhibited increased delta, theta, slow and fast alpha activity. Posthoc pair-wise comparison revealed increased fronto-central slow beta activity in SZ, PBP and their relatives. SZ and SZR exhibited augmented frontal delta activity, while PBP and PBPR showed augmented fast alpha activity.
Conclusions
SZ and PBP probands demonstrated aberrant low frequency activity. Slow beta activity was abnormal in SZ and PBP probands and their relatives perhaps indicating a common endophenotype for both disorders. Delta and fast alpha activity were unique endophenotypes for SZ and PBP respectively. EEG spectral activity exhibited moderate relative risk and heritability estimates, serving as intermediate phenotypes in future genetic studies for examining biological mechanisms underlying the pathogenesis of the two disorders.
Keywords: EEG, resting state, intermediate phenotypes, schizophrenia, bipolar disorder, psychosis
Introduction
Two common psychiatric illnesses schizophrenia (SZ) and bipolar disorder (BP) share significant overlapping symptomatology (1), brain structure and function (2), cognitive features (3), genetic risk (4) and medication response (5). The neurobiological underpinning of these disorders might provide a clearer basis for understanding their similarities and differences. Evidence suggests that SZ and psychotic BP (PBP) are strongly heritable (6); and have similar genetic risk loci (7). Intermediate phenotypes, sometimes referred to as endophenotypes, are heritable quantitative biological measures presumably related to disease risk rather than overt clinical disorders, presumed to have simpler genetic architecture and be closer to the causative genes than the clinical illness (8). Thus, intermediate phenotypes are believed to be modulated by the disease-related genes influencing biological risk and may be expressed in unaffected relatives of probands.
Neural oscillations represent one type of candidate intermediate phenotype for SZ and PBP. Such oscillations normally play a vital role in defining temporal communication between circuits that represent various brain functions such as information processing, memory, attention, perception and consciousness. Basic fundamental building blocks defining these oscillations are the neuronal firing within cohorts of neurons, which are described by the frequency characteristics of electroencephalogram recordings during “resting-state” (RS-EEG). Disturbances in the spectral behavior of the oscillation patterns in probands could indicate aberrant brain function via neural dysfunction (9). Although several different frequency bands have been examined in previous RS-EEG studies of SZ (10), the results are not consistent. Most prior investigations in SZ (11–13) show increased frontal slow wave activity, primarily assumed to reflect frontal lobe pathology (14). Augmented high-frequency beta (12) and gamma (12, 15) abnormalities are also often noted. The main purpose of this study is to examine electrophysiological phenotypes across psychotic disorders to dissect the common and distinct aspects of the psychosis dimension, an important clinical feature common to both SZ and PBP and to test the hypothesis that endophenotype characteristics are homogeneous within phenomenologically-derived DSM-IV diagnoses, rather than differentiating them (16). Therefore, we assessed RS-EEG spectral activity in SZ and PBP (major psychoses) and not in non-psychotic BP. In general, published RS-EEG studies are less common in both PBP (17) and non-psychotic BP (12).
As RS-EEG exhibits heritable characteristics (18, 19), several studies have examined RS-EEG abnormalities in unaffected relatives of SZ (12, 15, 17, 20) and PBP (17) probands to determine whether risk for each disorder is genetically determined. Some limitations with these studies include the use of few scalp leads, small study samples and uncertainty in identifying reliable intermediate phenotypes. To address the lack of spatial recording leads, Venables (12) conducted a study with 28 channels in both eyes open and closed condition and demonstrated that augmented gamma activity was expressed in SZ relatives (SZR) and non-psychotic BP relatives, while, increased beta was specific to SZR. Similar findings were reported in SZ siblings (20, 21). Another study (15) identified abnormal low frequency theta-alpha as a specific SZ risk marker. No RS-EEG abnormalities were present in relatives in the study (17) by Clementz that used only 3 central electrodes and may have lacked the ability to detect fronto-temporal-parietal differences. However, while that study clearly differentiated probands from nonpsychiatric controls, it did not distinguish between SZ and BP probands. Due to the heterogeneity of these illnesses and the variable findings in establishing neural oscillations as potential intermediate phenotypes for SZ and BP separately, we conducted a large scale multi-site study to directly test the hypotheses that RS-EEG frequency abnormalities are unique to SZ and PBP, and examine whether these abnormalities are expressed in their first degree relatives, to confirm genetic liability for these disorders.
The objectives of this study were to: 1) determine whether RS-EEG spectral profiles were unique to SZ and PBP or common to both illnesses, 2) examine whether abnormal spectral composition was present in SZR and PBPR to detect any genetic link to these disorders, 3) estimate heritability of frequency abnormalities present in probands and relatives, 4) compare SZ and PBP probands to relatives with DSM-IV cluster A (schizotypal, schizoid, paranoid) and cluster B personality disorders diagnoses regarded as formes frustes of the illnesses or psychosis spectrum personality disorders (PSPD), versus relatives with non-psychotic DSM-IV Axis I disorders and diagnosis-free relatives with neither Axis I nor cluster A or B diagnoses, and 5) correlate oscillatory activity in probands with symptom scores including Schizophrenia-Bipolar Scale (SBS) (1) and PANSS (22).
We predicted that frontal slow wave (delta, theta, slow alpha) oscillations would be disrupted in both SZ and PBP probands confirming to prior studies, with similar abnormalities likely manifesting in SZR and PBPR. From prior work, we expected augmented fast beta abnormalities in both SZ and SZR, while gamma abnormality would be detected in both SZR and PBPR.
Methods and Materials
Participant Recruitment
Probands were recruited from inpatient and outpatient units at the five centers (see online supplement 1) comprising the collaborative Bipolar & Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study (16). Inclusion criteria for probands were age between 15–65 years, meeting Structured Clinical Interview for DSM IV (SCID (23)) criteria for SZ or BP I disorder with psychosis (as defined by (24)) and having one or more eligible first-degree relatives participating in the study. Probands consisted of 225 SZ and 234 PBP. Other groups comprised 201 SZR, 231 PBPR, and 200 HC subjects. Table 1 shows demographic information and subject characteristics for the current study. All subjects had the study explained to them and written informed consent approved separately by individual sites IRB’s was obtained. Probands with schizoaffective disorder depressed and manic subtype diagnoses were classified as SZ and PBP respectively (1, 25, 26). Relatives of probands with schizoaffective disorder depressed and manic subtypes were classified as SZR and PBPR respectively. Relatives with lifetime Axis 1 psychoses, who met the SCID criteria for SZ or PBP disorder were assigned to the respective probands but those with non-psychotic Axis 1 disorders, e.g. major depression or anxiety disorder were included in the SZR or PBPR category respectively. Probands and relatives with psychotic psychiatric disorders were on stable doses of medication ≥4 weeks, while non-psychotic and unaffected relatives and HCs took no psychoactive medications (Table S1). HC included subjects not meeting DSM IV criteria for any Axis 1 disorder.
Table 1.
Demographic characteristics and clinical data for subjects (n=1091).
| HC | SZ | SZR | PBP | PBPR | Statistic | p value | |
|---|---|---|---|---|---|---|---|
| Subjects (N) | 200 | 225 | 201 | 234 | 231 | ||
| Mean age (years) (SD) | 37.53 (12.33) | 34.29 (12.17) | 44.19 (15.07) | 35.60 (12.55) | 41.19 (15.66) | F(4,1086)=19.2 | P<2.5e-15 |
| Sex (male/female) | 100/100 | 153/72 | 67/134 | 93/141 | 81/150 | χ2(4)=72.87 | P<5.6e-15 |
| Baltimore | 21 | 75 | 56 | 62 | 58 | - | - |
| Boston | 26 | 8 | 8 | 5 | 8 | - | - |
| Chicago | 36 | 22 | 25 | 44 | 51 | - | - |
| Dallas | 33 | 16 | 13 | 19 | 17 | - | - |
| Detroit | 29 | 30 | 27 | 26 | 17 | - | - |
| Hartford | 55 | 74 | 72 | 78 | 80 | - | - |
| Site effects | - | - | - | - | - | χ2(20)=93.65 | P<1.6e-11 |
| Mean epochs (SD) | 107.09 (35.14) | 119.9 (37.5) | 115.24 (37.63) | 112.32 (36.17) | 110.36 (34.38) | F(4,1086)=3.9 | P<0.004 |
| PANSS-positive Range Mean (SD) |
- | 7–34 16.15 (5.87) |
- | 7–38 14.4 (5.52) |
- | - | - |
| PANSS-negatives Range Mean (SD) |
- | 7–33 15.7 (5.51) |
- | 7–30 13.13 (4.7) |
- | - | - |
| PANSS-general Range Mean (SD) |
- | 16–56 30.95 (8.45) |
- | 16–57 30.23 (8.44) |
- | - | - |
| SBS Range Mean (SD) |
- | 4–9 7.69 (1.25) |
- | 0–7 2.46 (1.82) |
- | - | - |
| Cluster A & B personality disorder | 2.5% | 0% | 10% | 0% | 10% | - | - |
| Unaffected relatives | - | - | 47% | - | 45% | - | - |
| Schizoaffective disordera | 0% | 13.7% | 0% | 29.5% | 0% | ||
| Caucasian | 100 | 95 | 113 | 141 | 167 | ||
| African-American | 59 | 99 | 68 | 57 | 35 | ||
| Hispanic | 18 | 14 | 18 | 21 | 18 | ||
| Asian | 10 | 2 | 2 | 3 | 2 | ||
| American Indian | 3 | 0 | 0 | 0 | 0 | ||
| Mixed | 10 | 15 | 6 | 12 | 9 |
Schizoaffective disorder Probands with schizoaffective disorder depressed and manic subtype diagnoses were classified as SZ and PBP respectively. HC, healthy control; SZ, schizophrenia; SZR, first-degree relative of schizophrenia proband, PBP, psychotic bipolar disorder; PBPR, first degree relative of psychotic bipolar disorder proband; SD, Standard Deviation; PANSS, Positive and Negative Syndrome Scale; SBS Schizo-Bipolar Scale
EEG Data Collection and Processing
EEG recordings were collected (detailed in online supplemental data) in electrically shielded booth with electrodes placed in accordance with the International 10–10 system using a 66 electrode cap with ground electrode at mid forehead and nose as reference (Figure S1 in Supplement 1). EEG data were preprocessed (see supplement 1 for details) for generating artifact free epochs.
EEG Frequency Analysis
Frequency data for all the subjects were estimated by applying spectral transformation (detailed in supplement 1) to clean epochs. We included subjects with epochs ranging between 20 and 281 in the analysis.
Group Independent Component Analysis (GICA)
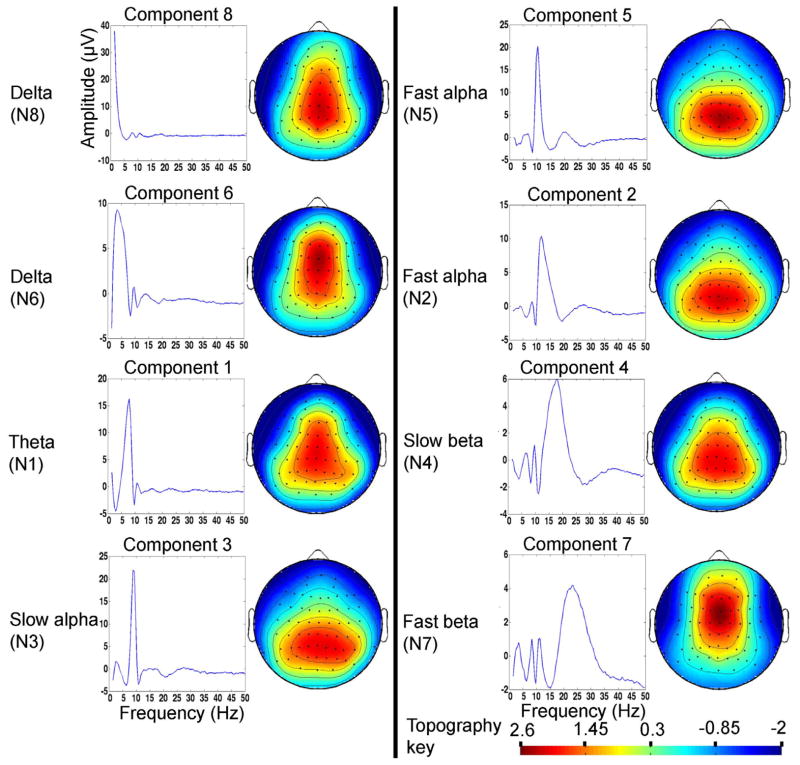
ICA is a data-driven multivariate tool that employs higher-order statistics for separating maximally independent sources from linear mixture, based on presumed EEG source independence, to provide better signal-to-noise ratio by identifying and eliminating unstructured noise sources from the data (27). GICA examines independent spatio-spectral components by treating spectral activity as a single effect across the entire group (probands, relatives and HC) data. Prior imaging studies have employed GICA (28): we extended this approach to RS- EEG (29) to identify spectral sources common to SZ, PBP, SZR, PBPR and HC subjects. The data organization for the GICA procedure is detailed in Supplement 1 (see Figure S2 in Supplement 1). The spectral data were decomposed into 8 (>95% reliability) independent frequency components, estimated using infomax ICA in GIFT. To facilitate the interpretability of GICA results, we also evaluated the spectral amplitude using the standard filtering by integrating the area under the spectral curve within various frequency bands and compared with those derived from the GICA.
Statistical Analysis
We examined group differences in scalp topographic weights using Analyses of Covariance (ANCOVA) (see Supplement 1). ANCOVA was carried out with 4 between subjects-factors (group: probands (SZ and PBP), relatives (SZR and PBPR) and HC; sex: (male/female); site: 6 levels and race: 6 categories (see Table. 1)). Age and number of epochs were included as covariates in all analyses. Post-hoc t-tests were conducted to assess pair-wise group comparisons. Similar tests were also conducted on the EEG spectral amplitude evaluated using the classical method. Additional post-hoc t-tests were conducted to compare probands vs relatives with and without PSPD (cluster-A and cluster-B diagnoses) for both SZ and PBP. Pearson correlations were computed between those topographic weights of the frequency components that were abnormal in probands and psychopathology ratings; PANSS positive, negative, total and SBS totals to detect associations between spectral components and psychopathology. Supplementary analyses including relative risk estimation (30, 31) and heritability evaluation using sequential oligogenic linkage analysis routines (SOLAR) (32) are detailed in Supplement 1.
Results
Resting State Oscillatory Activity
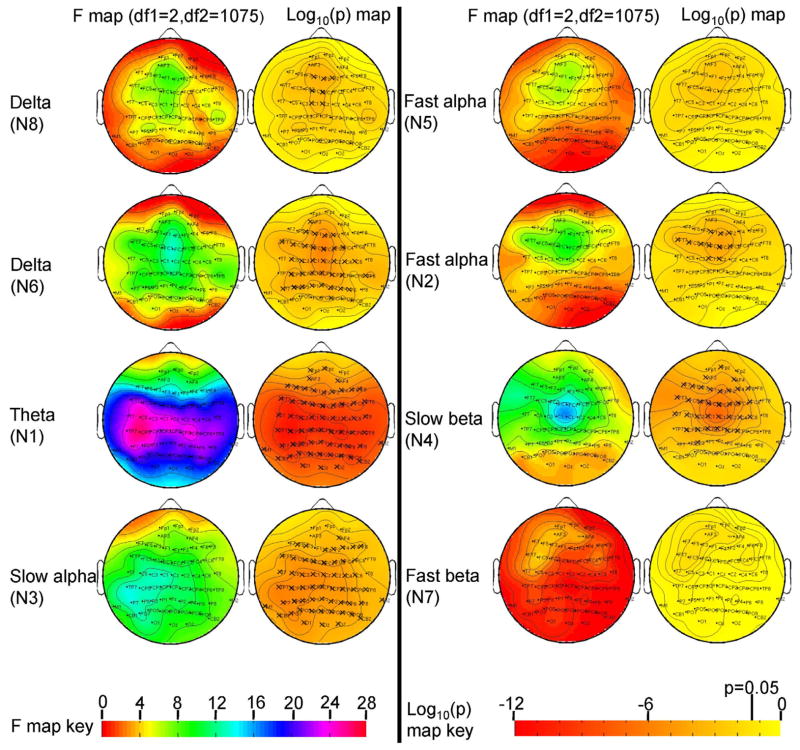
Spectral components from GICA were validated by visual inspection; such that each component (see Figure 1 and Supplement 1) best describes the brain activity underlying eyes open RS-EEG. Omnibus ANCOVA testing carried out at all 64 leads showed a main effect of group in 6 of the 8 components (Figure 2). There were significant site effects on the scalp weights, but no significant group x site interactions (see Figure S3 in supplement 1). Mean spatial weights and group comparisons (between probands, relative and HC) for various oscillatory networks are shown in Figures 3 and 4.
Figure 1.
Mean frequency component over epochs across all subjects (n=1271) and associated scalp topography from group independent component analysis (GICA). The spectral components are randomly ordered in GICA but for ease we have reordered the eight spectral components from low to high frequency (1.5–50 Hz). The spatial weights of each frequency component are dimensionless measure indicating the strength of the connection or association with that component. The spatial weights were transformed to Z-scores for visualization.
Figure 2.
F-maps and significance levels from the omnibus analysis of covariance test comparing spatial weights from GICA across three groups.
The three groups comprised schizophrenia and psychotic bipolar disorder probands (n=459) their relatives (n=432) and healthy controls (n=200). ‘X’ indicates significant after Bonferroni correction for 64 leads (p=0.05/64).
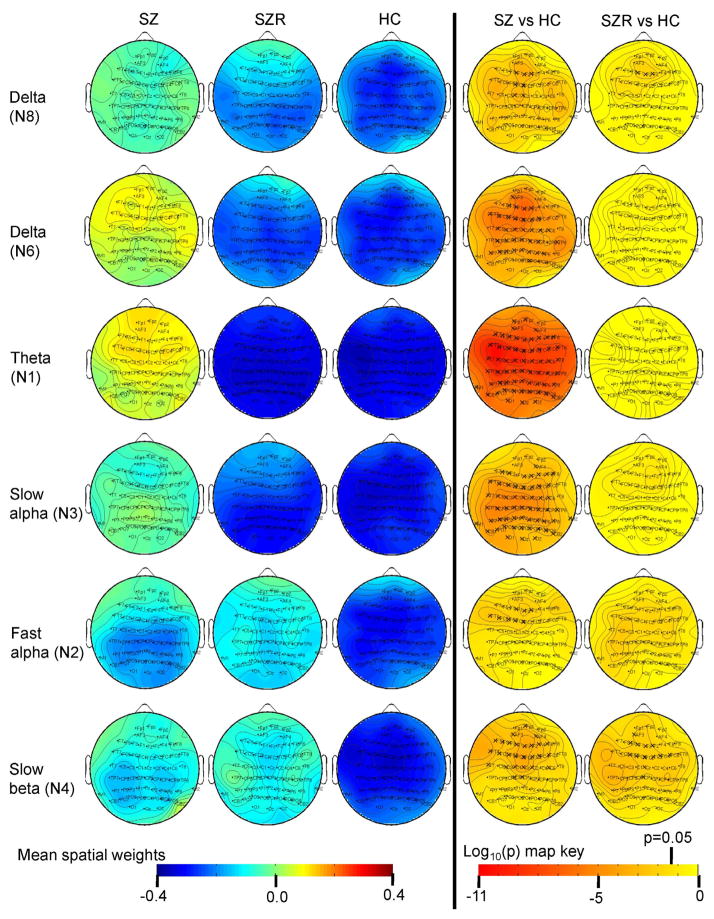
Figure 3.
Mean spatial weights in schizophrenia (SZ) probands, their relatives (SZR) and healthy controls (HC) and significance levels from pairwise post-hoc t-test.
The topographic weights are dimensionless quantity. ‘X’ indicates significant after multiple comparison correction for 5 comparisons (p=0.05/5). Activity and P maps are shown at all leads for continuity, but only leads significant in the analysis of covariance and significantly different in probands and relatives vs HC are highlighted in relatives.
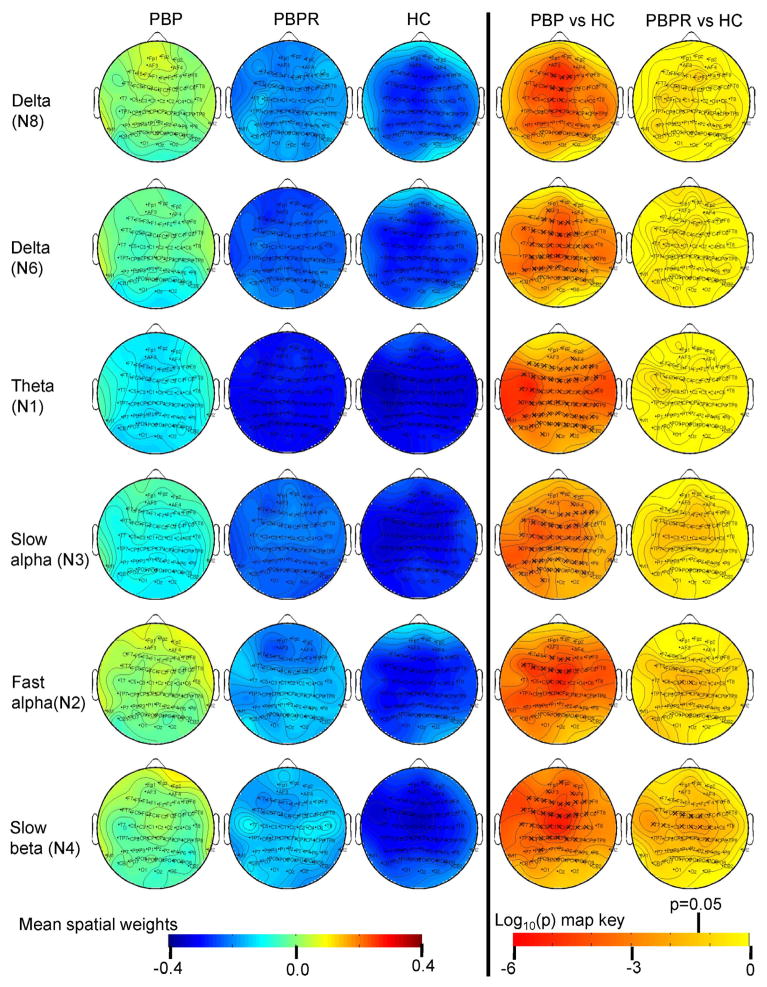
Figure 4.
Mean spatial weights in psychotic bipolar disorder (PBP) probands, their relatives (PBPR) and healthy controls (HC) and significance levels from pairwise post-hoc t-test.
The topographic weights are dimensionless quantity. ‘X’ indicates significant after multiple comparison correction for 5 comparisons (p=0.05/5). Activity and P maps are shown at all leads for continuity, but only leads significant in the analysis of covariance and significantly different in probands and relatives vs HC are highlighted in relatives.
Delta (N8)
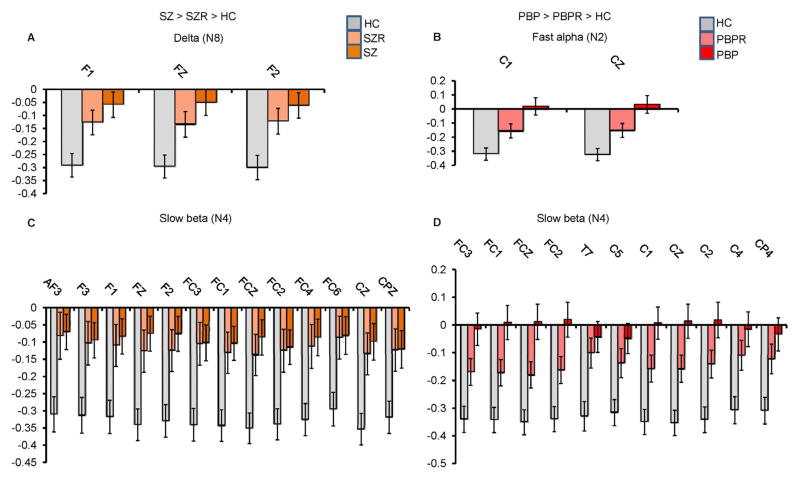
Several frontal and central sites showed a main effect of group (peak F(2,1075)=8.99, p<0.0001 at lead FZ). Post hoc t-tests revealed augmented activity in SZ and PBP probands versus HC. Additionally, SZR exhibited delta abnormality at frontal leads F1, FZ and F2 in the direction of probands versus HC.
Delta (N6)
Main effect of group for delta component was significant at anterior and posterior leads (peak F(2,1075)=14.12, p<0.0000008 at lead FCZ). Simple effects map showed both SZ and PBP probands exhibited increased activity at similar scalp locations versus HC.
Theta (N1)
Main effect of group for all spatial weights excluding FP2 from theta component was significant with a peak F value at lead CP5 (F(2,1075) = 26.7, p<4.8e-12) following Bonferonni correction. Simple effect analyses showed both SZ and PBP probands had significantly increased theta activity (Figures 3 and 4) at most scalp locations compared to HC. Theta activity also differed significantly between SZ and PBP probands (see Figure S4) in frontal, temporal and central regions with PBP showing reduced activity compared to SZ.
Slow alpha (N3)
The slow alpha component displayed significant group effect across most scalp locations except few frontal sites (with peak F(2,1075)=13.4, p<0.000001 at lead P7). Post-hoc t-tests showed both SZ and PBP probands had augmented activity at similar scalp sites compared to HC.
Fast alpha (N2)
Several frontal and central leads from the fast alpha component showed significant main effects of group (peak F(2,1075)=9.74, p<0.00006 at lead FCZ). Follow up contrast revealed both SZ and PBP probands had significantly increased activity at frontal and central channels compared to HC. Further, PBP relatives exhibited increased activity at two central leads (C1 and CZ) compared with HC. Abnormal fast alpha activity in PBPR was in the direction of PBP probands.
Fast alpha (N5)
Spatial weights from fast alpha component showed no significant group effects across any scalp region.
Slow beta (N4)
Frontal and central leads from the slow beta component differed significantly between groups (peak F(2,1075)=16.58, p<0.00000008 at lead CZ). Follow-up contrast revealed that both SZ and PBP probands exhibited increased activity at frontal and central leads. Increased activity was noted in SZR at subset of these leads compared to HC. Similarly, PBPR showed increased activity at subset of lead locations. For both groups, the abnormality in relatives was in the same direction as probands.
Fast beta (N7)
No significant group effect was detected in spatial weights from the fast beta component.
In summary, both SZ and BP probands showed abnormal low frequency activity compared to HC. Increased delta (N8) activity was common to SZ and SZR (Figure 5A). Theta (N1) activity significantly differed between HC and both the proband groups and between the two probands. Increased fast alpha (N2) activity was present in both PBP and PBPR (Figure 5B). Augmented slow beta (N4) activity at several frontal and central leads was present in SZ, PBP probands and their first-degree relatives (Figures 5C and 5D respectively). Results from supplementary analyses including relative risk and heritability are detailed in (see Table S2, S3 and S4) Supplement 1. ANCOVA and pairwise group comparisons on the spectral amplitude in various bands from standard EEG analysis revealed comparable results with augmented low frequency abnormalities in both proband groups (Figures S5, S6 & S7 in Supplement 1). Abnormality in relatives was similar to aforementioned results, except for minor differences in spatial locations where effects were noted.
Figure 5.
Mean spatial weights of clinically relevant leads significantly differing between healthy controls and both probands and relatives.
(A) Increased delta (N8) activity in schizophrenia (SZ) probands (n=225) and their relatives (SZR) (n=201) compared to healthy controls (HC) (n=200), (B) augmented fast alpha (N2) activity in psychotic bipolar disorder (PBP) probands (n=234) and their relatives (PBPR) (n=231) vs HC (n=200), (C) increased slow beta (N4) in SZ and SZR compared to HC and (D) increased slow beta (N4) in PBP and PBPR compared to HC. All the displayed leads were significantly different between HC and both probands and relatives after Bonferroni correction for pairwise comparison (p=0.05/5). Error bars represent SEM.
PSPD Analysis
Although only a minority of SZR and PBPR met criteria for PSPD, comparison of SZR and PBPR with PSPD to probands and HC (see Figures S8 (A–D)) showed a monotonic increasing pattern in delta and fast alpha activity from HC to other relatives to PSPD relatives to probands in SZ and PBP groups respectively. Similar patterns were observed in slow beta activity in PBPR, but not in SZR.
Clinical Correlation with Oscillatory Activity
Theta (N1) abnormality correlated positively with SBS scores at all 64 leads (see Figure S9, peak r = 0.16, p<0.0006 at lead F1), such that high SBS scores were associated with increased theta/slow alpha abnormalities. No other frequency abnormality correlated with SBS or PANSS positive, negative or total scores.
Discussion
The overall purpose of this multi-site study is to examine intermediate phenotypes in a large sample size cohort across the psychosis spectrum with generalizability. The main objectives of the current study were to examine whether similar RS-EEG oscillatory abnormalities were present in SZ and PBP probands, if similar patterns characterized their first degree relatives and to evaluate their heritability. Six of the 8 RS-EEG spectral components exhibited abnormalities in both SZ and PBP probands. Three abnormal spectral profiles were present in both probands and relatives; one was common to SZ and PBP while each of the remaining two were disorder-specific. To our knowledge this is the first large scale multi-site study with higher order spatial leads, to report neural oscillatory abnormalities in SZ, PBP probands and their first degree relatives using a novel, data-driven GICA approach with instantaneous RS-EEG spectral data.
Frequency abnormalities present in both SZ and PBP probands
Since SZ and PBP probands share psychotic features, we expected specific frequency abnormalities to characterize both disorders. Low frequency activity including delta, theta, slow alpha and slow beta were abnormal in both SZ and PBP. Low frequency abnormality (delta and theta) may be associated with genetic variants of COMT in SZ (12). Although spectral activity was not compared between schizoaffective, SZ and PBP probands, no frequency components (results not shown here) differed between schizoaffective depressive type vs SZ and schizoaffective mania type vs PBP proband groups.
Delta
Augmented delta activity (N6) was pronounced in frontal, left temporal, central and parietal locations in both SZ and PBP. Similarly, increased delta activity (N8) confined to frontal scalp locations was seen in both probands. Increased delta activity might be associated with reduced glucose metabolism (33); a reduction in brain metabolism in psychoses, consistent with the hypofrontality hypothesis advanced in prior studies (12, 13, 34).
Theta
Abnormally increased theta (N1) activity across the entire scalp was present in both proband groups. The neurobiological basis for abnormal theta in psychosis is unclear. However, one possible interpretation is that theta oscillations are hippocampally generated (35) and hippocampal cell discharges constitute a physiological model for psychosis (36). Thus, abnormal theta oscillations might play a common role in psychosis seen in SZ (11, 12), BP (17) and epilepsy (37). Venables (12) reported increased theta activity as a marker specific to SZ and not BP, but their BP subjects were non-psychotic, further suggesting that augmented theta activity is a psychosis biomarker. Our finding of increased theta activity is in agreement with prior similar studies (12, 15, 17). Theta activity (N1) was prominently increased in SZ compared to PBP, perhaps reflecting greater psychotic symptom severity in SZ. Additionally, clinical correlation with the SBS scale was weakly significant for frontal theta (N1) activity, suggesting that the frontal region differentiated SZ and PBP probands. The current findings suggest that a continuous rating such as the SBS revealed differences between proband groups.
Slow alpha and Fast alpha
Increased slow (N3) and fast alpha (N2) oscillatory activity was common to SZ and PBP probands. Slow alpha was more significant from the anterior to posterior brain regions including temporal locations in SZ probands compared to PBP. Fast alpha (N2) abnormality was confined to several fronto-central scalp locations in both SZ and PBP probands. Although it is unclear what specific neural mechanisms contribute to alpha oscillations, prior evidence shows that alpha activity is functionally correlated with arousal (38). Augmented alpha activity reflects reduced cortical activity and decreased arousal, generally being more pronounced in SZ compared to BP. Further, alpha rhythms are positively associated with Default Mode Network (DMN) activity (39) and negatively correlated with visual (40) and dorsal attentional activity (39). Increased alpha may indicate aberrant DMN activity and deficient visual and dorsal attention in probands, validated by fMRI studies (41, 42). Our findings are consistent with two studies (12, 15) but contradict two other previous reports (11, 34) of decreased alpha activity. Possible explanations might be between-study differences in methodology, sample distribution, processing parameters, diagnostic heterogeneity, illness chronicity or clinical subtypes.
Slow beta
Augmented fronto-central, high-frequency slow beta was common to SZ and PBP probands. Although the functional role of slow beta rhythms is unclear, in general they are associated with cognition (43) and neuronal excitability (44). Increased beta activity suggests excessive neuronal excitability in probands, consistent with prior study (12). Beta oscillations are related to the balance between excitatory pyramidal cells and inhibitory GABAA interneurons (45). Slow beta rhythms are genetically linked to a group of GABAA receptor genes that belong to the α2 subunit (46). Thus, abnormal beta activity may indicate dysfunctional GABA activity in SZ and PBP (47).
Fast beta and gamma
We detected no aberrant fast beta activity in probands or relatives despite prior reports. The omnibus ANCOVA test failed to show group differences; hence post-hoc tests were not carried out in probands and relatives. However, if the analysis was restricted separately to SZ, PBP probands vs controls then, only SZ and not PBP probands showed increased fast beta activity. In one prior study (12) abnormal fast beta activity was specific to SZ probands. Additionally, Venables used averaged spectral data, compared to the single trial analyses in our study.
The present study did not examine gamma activity. We were unable to extract a frontally active gamma component from GICA when the model order was varied from 2 to 20 likely due to the diminished amplitude of high frequency gamma activity covarying with dominant low frequency oscillations. Further, with increased model order, the estimated low frequency components generally implicated in SZ and PBP disintegrated into weak subcomponents.
Frequency abnormalities present in both probands and their relatives
Augmented fronto-central slow beta activity was detected in SZR and PBPR versus controls. As noted earlier, beta activity indexes neuronal excitability, suggesting altered cortical excitability in SZR and PBPR. Since abnormal beta activity was noted at same scalp locations in both proband groups, beta activity may serve as a potential intermediate phenotype reflecting genetic predisposition to both SZ and PBP. Our finding is contrary to prior studies (12, 20, 21) that reported fast beta abnormality in SZR. The present study was unable to examine fast beta activity due to methodological issues; omnibus ANCOVA failed to show group differences and no post-hoc tests were carried out, but increased fast beta activity was specific to SZ and not PBP probands. In general, beta activity is consistently identified as SZ intermediate phenotype indicating genetic liability for SZ.
Frequency abnormalities unique to SZR and PBPR
Abnormal delta activity was present in SZ probands and their relatives. Increased delta activity was localized frontally rather diffusely in both probands and relatives, indicating cortical hyperactivation. A prior study (20) failed to identify low frequency delta activity as an intermediate phenotype marker; SZR exhibited decreased delta power or cortical hyperactivation. However, that study identified reduced delta band coherence as a heritable trait related to SZ risk. Another prior report (15) revealed low frequency theta-alpha oscillation as a SZ intermediate phenotype. We did not observe theta-alpha abnormalities in SZR possibly due to methodological differences, but detected subtle excess fast alpha activity in two central leads in PBPR, perhaps reflecting weak liability to PBP illness.
Medication Effects
One challenge in interpreting our findings in probands is possible confounding effects of current medication treatment, illness chronicity, severity and duration on spectral activity. Although there is prior evidence (10, 14) for a lack of medication influence on RS-EEG spectra in probands, abnormalities detected in the present proband samples for e.g. increased delta, theta and alpha activity may be due to medications, similar to effects of clozapine on RS-EEG as reported in (48). The current literature has conflicting reports on medication effects. In our study probands were on several medications with varying dosages and durations, hence it was not feasible to account for medication effects. The study did not collect detailed longitudinal medication histories for probands to completely assess possible historical medication influence on RS-EEG spectral measures. However, slow beta (N4), fast alpha (N2) and delta (N8) abnormalities were present in both relatives and probands, suggesting that such abnormalities are unlikely to be due to antipsychotic medications, since the relatives were psychosis free and not taking psychotropic medications.
Relative Risk and Heritability
Delta and slow beta activity manifested moderate relative risk ratio suggesting familial characteristics associated with these measures and are candidate intermediate phenotypes for SZ. Similarly, fast alpha and slow beta activity exhibited a low relative risk for PBP. Oscillatory abnormalities in probands and relatives were further validated by our findings of moderate heritability. Our estimates were lower compared to pedigree based analyses, possibly due to less dense kinship structure in our sample (i.e. most probands were represented by only one relative); thus, “familiality” may be a more accurate descriptor. Our results are consistent with prior studies (18, 19) showing RS-EEG as a heritable trait across the frequency spectrum, suggesting a moderate amount of genetic influence in the variability of spectral activity.
Study Limitations
The current study has several limitations, including demographic data unbalanced in age and sex, which was accounted for in the analysis by regression at the cost of reduced statistical power. Second, we were unable to account for medication effects in probands, due to the multiple medications taken by probands and unavailability of longitudinal medication history. We were unable to extract a gamma component to investigate its role. There were significant site effects that had to be accounted for in the model. However, these did not significantly interact with clinical diagnoses. Some advantages of this study included large sample sizes from multiple sites, (hence generalizability) and high electrode density data covering the scalp.
Conclusion
Our current findings reveal similar low frequency abnormalities in SZ and PBP probands, consistent with prior reports. Delta abnormalities were present in both SZ and SZR, while PBP and PBPR revealed fast alpha abnormalities. Slow beta abnormality was present in both probands and their relatives. Future genetic investigations on the low frequency abnormalities would help clarify whether the biological mechanisms underlying these abnormalities are similar or different in these disorders. The oscillatory abnormalities in probands may provide insight into the underlying neurophysiologic problems. The weaker, familial frequency abnormalities identified in relatives may serve as intermediate phenotypes reflecting genetic liability for these illnesses and ultimately help identify the genetic architecture. Familiality for these intermediate phenotypes was moderate, but significant. Future studies need to investigate genetic underpinnings for spectral components and examine their functional implications by combining RS-EEG oscillations with functional MRI resting state networks.
Supplementary Material
Acknowledgments
This work was supported by funding from National Institute of Mental Health through linked R01 MH077851 to Dr. Carol Tamminga, MH077945 to Dr. Godfrey Pearlson, MH078113 to Dr. Matcheri Keshavan, MH077862 to Dr. John Sweeney and MH077852 to Dr. Gunavant Thaker.
Footnotes
Conflicts of Interest: All other authors report no biomedical financial interests or potential conflicts of interest.
This data were presented at 67th Society of Biological Psychiatry Meeting on May 3–5 2012, Philadelphia, Pennsylvania
Financial Disclosures: Dr. John Sweeney has received support from Takeda, BMS, Lilly, Roche and Janssen. Dr. Matcheri Keshavan has received support from Sunovion. Dr. Carol Tamminga has received funding from Astellas, Eli Lilly, Intracellular Therapies, Lundbeck and Pure Tech Ventures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keshavan MS, Morris DW, Sweeney JA, Pearlson G, Thaker G, Seidman LJ, et al. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo Bipolar Scale. Schizophr Res. 2011;133:250–254. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JD, Nieto Castanon A, McCarthy JM, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017. doi: 10.1038/npp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill SK, Reilly JL, Harris MS, Rosen C, Marvin RW, Deleon O, et al. A comparison of neuropsychological dysfunction in first-episode psychosis patients with unipolar depression, bipolar disorder, and schizophrenia. Schizophr Res. 2009;113:167–175. doi: 10.1016/j.schres.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bramon E, Sham PC. The common genetic liability between schizophrenia and bipolar disorder: a review. Curr Psychiatry Rep. 2001;3:332–337. doi: 10.1007/s11920-001-0030-1. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter WT, Bustillo JR, Thaker GK, van Os J, Krueger RF, Green MJ. The psychoses: cluster 3 of the proposed meta-structure for DSM-V and ICD-11. Psychol Med. 2009;39:2025–2042. doi: 10.1017/S0033291709990286. [DOI] [PubMed] [Google Scholar]
- 6.Potash JB, Willour VL, Chiu YF, Simpson SG, MacKinnon DF, Pearlson GD, et al. The familial aggregation of psychotic symptoms in bipolar disorder pedigrees. Am J Psychiatry. 2001;158:1258–1264. doi: 10.1176/appi.ajp.158.8.1258. [DOI] [PubMed] [Google Scholar]
- 7.Badner JA, Gershon ES. Meta analysis of whole genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7:405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- 8.Gottesman, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 9.Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G, Iacono W. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res. 2008;99:225–237. doi: 10.1016/j.schres.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sponheim SR, Clementz BA, Iacono WG, Beiser M. Resting EEG in first episode and chronic schizophrenia. Psychophysiology. 1994;31:37–43. doi: 10.1111/j.1469-8986.1994.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 12.Venables NC, Bernat EM, Sponheim SR. Genetic and disorder specific aspects of resting state EEG abnormalities in schizophrenia. Schizophr Bull. 2009;35:826–839. doi: 10.1093/schbul/sbn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karson CN, Coppola R, Daniel DG, Weinberger DR. Computerized EEG in schizophrenia. Schizophr Bull. 1988;14:193–197. doi: 10.1093/schbul/14.2.193. [DOI] [PubMed] [Google Scholar]
- 14.Gattaz WF, Mayer S, Ziegler P, Platz M, Gasser T. Hypofrontality on topographic EEG in schizophrenia. Correlations with neuropsychological and psychopathological parameters. Eur Arch Psychiatry Clin Neurosci. 1992;241:328–332. doi: 10.1007/BF02191956. [DOI] [PubMed] [Google Scholar]
- 15.Hong LE, Summerfelt A, Mitchell BD, O’Donnell P, Thaker GK. A shared low-frequency oscillatory rhythm abnormality in resting and sensory gating in schizophrenia. Clin Neurophysiol. 2012;123:285–292. doi: 10.1016/j.clinph.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, et al. Clinical Phenotypes of Psychosis in the Bipolar and Schizophrenia Network on Intermediate phenotypes (B-SNIP) American Journal of psychiatry. 2013 doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- 17.Clementz BA, Sponheim SR, Iacono WG, Beiser M. Resting EEG in first episode schizophrenia patients, bipolar psychosis patients, and their first-degree relatives. Psychophysiology. 1994;31:486–494. doi: 10.1111/j.1469-8986.1994.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 18.Smit DJ, Posthuma D, Boomsma DI, Geus EJ. Heritability of background EEG across the power spectrum. Psychophysiology. 2005;42:691–697. doi: 10.1111/j.1469-8986.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 19.van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. Am J Hum Genet. 1996;58:562–573. [PMC free article] [PubMed] [Google Scholar]
- 20.Winterer G, Egan MF, Radler T, Hyde T, Coppola R, Weinberger DR. An association between reduced interhemispheric EEG coherence in the temporal lobe and genetic risk for schizophrenia. Schizophr Res. 2001;49:129–143. doi: 10.1016/s0920-9964(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 21.Itil TM. Qualitative and quantitative EEG findings in schizophrenia. Schizophr Bull. 1977;3:61–79. doi: 10.1093/schbul/3.1.61. [DOI] [PubMed] [Google Scholar]
- 22.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbs P, Williams NM. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. New York: Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- 24.Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, et al. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry. 2005;57:633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Potash JB. Carving chaos: genetics and the classification of mood and psychotic syndromes. Harv Rev Psychiatry. 2006;14:47–63. doi: 10.1080/10673220600655780. [DOI] [PubMed] [Google Scholar]
- 26.Pawel S, David JS, Thaker GK, Stevens MC, Keshavan MS, Sweeney JA, et al. Diffusion Tensor Imaging White Matter Endophenotypes in Patients With Schzophrenia or Psychotic Bipolar Disorder and Their Relatives. American Journal of psychiatry. 2013 doi: 10.1176/appi.ajp.2013.12111448. [DOI] [PubMed] [Google Scholar]
- 27.Eichele T, Calhoun VD, Debener S. Mining EEG-fMRI using independent component analysis. Int J Psychophysiol. 2009;73:53–61. doi: 10.1016/j.ijpsycho.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45:S163–172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Congedo M, John RE, De Ridder D, Prichep L. Group independent component analysis of resting state EEG in large normative samples. Int J Psychophysiol. 2010;78:89–99. doi: 10.1016/j.ijpsycho.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR. Relative risk of attention deficits in siblings of patients with schizophrenia. Am J Psychiatry. 2000;157:1309–1316. doi: 10.1176/appi.ajp.157.8.1309. [DOI] [PubMed] [Google Scholar]
- 31.Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM, et al. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50:98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- 32.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guich SM, Buchsbaum MS, Burgwald L, Wu J, Haier R, Asarnow R, et al. Effect of attention on frontal distribution of delta activity and cerebral metabolic rate in schizophrenia. Schizophr Res. 1989;2:439–448. doi: 10.1016/0920-9964(89)90012-1. [DOI] [PubMed] [Google Scholar]
- 34.Sponheim SR, Clementz BA, Iacono WG, Beiser M. Clinical and biological concomitants of resting state EEG power abnormalities in schizophrenia. Biol Psychiatry. 2000;48:1088–1097. doi: 10.1016/s0006-3223(00)00907-0. [DOI] [PubMed] [Google Scholar]
- 35.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 36.Olypher AV, Klement D, Fenton AA. Cognitive disorganization in hippocampus: a physiological model of the disorganization in psychosis. J Neurosci. 2006;26:158–168. doi: 10.1523/JNEUROSCI.2064-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sachdev P. Schizophrenia-like psychosis and epilepsy: the status of the association. Am J Psychiatry. 1998;155:325–336. doi: 10.1176/ajp.155.3.325. [DOI] [PubMed] [Google Scholar]
- 38.Barry RJ, Clarke AR, Johnstone SJ, Magee CA, Rushby JA. EEG differences between eyes-closed and eyes-open resting conditions. Clin Neurophysiol. 2007;118:2765–2773. doi: 10.1016/j.clinph.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben Simon E, Podlipsky I, Arieli A, Zhdanov A, Hendler T. Never resting brain: simultaneous representation of two alpha related processes in humans. PLoS One. 2008;3:e3984. doi: 10.1371/journal.pone.0003984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 42.Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130:86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, et al. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci U S A. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Bauer LO, et al. Beta power in the EEG of alcoholics. Biol Psychiatry. 2002;52:831–842. doi: 10.1016/s0006-3223(02)01362-8. [DOI] [PubMed] [Google Scholar]
- 45.Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 46.Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci U S A. 2002;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 48.Knott V, Labelle A, Jones B, Mahoney C. Quantitative EEG in schizophrenia and in response to acute and chronic clozapine treatment. Schizophr Res. 2001;50:41–53. doi: 10.1016/s0920-9964(00)00165-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.