Abstract
This study aimed to investigate the correlation between the average iodine density (AID) detected by dual-energy computed tomography (DE-CT) and the maximum standardized uptake value (SUVmax) yielded by [18F] fluorodeoxyglucose positron emission tomography (18F-FDG PET) for non–small cell lung cancer (NSCLC) treated with stereotactic body radiotherapy (SBRT). Seventy-four patients with medically inoperable NSCLC who underwent both DE-CT and 18F-FDG PET/CT before SBRT (50‒60 Gy in 5‒6 fractions) were followed up after a median interval of 24.5 months. Kaplan–Meier analysis was used to determine associations between local control (LC) and variables, including AID, SUVmax, tumor size, histology, and prescribed dose. The median AID and SUVmax were 18.64 (range, 1.18–45.31) (100 µg/cm3) and 3.2 (range, 0.7–17.6), respectively. No correlation was observed between AID and SUVmax. Two-year LC rates were 96.2% vs 75.0% (P = 0.039) and 72.0% vs 96.2% (P = 0.002) for patients classified according to high vs low AID or SUVmax, respectively. Two-year LC rates for patients with adenocarcinoma vs squamous cell carcinoma vs unknown cancer were 96.4% vs 67.1% vs 92.9% (P = 0.008), respectively. Multivariate analysis identified SUVmax as a significant predictor of LC. The 2-year LC rate was only 48.5% in the subgroup of lower AID and higher SUVmax vs >90% (range, 94.4–100%) in other subgroups (P = 0.000). Despite the short follow-up period, a reduction in AID and subsequent increase in SUVmax correlated significantly with local failure in SBRT-treated NSCLC patients. Further studies involving larger populations and longer follow-up periods are needed to confirm these results.
Keywords: dual-energy CT, FDG-PET/CT, average iodine density, SUVmax, non–small cell lung cancer, stereotactic body radiotherapy
INTRODUCTION
Stereotactic body radiotherapy (SBRT) is considered a safe and effective treatment for medically inoperable patients with Stage I non–small cell lung cancer (NSCLC); however, ~10% of these patients develop local recurrences after high-dose SBRT [1, 2]. Several reports have described prognostic factors for local recurrence, including a lower biologically effective dose [3–5] and larger tumor size [6–9]. Furthermore, pretreatment glucose metabolism was found to have a significant impact on the biological behaviors of many malignancies, including NSCLC, and a number of studies have indicated that the maximum standardized uptake value (SUVmax) determined using [18F]-fluorodeoxyglucose positron emission tomography (18F-FDG PET) correlates with tumor aggressiveness and, therefore, is predictive of patient outcomes [10–14].
Tumor hypoxia also plays an important role in radioresistance [15, 16]. In recent studies, reductions in perfusion CT parameters suggestive of hypoxic conditions within a tumor, such as tumor blood volume, which is measured as the average iodine density (AID) via dual-energy computed tomography (DE-CT), have been shown to correlate with local recurrence [17]. However, the relationship between pretreatment glucose metabolism measurements and whole-tumor perfusion CT parameters has not yet been fully elucidated in NSCLC. The purpose of this study was to investigate a potential correlation between AID on DE-CT and SUVmax on 18F-FDG PET and the prognostic impacts of these parameters on local control (LC) in patients with SBRT-treated NSCLC.
MATERIALS AND METHODS
Patient, tumor and treatment characteristics
From March 2011 to July 2015, data of 74 patients [53 men and 21 women; median age, 77 (range, 57–87) years] with medically inoperable NSCLC who underwent both DE-CT and 18F-FDG PET/CT before SBRT were retrospectively reviewed. The median longest tumor diameter was 21 (range, 6–44) mm. Histological tumor confirmation was required for 54 (73.0%) patients. The remaining 20 (27%) patients were not confirmed histologically via bronchoscopy and/or CT-guided biopsy, and they received clinical diagnoses of NSCLC because of an increasing tumor size, elevated levels of tumor markers, and positive tracer accumulation on 18F-FDG PET/CT. Patient, tumor and treatment characteristics are summarized in Table 1. This study was approved by our institutional review board.
Table 1.
Patient, tumor and treatment characteristics
| Characteristics | |
|---|---|
| Age (years), median (range) | 77 (57–87) |
| Gender | |
| Male | 53 (71.6%) |
| Female | 21 (28.4%) |
| T-stage | |
| T1a | 36 (48.6%) |
| T1b | 27 (36.5%) |
| T2a | 11 (14.9%) |
| Longest tumor diameter (mm) | |
| ≤20 | 36 (48.6%) |
| >20 | 38 (51.4%) |
| Histology | |
| Adenocarcinoma | 35 (47.3%) |
| SCC | 19 (25.7%) |
| Unknown | 20 (27.0%) |
| Type of tumor | |
| Solid | 60 (81.1%) |
| GGA | 14 (18.9%) |
| Prescribed dose (Gy) | |
| 10 Gy × 5 | 63 (85.1%) |
| 10 Gy × 6 | 11 (14.9%) |
GGA = ground glass attenuation, SCC = squamous cell carcinoma.
Treatment procedure
Details regarding the performance of SBRT at our institution have been described previously [18]. Each patient was immobilized using a custom-made MoldCare head rest (ALCARE Co., Ltd, Tokyo, Japan) with an Esform shell (Engineering System Co., Ltd, Nagoya, Japan). Treatment-planning CT was performed using Optima (GE Healthcare, Milwaukee, WI, USA) with a 1.25 mm imaging slice thickness. If respiratory tumor movement was >1 cm, planning CT was performed using a breath-holding technique and an Abches device (APEX Medical Inc., Tokyo, Japan). If respiratory tumor movement was ≤1 cm, planning CT was performed according to the 4D-CT technique, using a real-time position management system (Varian Medical Systems, Palo Alto, CA, USA).
A 3D treatment-planning system (XiO, version 4.8; ELEKTA, Stockholm, Sweden) was applied for dose calculation according to the superposition method. The target margins were as follows: the clinical target volume (CTV) was equal to the gross tumor volume (GTV) or internal target volume (ITV) delineated on CT images displayed at a window level of −300 Hounsfield units (HU) and window width of 1700 HU. The planning target volume (PTV) was the CTV plus a median 8 mm (range, 5–10 mm) margin in all directions, and a 5 mm leaf margin was included around the PTV.
Irradiation was performed using 6-MV X-ray beams from a linear accelerator (Clinac iX; Varian Medical Systems) in 2–3 coplanar and 3–4 non-coplanar static ports. A total dose of 50–60 Gy at the isocenter in 5–6 single-dose fractions of 10 Gy was administered within 20% heterogeneity of the PTV dose. In most cases, the minimum dose to the PTV corresponded to 85–95% of the prescribed dose. Prescribed doses of 50 and 60 Gy were given for T1 tumors (≤30 mm longest tumor diameter) and T2 tumors (>30 mm), respectively.
Scanning procedure and image analyses for dual-energy computed tomography
Enhanced DE-CT imaging was performed using a dual-energy gemstone spectral CT scanner (Discovery CT 750 HD; GE Healthcare, Tokyo, Japan) and a fast kilovoltage (kV) switching method. Details of this imaging method have been previously reported [19]. Briefly, a non-ionic, low-osmolar contrast medium dose of 600 mg I/kg body weight, with an iodine content of 300 or 350 mgI/ml, was administered. The total amount of contrast medium was intravenously injected within 30 s. Scanning began 25 s after initiating the injection of contrast medium. Slices used for data analysis had a thickness of 0.63 mm.
All CT images were transferred to a workstation (GSI Viewer, GE Healthcare) and subjected to data analyses. Using a pulmonary window (window width, 1000 HU; window level, −700 HU), a region of interest (ROI) was set at the maximum cross-sectional diameter of the tumor to surround the entire tumor on the CT image; the image was subsequently converted to an iodine (water) image to obtain an AID.
Scanning procedure for [18F]-fluorodeoxyglucose positron emission tomography/computed tomography
All patients fasted for at least 4 h in preparation for FDG-PET/CT; water intake was encouraged [20]. Commercially produced and delivered 18F-FDG (FDG scan injectable, 185 MBq on assay date; Nihon Medi-Physics, Co., Ltd, Tokyo, Japan) was injected intravenously 60 min prior to the initiation of scanning. During the 60 min uptake period, patients drank a sufficient amount of water. A PET/CT system (Discovery ST Elite 16; GE Healthcare, Tokyo, Japan) was used to acquire data in 7‒8 bed positions, with an acquisition time of 2.5–3.0 min per bed position. CT was performed first (30–80 mA, 120 kV, 3.75–3.27 mm slice thickness). CT data were used for the attenuation correction of PET data, as well as for co-registration with attenuation-corrected PET images. Next, PET data were acquired immediately from the same body region. The reconstructed sectional images were evaluated visually and quantitatively using the SUVmax within an ROI placed on the lesions.
Follow-up and statistical analysis
Local tumor control was defined as an end point in this study. Follow-up CT scans were obtained at 3–6 month intervals and were used to assess tumor control. Local recurrence was diagnosed on the basis of local tumor enlargement on CT over a period of at least 6 months. 18F-FDG PET/CT and/or histological confirmation were recommended when local recurrence was suspected, but these were not mandatory. The LC rates from the first date of treatment until the date of local recurrence were calculated using the Kaplan–Meier method.
Differences in tumor characteristics between two patient groups (high and low groups) divided according to the median AID value and SUVmax of 4.0 were compared using the chi-square test. The log-rank test was used to evaluate statistically significant differences between Kaplan–Meier curves in univariate analyses. A Cox proportional hazard model was used for the multivariate analysis, which considered the following parameters: longest tumor diameter (≤20 vs >20 mm), histology [adenocarcinoma/unknown vs squamous cell carcinoma (SCC)], tumor type [solid vs ground glass attenuation (GGA)], prescribed dose (50 vs 60 Gy), AID [≤18.64 vs >18.64 (100 µg/cm3)], and SUVmax (≤4.0 vs >4.0). Differences were considered statistically significant at a probability (P) value of <0.05. All statistical analyses were performed using a commercially available statistical software package [Statistical Package for the Social Sciences (SPSS), version 22.0; IBM, Tokyo, Japan].
RESULTS
The patients in this study were subjected to follow-up for a median duration of 24.5 (range, 6.9–60.8) months. The median SUVmax and AID were 3.2 (range, 0.7–17.6) and 18.64 (range, 1.18–45.31) (100 µg/cm3), respectively.
Tumor characteristics
Patients were divided into high and low SUVmax and AID groups for comparisons of tumor characteristics; these analyses are summarized in Table 2. No significant correlation was found between SUVmax and AID; however, differences were observed between the SUVmax groups with respect to tumor type (solid vs GGA) and histology (adenocarcinoma vs SCC vs unknown) and between the AID groups in terms of prescribed dose.
Table 2.
Tumor and treatment characteristics according to SUVmax and AID
| Characteristics | SUVmax (n) | P-value | AID (n) | P-value | ||
|---|---|---|---|---|---|---|
| High | Low | High | Low | |||
| Longest tumor diameter (mm) | ||||||
| ≤20 | 13 | 23 | 0.598 | 21 | 15 | 0.163 |
| >20 | 16 | 22 | 16 | 22 | ||
| Histology | ||||||
| Adenocarcinoma | 11 | 24 | 0.001 | 17 | 18 | 0.869 |
| SCC | 15 | 4 | 9 | 10 | ||
| Unknown | 3 | 17 | 11 | 9 | ||
| Type of tumor | ||||||
| Solid | 29 | 31 | 0.001 | 32 | 28 | 0.235 |
| GGA | 0 | 14 | 5 | 9 | ||
| Prescribed dose (Gy) | ||||||
| 10 Gy × 5 | 25 | 38 | 0.835 | 35 | 28 | 0.022 |
| 10 Gy × 6 | 4 | 7 | 2 | 9 | ||
| AID | ||||||
| High | 12 | 25 | 0.234 | |||
| Low | 17 | 20 | ||||
GGA = ground glass attenuation, AID = average iodine density, SUVmax = maximum standard uptake value, SCC = squamous cell carcinoma.
LC
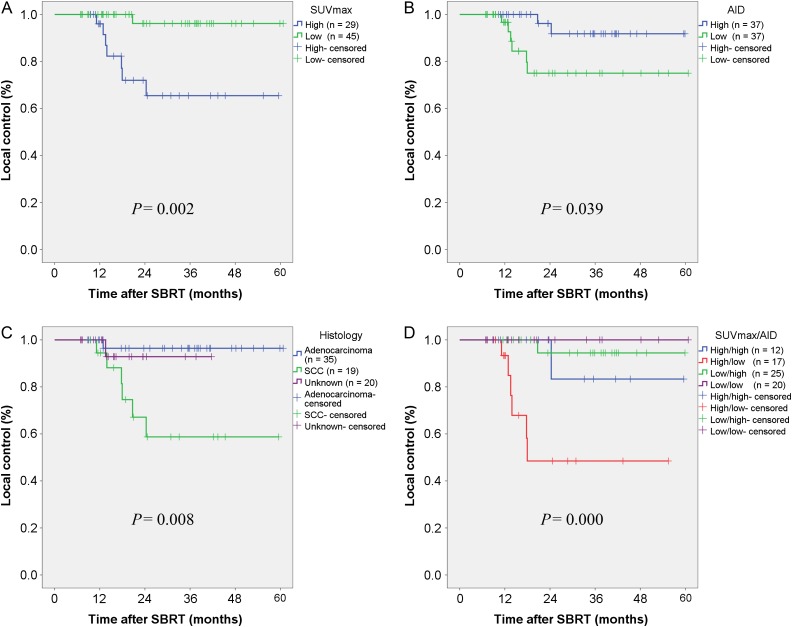
Local recurrence was observed in eight (10.8%) patients during the last follow-up, and an overall 2-year LC rate of 86.8% was calculated. The 2-year LC rates for the SUVmax high and low groups were 72.0% and 96.2% (P = 0.002), respectively (Fig. 1A); the corresponding rates for the AID high and low groups were 96.2% and 75.0% (P = 0.039), respectively (Fig. 1B). Tumors with a lower AID and higher SUVmax exhibited significantly poorer LC. Additionally, histological adenocarcinoma and unknown histology were associated with significantly better LC; the 2-year LC rates for adenocarcinoma, unknown and SCC tumors were 96.4%, 92.9% and 67.1% (P = 0.008), respectively (Fig. 1C). We also analyzed differences in LC stratified according to four groups according to SUVmax and AID (high/high, high/low, low/high and low/low). The 2-year LC rate for tumors in the high/low group was only 48.5% compared with >90% (range, 94.4%‒100%) in the other three groups (P = 0.000), as shown in Fig. 1D. Other factors, such as the longest tumor diameter, tumor type (solid vs GGA) and prescribed dose were not found to significantly affect LC. In the multivariate analysis, only SUVmax was selected as a significant prognostic factor with respect to LC, as shown in Table 3.
Fig. 1.
Kaplan–Meier curves of local control rates after stereotactic body radiotherapy (SBRT) according to maximum standard uptake value (SUVmax) (A), average iodine density (AID) (B), histology (C), and combined SUVmax/AID (D).
Table 3.
Univariate and multivariate analysis of local control
| Variables | n | 2y LC (%) | Univariate P-value | Multivariate HR (95% CI) | P-value |
|---|---|---|---|---|---|
| AID | |||||
| High | 37 | 96.2 | 0.039 | NS | |
| Low | 37 | 75.5 | |||
| SUVmax | |||||
| High | 29 | 72.0 | 0.002 | 12.96 (1.59–105.39) | 0.017 |
| Low | 45 | 96.2 | |||
| Histology | |||||
| Adenocarcinoma | 35 | 96.4 | 0.008 | NS | |
| SCC | 19 | 67.1 | |||
| Unknown | 20 | 92.9 | |||
| Longest tumor diameter (mm) | |||||
| ≤20 | 36 | 84.7 | 0.888 | ||
| >20 | 38 | 88.7 | |||
| Tumor type | |||||
| Solid | 60 | 83.8 | 0.165 | ||
| GGA | 14 | 100 | |||
| Prescribed dose (Gy) | |||||
| 10 Gy × 5 | 63 | 87.6 | 0.827 | ||
| 10 Gy × 6 | 11 | 80.0 |
2y = 2-year, LC = local control, AID = average iodine density, SUVmax = maximum standard uptake values, SCC = squamous cell carcinoma, GGA = ground glass attenuation, HR = hazard ratio, CI = confidence interval.
In addition, the 2-year LC rates in the combined SUVmax/AID groups (high/low vs other groups) were assessed in subgroups according to histology, longest tumor diameter, tumor type, and prescribed dose; these are listed in Table 4. Significant negative impacts of a high SUVmax/low AID on LC were observed in subgroups defined by histological adenocarcinoma (P = 0.004) and unknown histology (P = 0.000), longest tumor diameter of ≤20 mm (P = 0.001) and >20 mm (P = 0.004), solid tumor (P = 0.000), and prescribed dose of 50 Gy (P = 0.000) and 60 Gy (P = 0.046).
Table 4.
Two-year local control rates according to subgroups and SUVmax/AID (high/low vs other groups)
| Subgroups | n | % 2y LC (n) | P-value | |||
|---|---|---|---|---|---|---|
| High/low | Others | |||||
| Histology | ||||||
| Adenocarcinoma | 35 | 66.7 | (6) | 100 | (29) | 0.004 |
| SCC | 19 | 45.7 | (10) | 85.7 | (9) | 0.166 |
| Unknown | 20 | 0 | (1) | 100 | (19) | 0.000 |
| Longest tumor diameter (mm) | ||||||
| ≤20 | 36 | 42.9 | (7) | 93.3 | (29) | 0.001 |
| >20 | 38 | 51.4 | (10) | 100 | (28) | 0.004 |
| Tumor type | ||||||
| Solid | 60 | 48.5 | (17) | 96.3 | (43) | 0.000 |
| GGA | 14 | — | (0) | 100 | (14) | — |
| Prescribed dose (Gy) | ||||||
| 10 Gy × 5 | 63 | 53.8 | (14) | 96.8 | (49) | 0.000 |
| 10 Gy × 6 | 11 | 0 | (3) | 100 | (8) | 0.046 |
2y = 2-year, LC = local control, SUVmax = maximum standard uptake values, AID = average iodine density, SCC = squamous cell carcinoma, GGA = ground glass attenuation.
Patients who developed local recurrences are listed in Table 5. All recurrences occurred within 24 months after SBRT. Among the eight patients who developed local recurrences, 100% had solid tumors, 88% were male and had T1 tumors, and 75% had a high SUVmax combined with a low AID (high/low group) and had SCC.
Table 5.
List of patients who developed local recurrences
| Age (years) | Gender | T-stage | Histology | Tumor type | Tumor location | Dose (Gy) | SUVmax | Subgroup (SUVmax/AID) | Time to recurrence (months) | Treatment after recurrence | Status, months |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 77 | Male | T1b | SCC | Solid | Right upper | 50 | 5.2 | High/low | 18.0 | Chemotherapy | Dead, 32.8 |
| 83 | Male | T1b | Adenocarcinoma | Solid | Right lower | 50 | 5.5 | High/low | 12.9 | BSC | Alive, 41.9 |
| 64 | Male | T2a | SCC | Solid | Right upper | 60 | 13.7 | High/low | 17.8 | Surgery | Alive, 39.9 |
| 77 | Male | T1b | SCC | Solid | Right lower | 50 | 6.6 | High/high | 24.3 | Chemotherapy | Alive, 38.6 |
| 76 | Male | T1a | SCC | Solid | Right upper | 50 | 2.8 | Low/high | 20.7 | BSC | Alive, 32.9 |
| 57 | Female | T1a | SCC | Solid | Left upper | 50 | 5.9 | High/low | 11.1 | BSC | Dead, 22.4 |
| 57 | Male | T1a | NSCLC | Solid | Right upper | 50 | 4.4 | High/low | 13.6 | Surgery | Alive, 23.5 |
| 87 | Male | T1a | SCC | Solid | Right upper | 50 | 12.1 | High/low | 13.9 | BSC | Dead, 14.0 |
SUVmax = maximum standard uptake values, AID = average iodine density, SCC = squamous cell carcinoma, NSCLC = non-small cell lung carcinoma, BSC = best supportive care.
Among the eight patients who developed local recurrence, recurrence was confirmed by biopsy in three patients and was clinically diagnosed in five patients. After the diagnosis of local recurrence, salvage surgery was attempted for two patients following regrowth of the SBRT target lesion at 14 and 20 months after SBRT. These patients were still alive without any complications postoperatively. In contrast, six patients were treated with chemotherapy or best supportive care. Three of these patients had died from disease progression at the last follow-up.
DISCUSSION
We found strong associations of the pretreatment SUVmax and AID with LC, but did not observe a significant correlation between SUVmax and AID. We also found a significantly negative impact of tumors with a lower AID and higher SUVmax on LC, and this impact was independent of the tumor size, histology, tumor type, and prescribed dose. To our knowledge, this is the first report to assess LC in patients with NSCLC after SBRT through a pretreatment evaluation in combination with DE-CT and FDG-PET/CT.
Pretreatment glucose metabolism and outcomes
FDG-PET/CT has become widely used in diagnostic and pretreatment evaluations as well as staging for patients with malignant tumors. SUVmax is the most widely used PET parameter, and it has been shown to have potential prognostic value in patients undergoing SBRT for NSCLC. Several studies have reported the prognostic impact of pretreatment SUVmax on LC of NSCLC after SBRT. Takeda et al. [10] evaluated the FDG-PET SUVmax for localized NSCLC and observed that a higher SUVmax (>6.0) was a strong predictor of local recurrence after SBRT. The SUVmax thresholds and local recurrence rates differ among studies; however, similar findings have been reported by Clark et al. (SUVmax of 5.0) [11], Lee et al. (SUVmax of 6.8) [12], Hamamoto et al. (SUVmax of 5.0) [13] and Kohutek et al. (SUVmax of 3.0) [14].
In our study, a higher SUVmax (>4.0) was a strong predictor of local recurrence (P = 0.002), particularly when combined with a lower AID (P = 0.000); these findings suggest a decrease in tumor blood volume, reflective of a hypoxic cell population in the tumor. In other words, hypoxia activates the hypoxia inducible factor-1alfa (HIF-1α) pathway, which increases the expression of glucose transporters on the surface membranes of tumor cells [21], thus facilitating the uptake of FDG by tumor cells.
However, tumors with a higher SUVmax together with a higher AID were well controlled in our patient population. Furthermore, tumors with a lower SUVmax together with higher AID were also well controlled. These results suggest that both glucose metabolism measurements and tumor perfusion CT parameters play important roles in radiosensitivity.
Tumor perfusion computed tomography parameters and outcomes
Tumor hypoxia is a well-known and important determinant of the response to anti-tumor treatments, particularly radiotherapy [15, 16, 22]. Accordingly, techniques to directly determine the oxygen levels in human tumors have been developed, and the results of such measurements have been predictive of both response to therapy and patient survival [23]. However, the method for directly determining oxygen levels is generally technically demanding, invasive, and useful only for studying accessible tumors, and, therefore, it has not been accepted as a clinical tool for hypoxia measurement. Among non-invasive techniques, PET with fluorine-18 fluoromisonidazole (FMISO) has been the most extensively studied. However, FMISO has a number of limitations, such as slow accumulation in hypoxic tumors, a low level of target-to-background contrast, and a significant production of radioactive metabolites [24]. On the other hand, perfusion CT parameter assessment using DE-CT is a non-invasive and quantitative method for tumor blood volume evaluations. In our study, although oxygen pressure was not measured directly, a lower AID was associated with local recurrence after SBRT, indicating an association of radioresistance with low tumor blood volume and a probable association with a hypoxic condition.
Our study further revealed that tumors with a lower AID and lower SUVmax were well controlled after SBRT. Sato et al. [25] reported that a HIF-1 inhibitor selectively induced apoptosis in hypoxic cells through the depolarization of mitochondria in A549 human lung cancer cells. Although the results of in vitro studies do not necessarily reflect conditions in vivo, tumors with decreased glucose metabolism despite a hypoxic condition suggest the suppression of HIF-1 for an unclear reason.
Relationship between glucose metabolism and tumor perfusion computed tomography parameters
Some reports have described a relationship between SUVmax and perfusion CT parameters in patients with NSCLC; however, to our knowledge, no previous reports have described the relationships of LC and perfusion CT parameters with glucose metabolism measurements. Schmid-Bindert et al. [26] reported a positive correlation between SUVmax and iodine-related attenuation according to DE-CT in 37 patients with mostly Stage IV NSCLC. In contrast, Iwano et al. [27] reported that the iodine volume during the delayed phase of dual-phase dynamic DE-CT correlated negatively with SUVmax in 27 patients with primary lung cancer.
In our study, no correlation was observed between AID and SUVmax for 74 patients with early-stage NSCLC. A similar lack of correlation between the hypoxic cell fraction and glucose metabolic rate was previously reported by Cherk et al. [28] in 17 patients with early-stage NSCLC, a small cohort. Differences between studies with respect to patient and tumor characteristics as well as small numbers of patients appear to account for discrepancies regarding the relationship between AID and SUVmax.
Tumor size, BED10 and local control
A larger tumor size and lower BED10 have been reported as risk factors related to LC after SBRT; however, these were not risk factors in our patient population. In our study, the 2-year LC rate was 87.6% for T1 tumors and 80.0% for T2 tumors (P = 0.827). This observation might reflect the use of a higher BED10 for T2 tumors. In our study, the prescribed dose differed according to tumor size; 50 Gy (BED10 = 100 Gy) was administered for T1 tumors (≤30 mm), and 60 Gy (BED10 = 120 Gy) was administered for T2 tumors (>30 mm). Notably, since 2010, at our institution we also initiated a dose escalation study from 50 Gy to 60 Gy for lung oligo-recurrences from colorectal cancer [18], which has a poor LC rate.
Shibamoto et al. [29] previously reported an interesting treatment protocol involving SBRT with a radiobiology-based regimen for Stage I NSCLC and obtained reasonable LC rates using higher doses for T2 tumors and relatively long interfraction intervals. In our study, a higher SUVmax and lower AID according to pretreatment FDG-PET/CT and DE-CT evaluation for NSCLC was found to be a significant predictor of local recurrence after SBRT. We also observed a significant negative impact on LC in this subgroup (high/low group) for T1 tumors treated with a prescribed dose of 50 Gy. Our results suggest the need for dose escalation studies that account for a higher SUVmax with lower AID, even in T1 tumors.
This study had the following limitations. First, the follow-up period was relatively short, and it is unknown whether LC rates might decrease with a longer follow-up. Second, many factors influence the AID of a tumor, including scan delay, total contrast agent amount, injection rate, ROI settings with reference to the tumor, and patient characteristics. Finally, many factors influence the SUVmax of a tumor, including patient size, time from 18F-FDG injection to imaging, blood sugar and insulin levels, tumor size, imaging device, respiratory tumor movement, and ROI setting with reference to the tumor. Despite these limitations, however, this study provides a novel perspective on future directions in the development of local relapse predictions based on FDG-PET/CT and DE-CT after high-dose SBRT.
In conclusion, pretreatment glucose metabolism parameters and perfusion CT parameters throughout lung tumors were found to correlate with local relapse after SBRT. An altered fractionation schedule for SBRT should be considered for tumors with a higher SUVmax and a lower AID. Further studies will be required to confirm these results in larger populations and with longer follow-up periods.
ACKNOWLEDGEMENTS
This work was presented at the 28th Annual Meeting of the Japanese Society for Radiation Oncology, held 19–21 November 2015 in Maebashi, Japan.
FUNDING
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 24591830.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Baumann P, Nyman J, Hoyer M, et al.. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290‒6. [DOI] [PubMed] [Google Scholar]
- 2.Onishi H, Araki T.. Stereotactic body radiation therapy for stage I non-small-cell lung cancer: a historical overview of clinical studies. Jpn J Clin Oncol 2013;43:345‒50. [DOI] [PubMed] [Google Scholar]
- 3.Onishi H, Araki T, Shirato H, et al.. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623–31. [DOI] [PubMed] [Google Scholar]
- 4.Nagata Y, Takayama K, Matsuo Y, et al.. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427‒31. [DOI] [PubMed] [Google Scholar]
- 5.Chang JY, Roth JA.. Stereotactic body radiation therapy for stage I non-small cell lung cancer. Thorac Surg Clin 2007;17:251–9. [DOI] [PubMed] [Google Scholar]
- 6.Koto M, Takai Y, Ogawa Y, et al.. A phase II study on stereotactic body radiotherapy for stage I non-small cell lung cancer. Radiother Oncol 2007;85:429–34. [DOI] [PubMed] [Google Scholar]
- 7.Baumann P, Nyman J, Hoyer M, et al.. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290–6. [DOI] [PubMed] [Google Scholar]
- 8.Bral S, Gevaert T, Linthout N, et al.. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a phase II trial. Int J Radiat Oncol Biol Phys 2011;80;1343–9. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo Y, Shibuya K, Nagata Y, et al.. Prognostic factors in stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;79:1104–11. [DOI] [PubMed] [Google Scholar]
- 10.Takeda A, Yokosuka N, Ohashi T, et al.. The maximum standardized uptake value (SUVmax) on FDG-PET is a strong predictor of local recurrence for localized non-small-cell lung cancer after stereotactic body radiotherapy (SBRT). Radiother Oncol 2011;101:291–7. [DOI] [PubMed] [Google Scholar]
- 11.Clarke K, Taremi M, Dahele M, et al.. Stereotactic body radiotherapy (SBRT) for non-small cell lung cancer (NSCLC): is FDG-PET a predictor of outcome. Radiother Oncol 2012;104:62–6. [DOI] [PubMed] [Google Scholar]
- 12.Lee DS, Kim YS, Yoo Ie R, et al.. Long-term clinical experience of high-dose ablative lung radiotherapy: high pre-treatment [18F]fluorodeoxyglucose-positron emission tomography maximal standardized uptake value of the primary tumor adversely affects treatment outcome. Lung Cancer 2013;80:172–8. [DOI] [PubMed] [Google Scholar]
- 13.Hamamoto Y, Sugawara Y, Inoue T, et al. Relationship between pretreatment FDG uptake and local control after stereotactic body radiotherapy in stage I non-small-cell lung cancer: the preliminary results. Jpn J Clin Oncol 2011;41:543–7. [DOI] [PubMed] [Google Scholar]
- 14.Kohutek ZA, Wu AJ, Zhang Z, et al. FDG-PET maximum standardized uptake value is prognostic for recurrence and survival after stereotactic body radiotherapy for non–small cell lung cancer. Lung Cancer 2015;89:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson DJ, Yenice KM, Orton CG.. Tumor hypoxia is an important mechanism of radioresistance in hypofractionated radiotherapy and must be considered in the treatment planning process. Med Phys 2011;38:6347–50. [DOI] [PubMed] [Google Scholar]
- 16.Marie-Egyptienne DT, Lohse I, Hill RP.. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: potential role of hypoxia. Cancer Lett 2013;341:63–72. [DOI] [PubMed] [Google Scholar]
- 17.Aoki M, Hirose K, Sato M, et al. (28 January 2016) Prognostic impact of average iodine density assessed by dual-energy spectral imaging for predicting lung tumor recurrence after stereotactic body radiotherapy. J Radiat Res, 10.1093/jrr/rrv100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aoki M, Hatayama Y, Kawaguchi H, et al. Stereotactic body radiotherapy for lung metastases as oligo-recurrence: a single institutional study. J Radiat Res 2016;57:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoki M, Takai Y, Narita Y, et al. Correlation between tumor size and blood volume in lung tumors: a prospective study on dual-energy gemstone spectral CT imaging. J Radiat Res 2014;55:917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seino H, Ono S, Miura H, et al. Incidental prostate 18F-FDG uptake without calcification indicates the possibility of prostate cancer. Oncol Rep 2014;31:1517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res 2001;61:7992–8. [PubMed] [Google Scholar]
- 22.Dehdashti F, Mintun MA, Lewis JS, et al. In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur J Nucl Med Mol Imaging 2003;30:844–50. [DOI] [PubMed] [Google Scholar]
- 23.Nordsmark M, Overgaard M, Overgaard J.. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol 1996;41:31–9. [DOI] [PubMed] [Google Scholar]
- 24.Grönroos T, Minn H.. Imaging of tumour hypoxia using PET and 18F-labelled tracers: biology meets technology. Eur J Nucl Med Mon Imaging 2007;34:1563–5. [DOI] [PubMed] [Google Scholar]
- 25.Sato M, Hirose K, Kashiwakura I, et al.. LW6, a hypoxia inducible factor 1 inhibitor, selectively induces apoptosis in hypoxic cells through depolarization of mitochondria in A549 human lung cancer cells. Mol Med Rep 2015;12:3462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid-Bindert G, Henzler T, Chu TQ, et al. Functional imaging of lung cancer using dual energy CT: how does iodine related attenuation correlate with standardized uptake value of 18FDG-PET-CT. Eur Radiol 2012;22:93–103. [DOI] [PubMed] [Google Scholar]
- 27.Iwano S, Ito R, Umakoshi H, et al. Evaluation of lung cancer by enhanced dual-energy CT: association between three-dimensional iodine concentration and tumour differentiation. Br J Radiol 2015;88:20150224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherk MH, Foo SS, Poon AM, et al. Lack of correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in non-small cell lung cancer assessed by 18F-Fluoromisonidazole and 18F-FDG PET. J Nucl Med 2006:47:1921–6. [PubMed] [Google Scholar]
- 29.Shibamoto Y, Hashizume C, Baba F, et al. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I nonsmall cell lung cancer: a multicenter study. Cancer 2012;118:2078–84. [DOI] [PubMed] [Google Scholar]