Abstract
The purpose of this review was to evaluate the impact of epidermal growth factor receptor (EGFR) mutation status on disease recurrence in patients treated with chemoradiotherapy (CRT) for locally advanced non–small cell lung cancer (NSCLC). A literature search was conducted and a total of three studies were analyzed. There was no significant difference in the objective response rate between the EGFR mutation group and the EGFR wild-type group (odds ratios [OR] 1.46, 95% CI, 0.79–2.70, P = 0.228), and there was no significant difference in the incidence of disease recurrence (OR 1.37, 95% CI, 0.68–2.75, P = 0.379) between the two groups. There were significant difference in the incidence of local/locoregional progression (LP) (OR 0.35, 95% CI, 0.18–0.71, P = 0.003) and distant progression (DP) (OR 2.97, 95% CI, 1.59–5.54, P < 0.001). Brain metastasis (BM) was one of the main recurrence patterns of DP, and the incidence was significantly higher in the EGFR mutant group (OR 2.75, 95% CI, 1.43–5.31, P = 0.003). There were no statistically significant heterogeneities in these pooled analyses. The patterns of recurrence after CRT for locally advanced NSCLC were different according to EGFR mutation status. LP after CRT in patients with EGFR mutation was less frequent, but the high incidence of DP, especially BM, continued to be the major problem. On the other hand, LP continued to be the major problem in EGFR wild-type patients. In multimodality treatment for inoperable locally advanced NSCLC, we may need to consider different treatment strategies according to EGFR mutation status.
INTRODUCTION
Lung cancer is the most frequently diagnosed cancer in the world, and it accounts for 13% of all cancers diagnosed. It is estimated that lung cancer contributes to more than 1.6 million deaths each year [1]. Non–small cell lung cancer (NSCLC) accounts for ~90% of new lung cancer diagnoses, and approximately one-third of NSCLC patients present with locally advanced disease [2–4]. Concurrent chemoradiotherapy (CCRT) is considered to be the standard therapy for locally advanced and inoperable NSCLC patients [5], and sequential chemoradiotherapy (CRT) is considered to be one of the treatment options for elderly patients or those with poor performance status [6, 7].
Epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein and a member of the erbB receptor tyrosine kinase family, and it is commonly overexpressed in NSCLC [8, 9]. Following ligand-binding, EGFR receptors homo- and heterodimerize and promote autophosphorylation of the intracellular tyrosine kinase domain, and thus initiate a molecular cascade of events involved in growth, and cell proliferation, differentiation and survival [9–12]. It has been reported that EGFR mutations occur more frequently in Asian patients compared with European or North American patients, with mutation rates of ~30% and ~10%, respectively [13–15].
NSCLC cell lines with EGFR mutations have been reported to be more sensitive to radiation in an in vitro study [16]. It has also been reported that intracranial progression-free survival (or response rate) after cranial radiotherapy (RT) for brain metastases (BM) from NSCLC is favorable in patients with EGFR mutations [17–19]. A difference in the effectiveness of definitive CRT for locally advanced NSCLC according to EGFR mutation status in patients has not yet been established. The purpose of this study was to evaluate any association between EGFR mutation status and disease recurrence after CRT for NSCLC.
METHOD
A literature search, via PubMed and EMBASE, using the following terms and keywords: radiation therapy, radiotherapy, lung cancer, non–small cell lung cancer, non–small cell lung carcinoma, NSCLC, epidermal growth factor, EGFR, and a combination of these terms. The last research was conducted on 29 February 2016.
Data collection
For eligibility, studies were required to meet the following criteria: (i) studies which evaluated the effect of EGFR mutation status on the clinical outcome of locally advanced NSCLC; (ii) studies involving multimodality treatment including thoracic radiation therapy (RT); (iii) studies published in English, regardless of publication time; (iv) original papers containing a threshold amount of data. Studies failing to meet the eligibility criteria were excluded. Our focus was to evaluate the incidence of disease recurrence (DR) (local/locoregional progression [LP], distant progression [DP], BM) according to EGFR mutation status. Differences in patient characteristics (gender, smoking history) and tumor characteristics (clinical stage, clinical T stage and N stage), objective response rate (ORR), progression-free survival (PFS)/relapse-free survival (RFS), and overall survival (OS) were also compared in relation to EGFR mutation status. In this analysis, objective response was defined as complete response or partial tumor response according to the Response Evaluation Criteria for Solid Tumors (RECIST).
Statistical analysis
For each study, baseline characteristics, ORR and DR, were compared using Fisher's exact test. The results of studies were reported as pooled odds ratios (ORs) with the corresponding 95% confidence intervals (CIs). The Mantel–Haenszel method was used to estimate the pooled OR and its 95% CI in a fixed effect model. The homogeneity of the studies was tested by Q statistics and I^2 statistic (I^2 = 0–50% for no or moderate heterogeneity; I^2 > 50%, significant heterogeneity), which are quantitative measures of inconsistency across the studies [20]. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, The R Foundation for Statistical Computing) [21]. A P-value of <0.05 was inferred to be statistically significant.
RESULTS
A total of seven retrospective studies were identified [22–28]. RT was used as the only preoperative therapy in one study, which was thus excluded from further analysis [25]. One paper was in Chinese, and that study was also excluded from further analysis, based on the eligibility criteria [23]. One study included both patients who had received definitive CRT and others who had received perioperative CRT [22]. Evaluation of DR after CRT was difficult, and that study was also excluded. A fourth study included patients treated with RT or CRT [24]. It was difficult to evaluate the endpoints of this analysis separately for the RT and the CRT cohorts, and in light of the differences in treatment outcomes between RT and CRT [29], that study was also excluded. The data from the remaining three retrospective studies were evaluated in this article [26–28].
Patient, tumor and treatment characteristics
Patient, tumor and treatment characteristics of included studies according to EGFR mutation status are summarized in Table 1. Two studies [27, 28] contained enough data to compare smoking history, clinical T stage and clinical N stage, and these studies were included in our pooled analysis.
Table 1.
Summary of patient, tumor and treatment characteristics
| Study | EGFR mutation status | Akamatsu et al. (2014) [26] | Yagishita et al. (2015) [27] | Tanaka et al. (2015) [28] |
|---|---|---|---|---|
| Total no. of Pts | 44 | 198 | 104 | |
| Histology | Adc | nonsquamous NSCLC | Adc | |
| Concurrent administration of chemotherapy (%) | 44 (100) | 166 (84) | 104 (100) | |
| EGFR mutation (%) | ||||
| EGFR-m | 13 (30) | 34 (17) | 29 (28) | |
| EGFR-w | 31 (70) | 164 (83) | 75 (72) | |
| Median age, years (range) | ||||
| EGFR-m | 68 | 62 (46–75) | 62 (51–77) | |
| EGFR-w | 64 | 60 (32–76) | 62 (40–74) | |
| Median RT dose, Gy (range) | ||||
| EGFR-m | 60 (60–74) | 60 (60–72) | 60 (60–66) | |
| Concurrent CRT (%) | ||||
| EGFR-m | 13 (100) | 26 (76) | 29 (100) | |
| EGFR-w | 31 (100) | 140 (85) | 75 (100) | |
| P-value | >0.999 | 0.206 | >0.999 | |
| Gender, no. of females (%) | ||||
| EGFR-m | 5 (38) | 18 (54) | 20 (69) | |
| EGFR-w | 7 (23) | 28 (17) | 20 (27) | |
| P-value | 0.295 | <0.001 | <0.001 | |
| Never-smoker (%) | ||||
| EGFR-m | NA | 20 (59) | 18 (62) | |
| EGFR-w | NA | 18 (11) | 8 (11) | |
| P-value | <0.001 | <0.001 | ||
| Clinical Stage IIIB (%) | ||||
| EGFR-m | 4 (31) | 14 (41) | 16 (55) | |
| EGFR-w | 9 (69) | 78 (48) | 41 (55) | |
| P-value | 0.321 | 0.573 | >0.999 | |
| T3–4 (%) | ||||
| EGFR-m | NA | 1 (3) | 6 (21) | |
| EGFR-w | NA | 67 (41) | 37 (49) | |
| P-value | <0.001 | 0.008 | ||
| N3 (%) | EGFR-m | NA | 13 (38) | 14 (48) |
| EGFR-w | NA | 48 (29) | 27 (36) | |
| P-value | 0.313 | 0.662 |
EGFR = epidermal growth factor receptor, Pts = patients, EGFR-m = EGFR mutant group; EGFR-w = EGFR wild-type group, Adc = adenocarcinoma; NSCLC = non–small cell lung cancer, CRT = chemoradiotherapy, RT = radiation therapy, P-value: P-value of Fisher's exact test, NA = not available.
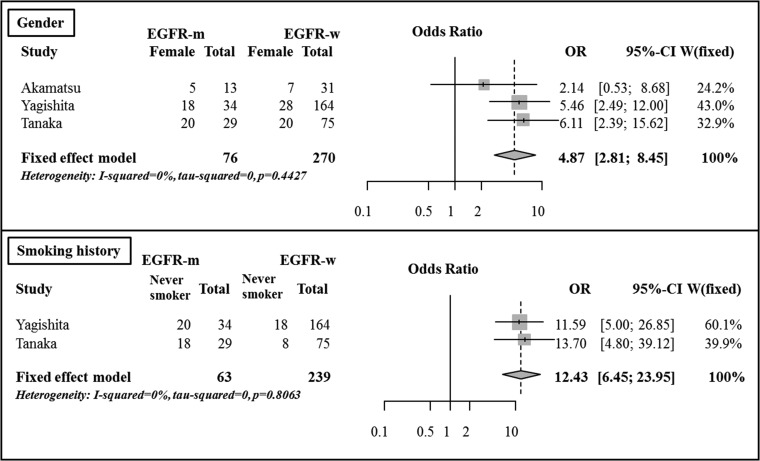
There was be no significant difference in the median patient age between the two groups. The median irradiated dose of all patients who underwent definitive CRT was 60 Gy, and there was no difference between that for the EGFR mutant group and that for the EGFR-wild type group. One study included both patients who underwent CCRT (84%) and others who underwent sequential CRT (16%) [27]. The proportion of patients who underwent CCRT was not statistically different between the EGFR mutant group (76%) and the EGFR wild-type group (85%) (P = 0.206). The other studies only included patients who underwent CCRT [26, 28]. Using Fisher's exact test, there was a significant difference between the gender ratio for the EGFR mutant group and the EGFR wild-type group in two of the studies [27, 28]. There was also a significant difference in the between the smoking history of the two groups in all of the evaluated studies [27, 28]. In the pooled analysis, females (OR 4.87, 95% CI, 2.81–8.45, P < 0.001) and never-smokers (OR 12.43, 95% CI, 6.45–23.95, P < 0.001) were more frequently observed in the EGFR mutant group than in the EGFR wild-type group (Fig. 1).
Fig. 1.
Comparison of patient characteristics between the EGFR mutant group and the EGFR wild-type group.
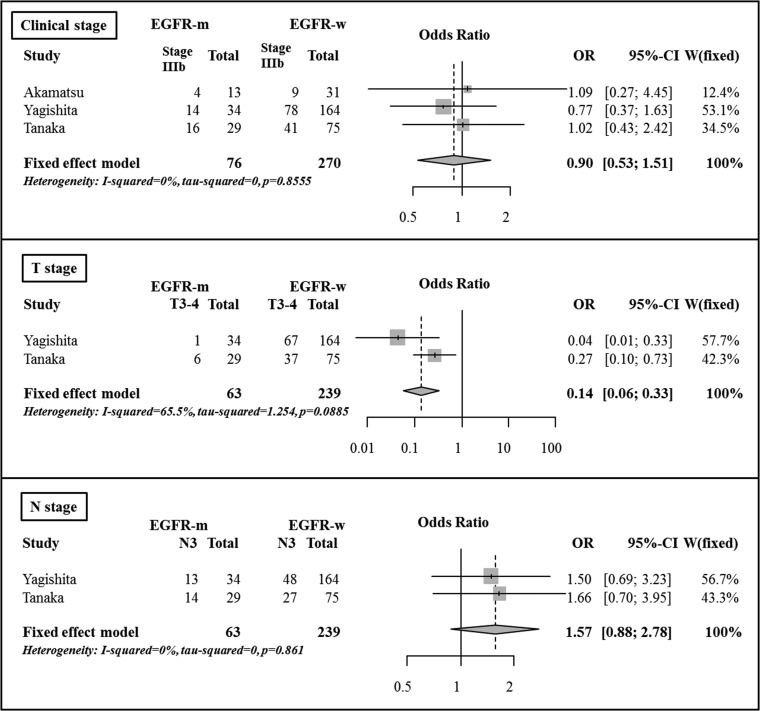
Based on Fisher's exact test, there were no significant differences in the clinical stage (Stage II–IIIA vs IIIB) or N stage (cN0–2 vs cN3) between the two groups. However, there were significant differences in the comparison of clinical T stage (cT1–2 vs cT3–4) between the two groups in both evaluated studies [27, 28]. In the pooled analysis, there was no significant difference in the clinical stage (OR 0.90, 95% CI, 0.53–1.51, P = 0.683) between the two groups. The patients with advanced T stage were less frequently observed in the EGFR mutant group compared with in the EGFR wild-type group (OR 0.14, 95% CI, 0.06–0.33, P < 0.001). The patients with advanced N stage tended to be more frequently observed in the EGFR mutant group, but the difference was not statistically significant (OR 1.57, 95% CI, 0.88–2.78, P = 0.126) (Fig. 2).
Fig. 2.
Comparison of tumor characteristics between the EGFR mutant group and the EGFR wild-type group.
Objective response rate
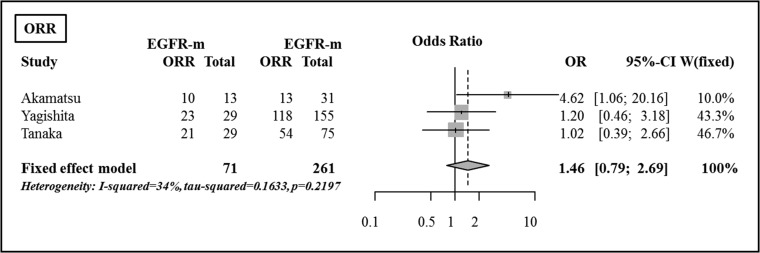
Although there were slight differences in the versions between the studies, ORRs were evaluated using RECIST criteria in all of the studies. RECIST verison 1.0 was used in one study [26], and version 1.1 was used in two studies [27, 28]. ORRs after CRT according to EGFR mutation status are summarized in Table 2. Based on Fisher's exact test, there were no significant differences in the comparison of ORR between the EGFR mutant group and the EGFR wild-type group. There were no significant differences in the ORR between the two groups (OR 1.46, 95% CI, 0.79–2.70, P = 0.228) (Fig. 3).
Table 2.
Objective response rate
| EGFR mutation status | Akamatsu et al. (2014) [26] | Yagishita et al. (2015) [27]a | Tanaka et al. (2015) [28] | |
|---|---|---|---|---|
| Total no. of evaluated Pts | 44 | 184 | 104 | |
| EGFR mutant (%) | 13 (30) | 29 (16) | 29 (28) | |
| Objective response (%) | ||||
| EGFR-m | 10 (77) | 23 (79) | 21 (72) | |
| EGFR-w | 13 (43) | 118 (76) | 54 (72) | |
| P-value | 0.185 | 0.678 | >0.999 |
aPatients who were enrolled in the JCOG 0402 trial, had received RT at a total dose of <50 Gy, or who received epidermal growth factor receptor–tyrosine kinase inhibitor therapy before CRT were excluded. EGFR = epidermal growth factor receptor, Pts = patients, EGFR-m = EGFR mutant group, EGFR-w = EGFR wild-type group, P-value = P-value of Fisher's exact test.
Fig. 3.
Comparison of objective response rate between the EGFR mutant group and the EGFR wild-type group.
Disease recurrence
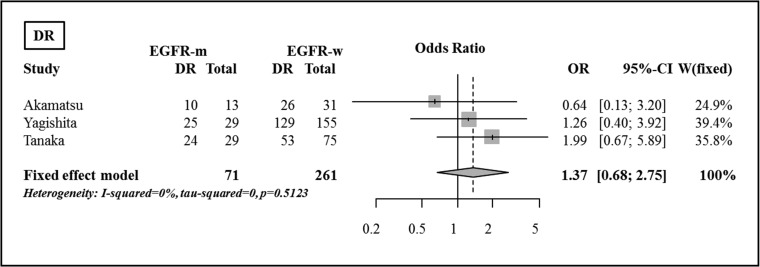
The rates of DR after definitive CRT are summarized in Table 3. There were no significant differences between the rates of DR for the EGFR mutant and the EGFR wild-type groups, using Fisher's exact tests and the pooled analysis (OR 1.37, 95% CI, 0.68–2.75, P = 0.379) (Fig. 4).
Table 3.
Disease recurrence
| EGFR mutation status | Akamatsu et al. (2014) [26] | Yagishita et al. (2015) [27]a | Tanaka et al. (2015) [28] | |
|---|---|---|---|---|
| Total no. of evaluated Pts | 44 | 184 | 104 | |
| No. of Pts (%) | ||||
| EGFR-m | 13 (30) | 29 (16) | 29 (28) | |
| EGFR-w | 31 (70) | 155 (84) | 24 (83) | |
| Disease recurrence (%) | ||||
| EGFR-m | 10 (77) | 25 (86) | 24 (83) | |
| EGFR-w | 26 (84) | 129 (83) | 53 (71) | |
| P-value | 0.676 | 0.503 | 0.318 | |
| Local/locoregional progression (%) | ||||
| EGFR-m | 2 (15) | 5 (17) | 4 (14) | |
| EGFR-w | 10 (32) | 54 (35) | 26 (35) | |
| P-value | 0.459 | 0.039 | 0.052 | |
| Distant progression (%) | ||||
| EGFR-m | 9 (69) | 24 (83) | 22 (76) | |
| EGFR-w | 18 (58) | 102 (66) | 30 (40) | |
| P-value | 0.735 | 0.435 | 0.002 | |
| Brain metastases (%) | ||||
| EGFR-m | 6 (46) | 4 (14) | 10 (35) | |
| EGFR-w | 4 (13) | 15 (10) | 11 (15) | |
| P-value | 0.043 | 0.748 | 0.031 |
aPatients who were enrolled in the JCOG 0402 trial, had received RT at a total dose of <50 Gy, or who received epidermal growth factor receptor–tyrosine kinase inhibitor therapy before CRT were excluded. EGFR = epidermal growth factor receptor, Pts = patients, EGFR-m = EGFR-mutant group, EGFR-w = EGFR wild-type group, P-value = P-value of Fisher's exact test.
Fig. 4.
Comparison of disease recurrence between the EGFR mutant group and the EGFR wild-type group.
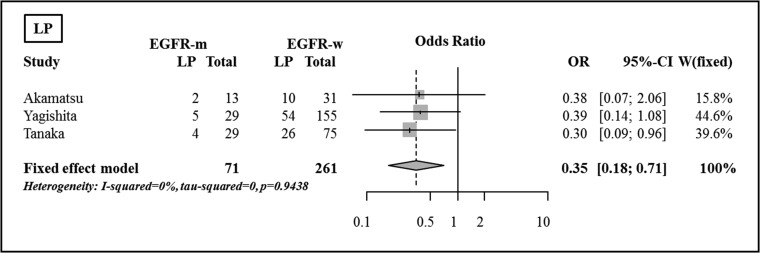
Local/locoregional progression
There was a significant difference between the groups with respect to the incidence of LP for one study, using Fisher's exact test (P = 0.039); the rate of LP was less frequent in the EGFR mutant group (17% vs 35%). In the pooled analysis, LP was shown to be significantly less frequent in the EGFR mutant group compared with the EGFR wild-type group (OR 0.35, 95% CI, 0.18–0.71, P = 0.003) (Fig. 5).
Fig. 5.
Comparison of local/locoregional progression between the EGFR mutant group and the EGFR wild-type group.
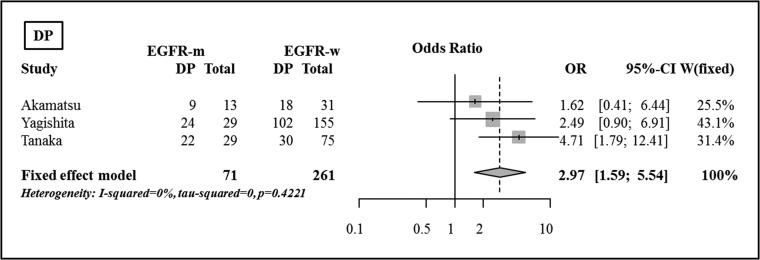
Distant progression
There were significant differences in the incidences of DP between the two groups in one study, based on Fisher's exact test [28]. DP was reported to be more frequent in the EGFR mutant group (76% vs 40%, P = 0.002) [28]. In the pooled analysis, DP was shown to be significantly more frequent in the EGFR mutant group compared with in th EGFR wild-type group (OR 2.97, 95% CI, 1.59–5.54, P < 0.001), and the incidence of DP was significantly higher in the EGFR mutant group compared with in the EGFR wild-type group (Fig. 6).
Fig. 6.
Comparison of distant progression between the EGFR mutant group and the EGFR wild-type group.
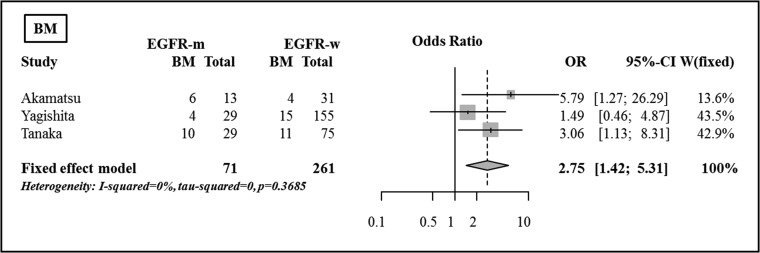
Brain metastases
For the BM evaluation, three studies contained enough data to be included [26–28]. There were significant differences between the two groups in the incidence of BM in two of the studies, based on Fisher's exact test [26, 28]. The incidence of BM was higher in the EGFR mutant group in both studies (46% vs 13%, P = 0.043; 35% vs 11%, P = 0.031). In the pooled analysis, the incidence of BM was shown to be significantly higher in the EGFR mutant group compared with in the EGFR wild-type group (OR 2.75, 95% CI, 1.43–5.31, P = 0.003) (Fig. 7).
Fig. 7.
Comparison of brain metastases between the EGFR mutant group and the EGFR wild-type group.
Progression/relapse-free survival
The terms and definitions used for these endpoints differed between the studies. The term ‘PFS’ was used in two of the studies [26, 28], but ‘RFS’ was used in another trial [27]. Although there were slight differences in the definitions used, these endpoints were calculated from the date of initiation of CRT to detection of DR or death from any cause in the two studies [26, 27]. PFS was calculated from the date of initiation of CCRT to either the date of recurrence or the date of last contact [28]. The reported results for RFS/PFS are summarized in Table 4. The 2-year estimated probabilities were based on Kaplan–Meier plots in one study [26]. There were no statistically significant differences in RFS/PFS between the EGFR mutant group and the EGFR wild-type group, except for in one study [28], in which the definition of PFS was different from that of the other studies.
Table 4.
Progression/relapse-free survival and overall survival
| Study | EGFR mutation status | Akamatsu et al. (2014) [26] | Yagishita et al. (2015) [27]a | Tanaka et al. (2015) [28] |
|---|---|---|---|---|
| Evaluated outcome | PFS | RFS | PFS | |
| Median time (mo) | ||||
| EGFR-m | 9.6 | 12.1 | 9.8 | |
| EGFR-w | 13.2 | 10.9 | 16.5 | |
| 2-year estimated probability | ||||
| EGFR-m | (24)b | 22 | 7.7 | |
| EGFR-w | (29)b | 30 | 28.1 | |
| Reported P-value | 0.78 | 0.545 | 0.028 | |
| Median survival time (mo) | ||||
| EGFR-m | 57 | 46.9 | 51.1 | |
| EGFR-w | 30.7 | 33.3 | 42.9 | |
| Reported P-value | NA | 0.158 | 0.637 |
aPatients who were enrolled in JCOG 0402 trial, had received RT at a total dose of <50 Gy, or who had received epidermal growth factor receptor–tyrosine kinase inhibitor therapy before CRT were excluded.
bThe values were estimated using reported Kaplan–Meier plots. EGFR = epidermal growth factor receptor, PFS = progression-free survival, RFS = relapse-free survival, mo = months, EGFR-m = EGFR-mutant group, EGFR-w = EGFR wild-type group, P-value = P-value of log-rank test, NS = not statistically significant, NA = not available.
Overall survival
Although there were slight differences in the definition, this endpoint was calculated from the initiation of CRT to the date of death from any cause in three studies [26–28]. The reported results of OS are summarized in Table 4. There were no significant differences in OS between the EGFR mutant group and the EGFR wild-type group.
DISCUSSION
In this article, we evaluated the differences between the EGFR mutant group and the EGFR wild-type group in the setting of definitive CRT for locally advanced NSCLC. Females (OR 4.94, P < 0.001) and never-smokers (OR 11.10, P < 0.001) were more frequently observed in the EGFR mutant group compared with in the EGFR wild-type group. It has been reported that EGFR mutation is seen more frequently in non-smoking females with adenocarcinoma, and our results were compatible with those findings [30–32]. There were no significant differences in clinical stage (OR 0.90, P = 0.683) or N stage (OR 1.57, P = 0.126) between the EGFR mutant group and the EGFR wild-type group. However, patients with advanced T stage were less frequently observed in the EGFR mutation group (OR 0.14, P < 0.001).
In ORR, there was no significant difference between the EGFR mutant group and the EGFR wild-type group in the pooled analysis (OR 1.46, P = 0.228). Gow et al. reported the clinical response to whole brain radiation therapy (WBRT) for BM from lung adenocarcinoma [17]. In their analysis, the response rate was more favorable in the EGFR mutant group compared with in the EGFR wild-type group (54% vs 24%, P = 0.045), and administration of EGFR tyrosine kinase inhibitor (TKI) (P = 0.034) and an EGFR mutation (P = 0.029) were shown to be independent factors associated with response to WBRT. Thus, the ORR after CRT for locally advanced NSCLC and BM could be different. BM could be resistant to systemic chemotherapy due to the blood–brain barrier [33]. On the other hand, all the patients included in our analysis were administered chemotherapy. The effect of chemotherapy might contribute to minimizing the difference in response to RT in locally advanced NSCLC.
Overall, the incidence of DR did not differ between the EGFR mutant group and the EGFR wild-type group (OR 1.37, P = 0.379). However, the patterns of recurrence did differ between the two groups. The incidence of LP was shown to be significantly less frequent in the EGFR mutant group (OR 0.35, P = 0.003) in our pooled analysis. One possible explanation for the difference in LP is the difference in sensitivity to radiation as shown in the in vitro study [16]. Another possible explanation is the difference in T stage between the EGFR mutant group and the EGFR wild-type group. Patients with advanced T stage were less frequently observed in the EGFR mutant group compared with in the EGFR wild-type group. Although advanced T stage could be a risk factor for LP after CRT for NSCLC, this is still controversial [34]. Among the studies that were included in our analysis, risk factors for LP were investigated in one study [27]. Neither T stage nor diameter of the primary tumor were significant factors for time to local relapse in univariate and multivariate analysis. Schytte et al. investigated risk factors for LP after definitive RT for NSCLC using logistic regression analysis [34]. Gross tumor volume was the only significant factor for intrapulmonary failure (P = 0.04), and advanced T stage (T3/4) had borderline significance (P = 0.06) in their study. Although there could be a significant correlation between T stage and gross tumor volume, gross tumor volume was reported to be a risk factor for locoregional failure after CCRT for NSCLC in another study [35]. The Cox proportional hazard model was used to investigate risk factors for locoregional failure in the analysis, and log10 (volume) was shown to be the only significant factor for locoregional failure in multivariate analysis. These findings indicate that tumor volume is a more important factor than T stage or N stage for LP after CRT for NSCLC. Correlation between tumor volume and LP was not evaluated in the studies that were included in our analysis, and this needs further investigation. On the other hand, Yagishita et al. reported that timing of chemotherapy (sequential vs concurrent) was the significant factor correlated with time to local relapse in univariate analysis (P = 0.036), and this correlation had borderline significance in multivariate analysis (P = 0.054) [27]. This was compatible with the results of several randomized trials and meta-analyses [36, 37].
The incidence of DP was significantly higher in the EGFR mutant group (OR 2.97, P < 0.001) compared with in the EGFR-wild type group. The brain has been reported to be the site most often affected in EGFR-mutant patients after CRT for locally advanced NSCLC [28]. In our pooled analysis, the incidence of BM was shown to be significantly higher in the EGFR mutant group compared with in the EGFR-wild type group (OR 2.75, P = 0.003).
Considering these results, LP after CRT for NSCLC is less frequent in patients with EGFR mutations, and CRT is considered to be the effective local treatment. On the other hand, a high incidence of DP, especially BM, is still a major problem. In patients with EGFR mutations, the benefit of administration of TKI as adjuvant or maintenance therapy has been reported in patients with locally advanced or metastatic NSCLC [38, 39], and this could be promising in decreasing DP, including BM, after CRT. Although the considerations for prognosis of patients with BM from NSCLC had been insufficient [40, 41], the survival time after diagnosis of BM has been reported to be longer in patients with EGFR mutations compared with those without EGFR mutations. [42, 43]. In the treatment of BM, upfront cranial RT for patients has been reported to improve intracranial disease PFS and OS compared with TKI alone [44]. However, neurological adverse events are still a problem. Considering the relatively favorable prognosis of these patients, concomitant use of memantine or hippocampal sparing during WBRT would be beneficial to reduce the potentially negative effects of WBRT on cognitive function [45–48].
On the other hand, local control after CRT is far from satisfactory in patients in the EGFR wild-type group, and LP after CRT is still a major problem. At this time, the benefits int terms of survival or local control from dose escalation with conventionally fractionated RT above 60 Gy in unselected locally advanced NSCLC are unclear according to the results of RTOG 0617 [49]. In the trial, the irradiated dose to the normal organs was higher in the dose-escalated group (74 Gy) compared with inn the standard-dose group (60 Gy) [50, 51]. Liao et al. reported that the irradiated dose to the heart and lung correlated with OS after CRT for locally advanced NSCLC, and the survival of patients who were irradiated with a higher dose to these organs was unfavorable [52]. To reduce the radiation dose to normal organs, irradiation with intensity-modulated radiation therapy (IMRT) would be useful. Indeed, IMRT was reported to be associated with an improvement in the median OS and 5-year survival rate, in patients with T3 and T4 disease, compared with 3D conformal radiation therapy (P = 0.021) [53]. This association was also confirmed in a propensity score–matched cohort of T3 and T4 patients (hazard ratio: 0.80, 95% CI, 0.64–1.00, P = 0.048). Patients with EGFR wild-type more frequently had an advanced tumor, and IMRT might provide a survival benefit for this population.
The analyses of this article had several limitations. The studies included in our analyses were all retrospective studies and from a single institution. The policies of treatment and follow-up might vary among institutions. The differences in histology included in the studies also pose a limitation. The treatment efficacies and patterns of recurrence after CRT might be differ between the histologies of NSCLC. The studies included in our analysis were all from Japan and most of the included patients were Asian. Difference between ethnicities could not be evaluated. Despite these limitations, our analyses contribute to understanding the effects of EGFR mutation status on patterns of recurrence after CRT for locally advanced NSCLC.
In conclusion, we reviewed literature that compared the outcomes according to EGFR mutation status after CRT for locally advanced NSCLC. In patients with EGFR mutation, CRT seemed to be a highly effective treatment for local control, and RT is essential in multimodality treatment. DP, especially BM, is the major problem in this population. In patients with EGFR wild-type, LP still remains a major problem. Patients frequently have a tumor with advanced T stage, and the application of advanced RT technique would contribute to an improved outcome by reducing the radiation dose to the normal organs in this population.
FUNDING
This work was supported by Matsusaka Central Hospital.
CONFLICT OF INTEREST
There is no conflict of interest, grant or any other assistance to be disclosed for any of the authors.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. . Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Groome PA, Bolejack V, Crowley JJ, et al. . The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:694–705. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, DeSantis C, Virgo K, et al. . Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220–41. [DOI] [PubMed] [Google Scholar]
- 4.Yalman D. Neoadjuvant radiotherapy/chemoradiotherapy in locally advanced non–small cell lung cancer. Balkan Med J 2015;32:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schild SE, Vokes EE.. Pathways to improving combined modality therapy for stage III nonsmall-cell lung cancer. Ann Oncol 2016;27:590–9. [DOI] [PubMed] [Google Scholar]
- 6.Pallis AG, Gridelli C, van Meerbeeck JP, et al. . EORTC Elderly Task Force and Lung Cancer Group and International Society for Geriatric Oncology (SIOG) experts’ opinion for the treatment of non–small-cell lung cancer in an elderly population. Ann Oncol 2010;21:692–706. [DOI] [PubMed] [Google Scholar]
- 7.Blanco R, Maestu I, de la Torre MG, et al. . A review of the management of elderly patients with non–small-cell lung cancer. Ann Oncol 2015;26:451–63. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch FR, Scagliotti GV, Langer CJ, et al. . Epidermal growth factor family of receptors in preneoplasia and lung cancer: perspectives for targeted therapies. Lung Cancer 2003;41:S29–42. [DOI] [PubMed] [Google Scholar]
- 9.Scagliotti GV, Selvaggi G, Novello S, et al. . The biology of epidermal growth factor receptor in lung cancer. Clin Cancer Res 2004;10:4227s–32s. [DOI] [PubMed] [Google Scholar]
- 10.Sharma SV, Bell DW, Settleman J, et al. . Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169–81. [DOI] [PubMed] [Google Scholar]
- 11.Zheng DJ, Yu GH, Gao JF, et al. . Concomitant EGFR inhibitors combined with radiation for treatment of non–small cell lung carcinoma. Asian Pac J Cancer Prev 2013;14:4485–94. [DOI] [PubMed] [Google Scholar]
- 12.Korpanty GJ, Graham DM, Vincent MD, et al. . Biomarkers that currently affect clinical practice in lung cancer: EGFR, ALK, MET, ROS-1, and KRAS. Front Oncol 2014;4:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch TJ, Bell DW, Sordella R, et al. . Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–39. [DOI] [PubMed] [Google Scholar]
- 14.Paez JG, Jänne PA, Lee JC, et al. . EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–500. [DOI] [PubMed] [Google Scholar]
- 15.Mitsudomi T, Yatabe Y.. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci 2007;98:1817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das AK, Sato M, Story MD, et al. . Non–small-cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res 2006;66:9601–8. [DOI] [PubMed] [Google Scholar]
- 17.Gow CH, Chien CR, Chang YL, et al. . Radiotherapy in lung adenocarcinoma with brain metastases: effects of activating epidermal growth factor receptor mutations on clinical response. Clin Cancer Res 2008;14:162–8. [DOI] [PubMed] [Google Scholar]
- 18.Lee HL, Chung TS, Ting LL, et al. . EGFR mutations are associated with favorable intracranial response and progression-free survival following brain irradiation in non–small cell lung cancer patients with brain metastases. Radiat Oncol 2012;7:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsiao SH, Lin HC, Chou YT, et al. . Impact of epidermal growth factor receptor mutations on intracranial treatment response and survival after brain metastases in lung adenocarcinoma patients. Lung Cancer 2013;81:455–61. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, et al. . Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mak RH, Doran E, Muzikansky A, et al. . Outcomes after combined modality therapy for EGFR-mutant and wild-type locally advanced NSCLC. Oncologist 2011;16:886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F, Bai H, Li X, et al. . Role of EGFR mutation status in patients with stage III non-squamous non–small cell lung cancer treated with chemoradiotherapy. Zhongguo Fei Ai Za Zhi 2011;14:715–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi H, Okamoto I, Kimura H, et al. . Clinical outcomes of thoracic radiotherapy for locally advanced NSCLC with EGFR mutations or EML4-ALK rearrangement. Anticancer Res 2012;32:4533–7. [PubMed] [Google Scholar]
- 25.Ahn HK, Choi YL, Han JH, et al. . Epidermal growth factor receptor mutation and treatment outcome of mediastinoscopic N2 positive non–small cell lung cancer patients treated with neoadjuvant chemoradiotherapy followed by surgery. Lung Cancer 2013;79:300–6. [DOI] [PubMed] [Google Scholar]
- 26.Akamatsu H, Kaira K, Murakami H, et al. . The impact of clinical outcomes according to EGFR mutation status in patients with locally advanced lung adenocarcinoma who recieved concurrent chemoradiotherapy. Am J Clin Oncol 2014;37:144–7. [DOI] [PubMed] [Google Scholar]
- 27.Yagishita S, Horinouchi H, Katsui Taniyama T, et al. . Epidermal growth factor receptor mutation is associated with longer local control after definitive chemoradiotherapy in patients with stage III nonsquamous non–small-cell lung cancer. Int J Radiat Oncol Biol Phys 2015;91:140–8. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka K, Hida T, Oya Y, et al. . EGFR mutation impact on definitive concurrent chemoradiation therapy for inoperable stage iii adenocarcinoma. J Thorac Oncol 2015;10:1720–5. [DOI] [PubMed] [Google Scholar]
- 29.Glatzer M, Elicin O, Ramella S. Radio(chemo)therapy in locally advanced nonsmall cell lung cancer. Eur Respir Rev 2016;25:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shigematsu H, Lin L, Takahashi T, et al. . Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339–46. [DOI] [PubMed] [Google Scholar]
- 31.Usui K, Ushijima T, Tanaka Y, et al. . The frequency of epidermal growth factor receptor mutation of nonsmall cell lung cancer according to the underlying pulmonary diseases. Pulm Med 2011;2011:290132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prabhakar CN. Epidermal growth factor receptor in non–small cell lung cancer. Transl Lung Cancer Res 2015;4:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ba JL, Jandial R, Nesbit A, et al. . Current and emerging treatments for brain metastases. Oncology (Williston Park) 2015;29:250–7. [PubMed] [Google Scholar]
- 34.Schytte T, Nielsen TB, Brink C, et al. . Pattern of loco-regional failure after definitive radiotherapy for non–small cell lung cancer. Acta Oncol 2014;53:336–41. [DOI] [PubMed] [Google Scholar]
- 35.van Diessen JN, Chen C, van den Heuvel MM, et al. . Differential analysis of local and regional failure in locally advanced non–small cell lung cancer patients treated with concurrent chemoradiotherapy. Radiother Oncol 2016;118:447–52. [DOI] [PubMed] [Google Scholar]
- 36.Furuse K, Fukuoka M, Kawahara M, et al. . Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non–small-cell lung cancer. J Clin Oncol 1999;17:2692–9. [DOI] [PubMed] [Google Scholar]
- 37.Aupérin A, Le Péchoux C, Rolland E, et al. . Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non–small-cell lung cancer. J Clin Oncol 2010;28:2181–90. [DOI] [PubMed] [Google Scholar]
- 38.Kelly K, Altorki NK, Eberhardt WE, et al. . Adjuvant Erlotinib Versus Placebo in Patients With Stage IB-IIIA Non-Small-Cell Lung Cancer (RADIANT): a randomized, double-blind, phase iii trial. J Clin Oncol 2015;33:4007–14. [DOI] [PubMed] [Google Scholar]
- 39.Zhao H, Fan Y, Ma S, et al. . Final overall survival results from a phase III, randomized, placebo-controlled, parallel-group study of gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804). J Thorac Oncol 2015;10:655–64. [DOI] [PubMed] [Google Scholar]
- 40.Nieder C, Bremnes RM, Andratschke NH.. Prognostic scores in patients with brain metastases from non–small cell lung cancer. J Thorac Oncol 2009;4:1337–41. [DOI] [PubMed] [Google Scholar]
- 41.Sperduto PW, Chao ST, Sneed PK, et al. . Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 2010;77:655–61. [DOI] [PubMed] [Google Scholar]
- 42.Eichler AF, Kahle KT, Wang DL, et al. . EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol 2010;12:1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sekine A, Satoh H, Iwasawa T, et al. . Prognostic factors for brain metastases from non–small cell lung cancer with EGFR mutation: influence of stable extracranial disease and erlotinib therapy. Med Oncol 2014;31:228. [DOI] [PubMed] [Google Scholar]
- 44.Soon YY, Leong CN, Koh WY, et al. . EGFR tyrosine kinase inhibitors versus cranial radiation therapy for EGFR mutant non–small cell lung cancer with brain metastases: a systematic review and meta-analysis. Radiother Oncol 2015;114:167–72. [DOI] [PubMed] [Google Scholar]
- 45.Brown PD, Pugh S, Laack NN, et al. . Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gondi V, Pugh SL, Tome WA, et al. . Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 2014;32:3810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caine C, Deshmukh S, Gondi V, et al. . CogState computerized memory tests in patients with brain metastases: secondary endpoint results of NRG Oncology RTOG 0933. J Neurooncol 2016;126:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owen S, Souhami L.. The management of brain metastases in non–small cell lung cancer. Front Oncol 2014;4:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bradley JD, Paulus R, Komaki R, et al. . Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non–small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong JC, Salama JK.. Dose escalation for unresectable locally advanced non–small cell lung cancer: end of the line. Transl Lung Cancer Res 2016;5:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao Z, Tucker SL, Gomez D, et al. . Heart and lung radiation and overall survival in non–small cell lung cancer patients after chemoradiation therapy. Int J Radiat Oncol Biol Phys 2012;84:S578. [Google Scholar]
- 52.Jegadeesh N, Liu Y, Gillespie T, et al. . Evaluating intensity-modulated radiation therapy in locally advanced non–small-cell lung cancer: results from the National Cancer Data Base. Clin Lung Cancer, 10.1016/j.cllc.2016.01.007 (2 February 2016, date last accessed). [DOI] [PubMed] [Google Scholar]
- 53.Jegadeesh N, Liu Y, Gillespie T, et al. . Evaluating Intensity-Modulated Radiation Therapy in Locally Advanced Non-Small-Cell Lung Cancer: Results From the National Cancer Data Base. Clin Lung Cancer 2016. Feb 2. pii: S1525-7304(16)30004-3. 10.1016/j.cllc.2016.01.007. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]