Abstract
Impulsive choice behavior occurs when individuals make choices without regard for future consequences. This behavior is often maladaptive and is a common symptom in many disorders, including drug abuse, compulsive gambling, and obesity. Several proposed mechanisms may influence impulsive choice behavior. These mechanisms provide a variety of pathways that may provide the basis for individual differences that are often evident when measuring choice behavior. This review provides an overview of these different pathways to impulsive choice, and the behavioral intervention strategies being developed to moderate impulsive choice. Because of the compelling link between impulsive choice behavior and the near-epidemic pervasiveness of obesity in the United States, we focus on the relationship between impulsive choice behavior and obesity as a test case for application of the multiple pathways approach. Choosing immediate gratification over healthier long term food choices is a contributing factor to the obesity crisis. Behavioral interventions can lead to more self controlled choices in a rat pre-clinical model, suggesting a possible gateway for translation to human populations. Designing and implementing effective impulsive choice interventions is crucial to improving the overall health and well-being of impulsive individuals.
Keywords: impulsive choice, individual differences, intervention, rat
Everyday life is comprised of many choices. Decisions must be made about how to act, what to buy, what to eat, and so on. Some of these daily choices may be deliberate, whereas others are unplanned and impulsive. It is essential to better understand those impulsive decisions, as impulsive behavior is associated with many disorders including attention deficit hyperactivity disorder (ADHD; Neef et al. 2005), schizophrenia (Heerey et al. 2007), drug abuse (Bickel and Marsch 2001), smoking (Mitchell 1999), compulsive gambling (Alessi and Petry 2003, Petry and Casarella 1999), and obesity (Boomhower, Rasmussen, and Doherty 2013, Jarmolowicz et al. 2014, Weller et al. 2008). Impulsive behavior often involves making choices without considering the future consequences (Madden and Bickel 2010), for instance, choosing to purchase and devour a tempting chocolate bar at the checkout line in the grocery store, instead of waiting to prepare and eat the more healthy options in your shopping cart.
Much of the current impulsive choice research endeavors to understand mechanisms and means of moderating impulsive choice through the use of laboratory animals. Animals provide a behavioral analog that enables researchers to assess impulsivity under a number of varied conditions and control for many outside influences that cannot necessarily be controlled in human samples. Impulsive choice is the propensity to choose a smaller reward that will be obtained sooner (smaller-sooner, SS) rather than waiting for a larger reward that would be obtained later (larger-later, LL; Cardinal et al. 2001, Evenden and Ryan 1996). For example, a rat may choose to wait 5-s for 1 food pellet (the SS) or to wait 30-s for 2 food pellets (the LL). The rat then has to wait 60-s before being able to choose again. Here, the LL food reward is the optimal choice in the long run, resulting in more total food earned, compared to the SS reward. Thus, predominant choices of the SS are sub-optimal, and hence impulsive. The amount of food reward and/or the delay until reward can be manipulated to assess the rat's self-control and ability to wait for a larger reward.
The basic testing paradigm for assessing impulsive choice consists of placing the rat in an operant conditioning box (i.e., an experimental chamber) and giving it repeated choices of different delays and food reward amounts. For each individual choice, the rat makes its selection of either the SS or LL reward by pressing one of two levers that it has learned to associate with the food-delay combinations of the rewards. Testing of impulsive choice behavior in human participants can be conducted using a similar task, though there are several other available methods. One common test entails giving people choices between SS and LL choices that involve monetary rewards delivered at different delays (Madden et al. 2004). Human testing is generally conducted in a single session of shorter duration than the comparable lab animal testing sessions.
These testing procedures in rats and humans have identified a broad spectrum of individual differences in impulsive choice behavior (Marshall, Smith, and Kirkpatrick 2014, Navarick 1998, Galtress, Garcia, and Kirkpatrick 2012). For instance, there are individual differences in the rate at which one devalues future rewards and views the immediate reward as more valuable, a phenomenon referred to as delay discounting. The discounting rate is a common measure of impulsive behavior in rats and humans; specifically, steeper discounting functions reflect greater impulsivity. The hyperbolic discounting equation is commonly used to predict the subjective value (V) of reward as a function of the amount (A) of the reward and the delay (D):
Here, k represents the discounting rate (i.e., an individual difference parameter in impulsive choice behavior). An updated model includes an exponent to the denominator of the previous equation, s, in which individual differences in s account for individual differences in sensitivity to time; this model has been shown to more accurately fit individual differences in choice data in some cases (Myerson and Green 1995):
In accordance with the modified hyperbolic discounting equation, many recent studies have indicated that temporal processing, for example, may play a critical role in impulsive choice behavior (Marshall, Smith, and Kirkpatrick 2014, Baumann and Odum 2012, Cheng 1992, Galtress, Garcia, and Kirkpatrick 2012, McClure, Podos, and Richardson 2014, McGuire and Kable 2012, Wittmann and Paulus 2008, Smith, Marshall, and Kirkpatrick 2015, Zauberman et al. 2009). How one understands and perceives time, may affect timing abilities and the subjective value of rewards. Temporal myopia, making poor long-term choices, is a result of the inability to find appropriate value in long-term consequences (Rachlin 2000). Additionally, stable levels of delay discounting have been found in humans and rats (Dellu-Hagedorn, Trunet, and Simon 2004, Galtress, Garcia, and Kirkpatrick 2012, Jimura et al. 2011a, Marshall, Smith, and Kirkpatrick 2014, Peterson, Hill, and Kirkpatrick 2015), indicating that delay discounting/impulsive choice is a stable trait variable (Odum 2011a, b, Odum and Baumann 2010). Although individual discounting rates remain relatively stable across time, recent research has indicated that time-based interventions may effectively change these responses. This suggests that impulsive choice behavior is a stable, yet somewhat malleable trait. This review examines the role of individual differences in impulsive choice, the possible mechanisms involved in impulsive choice behavior, and how intervention strategies may alter choice behavior.
With impulsive choice playing such an important role in our lives and health, behavioral interventions have become essential tools for changing impulsive choice. For laboratory animals, interventions include exposing animals to extended or uncertain delays. Experience with long delays and/or dynamically changing delays increases self-control in lab rats (Smith, Marshall, and Kirkpatrick 2015, Stein, Johnson, et al. 2013, Stein et al. 2015). Experience with delay has also been used as an effective intervention in humans, leading to more self-controlled choices (e.g., Eisenberger and Adornetto 1986). Intervention strategies also incorporate improvement of key components of choice behavior, such as working memory and delay discrimination ability (Bobova et al. 2009, Marshall, Smith, and Kirkpatrick 2014). Intervention strategies must account for the vast individual differences in impulsive choice behavior; strategies that are successful for one person (or rat) may not be useful for another. Although some neurocognitive, pharmacological, and behavioral interventions have been utilized in humans, more work is needed. Designing and implementing targeted interventions to increase self-control in lab animals can potentially lead to effective translational applications for the human population. The ability to increase self-control and decrease impulsive choice behavior in humans could minimize health risks in impulsive individuals, such as drug abuse, pathological gambling, and obesity-related disease as well as improve overall health.
Mechanisms of Impulsive Choice
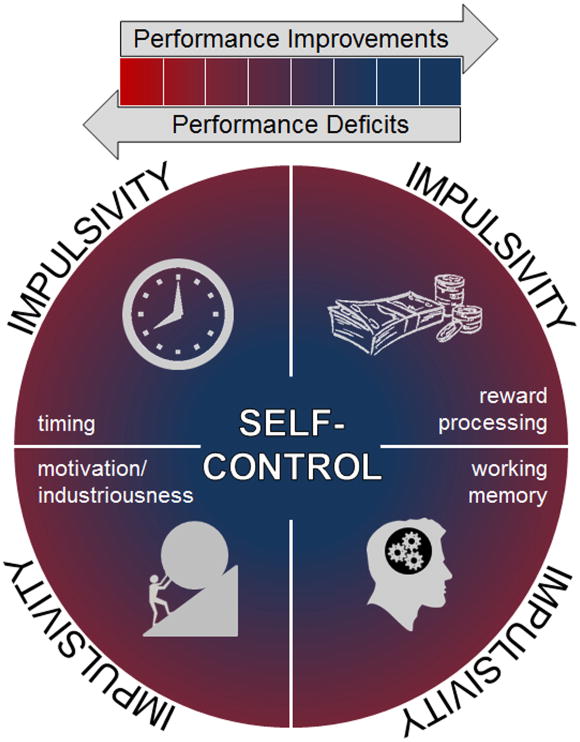
There are several mechanisms which affect an individual's level of impulsive choice, such as timing, reward processing, motivation/industriousness, and working memory (Figure 1). Individual differences in impulsive choice may be a result of different mechanisms attributing to their impulsive choice (Kirkpatrick, Marshall, and Smith 2015). Thus, impulsive choice could emerge from deficits in one or more pathways. What this means is that two individuals could be equally impulsive, but for totally different reasons. This may be one reason why impulsive choice is a trans-disease process (Bickel and Mueller 2009), because there are multiple causal pathways. It is essential to understand the mechanisms leading to impulsive choice as these can be potential avenues to moderate impulsive choice through behavioral interventions (see Moderating Impulsive Choice through Behavioral Interventions). It is critical to understand not only whether an individual is impulsive, but also why they are impulsive.
Figure 1.
Deficits in an individual's ability to process and perceive time are thought to contribute to individual differences in impulsive choice (see Navarick 1998), providing one potential pathway to impulsivity (see Figure 1). Previous research in humans has suggested that impulsivity is related to deficits in interval timing, or the perception of time on a scale of seconds to minutes (Baumann and Odum 2012, Darcheville, Rivière, and Wearden 1992, van den Broek, Bradshaw, and Szabadi 1992). Moreover, rats that made more self-controlled (LL) choices also showed better temporal discrimination abilities (Marshall, Smith, and Kirkpatrick 2014, McClure, Podos, and Richardson 2014). Another component of timing contributing to impulsive choice behavior is the extent to which an individual considers future consequences. The more self-controlled (LL) choices an individual makes, the more likely they are to focus on the future (Daugherty and Brase 2010). Together, more self-controlled behavior and greater consideration of future consequences predict more healthy behaviors such as less frequency of alcohol use and wearing a seat belt (Daugherty and Brase 2010). Shifting one's perspective to a more future-oriented mindset could be an avenue for treatment programs for obesity, substance abuse, and gambling (Wittmann and Paulus 2009, Rachlin 2000). Overall, these results suggest that one's perception of the passage of time may be strongly related to his or her propensity to make impulsive decisions (Takahashi 2005, Takahashi, Oono, and Radford 2008, Wittmann and Paulus 2008, Kim and Zauberman 2009).
Similar to the evidence suggesting that poor timing may underlie impulsive choice behavior (Marshall, Smith, and Kirkpatrick 2014, McClure, Podos, and Richardson 2014), recent research has suggested that poor reward discrimination may also contribute to impulsive choice (Marshall and Kirkpatrick under review). Here, rats chose between SS and LL outcomes, and the magnitude of the LL reward was manipulated. Subsequently, the rats experienced a reward discrimination task, in which the reward magnitude for making a certain response was manipulated. The rats that made more LL choices in the impulsive choice task also made more responses for the larger reward magnitude in the reward discrimination task, suggesting that rats that are better at discriminating differences in amount are also more self-controlled (Marshall and Kirkpatrick under review). This mirrors the findings with temporal discrimination and suggests that poor discrimination of the components of the SS and LL outcomes drive impulsive choices. In other words, self-control may relate to the ability to make well informed choices. Thus, deficits in reward processing represent another pathway to impulsivity, as depicted in Figure 1.
In addition to mechanisms related to interval timing and reward processing, motivation and industriousness are a third potential pathway to impulsivity. Incentive motivation refers to the willingness to work for rewards. Examinations of individual differences in rats have indicated that willingness to work for food rewards, measured by a progressive ratio schedule, related to adaptability in choice behavior with changes in choice parameters (Kirkpatrick et al. 2014). In addition, the nucleus accumbens, a brain region that is implicated in regulating incentive motivation (Balleine and Killcross 1994, Corbit, Muir, and Balleine 2001), also plays an important role in impulsive choice through effects on reward processing (e.g., Galtress and Kirkpatrick 2010). Similar to motivation, industriousness refers to the effort put forth to obtain a reward. Eisenberger and colleagues posit that learned industriousness, the process of learning that high effort behaviors are associated with high reward, can improve self-control (e.g., Eisenberger et al. 1989, Eisenberger, Mitchell, and Masterson 1985). Understanding the motivational levels exhibited by impulsive individuals and learning how to manipulate motivation may be an avenue to moderating impulsive choice.
A final pathway to impulsivity is working memory which is presumably important for maintaining an active expectation of reward delivery during the delay to receipt of reward (see Figure 1). Bobova et al. (2009) demonstrated that individuals who were more impulsive also exhibited poorer working memory (or short-term memory) capacity, suggesting that more impulsive individuals are less proficient at maintaining, storing, and manipulating information (see Baddeley 1992). Moreover, individuals tend to make more impulsive choices when more information has to be maintained in working memory (Hinson, Jameson, and Whitney 2003). Additionally, when working memory is taxed, food choices are strongly driven by implicit attitudes that have been suggested to relate to impulsive behavior (see Friese, Hofmann, and Wänke 2008). Thus, deficits in working memory may be related to individual differences in impulsive choice behavior, creating another potential pathway to impulsive decisions.
Collectively, these results indicate that deficits in interval timing, reward processing, motivation (and industriousness), and working memory are separate pathways leading to individual differences in impulsive choice behavior. As exemplified in Figure 1, there is a broad spectrum of performance levels across these four domains that may produce the same overall degree of impulsivity, indicating that a comprehensive reductionist/mechanistic approach to the analysis of impulsive choice should be implemented so as to best understand why certain individuals can wait for a larger reward, while other individuals simply cannot.
Moderating Impulsive Choice through Behavioral Interventions
In addition to gaining insight into mechanisms of individual differences, examination of the specific factors that govern individual differences can provide a gateway to developing neurocognitive and/or pharmacological interventions to alleviate the impulsivity-based behavioral deficits associated with diseases and disorders such as drug abuse, pathological gambling and obesity (e.g., Bickel and Marsch 2001, Bruce et al. 2011, Dixon, Marley, and Jacobs 2003, Weller et al. 2008). The preceding section described how individual differences in specific processes could create different pathways to impulsive choice behavior (see Figure 1). Accordingly, these mechanisms can be targeted by specific interventions to promote self-control. In other words, if the corresponding performance levels within the underlying mechanisms improve, then self-control may be a direct result of such improvements (see Figure 1).
The first pathway to impulsivity noted above is through deficits in timing processes. Temporal discrimination is an important feature of decisions between differentially-delayed rewards; indeed, rats that are more self-controlled are also more tolerant of longer delays to reward and show better temporal discrimination (Marshall, Smith, and Kirkpatrick 2014). Accordingly, previous research has employed targeted interventions focused on improving how well an individual processes time (i.e., temporal processing); these interventions will be subsequently referred to as time-based interventions to improve self-control (i.e., reduce impulsive choice). Simple exposure to delayed rewards results in a reduction in impulsive choice behavior in rats (Madden et al. 2011, Stein, Johnson, et al. 2013, Stein et al. 2015) and humans (Eisenberger and Adornetto 1986). Additionally, exposure to long reward delays results in less impulsive choice behavior than exposure to short reward delays (Eisenberger, Masterson, and Lowman 1982, also see Stein, Johnson, et al. 2013). Moreover, in pigeons, Mazur and Logue (1978) showed that gradual shortening (“fading”) of the SS delay to 0 s (after initially setting the SS and LL delays equal at 6-s) maintained increased preference for the LL outcome compared to a group of pigeons that simply chose between an immediate (0-s) SS outcome and the delayed (6-s) LL outcome. It has been subsequently shown that gradually increasing the delay to the LL reward while participants engaged in a secondary task maintained preference for the LL outcome in adults with development disabilities (Dixon et al. 1998), children with ADHD (Binder, Dixon, and Ghezzi 2000, also see Neef, Bicard, and Endo 2001), and adults with moderate to severe intellectual disabilities (Dixon, Rehfeldt, and Randich 2003). Moreover, impulsive choices in delay discounting paradigms are reduced when the decision questions are framed in different ways. For instance, delayed rewards are perceived as more valuable when the delay is specified in terms of the date of receipt rather than the amount of time until receipt (see Koffarnus et al. 2013). Overall, there is clear indication that temporal processes are important in impulsive choice, either through the direct experience of delays or through framing of delay information.
Recent research in our laboratory has investigated the effects of different types of time-based interventions on impulsive choices and timing processes in rats (Smith, Marshall, and Kirkpatrick 2015). For one group of rats, the intervention required the rats to inhibit their responses for reward for a programmed interval of time (e.g., 30-s), a differential reinforcement of low rate schedule. Two other groups of rats were exposed to the same reward delays as they received in the impulsive choice task; half of these rats were exposed to exactly the same reward delays as the impulsive choice task, while the other half were exposed to reward delays that averaged the same interval as the impulsive choice task. Interestingly, all three of these different interval-timing-based interventions promoted self-control and also led to improved temporal discrimination (Smith, Marshall, and Kirkpatrick 2015). Such improved timing and choice performances following the time-based interventions confirm the relationship between interval timing and impulsive choice (see Kirkpatrick, Marshall, and Smith 2015) as a potential causal pathway. The ability of time-based interventions to alleviate impulsive choice tendencies reflects a crucial step in treating maladaptive behavioral tendencies in populations that also exhibit temporal processing deficits, such as those characterized by schizophrenia, ADHD, and drug abuse (e.g., Allman and Meck 2012, Wittmann and Paulus 2008).
In contrast to the relationship between temporal processing and impulsive choice, further evidentiated by the impact of temporal interventions on choice behavior, the employment of reward-based interventions to improve impulsive choice has been relatively absent from the literature. Stein, Smits, et al. (2013) implemented a reward bundling procedure between two phases of an impulsive choice task. In this reward procedure (see Ainslie and Monterosso 2003), smaller-sooner (i.e., impulsive) and large-later (i.e., self-controlled) rewards were delivered throughout a trial. For example, if the size of the bundle was three, then a smaller-sooner choice resulted in three smaller-sooner rewards (e.g., 1 pellet × 3 deliveries), while a larger-later choice resulted in three later-later rewards (e.g., 3 pellets × 3 deliveries). In the bundle conditions, each of the smaller-sooner and larger-later rewards were separated by the length of the larger-later delay; that is, the greater the bundle, the more exposure to larger-later delays. Stein, Smits, et al. (2013) reported that the greater the reward bundling (i.e., 1 vs. 3 vs. 9 deliveries of reward), the more often rats chose the larger-later outcome in the impulsive choice task following the reward bundling procedure. Interestingly, these results were explained not in terms of reward bundling, but in terms of more exposure to the LL delays throughout the reward bundling procedure for the rats that received larger bundles of reward (Stein, Smits, et al. 2013). Essentially, this explanation corroborates the impact of a time-based intervention on impulsive choice (e.g., Smith, Marshall, and Kirkpatrick 2015), but did little to suggest whether a reward-based intervention would be able to reduce impulsive choice via improvements in reward processing.
Such a question was addressed by Marshall and Kirkpatrick (under review) by delivering a reward magnitude intervention and assessing the effects on impulsive choice behavior. During the intervention, the Intervention Group responded for different magnitudes of reward, while the Control Group responded for identical magnitudes, with the goal that the Intervention Group would become better at discriminating reward magnitudes. Following this behavioral task, the rats were re-exposed to the impulsive choice task and then tested for their reward discrimination ability. The rats in the Intervention Group showed decreases in impulsive choice and greater discriminability of reward magnitudes. Thus, these results suggest that targeting an individual's reward processing ability through a reward-based intervention promotes more self-controlled choices, minimizing any suboptimal/maladaptive behavioral propensities. These results also confirm reward magnitude processing as a second viable pathway to impulsive choice.
A successful program of intervention development in humans has been Eisenberger's research on learned industriousness, focused on training individuals to tolerate more intensive work demands (e.g., Eisenberger et al. 1989, Eisenberger, Mitchell, and Masterson 1985). This line of research provides evidence that behavioral training methods can prove effective as interventions for impulsive choice in humans and has implications for the interaction between motivation and impulsivity. However, self-control engendered by industriousness training appears to be largely independent from self-control in delay discounting situations, where the focus is on delay tolerance rather than work load (see for example, Eisenberger, Masterson, and Lowman 1982, Walton et al. 2006). Ultimately, self-control may be at least partially promoted via implementation of programs that encourage persistence and tolerance to more laborious tasks, thereby motivating individuals toward a delayed yet more rewarding outcome.
A final targeted mechanism of behavioral interventions is working memory, as poor working memory has been shown to predict impulsive choice. Improvements in working memory capacity may promote greater self-control in sub-populations that exhibit working memory deficits, such as those characterized by drug abuse (Bechara and Martin 2004), schizophrenia (Lee and Park 2005), and ADHD (Martinussen et al. 2005). Indeed, Bickel et al. (2011) exposed substance abusers to a computerized working memory training program (PSSCogReHab), in which the participants were required to recall sequences of digits or words (i.e., a behavioral intervention), or a control program in which the participants were provided the answers to the same series of task questions in the working memory training program. The individuals who experienced the working memory training decreased their impulsive choices, while the individuals who experienced the control task increased their impulsive choices (Bickel et al. 2011). In an attempt to extend these findings to non-human animals, Renda, Stein, and Madden (2015) presented rats with either a working memory intervention or a non-intervention control task and assessed the effects on impulsive choice behavior. While the training improved working memory performance in the rats exposed to the intervention, it did not reduce impulsive choice relative to that of the control task. Therefore, given the discrepancy in results in different species, future research is needed to better elucidate the interaction between these psychological mechanisms (see Bickel, Moody, and Quisenberry 2014).
Overall, the aforementioned results speak to malleability of individual differences in impulsive choice. While individual differences are relatively stable across long stretches of time in both humans and rats (e.g., Jimura et al. 2011b, Odum 2011c, Peterson, Hill, and Kirkpatrick 2015), it is possible to intervene and adjust behavior. This may be done via improvements in core processes that create specific pathways to impulsive choice, and the success of the interventions further attests to the involvement of these specific mechanisms in impulsive choice (see Mechanisms of Impulsive Choice). However, it is important to note that such targeted interventions may be more or less successful depending on the individual or group of individuals. For instance, a time-based intervention was successful, albeit more transient and weaker, in the Lewis strain of rats (Smith, Marshall, and Kirkpatrick 2015), which has traditionally shown a strong propensity to make impulsive choices (e.g., Anderson and Diller 2010, Anderson and Woolverton 2005, Garcia and Kirkpatrick 2013, Madden et al. 2008). Lewis rats also are known to self-administer a variety of psychoactive compounds at a higher rate than control strains (García-Lecumberri et al. 2010, Kosten et al. 1997, Picetti et al. 2012, Suzuki, George, and Meisch 1988). Their higher rates of self-administration are likely due to deficits in reward system functioning (Flores et al. 1998, Harris and Nestler 1996, Higuera-Matas et al. 2011, Martin et al. 1999). Thus, a reward-based intervention may provide a better outcome for these rats, a possibility that remains to be tested. In general, the effectiveness of any intervention will likely depend on the target mechanism and the target population.
Impulsive Choice and Obesity
The mechanistic approach to understanding impulsive choice and the methods to moderate impulsive choice proposed in this paper can be elucidated through an example involving obesity. Although there are many negative consequences associated with impulsive choice, obesity is one of the most prevalent. Over the past decade, the relationship between obesity and impulsive choice has received growing attention. Body mass index (BMI; i.e., body weight divided by height) and body fat percentage are two measures that are often used to define obesity, and the two measures have been used to understand the relationship between obesity and impulsive choice. Specifically, BMI (Jarmolowicz et al. 2014) and higher body fat percentage (Hendrickson, Rasmussen, and Lawyer 2015, Rasmussen, Lawyer, and Reilly 2010) are correlated with greater impulsive choice behavior. Obesity can lead to a myriad of negative health and economic consequences such as weight related illnesses, increased health insurance costs, lost productivity, absenteeism, and premature death (Hammond and Levine 2010). According to the CDC, health problems associated with excess body weight include heart disease, stroke, cancer, and type 2 diabetes (Centers for Disease Control). Obesity-related medical costs in the United States were $147 billion in 2008 alone (Centers for Disease Control, Finkelstein et al. 2009). Moreover, it is estimated that by 2030, as much as 86% of the adult population in the United States will be overweight or obese (Wang et al. 2008). These dire projections make the study of impulsive food choices a critical component of improving the physical and economic health of the United States.
While diet and exercise are common approaches used for treatment of obesity, one contributor that is often overlooked in obesity treatment programs is impulsive choice, which can be moderated through behavioral interventions. Impulsive choice behavior may be a possible mechanism by which individuals develop obesity, as individuals with greater impulsive choice behavior have been found to overeat (Guerrieri et al. 2007). In a task where normal weight women could eat as many snacks as they wanted, those who were more impulsive and more motivated to work for food consumed more snacks than women who were similarly motivated to work for food yet not impulsive (Rollins, Dearing, and Epstein 2010). This suggests that the combination of greater impulsivity and motivation to work for food influences food choice and food intake. This finding has important implications as it shows that normal weight women with high scores on both tasks may be more at risk for becoming obese as a result of the increased food intake. Intervention strategies aimed at decreasing impulsive choice provide a possible avenue for preventing obesity and treating overweight persons.
In order to appropriately address the underlying behavioral issues that contribute to poor food choices, the mechanisms leading to impulsive choice for people with obesity must be better understood. For example, if individuals with obesity typically have deficits in temporal processing, then a time-based intervention may be most appropriate for improving self-control in obese individuals. As obese individuals have been found to be more impulsive than normal weight individuals, one might expect this to be a result of poor temporal processing (i.e., timing). However, obese Zucker rats, an animal model for genetic obesity, were more sensitive to the rate at which they received reward compared with their lean counterparts, which suggests that they are sensitive to time (Buckley and Rasmussen 2012). The contribution of time discrimination to impulsive choice in obese individuals needs to be investigated further to determine if this is a deficit that can be addressed to improve impulsive choice behavior. However, the role of consideration of future consequences in food choices is clear. Those who reported healthier eating were more likely to consider future consequences and focus less on immediate rewards (Dassen, Houben, and Jansen 2015). Behavioral interventions with a focus on changing a person's perspective of time may moderate impulsive choice, thus decreasing overeating and potentially leading to sustained weight loss.
Alternatively, targeting reward and/or motivational processes may be a fruitful route given the relationship between obesity and motivation to earn food reward. Applying learned industriousness to address impulsive behavior is a possibility, but it would need to be used cautiously, as improving the motivation to work in an impulsive individual may result in greater food intake if that effort is placed on working for food rewards. Rather, learned industriousness may be a potential avenue if the effort can be directed towards healthy behaviors intended to help with weight loss such as exercising. Finding a way to direct effort towards particular behaviors could be one possible way to address impulsive choice to treat obesity.
Ultimately, any successful intervention needs to be well informed by an understanding of the mechanisms that result in obesity through impulsive choices. Moreover, by focusing on those underlying mechanisms, treatments that decrease impulsive food choices in obese individuals could also generalize to other disorders associated with the same mechanism (e.g., Bickel and Mueller 2009). Thus, a more mechanistic focus could result in the ability for interventions to simultaneously treat multiple co-morbid disorders within the same individual.
Discussion
Impulsivity is a trait behavior (Jimura et al. 2011b, Kirby 2009) with the potential to lead to many maladaptive behaviors such as addiction, gambling, and obesity. Due to the copious health implications associated with impulsive choice behavior, gaining a deeper understanding through the parsing out of its infrastructure (timing, reward processing, motivation/industriousness, and working memory), as well as addressing deficits via the development of targeted behavioral and pharmaceutical interventions is essential to decreasing impulsive choice behavior and improving physical, mental, and economic health.
One common method for measuring impulsivity and differences therein, is through a SS versus LL impulsive choice task. In humans, the iconic marshmallow task, in which a child is told s/he can have one marshmallow now, or wait for a second marshmallow (Mischel and Underwood 1974), and variations thereof can be used to assess impulsive choice. This task can be mimicked in rats with food pellets via SS and LL choices, which gives us novel insight into impulsive choice, decision-making processes, and their various components. The use of these tasks also highlights the individual differences in constituents of impulsive choice behavior, including the valuation of reward, the gravity of delay, and the impacts of diet on choice, as differences therein expound upon individual propensities in impulsive choice.
The maladaptive behaviors that can stem from increased impulsive choice can be attributed to deficits in one, or multiple, components of impulsivity, leading to different potential pathways to impulsive choice. Deficits stemming from these various mechanisms (timing, reward processing, motivation/industriousness, and working memory; see Figure 1) manifest themselves in unique behavioral properties when a choice paradigm is manipulated, potentially resulting in lack of patience, poor valuation of outcomes, weak diligence, or faulty memory processes. This provides the opportunity to discretely deconstruct, evaluate, and target the building blocks of impulsivity, as well as assess individual propensities to succumb to the maladaptive behaviors exacerbated by inherent impulsive tendencies.
Fortunately, copious research advocates that although certain individuals may be inherently more impulsive, more prone to making myopic decisions, and more at risk for developing maladaptive behaviors and health problems, their choice patterns do not have to be stagnant. Quite the contrary, through behavioral interventions that improve timing, reward processing, industriousness, or working memory impulsive choice behavior can be reduced. However, as described above, interspecies and individual discrepancies between the effects of certain behavioral interventions attests to the continuing urgency of further research.
Conclusion
With impulsive choice moderating everything from meal selections to lifelong paths, understanding the mechanisms and components thereof is essential for the overall physical, mental, and economic health of society. And, as current knowledge is still in its infancy, more growth must occur via the analysis of the mechanisms and pathways that affect impulsive choice. Furthering such knowledge will offer new insight into the behavioral interventions used to attenuate impulsive choice and allow for the development of more specifically targeted interventions. Current research should continue to focus on creating interventions tailored to the individual constructs of impulsive choice with the ultimate goal of producing targeted behavioral interventions for preventative and therapeutic use in humans.
References
- Ainslie G, Monterosso JR. Building blocks of self-control: Increased tolerance for delay with bundled rewards. Journal of the Experimental Analysis of Behavior. 2003;79(1):37–48. doi: 10.1901/jeab.2003.79-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behavioural Processes. 2003;64(3):345–354. doi: 10.1016/S0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Allman MJ, Meck WH. Pathophysiological distortions in time perception and timed performance. Brain. 2012;135:656–677. doi: 10.1093/brain/awr210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Diller JW. Effects of acute and repeated nicotine administration on delay discounting in Lewis and Fischer 344 rats. Behavioural Pharmacology. 2010;21(8):754–764. doi: 10.1097/FBP.0b013e328340a050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Woolverton WL. Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacology, Biochemistry and Behavior. 2005;80:387–393. doi: 10.1016/j.pbb.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Balleine B, Killcross S. Effects of ibotenic acid lesions of the nucleus accumbens on instrumental action. Behavioural Brain Research. 1994;65(2):181–193. doi: 10.1016/0166-4328(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Baumann AA, Odum AL. Impulsivity, risk taking, and timing. Behavioural Processes. 2012;90:408–414. doi: 10.1016/j.beproc.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18(1):152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Moody L, Quisenberry A. Computerized working-memory training as a candidate adjunctive treatment for addiction. Alcohol Research Current Reviews. 2014;36(1):123–126. [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Mueller ET. Toward the Study of Trans-Disease Processes: A Novel Approach With Special Reference to the Study of Co-morbidity. Journal of dual diagnosis. 2009;5(2):131–138. doi: 10.1080/15504260902869147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96(1):73–86. doi: 10.1080/09652140020016978. [DOI] [PubMed] [Google Scholar]
- Binder LM, Dixon MR, Ghezzi PM. A procedure to teach self-control to children with attention deficit hyperactivity disorder. Journal of Applied Behavior Analysis. 2000;33(2):233–237. doi: 10.1901/jaba.2000.33-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobova L, Finn PR, Rickert ME, Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Experimental and Clinical Psychopharmacology. 2009;17(1):51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomhower SR, Rasmussen EB, Doherty TS. Impulsive-choice patterns for food in genetically lean and obese Zucker rats. Behavioral Brain Research. 2013;241:214–21. doi: 10.1016/j.bbr.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Black WR, Bruce JM, Daldalian M, Martin LE, Davis AM. Ability to delay gratification and BMI in preadolescence. Obesity (Silver Spring) 2011;19(5):1101–2. doi: 10.1038/oby.2010.297. [DOI] [PubMed] [Google Scholar]
- Buckley JL, Rasmussen EB. Obese and lean Zucker rats demonstrate differential sensitivity to rates of food reinforcement in a choice procedure. Physiology & Behavior. 2012;108:19–27. doi: 10.1016/j.physbeh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Adult obesity facts. Centers for Disease Control and Prevention; 2014. Web page. Last Modified September 9, 2014. [Google Scholar]
- Cheng K. The form of timing distributions in pigeons under penalties for responding early. Animal Learning & Behavior. 1992;20(2):112–120. [Google Scholar]
- Corbit LH, Muir JL, Balleine B. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. The Journal of Neuroscience. 2001;21(9):3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcheville JC, Rivière V, Wearden JH. Fixed-interval performance and self-control in children. Journal of the Experimental Analysis of Behavior. 1992;57(2):187–199. doi: 10.1901/jeab.1992.57-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassen FC, Houben K, Jansen A. Time orientation and eating behavior: Unhealthy eaters consider immediate consequences, while healthy eaters focus on future health. Appetite. 2015;91:13–9. doi: 10.1016/j.appet.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Daugherty James R, Brase Gary L. Taking time to be healthy: Predicting health behaviors with delay discounting and time perspective. Personality and Individual Differences. 2010;48(2):202–207. doi: 10.1016/j.paid.2009.10.007. [DOI] [Google Scholar]
- Dellu-Hagedorn F, Trunet S, Simon H. Impulsivity in youth predicts early age-related cognitive deficits in rats. Neurobiol Aging. 2004;25(4):525–37. doi: 10.1016/j.neurobiolaging.2003.06.006. [DOI] [PubMed] [Google Scholar]
- Dixon MR, Hayes LJ, Binder LM, Manthey S, Sigman C, Zdanowski DM. Using a self-control training procedure to increase appropriate behavior. Journal of Applied Behavior Analysis. 1998;31(2):203–210. doi: 10.1901/jaba.1998.31-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MR, Marley J, Jacobs EA. Delay discounting by pathological gamblers. Journal of Applied Behavior Analysis. 2003;36(4):449–458. doi: 10.1901/jaba.2003.36-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MR, Rehfeldt RA, Randich L. Enhancing tolerance to delayed reinforcers: The role of intervening activities. Journal of Applied Behavior Analysis. 2003;36(2):263–266. doi: 10.1901/jaba.2003.36-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger R, Masterson FA, Lowman K. Effects of previous delay of reward, generalized effort, and deprivation on impulsiveness. Learning and Motivation. 1982;13:378–389. doi: 10.1016/0023-9690(82)90016-9. [DOI] [Google Scholar]
- Eisenberger R, Mitchell M, Masterson FA. Effort training increases generalized self-control. Journal of Personality and Social Psychology. 1985;49:1294–1301. [Google Scholar]
- Eisenberger R, Weir F, Masterson FA, Theis F. Fixed ratio schedules increase generalized self-control: Preference for large rewards despite high effort or punishment. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:383–392. [Google Scholar]
- Eisenberger R, Adornetto M. Generalized Self-Control of Delay and Effort. Journal of Personality and Social Psychology. 1986;51(5):1020–1031. doi: 10.1037//0022-3514.51.5.1020. [DOI] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behavior in rats: the effects of drugs on response choice with varying delays to reinforcement. Psychopharmacology (Berlin) 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual Medical Spending Attributable To Obesity: Payer- And Service-Specific Estimates. Health Affairs. 2009;28(5):W822–W831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- Flores Gonzalo, Wood Graham K, Barbeau David, Quirion Rémi, Srivastava Lalit K. Lewis and Fischer rats: a comparison of dopamine transporter and receptors levels. Brain Research. 1998;814:34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- Friese M, Hofmann W, Wänke M. When impulses take over: moderated predictive validity of explicit and implicit attitude measures in predicting food choice and consumption behaviour. British Journal of Social Psychology. 2008;47:397–419. doi: 10.1348/014466607X241540. [DOI] [PubMed] [Google Scholar]
- Galtress T, Garcia A, Kirkpatrick K. Individual differences in impulsive choice and timing in rats. Journal of the Experimental Analysis of Behavior. 2012;98(1):65–87. doi: 10.1901/jeab.2012.98-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtress T, Kirkpatrick K. The role of the nucleus accumbens core in impulsive choice, timing, and reward processing. Behavioral Neuroscience. 2010;124(1):26–43. doi: 10.1037/a0018464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Lecumberri C, Torres I, Martín S, Crespo JA, Miguéns M, Nicanor C, Higuera-Matas A, Ambrosio E. Strain differences in the dose-response relationship for morphine self-administration and impulsive choice between Lewis and Fischer 344 rats. Journal of Psychopharmacology. 2010;25(6):783–791. doi: 10.1177/0269881110367444. [DOI] [PubMed] [Google Scholar]
- Garcia A, Kirkpatrick K. Impulsive choice behavior in four strains of rats: Evaluation of possible models of Attention-Deficit/Hyperactivity Disorder. Behavioural Brain Research. 2013;238:10–22. doi: 10.1016/j.bbr.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Stankiewicz K, Alberts H, Geschwind N, Martijn C, Jansen A. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite. 2007;49(1):66–73. doi: 10.1016/j.appet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Hammond RA, Levine R. The economic impact of obesity in the United States. Diabetes Metab Syndr Obes. 2010;3:285–95. doi: 10.2147/DMSOTT.S7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris Herbert W, Nestler Eric J. Immunohistochemical studies of mesolimbic dopaminergic neurons in Fischer 344 and Lewis rats. Brain Research. 1996;706:1–12. doi: 10.1016/0006-8993(95)01088-2. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cogn Neuropsychiatry. 2007;12(3):213–21. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson Kelsie L, Rasmussen Erin B, Lawyer Steven R. Measurement and validation of measures for impulsive food choice across obese and healthy-weight individuals. Appetite. 2015;90:254–263. doi: 10.1016/j.appet.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Higuera-Matas A, Montoya GL, Coria SM, Miguens M, Garcia-Lecumberri C, Ambrosio E. Differential gene expression in the nucleus accumbens and frontal cortex in Lewis and Fischer 344 rats relevant to drug addiction. Current Neuropharmacology. 2011;9:143–150. doi: 10.2174/157015911795017290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29(2):298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Jarmolowicz DP, Cherry JBC, Reed DD, Bruce JM, Crespi JM, Lusk JL, Bruce AS. Robust relation between temporal discounting rates and body mass. Appetite. 2014;78:63–67. doi: 10.1016/j.appet.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Myerson J, Hilgard J, Keighley J, Braver TS, Green L. Domain independence and stability in young and older adults’ discounting of delayed rewards. Behavioural Processes. 2011a;87(3):253–259. doi: 10.1016/j.beproc.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Myerson J, Hilgard J, Keighley J, Braver TS, Green L. Domain independence and stability in young and older adults' discounting of delayed rewards. Behav Processes. 2011b;87(3):253–9. doi: 10.1016/j.beproc.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BK, Zauberman G. Perception of anticipatory time in temporal discounting. Journal of Neuroscience, Psychology, and Economics. 2009;2(2):91–101. [Google Scholar]
- Kirby KN. One-year temporal stability of delay-discount rates. Psychonomic Bulletin & Review. 2009;16(3):457–462. doi: 10.3758/Pbr.16.3.457. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K, Marshall AT, Smith AP. Mechanisms of individual differences in impulsive and risky choice in rats. Comparative Cognition & Behavior Reviews. 2015;10:45–72. doi: 10.3819/CCBR.2015.100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick K, Marshall AT, Smith AP, Koci J, Park Y. Individual differences in impulsive and risky choice: Effects of environmental rearing conditions. Behavioural Brain Research. 2014;269(115-127) doi: 10.1016/j.bbr.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus Mikhail N, Jarmolowicz David P, Terry Mueller E, Bickel Warren K. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: A review. Journal of the Experimental Analysis of Behavior. 2013;99(1):32–57. doi: 10.1002/jeab.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Research. 1997;778:418–429. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. Journal of Abnormal Psychology. 2005;114(4):599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Francisco MT, Brewer AT, Stein JS. Delay discounting and gambling. Behavioural Processes. 2011;87(1):43–49. doi: 10.1016/j.beproc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Smith NG, Brewer AT, Pinkston JW, Johnson PS. Steady-state assessment of impulsive choice in Lewis and Fischer 344 rats: between-condition delay manipulations. Journal of the Experimental Analysis of Behavior. 2008;90(3):333–344. doi: 10.1901/jeab.2008.90-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden Gregory J, Bickel Warren K. Impulsivity : the behavioral and neurological science of discounting. 1st. Washington, DC: American Psychological Association; 2010. [Google Scholar]
- Madden Gregory J, Raiff Bethany R, Lagorio Carla H, Begotka Andrea M, Mueller Angela M, Hehli Daniel J, Wegener Ashley A. Delay discounting of potentially real and hypothetical rewards: II. Between- and within-subject comparisons. Experimental and Clinical Psychopharmacology. 2004;12(4):251–261. doi: 10.1037/1064-1297.12.4.251. [DOI] [PubMed] [Google Scholar]
- Marshall AT, Kirkpatrick K. Mechanisms of impulsive choice: III. The role of reward processes. doi: 10.1016/j.beproc.2015.10.013. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AT, Smith AP, Kirkpatrick K. Mechanisms of impulsive choice: I. Individual differences in interval timing and reward processing. Journal of the Experimental Analysis of Behavior. 2014;102(1):86–101. doi: 10.1002/jeab.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Manzanares J, Corchero J, Garcia-Lecumberri C, Crespo JA, Fuentes JA, Ambrosio E. Differential basal proenkaphalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Research. 1999;821:350–355. doi: 10.1016/s0006-8993(99)01122-1. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in childrewn with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(4):377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Mazur JE, Logue AW. Choice in a “self-control” paradigm: Effects of a fading procedure. Journal of the Experimental Analysis of Behavior. 1978;30(1):11–17. doi: 10.1901/jeab.1978.30-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure J, Podos J, Richardson HN. Isolating the delay component of impulsive choice in adolescent rats. Frontiers in Integrative Neuroscience. 2014;8(3):1–9. doi: 10.3389/fnint.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JT, Kable JW. Decision makers calibrate behavioral persistence on the basis of time-interval experience. Cognition. 2012;124(2):216–226. doi: 10.1016/j.cognition.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Underwood B. Instrumental ideation in delay of gratification. Child Dev. 1974;45(4):1083–8. [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berlin) 1999;146(4):455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L. Discounting of delayed rewards: Models of individual choice. Journal of the Experimental Analysis of Behavior. 1995;64:263–276. doi: 10.1901/jeab.1995.64-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarick DJ. Impulsive choice in adults: How consistent are individual differences? The Psychological Record. 1998;48:665–674. [Google Scholar]
- Neef NA, Bicard DF, Endo S. Assessment of impulsivity and the development of self-control in students with attention deficit hyperactivity disorder. Journal of Applied Behavior Analysis. 2001;34(4):397–408. doi: 10.1901/jaba.2001.34-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef NA, Marckel J, Ferreri SJ, Bicard DF, Endo S, Aman MG, Miller KM, Jung S, Nist L, Armstrong N. Behavioral assessment of impulsivity: A comparison of children with and without attention deficit hyperactivity disorder. Journal of Applied Behavior Analysis. 2005;38(1):23–37. doi: 10.1901/jaba.2005.146-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL. Delay discounting: I'm a k, you're a k. Journal of the Experimental Analysis of Behavior. 2011a;96(3):427–439. doi: 10.1901/jeab.2011.96-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL. Delay discounting: Trait variable? Behavioural Processes. 2011b;87(1):1–9. doi: 10.1016/j.beproc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, Baumann AAL. Delay discounting: state and trait variable. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. Washington,DC,: APA Books; 2010. pp. 39–65. [Google Scholar]
- Odum AL. Delay discounting: trait variable? Behav Processes. 2011c;87(1):1–9. doi: 10.1016/j.beproc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JR, Hill CC, Kirkpatrick K. Measurement of impulsive choice in rats: Same- and alternate-form test-retest reliability and temporal tracking. Journal of the Experimental Analysis of Behavior. 2015;103(1):166–179. doi: 10.1002/jeab.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Casarella T. Excessive discounting of delayed rewards in substance abusers with gambling problems. Drug and Alcohol Dependence. 1999;56:25–32. doi: 10.1016/s0376-8716(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Picetti R, Caccavo JA, Ho A, Kreek MJ. Dose escalation and dose preference in extended-access heroin self-administration in Lewis and Fischer rats. Psychopharmacology (Berlin) 2012;220:163–172. doi: 10.1007/s00213-011-2464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H. The science of self-control. Cambridge, MA: Harvard University Press; 2000. [Google Scholar]
- Rasmussen EB, Lawyer SR, Reilly W. Percent body fat is related to delay and probability discounting for food in humans. Behavioural Processes. 2010;83(1):23–30. doi: 10.1016/j.beproc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Renda CR, Stein JS, Madden GJ. Working-memory training: effects on delay discounting in male Long Evans rats. Journal of the Experimental Analysis of Behavior. 2015;103(1):50–61. doi: 10.1002/jeab.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. 2010;55(3):420–5. doi: 10.1016/j.appet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Marshall AT, Kirkpatrick K. Mechanisms of impulsive choice: II. Time-based interventions to improve self-control. Behavioural Processes. 2015;112:29–42. doi: 10.1016/j.beproc.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Johnson PS, Renda CR, Smits RR, Liston KJ, Shahan TA, Madden GJ. Early and prolonged exposure to reward delay: Effects on impulsive choice and alcohol self-administration in male rats. Experimental and Clinical Psychopharmacology. 2013;21(2):172–180. doi: 10.1037/a0031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Renda CR, Hinnenkamp JE, Madden GJ. Impulsive choice, alcohol consumption, and pre-exposure to delayed rewards: II. Potential mechanisms. Journal of the Experimental Analysis of Behavior. 2015;103(1):33–49. doi: 10.1002/jeab.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Smits RR, Johnson PS, Liston KJ, Madden GJ. Effects of reward bundling on male rats' preference for larger-later food rewards. Journal of the Experimental Analysis of Behavior. 2013;99(2):150–158. doi: 10.1002/jeab.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, George FR, Meisch RA. Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. Journal of Pharmacology and Experimental Therapeutics. 1988;245:164–170. [PubMed] [Google Scholar]
- Takahashi T. Loss of self-control in intertemporal choice may be attributable to logarithmic time-perception. Medical Hypotheses. 2005;65:691–693. doi: 10.1016/j.mehy.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Oono H, Radford MHB. Psychophysics of time perception and intertemporal choice models. Physica A: Statistical and Theoretical Physics (Amsterdam) 2008;387:2066–2074. doi: 10.1016/j.physa.2007.11.047. [DOI] [Google Scholar]
- van den Broek MD, Bradshaw CM, Szabadi E. Performance of impulsive and non-impulsive subjects on two temporal differentiation tasks. Personality and Individual Differences. 1992;13(2):169–174. doi: 10.1016/0191-8869(92)90039-R. [DOI] [Google Scholar]
- Walton ME, Kennerley SW, Bannerman DM, Phillips PEM, Rushworth MFS. Weighing up the benefits of work: Behavioral and neural analyses of effort-related decision making. Neural Networks. 2006;19:1302–1314. doi: 10.1016/j.neunet.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity. 2008;16(10):2323–2330. doi: 10.1038/Oby.2008.351. [DOI] [PubMed] [Google Scholar]
- Weller RE, Cook EW, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51(3):563–569. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trends in Cognitive Sciences. 2008;12(1):7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Wittmann Marc, Paulus Martin P. Temporal horizons in decision making. Journal of Neuroscience, Psychology, and Economics. 2009;2(1):1–11. doi: 10.1037/a0015460. [DOI] [Google Scholar]
- Zauberman G, Kim BK, Malkoc SA, Bettman JR. Discounting Time and Time Discounting: Subjective Time Perception and Intertemporal Preferences. Journal of Marketing Research. 2009;46(4):543–556. [Google Scholar]