Abstract
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcriptional factor that regulates many antioxidants, and we have recently succeeded in obtaining a novel Nrf2 activator, RS9, from microbial transformation. RS9 is categorized as a triterpenoid, and well-known triterpenoids such as RTA 402 (bardoxolone methyl) and RTA 408 have been tested in clinical trials. RTA 408 lotion is currently being tested in patients at risk for radiation dermatitis. This prompted us to study the profiles of RS9 in the skin. All the above triterpenoids increased the level of an Nrf2-targeted gene, NADPH:quinone oxidoreductase-1, in normal human epidermal keratinocytes. Among them, the activity of RS9 was prominent; furthermore, the cellular toxicity was less compared with RTA compounds. BALB/c mice were irradiated with 30 Gy/day on Day 0, and compounds were topically applied on the back once daily from Day 1 to Day 30. Dermatitis scores peaked on Day 18, with a score of 2.6 in vehicle-treated mice, and topical applications of 0.1% RTA 402, RTA 408 and RS9 reduced the scores to 1.8, 2.0 and 1.4, respectively. Moreover, the percentage of animals with scores ≥2 was analyzed, and 0.1% RS9 suppressed the percentage from 100% to 47%. These results imply that RS9 has potential efficacy for treating radiation dermatitis.
Keywords: Nrf2, RS9, microbial transformation, radiation dermatitis, antioxidant
INTRODUCTION
Nuclear factor erythroid 2-related factor 2 (Nrf2) is expressed in many tissues, particularly those that are exposed to the external environment (skin, lungs and gastrointestinal tract) and those associated with detoxification (liver and kidney) [1]. Under the basal resting condition, Nrf2 is trapped within the cytosol by an adaptor protein, Kelch-like ECH-associated protein 1 (Keap1). Several cysteine residues in Keap1 serve as the primary sensors for stress signals. This interaction disrupts the Nrf2–Keap1 complex, and liberated Nrf2 is then translocated to the nucleus. The liberated Nrf2 binds to antioxidant response elements, which leads to the induction of many antioxidant enzymes and phase II detoxifying enzymes [2]. Activation of the signaling is expected to rescue cells from a variety of reactive toxicants, pro-inflammatory stimuli, etc. In addition, Nrf2-deficient mice exhibit profound sensitivity to chemical and biological stress in multiple target organs.
Some synthetic triterpenoids are known to react with the cysteine of Keap1 as Michael acceptors and thereby activate the Keap1–Nrf2 signaling [3, 4]. One of the triterpenoids, RTA 402 [2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) methyl ester/CDDO-Me/bardoxolone methyl], was tested as a therapy for chronic kidney disease (CKD) with type 2 diabetes [5]; the Bardoxolone Methyl Treatment: Renal Function in CKD/Type 2 Diabetes (BEAM) Phase 2 trial evaluated the safety and efficacy in patients with moderate (Stage 3b) to severe (Stage 4) CKD associated with type 2 diabetes. Patients receiving RTA 402 showed an increase in the estimated glomerular filtration rate at 24 weeks, and the improvement persisted at 52 weeks. However, a higher cardiovascular mortality in the treatment arm in Phase 3 caused the Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Mellitus: the Occurrence of Renal Events (BEACON) study to be terminated in 2012 [6]. The controversy has not been fully settled concerning whether both the efficacy and adverse events are the results of Nrf2 activation. Another compound, RTA 408, is currently being tested in lotion form in clinical trials for radiation-induced dermatitis with breast cancer (ClinicalTrials.gov Identifier: NCT02142959) [7]. We have also identified a novel potent Nrf2 activator, RS9, using a biotransformation technique, and demonstrated that RS9 inhibits blood–retinal barrier permeability in rabbits [8].
Acute radiation damage to the skin is manifested by early erythema and desquamation, in part due to depopulation of the acutely responding basal epithelial cells. Accumulated data indicate that the injury is initiated by stimulation of the innate immune response and oxidative stress [9]. It is well accepted that ionizing radiation induces damage by generating reactive oxygen species such as peroxides, superoxide and the highly reactive hydroxyl and hydrogen radicals. This might explain how application of Nrf2 activators might alleviate radiation damage. In consideration of the above information, in this study we compared the efficacy of RTA 402, RTA 408 and RS9 in a radiation dermatitis model.
MATERIALS AND METHODS
Animals and reagents
Ten-week-old female BALB/c mice were purchased from Japan SLC (Shizuoka, Japan). Experimental procedures were performed in accordance with the in-house guideline of the Institutional Animal Care and Use Committee of Daiichi Sankyo. All animals received a standard laboratory diet and filtered water ad libitum. The following cells and medium-related materials were purchased: normal human epidermal keratinocytes (NHEKs, adult), HuMedia-KB2 (cat. No. KK-6150) and Humedia-KG2 (cat. No. KK-2150S) from Kurabo Industries (Osaka, Japan). RTA 402 and RTA 408 were synthesized, and RS9 was purified from fermentation products at Daiichi Sankyo RD Novare Co. The following materials were purchased: RNeasy® Fibrous Tissue Mini Kit from Qiagen (Valencia, CA, USA); First-Strand cDNA Synthesis Kit from GE Healthcare (Buckinghamshire UK); TaqMan® Universal PCR Master Mix, TaqMan® Gene Expression Assays for human Nqo1 (NADPH:quinone oxidoreductase-1, ID: Hs02512143_s1), human Actb (beta-actin, ID: Hs0160665_g1), mouse nqo1 (ID: Mm01253561_m1), mouse gclm (glutamate-cysteine ligase, modifier subunit, ID: Mm00514996_m1), mouse hmox1 (heme oxygenase 1, ID: Mm00516005_m1), mouse actb (ID: m01205647_g1), mouse ccl2 (chemokine (C-C motif) ligand 2, ID: Mm00441242_m1), mouse il1b (interleukin 1 beta, ID: Mm00434228_m1) and mouse il6 (interleukin 6, ID: Mm00446190_m1) from Thermo Fisher Scientific (Waltham, MA, USA); LDH-Cytotoxic Test (LDH: lactate dehydrogenase) from Wako Pure Chemical Industries (Osaka, Japan); and sesame oil and Triton-X100 from Sigma-Aldrich (St Louis, MO, USA).
Measurement of Nrf2-targeted genes in NHEKs
NHEKs were cultured in HuMedia-KB2 supplemented with Humedia-KG2 containing epidermal growth factor insulin, hydrocortisone, and bovine pituitary extract. Cells were plated in 24-well plates at 25 000 cells/well with 750 μl of HuMedia-KB2 (12 500 cells/cm2) and cultured for 4 days. The medium was changed to fresh medium containing compounds and then maintained for 6 h. The cells were used for collecting mRNA, and cDNA was synthesized from the mRNA. A quantitative polymerase chain reaction (PCR) was performed using the Real-time PCR HT7900 (Thermo Fisher Scientific), and the threshold cycle (Ct) of each transcript was normalized to the average Ct for housekeeping genes. Fold differences were determined by the 2−ΔΔCt method.
An LDH assay
NHEKs were plated in 96-well plates at 10 000 cells/well in 100 μl of medium and cultured for 24 h. The medium was changed to fresh medium containing compounds and then maintained for another 24 h. The amount of LDH released reflects the degree of plasma membrane damage in cells, and was measured according to the manufacture's manual using 50 μl of supernatant. Cell lysate was prepared by adding 0.8% (w/v) Triton-X100 and this value was normalized to 100%.
Radiation-induced dermatitis
On Day −1, the skin on the back was shaved by an animal shaver. On Day 0, mice were deeply anesthetized by intraperitoneal injection (ketamine 25 mg/kg and medetomidine 0.1 mg/kg), and dermatitis was induced by a one-time radiation dose of 30 Gy to a circle area (1 cm diameter) on the back. A lead shield was employed to prevent radiation outside the targeted area, and a radiation probe was set at the same height as the back of mice to monitor the radiation dose. The radiation was conducted at a rate of 1.42 Gy/min at the setting of 150 kVp, 15 mA (X-ray irradiator, Hitachi, model No. MBR-1520R-3). The distance between the X-ray source and the target was 30 cm, and the X-ray beam was hardened with a 0.5 mm aluminum filter. The irradiated sites were topically treated by vehicle (sesame oil) or compounds (RTA 402: 0.1%, RTA 408 0.1%, and RS-0009: 0.01% and 0.1%) with a volume of 50 µl once daily from Day 1 to Day 30. The severity of dermatitis was scored every 2 days from Day 2 to Day 30 by combining the criteria of The Common Terminology Criteria for Adverse Events (CTCAE) [10] and the practical symptoms for erythema, hemorrhage, ulceration and inflammation [7]: Score 1: minimally detectible, Score 2: mild, Score 3: moderate, Score 4: marked and Score 5: severe. All mice were euthanized on Day 30, and 8 mm dermal punches of the skin were collected. mRNA purification and cDNA synthesis were conducted according to the manufacturer's instructions.
Statistical analysis
Data are shown as means ± S.E.M., and significance tests were conducted using SAS System Release 9.2 (SAS Institute, Cary, NC, USA). Statistical analyses among multiple groups were performed using one-way ANOVA, followed by the Tukey's test, and P < 0.05 was considered significant.
RESULTS
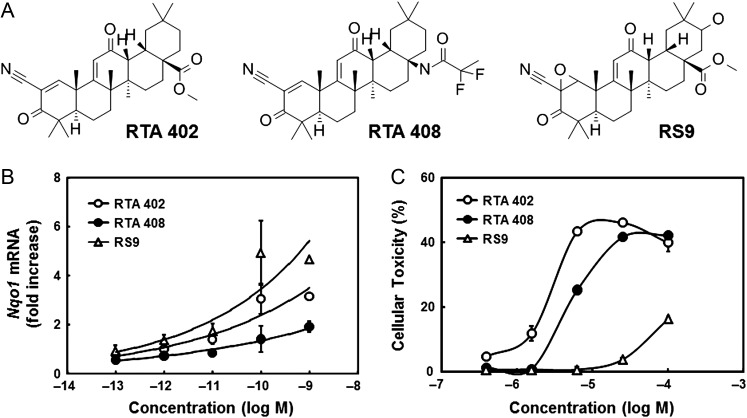
Chemical structures of the tested compounds are shown in Fig. 1A. Biotransformation of RTA 402 resulted in obtaining a novel Nrf2 activator, RS9; the main differences between the RTA compounds and RS9 were epoxidation at the A-ring and hydroxylation/ketonization at the E-ring. The effects on a typical Nrf2-targeted gene, Nqo1, were tested in RTA 402, RTA 408 or RS9-treated NHEKs. Increased levels of Nqo1 mRNA were detected in all compounds (Fig. 1B), and the change was most apparent in RS9. Cytotoxicity of Nrf2 activators was tested in NHEKs at higher concentrations, and RS9 showed less cytotoxicity than RTA compounds (Fig. 1C). Released LDH was observed in large quantities with 100 μM RTA 402 and RTA 408.
Fig. 1.
Profiles of Nrf2 activators. (A) Chemical structures of Nrf2 activators. (B and C) Fold increases of Nqo1 mRNA and cellular toxicity of Nrf2 activators in NHEKs. Open circles: RTA 402, closed circles: RTA 408 and open triangles: RS9, n = 3–4 wells.
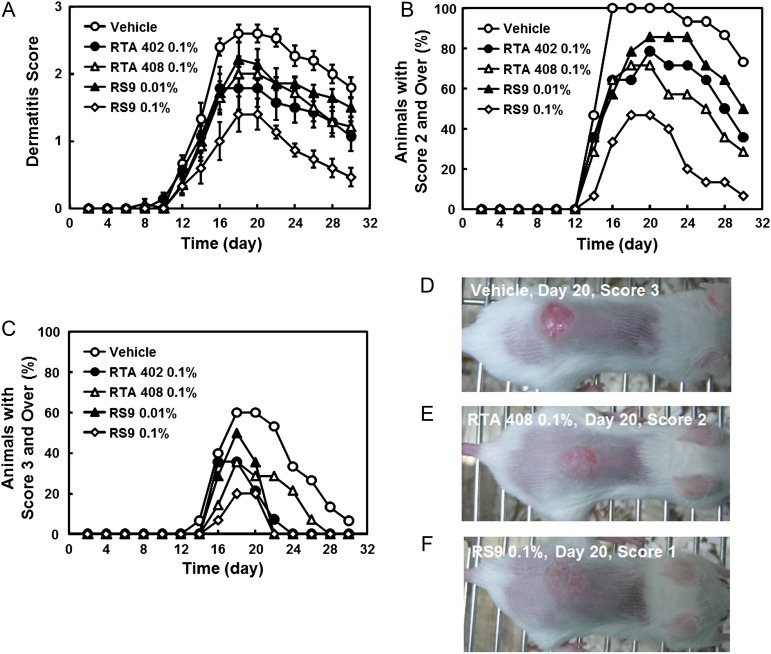
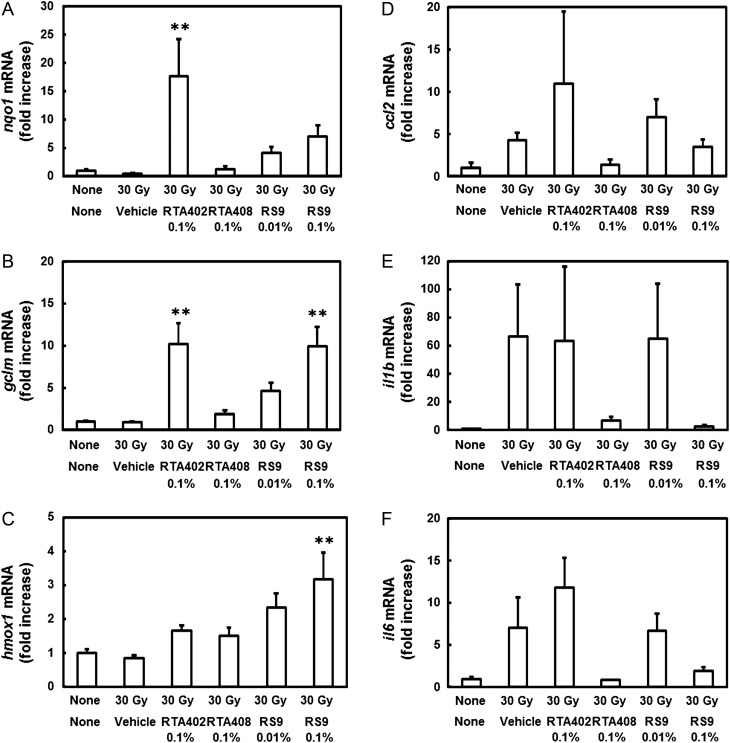
Dermatitis scores were gradually increased 10 days after X-ray radiation and reached a peak on Day 18 or Day 20 in all groups (Fig. 2A). The score in a vehicle-applied group on Day 18 was 2.6, but all compounds inhibited the dermatitis. Among tested compounds, RS9 at a dose of 0.1% showed superior efficacy to RTA 402 and RTA 408 through the observation period. The dermatitis was also analyzed by counting the number of mice that were assessed at score 2 and over (Fig. 2B) or score 3 and over (Fig. 2C). The percentage was also lower in RS9 compared with that in RTA compounds, and macroscopic views of radiation images are shown in Fig. 2D–F. The mRNA changes were examined in the back skin of mice to which Nrf2 activators had repeatedly been applied. The tissues were collected in the radiation-induced dermatitis experiment on Day 30. The increases in nqo1 and gclm in the RTA 402–treated group, and gclm and hmox1 in the RS9-treated group were significant among the tested Nrf2-targeted genes (Fig. 3A–C). Inflammatory genes were also tested, and all of the genes ccl2, il1b and il6 showed a tendency to decrease in the RTA 408– and RS9-treated groups; however, this did not reach statistical significance (Fig. 3D–E).
Fig. 2.
Effects of Nrf2 activators on radiation-induced dermatitis. (A) Effects of Nrf2 activators on skin dermatitis scoring. (B) The percentage of animals with a dermatitis score of ≥2. (C) The percentage of animals with a dermatitis score of ≥3 in the vehicle-treated (n = 15), RS 0.1%-treated (n = 15), and other groups (n = 14). (D–F) Macroscopic images of radiation dermatitis on Day 20.
Fig. 3.
Effects of Nrf2 activators on mRNA in the back skin of radiation-irradiated BALB/c mice. (A–C) Changes in Nrf2-targeted genes in the back skin. (D–F) Changes in inflammatory genes in the back skin. Compounds were topically applied every day throughout experiments, and the tissue was collected on Day 30. **P < 0.01 vs the irradiated vehicle-applied group, Tukey's test, n = 4.
DISCUSSION
We have demonstrated that our novel Nrf2 activator, RS9, alleviates radiation-induced dermatitis and that the efficacy is superior to that of RTA compounds. The efficacy is consistent with in vitro data indicating that the induction of Nrf2-targeted genes was more prominent in response to RS9 compared with RTA compounds in NHEKs. The cellular toxicity of RTA compounds was also strong in vitro; it is likely that if animals were treated with compounds for a longer time or at higher doses, the safety margin between RS9 and RTA compounds would become more apparent.
Kyowa Hakko Kirin announced the recommencement of Phase 2 of RTA 402 in CKD patients with type 2 diabetes in Japan on 5 March 2015. RTA 402 is also being tested in pulmonary arterial hypertension (ClinicalTrials.gov Identifier: NCT02036970). Another compound, RTA 408, is currently being clinically tested in not only radiation-induced dermatitis with breast cancer (NCT02142959), but also post-surgical corneal endothelial cell loss (NCT02128113), Friedreich's ataxia (NCT02255435), mitochondrial myopathies (NCT02255422) and melanoma (NCT02259231). Though there have been disappointing results in clinical trials of Nrf2 activators, these ongoing activities imply that Nrf2 activators are promising therapeutic agents if the correct indications are selected and appropriate doses are prescribed. In addition, the above activities might be influenced by the approval of dimethyl fumarate (Tecfidera, BG-12) for relapsing remitting multiple sclerosis, which is a weak electrophile and expected to be a weak Nrf2 activator.
In conclusion, we found out that RS9 is effective in an authentic radiation-induced dermatitis model. Radiation dermatitis is one of the most common adverse events in radiotherapy for cancer, and 95% of patients receiving radiotherapy show this symptom [11]. The dermatitis not only decreases quality of life, but also hampers the conducting of necessary additional radiotherapy. Moreover, there is no effective drug therapy, except for topical steroids and emollient creams. Radiation is thought to damage the skin in two ways: by generating reactive oxygen species after reacting with water in the cytosol [12], and by consequent indirect inflammation. Considering the above facts, the mechanisms of action of Nrf2 activators seem appropriate for combating the progress of radiation dermatitis, and this is worth investigating further.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
This study was conducted without funding.
REFERENCES
- 1.Itoh K, Mimura J, Yamamoto M.. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid Redox Signal 2010;13:1665–78. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki T, Motohashi H, Yamamoto M.. Toward clinical application of the Keap1–Nrf2 pathway. Trends Pharmacol Sci 2013;34:340–6. [DOI] [PubMed] [Google Scholar]
- 3.Sporn MB, Liby KT, Yore MM, et al. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod 2011;74:537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abboud HE. Synthetic oleanane triterpenoids: magic bullets or not. Kidney Int 2013;83:785–7. [DOI] [PubMed] [Google Scholar]
- 5.Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 2011;365:327–36. [DOI] [PubMed] [Google Scholar]
- 6.de Zeeuw D, Akizawa T, Audhya P, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 2013;369:2492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reisman SA, Lee CY, Meyer CL, et al. Topical application of the synthetic triterpenoid RTA 408 protects mice from radiation-induced dermatitis. Radiat Res 2014;181:512–20. [DOI] [PubMed] [Google Scholar]
- 8.Nakagami Y, Masuda K, Hatano E, et al. Novel Nrf2 activators from microbial transformation products inhibit blood–retinal barrier permeability in rabbits. Br J Pharmacol 2015;172:1237–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JH, Kolozsvary AJ, Jenrow KA, et al. Mechanisms of radiation-induced skin injury and implications for future clinical trials. Int J Radiat Biol 2013;89:311–8. [DOI] [PubMed] [Google Scholar]
- 10.Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol 2012;67:1025–39. [DOI] [PubMed] [Google Scholar]
- 11.Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol 2012;132:985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doctrow SR, Lopez A, Schock AM, et al. A synthetic superoxide dismutase/catalase mimetic EUK-207 mitigates radiation dermatitis and promotes wound healing in irradiated rat skin. J Invest Dermatol 2013;133:1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]