Abstract
Pretreatment characteristics are suggested as predictive and/or prognostic factors for nasopharyngeal carcinoma (NPC); however, individual tumor radiosensitivities have previously not been considered. As boost planning is recommended for NPC, we performed interim assessments of magnetic resonance (MR) images for boost planning and retrospectively evaluated their predictive value for the survival of NPC patients. Radiation therapy via elective nodal irradiation (median dose: 39.6 Gy) with/without chemotherapy was used to treat 63 NPC patients. Boost irradiation (median total dose: 70 Gy) was performed based on the interim assessment. The largest lymph node (LN) was measured on MR images acquired at the time of interim assessment. The site of first failure was local in 8 (12.7%), regional in 7 (11.1%), and distant in 12 patients (19.0%). All 7 patients with regional failure harbored LNs ≥15 mm at interim assessment. We divided the 63 patients into two groups based on LN size [large (≥15 mm), n = 10 and small (<15 mm), n = 53]. Univariate analysis showed that 5-year overall survival (OS) and cause-specific survival (CSS) rates for large LNs were significantly lower than for small LNs (OS: 12.5% vs 70.5%, P < 0.001 and CSS: 25.0% vs 80.0%, P < 0.001). Multivariate analysis showed that large LNs were a significantly unfavorable factor for both OS (hazard ratio = 4.543, P = 0.002) and CSS (hazard ratio = 6.020, P = 0.001). The results suggest that LN size at interim assessment could predict survival in NPC patients.
Keywords: nasopharyngeal carcinoma, radiation therapy, lymph node metastasis, adaptive radiation therapy, predictive factor, tailored therapy
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is a malignant entity distinct from other head and neck cancers and may be associated with Epstein–Barr virus (EBV) infection. Most NPCs are poorly differentiated or undifferentiated radiosensitive carcinomas. Because of its anatomic characteristics, NPC is rarely treated surgically; radiation therapy (RT) is generally delivered with or without chemotherapy [1, 2].
In patients with head and neck cancer, RT elicits substantial anatomic changes due to tumor shrinkage, decreased salivary gland volume, and body weight loss [3, 4]. Because these changes occur during the first 3–4 weeks of treatment and affect the dose distribution to the target volume and organs at risk (OARs), replanning based on repeated computed tomography (CT) simulations at 30–40 Gy for boost irradiation is recommended, particularly for patients with NPC [3–5]. For RT planning, particularly high-precision RT used to treat NPC, such as 3D conformal RT (3D-CRT) and intensity-modulated RT (IMRT), the target volume must be clearly identified. Magnetic resonance (MR) imaging, which has excellent soft tissue contrast resolution, facilitates accurate tumor delineation and is, therefore, an essential imaging modality for treatment planning [6]. Accordingly, we routinely use interim assessments of NPCs on MR images for boost planning.
Although the standard NPC treatment is RT at 66–70 Gy with cisplatin-based chemotherapy, more intensive treatment options such as RT dose escalation and adjuvant chemotherapy have been recommended [2, 7, 8]. However, these aggressive options have adverse effects and should only be used in selected patients with poor outcomes. Age, performance status (PS), histology, and TNM stage are suggested as predictive and/or prognostic factors for NPC [9–11]; however, individual tumor radiosensitivities have previously not been considered. Prediction of the treatment outcomes during RT through interim assessments may facilitate treatment modifications based on tumor radiosensitivity.
The predictive value of interim assessment in patients with head and neck cancer has been reported. According to Jaulerry et al. [12], tumor regression at 55 Gy is an independent predictive factor of local control, whereas Ohizumi et al. [13] found a locoregional response at 40 Gy to independently predict locoregional control. However, these reports were mainly based on clinical and endoscopic examinations and may have lacked objectivity. Hong et al. [14], who evaluated the predictive value of MR imaging for NPC, found that fractional changes in the apparent diffusion coefficient of the primary lesion from pretreatment to 2 weeks after RT initiation could predict the presence of a residual tumor 3 months after the end of RT. However, its predictive value for survival was not assessed. Here we investigated the value of interim assessment for predicting survival in patients with NPC.
MATERIALS AND METHODS
Patients
This retrospective study was approved by the institutional review board of the Kumamoto University Hospital. Prior informed consent for treatment was obtained from all patients. Between February 1996 and October 2012, 66 patients with pathologically diagnosed NPC who had undergone pretreatment physical, endoscopic and radiological examinations were treated with RT with or without chemotherapy. Of these, 63 were included in this study (Table 1); 3 patients were excluded because of distant metastasis at initial diagnosis, neck dissection for diagnostic purposes before treatment, or coexisting thyroid cancer. The remaining patients were staged or re-staged according to the 7th edition of the Union for International Cancer Control.
Table 1.
Patient characteristics (n = 63)
| Characteristics | n | (%) |
|---|---|---|
| Age (years) | Median 63 (range 17–85) | |
| Gender | ||
| Male | 49 | 78 |
| Female | 14 | 22 |
| WHO histology | ||
| Type I | 10 | 16 |
| Type II | 25 | 40 |
| Type III | 28 | 44 |
| ECOG PS | ||
| 0 | 25 | 40 |
| 1 | 31 | 49 |
| 2 | 7 | 11 |
| T stage | ||
| T1 | 7 | 11 |
| T2 | 24 | 38 |
| T3 | 6 | 10 |
| T4 | 26 | 41 |
| N stage | ||
| N0 | 13 | 21 |
| N1 | 13 | 21 |
| N2 | 31 | 49 |
| N3 | 6 | 10 |
| Clinical stage | ||
| I | 2 | 3 |
| II | 11 | 17 |
| III | 21 | 33 |
| IVA–IVB | 29 | 46 |
WHO = World Health Organization, ECOG = Eastern Cooperative Oncology Group, PS = performance status.
Radiological examinations
Pretreatment examinations included ultrasonography (US), MR imaging, CT and/or 2-deoxy-2-[18F]fluoro-D-glucose (FDG)-positron emission tomography (PET)/CT. MR studies were performed before RT and before boost irradiation on a 1.5-T MR scanner (Magnetom Symphon; Siemens, Erlangen, Germany). A neck coil was used for pretreatment, interim, and post-treatment assessments. Axial T1-weighted spin-echo (SE) [repetition time (TR)/echo time (TE): 600–670/14–17], T2-weighted fast SE (TR/TE: 3700–4000/96–102), and triplanar contrast-enhanced T1-weighted SE sequences were obtained after bolus injection of gadopentetate dimeglumine (0.1 mmol/kg body weight; Magnevist; Bayer HealthCare Pharmaceuticals, Osaka, Japan). The MR images were acquired at a section thickness of 4 mm and an intersection gap of 0.8 mm. All patients were instructed to breathe quietly and refrain from coughing or swallowing.
Treatment
Of the 63 patients, 52 received chemoradiotherapy (CRT), whereas 11 underwent RT alone (because of old age in 7, impaired renal function in 2, hepatitis in 1, and patient's refusal in 1). Chemotherapy consisted of 1–3 cycles of cisplatin and 5-FU [cisplatin 50–70 mg/m2/d intravenously (i.v.) for 1–2 days, 5-FU 500–800 mg/m2/d i.v. for 4–5 days]. Elective nodal irradiation was delivered to the upper or whole neck at a median dose of 39.6 Gy. Interim MR assessment was performed before boost irradiation (i.e. 4 weeks after RT initiation). Based on this assessment, boost irradiation (median total dose: 70 Gy) was delivered to the primary lesion and suspicious metastatic lymph nodes (LNs). Of the 63 patients, 43 underwent 3D-CRT and 20 received IMRT. Post-RT neck dissection, defined as neck dissection within 6 months after RT, was performed in 9 patients with suspicious residual LN metastases, despite a complete response (CR) of the primary lesion.
Interim assessment
During the interim assessment, we recorded our findings based on the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1). CR was defined as the disappearance of all target lesions and reduction in the short axis of any pathological (≥10 mm) LNs to <10 mm, partial response (PR) as a ≥30% decrease in the summed diameter of the target lesions, progressive disease (PD) as a ≥20% increase in the summed diameter of the target lesions, and stable disease (SD) as insufficient shrinkage or increase to qualify as PR or as PD. Because pretreatment LN images were not available for some patients, we defined CR as disappearance of the primary lesion and a shortening of the short axes of all LNs to <10 mm. Non-CR was recorded when CR criteria were not fulfilled. Only the treatment responses of primary lesions were assessed based on RECIST criteria [15]. Because no precise criteria for CR of the LNs are available, we measured the short axis of the largest LN.
Follow-up
After treatment completion, patients were evaluated every 1–2 months during the first year, every 3 months during the second year, and every 6 months thereafter. All patients underwent physical and endoscopic examinations at each follow-up visit. Post-treatment US, MR imaging, and/or CT studies were performed within 1–2 months after treatment completion and every 6 months thereafter or when clinically indicated. A diagnosis of treatment failure was based on the results of follow-up studies or neck dissection specimens indicating persistent disease, relapse, or disease progression. Failures were classified as involving the primary lesion (local), neck LN metastases (regional), or distant metastases (distant).
Statistical analysis
Overall survival (OS), cause-specific survival (CSS), local control (LC), regional control (RC), and distant control (DC) rates were calculated from treatment initiation using the Kaplan–Meier method. Candidate variables for predictive factors of survival, including age, World Health Organization histology, Eastern Cooperative Oncology Group performance status, T stage, N stage, chemotherapy, radiation type, interim assessment results based on RECIST, interim assessment of the primary lesion, and interim LN size were evaluated using log-rank statistics. Multivariate analyses were performed to test the independent significance of variables, using the Cox proportional hazards model. Differences with P-values <0.05 were considered statistically significant. Statistical calculations were performed with SPSS software, version 22.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
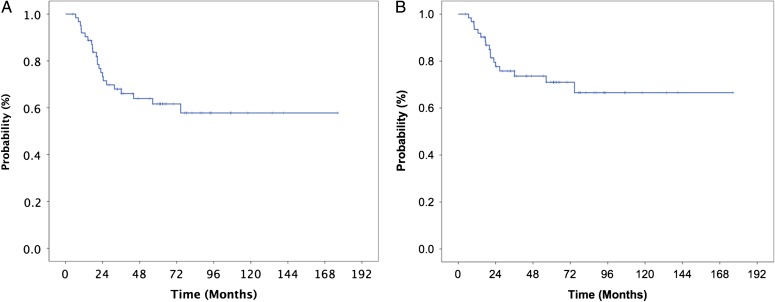
The median follow-up duration was 43.5 (range: 4.6–176.4) months. During the follow-up period, 36 (57.1%) patients experienced tumor control and 27 (42.9%) suffered treatment failure. The 5-year OS and CSS rates were 61.6% and 71.0%, respectively (Fig. 1). The site of first failure was local in 8 (12.7%), regional in 7 (11.1%), and distant in 12 (19.0%) patients. In the 7 patients with regional failure, the LN size was ≥15 mm at the interim assessment. Among 9 patients who underwent post-RT neck dissection for their suspicious residual LN metastases, a LN ≥15 mm at the interim assessment was observed in 6 (66.7%) patients. The current study did not show the benefit of post-RT neck dissection on the outcome; 6 (66.7%) suffered treatment failure.
Fig. 1.
Overall (A) and cause-specific (B) survival curves for patients with nasopharyngeal carcinoma treated with radiation therapy and with or without chemotherapy.
We divided the patients into large and small LN groups (largest LN ≥ 15 mm, n = 10 and smallest LN < 15 mm, n = 53). Among 10 patients of the large LN group, at initial diagnosis, 9 patients were classified as N2 and 1 as N3. A univariate analysis found that age, PS, chemotherapy, and interim LN size were significant variables for OS, and that PS, chemotherapy, and interim LN size were significant for CSS (Table 2). PS, chemotherapy, and interim LN size remained independent variables for both OS and CSS in a multivariate analysis (Table 3, Fig. 2).
Table 2.
Univariate analysis of the survival of 63 patients with nasopharyngeal carcinoma
| Variable | n | 5-year OS (%) | P-value | 5-year CSS (%) | P-value |
|---|---|---|---|---|---|
| Age | |||||
| <65years | 32 | 83.3 | 0.004 | 83.3 | 0.096 |
| ≥65years | 31 | 39.6 | 55.9 | ||
| Histology (WHO) | |||||
| Type I | 10 | 60.0 | 0.785 | 60.0 | 0.358 |
| Type II–III | 53 | 62.0 | 73.2 | ||
| ECOG PS | |||||
| 0–1 | 56 | 66.1 | <0.001 | 75.1 | 0.001 |
| 2 | 7 | 28.6 | 33.3 | ||
| T stage | |||||
| 1–2 | 31 | 66.3 | 0.220 | 75.8 | 0.170 |
| 3–4 | 32 | 57.4 | 67.0 | ||
| N stage | |||||
| 0 | 13 | 64.8 | 0.926 | 81.5 | 0.448 |
| 1–3 | 50 | 61.4 | 69.0 | ||
| N stage | |||||
| 0–2 | 57 | 61.0 | 0.789 | 71.5 | 0.819 |
| 3 | 6 | 66.7 | 66.7 | ||
| Chemotherapy | |||||
| Yes | 52 | 71.8 | <0.001 | 78.2 | 0.010 |
| No | 11 | 0 | 32.0 | ||
| Radiation type | |||||
| 3D-CRT | 43 | 59.7 | 0.470 | 72.7 | 0.926 |
| IMRT | 20 | 63.4 | 63.4 | ||
| Interim assessment by RECIST | |||||
| CR | 7 | 71.4 | 0.518 | 100 | 0.108 |
| Non-CR | 56 | 60.1 | 67.3 | ||
| Interim assessment of primary lesion | |||||
| CR | 10 | 70.0 | 0.633 | 87.5 | 0.346 |
| PR | 33 | 60.8 | 69.3 | ||
| SD | 20 | 58.7 | 66.9 | ||
| Interim assessment of primary lesion | |||||
| CR + PR | 43 | 62.5 | 0.451 | 72.4 | 0.431 |
| SD | 20 | 58.7 | 66.9 | ||
| Interim LN size | |||||
| <10 mm | 32 | 69.8 | <0.001 | 78.8 | <0.001 |
| ≥10 mm to <15 mm | 21 | 73.0 | 82.5 | ||
| ≥15 mm | 10 | 12.5 | 25.0 | ||
| Interim LN size | |||||
| Small group (<15 mm) | 53 | 70.5 | <0.001 | 80.0 | <0.001 |
| Large group (≥15 mm) | 10 | 12.5 | 25.0 | ||
| Total | 63 | 61.6 | 71.0 | ||
OS = overall survival, CSS = cause-specific survival, WHO = World Health Organization, ECOG = Eastern Cooperative Oncology Group, PS = performance status, 3D-CRT = 3D conformal radiation therapy, IMRT = intensity-modulated radiation therapy, CR = complete response, PR = partial response, SD = stable disease, LN = lymph node.
Table 3.
Multivariate analysis of the survival of 63 patients with nasopharyngeal carcinoma
| Variable | n | OS | CSS | ||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Age | |||||
| <65 years | 32 | 1 | 0.172 | NA | |
| ≥65 years | 31 | 2.025 (0.736–5.572) | |||
| ECOG PS | |||||
| 0–1 | 56 | 1 | 0.018 | 1 | 0.024 |
| 2 | 7 | 3.938 (1.264–12.274) | 4.560 (1.218–17.071) | ||
| Chemotherapy | |||||
| Yes | 52 | 1 | 0.008 | 1 | 0.040 |
| No | 11 | 3.487 (1.376–8.832) | 3.153 (1.057–9.409) | ||
| Interim LN size | |||||
| Small group (<15 mm) | 53 | 1 | 0.002 | 1 | 0.001 |
| Large group (≥15 mm) | 10 | 4.543 (1.764–11.699) | 6.020 (2.118–17.112) | ||
| Total | 63 | ||||
OS = overall survival, CSS = cause-specific survival, HR = hazard ratio, CI = confidence interval, ECOG = Eastern Cooperative Oncology Group, PS = performance status, LN = lymph node, NA = not applicable.
Fig. 2.
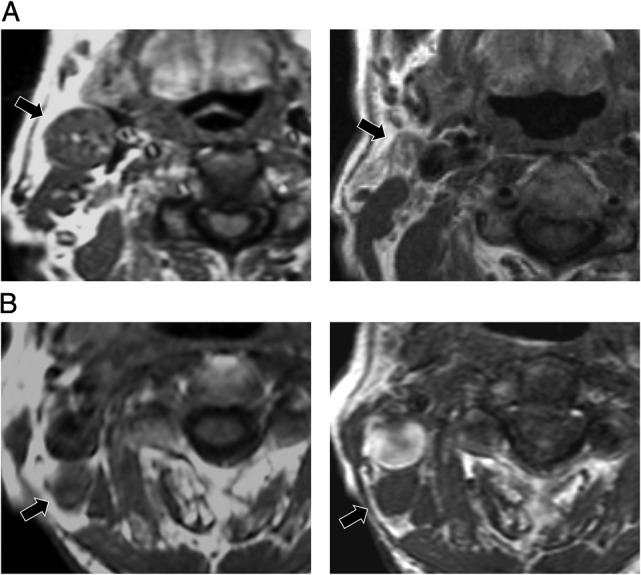
Pretreatment and interim magnetic resonance (MR) images of two representative patients with T4N2M0 Stage IVA nasopharyngeal carcinoma. Their lymph nodes (LNs) were similarly sized before treatment. (A) The largest pretreatment LN was 24 mm (left). This LN measured 11 mm at the interim assessment (right). This patient remains alive without recurrence 62 months after therapy. (B) The largest pretreatment LN was 22 mm (left). This LN measured 19 mm at the interim assessment (right). This patient suffered multiple regional failures and died 9 months after treatment initiation.
The site of overall failure was local in 10 (15.9%), regional in 7 (11.1%) and distant in 15 (23.8%) patients. The 5-year LC rate was 84.6%; there were no significant differences between patients with large and small LNs (77.1% vs 86.0%, P = 0.485). The overall 5-year RC and DC rates were 87.1% and 72.4%, respectively; they were significantly lower among patients with large LNs than among those with small LNs (RC: 0% vs 100%, P < 0.001 and DC: 31.1% vs 78.7%, P = 0.005).
DISCUSSION
Our study, which was based on long-term follow-up data, revealed that the LN size measured during the interim assessment was predictive of treatment outcomes in patients with NPC. Our findings are similar to those of Jang et al. [15], who reviewed 50 patients with node-positive head and neck cancer, including 25 patients with NPC who underwent RT, and found that a post-RT nodal size >15 mm was associated with poor RC. Our study further suggests that the LN size at interim assessment predicts not only RC but also survival. Accordingly, interim assessment could be used as a predictive surrogate imaging marker of survival in patients with NPC. In addition, the risks of distant failure and poor survival have been more strongly correlated with LN metastasis than with the primary lesion [16]. Our findings similarly suggest a significant association between large LNs and poor DC. Large LNs appear to reflect poor tumor radiosensitivity and a strong potential for distant metastasis, resulting in poor survival. As previously reported [1, 2, 8], our results also suggest the significant survival benefit of the addition of chemotherapy to RT. The use of chemotherapy might be essential for reduced distant metastasis, which improves the survival of NPC patients.
Meanwhile, the treatment response of the primary lesion at interim assessment was not found to predict survival. Until the 1990s, NPC was evaluated using conventional 2D RT. At this time, the LC of patients with NPC was poor, with rates of 75–85% at disease Stages T1 and T2 and 40–65% at Stages T3 and T4, and the primary lesion status was considered to be among the most important factors for survival [17, 18]. Recent advances in irradiation techniques for 3D-CRT and IMRT provide highly conformal dose distribution to the planning target volume while minimizing doses to the OAR, resulting in dose escalation to the target volume and excellent tumor control, with a reported LC of 80–95%, regardless of T stage [16, 19]. Early results of these treatments suggested that the primary lesion has no significant impact on survival [16]. Because of the improvements in LC, which are attributable to advanced RT techniques, the impact of the primary lesion on survival appears to be decreasing. Similarly, RECIST, which is generally used for post-treatment assessment and requires evaluation of multiple target lesions, cannot predict survival outcomes. In addition to the decreasing impact of the primary lesion on survival, the cut-off value of 10 mm for LN size appears inadequate for predicting survival at interim assessment. van den Brekel et al. [20] used dissected specimens to evaluate the accuracy of radiological criteria for detecting cervical LN metastases in patients with head and neck cancer. They reported that the rates of metastasis and non-metastasis in LNs with sizes of 11–15 mm were 75% and 25%, respectively, indicating that non-metastatic LNs may be larger than 10 mm during treatment.
Patients with head and neck cancer were subjected to prediction of treatment outcomes at different time points during RT at doses of 20–55 Gy [12–14]. It may be too late to change the radiation dose if assessments are performed at doses exceeding 50 Gy because the NPC target volume is surrounded by several radiosensitive structures (e.g. spinal cord, brain stem, optic chiasm, optic nerves, retina, and lenses) with tolerance doses ranging from 10 to 50 Gy. In addition, because significant anatomical changes occur during the first 3–4 weeks of NPC treatment [3, 4], MR imaging at a dose of ~40 Gy appears appropriate for both interim assessments and changes in therapeutic strategies.
As MR imaging has better soft tissue contrast resolution than CT, it is essential for boost planning [6]. The use of MR imaging at interim assessment provides information about anatomical changes and prediction of survival in one examination. Our method of predicting survival is easy and practical because it does not require the evaluation of multiple target lesions or the addition of imaging studies to routine MR studies for boost planning. Based on the interim assessment findings, RT dose escalation and/or adjuvant chemotherapy should be considered for patients with NPC who have a poor prognosis [2, 7, 8].
Our study has some limitations, including the retrospective design, relatively small number of patients, and variability in RT doses, radiation types, and chemotherapy regimens. We also cannot rule out physician bias with respect to post-RT neck dissection. These factors may affect treatment outcomes. In addition, we divided the interim responses, recorded based on RECIST, into CR and non-CR because no pretreatment LN images were available for some patients. This categorization may have resulted in a failure to detect patients with poor outcomes. Further investigations are underway to address this issue.
In conclusion, the interim assessment of LNs provides information that may serve as a surrogate imaging marker predictive of treatment outcomes in patients with NPC, specifically that a large LN size at interim assessment is predictive of an unfavorable survival outcome.
ACKNOWLEDGEMENTS
This work was presented in part at the 56th Annual Meeting of the American Society for Radiation Oncology (ASTRO), San Francisco, CA, held 14–16 September 2014, and at the 15th Asian Oceanian Congress of Radiology (AOCR), Kobe, Japan, held 24–28 September 2014.
FUNDING
This work was supported by JSPS KAKENHI Grant Number 25461918.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
- 1.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet 2005;365:41–54. [DOI] [PubMed] [Google Scholar]
- 2.Lee AW, Ng WT, Chan YH, et al. . The battle against nasopharyngeal cancer. Radiother Oncol 2012;104:272–8. [DOI] [PubMed] [Google Scholar]
- 3.Barker JL Jr, Garden AS, Ang KK, et al. . Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys 2004;59:960–70. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz DL, Garden AS, Shah SJ, et al. . Adaptive radiotherapy for head and neck cancer—dosimetric results from a prospective clinical trial. Radiother Oncol 2013;106:80–4. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura Y, Shibata T, Nakamatsu K, et al. . A two-step intensity-modulated radiation therapy method for nasopharyngeal cancer: the Kinki University experience. Jpn J Clin Oncol 2010;40:130–8. [DOI] [PubMed] [Google Scholar]
- 6.Lai V, Khong PL. Updates on MR imaging and F-FDG PET/CT imaging in nasopharyngeal carcinoma. Oral Oncol 2013;50:539–48. [DOI] [PubMed] [Google Scholar]
- 7.Kwong DL, Sham JS, Leung LH, et al. . Preliminary results of radiation dose escalation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2006;64:374–81. [DOI] [PubMed] [Google Scholar]
- 8.Lee AW, Tung SY, Ngan RK, et al. . Factors contributing to the efficacy of concurrent–adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer 2011;47:656–66. [DOI] [PubMed] [Google Scholar]
- 9.Toh CK, Heng D, Ong YK, et al. . Validation of a new prognostic index score for disseminated nasopharyngeal carcinoma. Br J Cancer 2005;92:1382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy SP, Raslan WF, Gooneratne S, et al. . Prognostic significance of keratinization in nasopharyngeal carcinoma. Am J Otolaryngol 1995;16:103–8. [DOI] [PubMed] [Google Scholar]
- 11.Xu L, Pan J, Wu J, et al. . Factors associated with overall survival in 1706 patients with nasopharyngeal carcinoma: significance of intensive neoadjuvant chemotherapy and radiation break. Radiother Oncol 2010;96:94–9. [DOI] [PubMed] [Google Scholar]
- 12.Jaulerry C, Dubray B, Brunin F, et al. . Prognostic value of tumor regression during radiotherapy for head and neck cancer: a prospective study. Int J Radiat Oncol Biol Phys 1995;33:271–9. [DOI] [PubMed] [Google Scholar]
- 13.Ohizumi Y, Tamai Y, Imamiya S, et al. . Prediction of tumor control by tumor regression at 40 Gy/4 weeks of external beam irradiation for oropharyngeal carcinoma. Radiat Med 2004;22:324–31. [PubMed] [Google Scholar]
- 14.Hong J, Yao Y, Zhang Y, et al. . Value of magnetic resonance diffusion-weighted imaging for the prediction of radiosensitivity in nasopharyngeal carcinoma. Otolaryngol Head Neck Surg 2013;149:707–13. [DOI] [PubMed] [Google Scholar]
- 15.Jang NY, Lee KW, Ahn SH, et al. . Neck control after definitive radiochemotherapy without planned neck dissection in node-positive head and neck cancers. BMC Cancer 2012;12:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S, Pan J, Han L, et al. . Nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the 3-year outcome of a prospective series. Int J Radiat Oncol Biol Phys 2009;75:1071–8. [DOI] [PubMed] [Google Scholar]
- 17.Perez CA, Devineni VR, Marcial-Vega V, et al. . Carcinoma of the nasopharynx: factors affecting prognosis. Int J Radiat Oncol Biol Phys 1992;23:271–80. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita S, Kondo M, Hashimoto S. Squamous cell carcinoma of the nasopharynx. An analysis of failure patterns after radiation therapy. Acta Radiol Oncol 1985;24:315–20. [DOI] [PubMed] [Google Scholar]
- 19.Luo W, Ye L, Yu Z, et al. . Effectiveness of three-dimensional conformal radiotherapy for treating early primary nasopharyngeal carcinoma. Am J Clin Oncol 2010;33:604–8. [DOI] [PubMed] [Google Scholar]
- 20.van den Brekel MW, Stel HV, Castelijns JA, et al. . Cervical lymph node metastasis: assessment of radiologic criteria. Radiology 1990;177:379–84. [DOI] [PubMed] [Google Scholar]