Abstract
Objective
To determine the impact of tocilizumab on physical function and quality of life in patients diagnosed with rheumatoid arthritis.
Methods
A systematic literature review was performed to select for trials that could be used to examine the impact of tocilizumab on patients in terms of health-related physical function, quality of life, and quality of sleep. By examining background therapy, disease duration, and remission rates, we were able to determine the impact that a dose of tocilizumab has on various patients.
Results
A total of 2617 tocilizumab-treated patients and 1271 controls were available for this study. Tocilizumab improved the Health Assessment Questionnaire Disability Index score statistically in comparison to the controls, with odds ratios from 1.4 to 7.0. Tocilizumab improved the physical function measure substantially more than the minimal clinically important difference (MCID) (5 units) – 8.9 and 9.7 – compared to 4.1 and 5.0 for controls. Seven and nine units of improvement were observed when measuring fatigue in rheumatoid arthritis patients. Using the Epworth Sleepiness Scale, we found that sleep improved (from 7.7 [3.1] to 3.4 [2.2]).
Conclusion
Tocilizumab improves function and quality of life and decreases fatigue in patients with rheumatoid arthritis.
Keywords: tocilizumab, rheumatoid arthritis, quality of life, sleep, randomized trials
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by joint swelling, joint pain, fatigue, and even loss of certain crucial physical functions. Disease-modifying antirheumatic drugs are standard care for RA. Recent studies have shown that interleukin 6 (IL-6) plays an important role in inflammation. IL-6 is a membrane receptor produced by cells such as lymphocytes and monocytes, as well as other cell types. IL-6 activates T cells and catalyses B cell proliferation. IL-6 also stimulates osteoclast differentiation, leading to joint destruction.
Tocilizumab (TCZ) is a humanized anti-IL-6 receptor (anti-IL-6R) monoclonal antibody. TCZ can bind to either soluble or membrane-bound IL-6 receptors. When bound, TCZ blocks the IL-6 receptor and prevents or decreases inflammation. It has been approved by the FDA for use in patients diagnosed with moderate to severe rheumatoid arthritis.1 The purpose of this article is to review the effect that TCZ has on patients’ physical function, quality of life (QoL), level of fatigue, and sleep patterns.1,2
Materials and methods
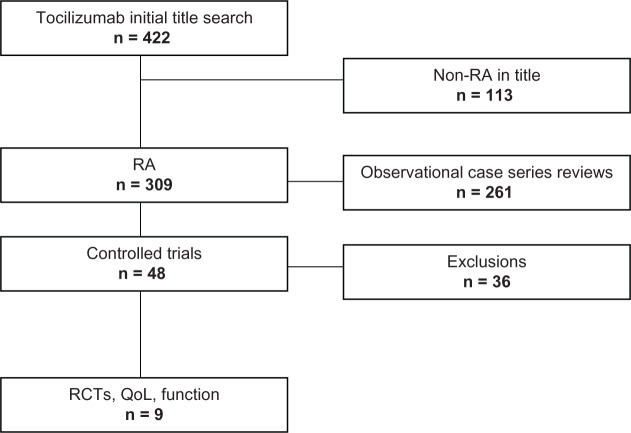
As seen in Figure 1, a systematic review of TCZ was undertaken with the following keywords used as criteria for the search: TCZ, clinical trial, rheumatoid arthritis, human, and English. Also of interest were the following: health-related quality of life, activities of daily living, quality of life, fatigue, sleep, Health Assessment Questionnaire Disability Index (HAQ-DI), Short Form 36 (SF-36), European Quality of Life-5 Dimensions (EQ-5D), Functional Assessment of Chronic Illness Therapy (FACIT), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Pittsburgh Sleep Quality Index (PSQI), and Epworth Sleepiness Scale (ESS). Exclusions included reviews, editorials, non-RA studies, case series, and randomized non-trials that did not produce quantifiable data for any one of the following factors: functionality, QoL, fatigue, or sleep. PubMed and the Cochrane databases were queried and bibliographies in all articles were examined. Two individuals extracted data independently from the final articles and any disagreements regarding whether or not the articles stayed within the criteria were discussed. An agreement was reached, resulting in eight relevant articles.
Figure 1.
A systematic literature review was undertaken.
Abbreviations: RA, rheumatoid arthritis; RCTs, randomized controlled trials; QoL, quality of life.
Because randomized controlled trials (RCTs) are of higher quality, this review concentrates on the RCTs, while the remaining open-label trials are used for support. For one domain, sleep, only a single, open-label study was available.
Table 1 describes the validated measures of function, quality of life, fatigue, and sleep used in this study.3–10
Table 1.
Descriptions of the most commonly used measurements of function, quality of life, fatigue, and sleep
| Method | Measures | Domains | Range | MCID |
|---|---|---|---|---|
| HAQ-DI3 | Health-related physical function | Dressing, rising, eating, walking, hygiene, reach, grip, and daily activity | 0 to 3 | 0.22 |
| SF-364 | Quality of life | Physical activities, social activities, role functioning due to emotional distress, role functioning due to physical impairment, bodily pain, general mental health, general health perceptions, and vitality | 0 to 100 | 5–10 |
| EQ-5D5,6 | Differences in health states in RA | Mobility, self-care, usual activities, pain discomfort and anxiety/depression (mood disorders) | 0 to 1 | – |
| FACIT7–9 | Quality of life | Physical wellbeing, social/family wellbeing, emotional wellbeing, functional wellbeing | 0 to 100 | – |
| FACIT-F7–9 | Fatigue | 13 items on a 0–4 scale; ie, fatigue, weakness, listlessness, tiredness, energy, difficulty starting things, difficulty finishing things, able to do usual activities, need for assistance in doing usual activities, sleeping during the day, too tired to eat, frustration due to feeling too tired to do things you want, limitation of social activity due to tiredness | 0 to 52 | 4 |
| PSQI10 | Quality of sleep | Sleep quality, sleep latency, sleep duration, habitual sleep disturbance, sleep disturbance, use of sleep medication, daytime dysfunction | 0 to 21 | – |
| ESS10 | Sleepiness | Tendency to fall asleep in certain situations of daily life: reading, watching TV, sitting in public place, sitting in the car as passenger, resting in the afternoon, talking, sitting after lunch, sitting in car after stopping for traffic | 0 to 24 | – |
Abbreviations: HAQ-DI, Health Assessment Questionnaire Disability Index; SF-36, Short Form 36; EQ-5D, European Quality of Life-5 Dimensions; FACIT, Functional Assessment of Chronic Illness Therapy; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; PSQI, Pittsburgh Sleep Quality Index; ESS, Epworth Sleepiness Scale.
Results and discussion
Table 2 reviews the eight RCTs included in the systematic review.1,2,11–16 They include 2617 TCZ-treated patients and 1271 controls. Most of the patients were followed in 24-week studies, while one study was 16 weeks, one was 52 weeks, and one was 12 weeks. The patients were quite heterogeneous, with disease duration lasting from less than one year to more than eleven years. Patients had used a wide variety of background therapy and/or previous medications. The background therapies ranged from an 8 mg per week dosage of methotrexate to having had an incomplete response to two tumor necrosis factor inhibitors. Nonetheless, all of the patients’ RA activity was very vigorous, with disease activity scores, using 28 joint counts, between 6.1 and 6.8. This degree of heterogeneity precludes a credible meta-analysis.
Table 2.
Description of RCTs
| Study | Trial duration | Rx (dose) | Background therapy | n | Disease duration | RF +ve | Baseline DAS28 |
|---|---|---|---|---|---|---|---|
| Nishimoto et al11 | 24 wks | TCZ 8.00 mg/kg every 4 weeks | MTX-IR | 61 | 8.50 yrs | NA | 6.10 |
| Placebo + MTX 8.00 mg/week | 64 | 8.70 yrs | NA | 6.20 | |||
| Maini et al12 | 16 wks | TCZ 2.00 mg/kg | MTX-IR | 53 | 9.19 mos | 83.0% | 6.48 |
| TCZ 4.00 mg/kg | 54 | 9.79 mos | 72.2% | 6.55 | |||
| TCZ 8.00 mg/kg (every 4 weeks) | 52 | 9.21 mos | 82.7% | 6.43 | |||
| TCZ 2.00 mg/kg + MTX | 52 | 9.33 mos | 88.5% | 6.58 | |||
| TCZ 4.00 mg/kg + MTX | 49 | 7.82 mos | 77.6% | 6.34 | |||
| TCZ 8.00 mg/kg + MTX | 50 | 10.62 mos | 80.0% | 6.47 | |||
| Placebo + MTX (MTX 10.00–25.00 mg/wk) | 49 | 11.24 mos | 95.9% | 6.75 | |||
| Genovese et al1 | 24 wks | TCZ 8.00 mg/kg + DMARD | DMARD-IR | 803 | 9.80 yrs | NA | 6.70 |
| DMARD | 413 | 9.80 yrs | NA | 6.60 | |||
| Jones et al13 | 24 wks | TCZ 8.00 mg/kg every 4 wks | Never failed MTX or biological agents in the past | 288 | 6.40 yrs | NA | 6.80 |
| MTX 7.50 mg/wk (titrated to 20.00 mg/kg at 8 wks) | 284 | 6.20–6.30 yrs | NA | 6.80 | |||
| Placebo (first 8 wks), then TCZ for 16 wks | 101 | NA | NA | NA | |||
| Emery et al14 | 24 wks | TCZ 8.00 mg/kg + MTX | TNFi-IR | 170 | 12.60 yrs | 79.00% | 6.79 |
| TCZ 4.00 mg/kg + MTX | 161 | 11.00 yrs | 73.00% | 6.78 | |||
| Placebo + MTX | 158 | 11.40 yrs | 75.00% | 6.80 | |||
| Nishimoto et al15 | 52 wks | TCZ 8.00 mg/kg every 4 weeks | DMARD-IR or failed immunosuppressant | 157 | 2.20 yrs | NA | 6.50 |
| DMARD | 145 | 2.40 yrs | NA | 6.40 | |||
| Smolen et al2 | 24 wks | TCZ 4.00 mg/kg + MTX | MTX discontinued ≥12 weeks before study | 214 | 7.40 yrs | 78.00% | 6.80 |
| TCZ 8.00 mg/kg + MTX (every 4 wks) | 205 | 7.50 yrs | 83.00% | 6.80 | |||
| Placebo + MTX (MTX 10.00–25.99 mg/wk) | 204 | 7.80 yrs | 71.00% | 6.80 | |||
| Nishimoto et al16 | 12 wks | TCZ 4.00 mg/kg | DMARD-IR or failed immunosuppressant | 54 | 7.30 yrs | NA | NA |
| TCZ 8 mg/kg (every 4 wks) | 55 | 8.30 yrs | NA | NA | |||
| Placebo | 53 | 8.40 yrs | NA | NA | |||
| Choy et al17 | 8 wks | TCZ 0.1 mg/kg | DMARD-IR or failed immunosuppressant | 9 | 17.00 yrs | NA | NA |
| TCZ 1 mg/kg | 9 | 6.00 yrs | NA | NA | |||
| TCZ 5 mg/kg | 9 | 14.00 yrs | NA | NA | |||
| TCZ 10 mg/kg | 7 | 13.00 yrs | NA | NA | |||
| Placebo | 11 | 14.00 yrs | NA | NA |
Abbreviations: TCZ, tocilizumab; MTX, methotrexate; DMARD, disease-modifying antirheumatic drug; RCT, randomized controlled trial.
Table 3 displays the results of the health questionnaires’ disability index (HAQ-DI) responses in the eight RCTs, where such data were available.1,2,11,13–17 TCZ improved the HAQ-DI score statistically in comparison to the controls, with odds ratios from 1.4 to 7.0, favoring TCZ. The percent of patients who improved more than the MCIDs (≥0.22) were noted in four studies, and the TCZ groups improved by at least that amount in 60%–68% of the patients, compared to 32%–48% of the controls. It is clear that TCZ improves disease-related function significantly and more frequently than the controls, in turn making the amount of improvement clinically important. Open-label studies using TCZ supported the RCT findings. HAQ-DI normalization (<0.5) occurred in 23%–48% of TCZ-treated patients in four trials.18–22
Table 3.
Double-blind studies examining HAQ-DI
| Study | Treatment dose | Baseline HAQ-DI | Changes from baseline | P-value | MCID (≥0.22) | P-value |
|---|---|---|---|---|---|---|
| Nishimoto et al11 | TCZ | NA | NA | NA | 67% | P < 0.001 |
| Control | NA | NA | NA | 34% | NA | |
| Genovese et al1 | TCZ 8 mg/kg | 1.50 | −0.50 | P < 0.0001 | 60% | NA |
| Placebo + DMARDS | 1.50 | −0.20 | NA | 34%b | NA | |
| Jones et al13 | TCZ | 1.50 | −0.70 | NA | NA | NA |
| MTX | 1.60 | −0.50 | NA | NA | NA | |
| Emery et al14 | TCZ 4 | 1.70 | −0.31 | P = 0.003 | NA | NA |
| TCZ 8 | 1.70 | −0.39 | Less than P < 0.001 | NA | NA | |
| Control | 1.70 | −0.05 | NA | NA | NA | |
| Nishimoto et al15 | TCZ 8 mg/kg every 4 weeks | 0.80 | −0.50 | P < 0.001 | 68% | P < 0.001 |
| DMARD | 0.90a | −0.30a | NA | 40% | NA | |
| Smolen et al2 | TCZ 4.00 mg/kg | 1.60 | −0.52 | P = 0.0296 | 61% | NA |
| TCZ 8.00 mg/kg | 1.60 | −0.55 | P = 0.0082 | 59% | NA | |
| Control | 1.50 | −0.34 | NA | 47%b | NA | |
| Nishimoto et al16 | TCZ 4.00 mg/kg | 1.00 | −0.01 | P < 0.0100 | NA | NA |
| TCZ 8.00 mg/kg | 1.00 | −0.25 | NA | NA | NA | |
| Control | 1.00a | −0.35a | NA | NA | NA | |
| Choy et al17 | TCZ 0.10 mg/kg | 2.30 | −0.30 | NA | NA | NA |
| TCZ 1.00 mg/kg | 2.40 | −0.10 | NA | NA | NA | |
| TCZ 5.00 mg/kg | 2.00 | −0.80 | NA | NA | NA | |
| TCZ 10.00 mg/kg | 2.60 | −0.60 | NA | NA | NA | |
| Placebo | 2.60a | −0.10a | NA | NA | NA |
Notes:
Approximately;
HAQ ≥ 0.30.
Abbreviations: TCZ, tocilizumab; MTX, methotrexate; DMARD, disease-modifying antirheumatic drug; HAQ-DI, Health Assessment Questionnaire Disability Index; MCID, minimal clinically important difference.
Table 4 displays the results regarding QoL (SF-36). There were only two double-blind RCTs of TCZ that described this measure.1,2 The Physical Component Summary comprises4 the four domains of the SF-36 pertaining to physical function, and it is statistically clear that TCZ improved this measure substantially more than the MCID (5 units) – 8.9 and 9.7 – compared to 4.1 and 5.0 for controls. Unusually, TCZ also statistically improved the Mental Component Summary4 versus control, implying that TCZ may have a positive effect on mood and mental status. As for the HAQ-DI, it is reasonably clear that TCZ both physically and mentally improves QoL in patients diagnosed with RA. The strength of this conclusion is less than that for the HAQ-DI, because there are only two controlled studies measuring QoL.
Table 4.
Double-blind studies examining SF-36 and FACIT-F
| Study | Treatment Dose | Baseline FACIT-F | Changes in FACIT-F from baseline | P-value | Baseline SF-36 scores
|
Changes from baseline
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Physical | Mental | Physical | P-value | Mental | P-value | |||||
| Genovese et al1 | TCZ 8.00 mg/kg | NA | 8.0 | P < 0.0001 (>MCID) | NA | NA | 8.9 | P < 0.0001 | 5.3 | P < 0.0001 |
| Placebo + DMARDS | NA | 3.6 | NA | NA | NA | 4.1 | P < 0.0001 | 2.3 | P < 0.0001 | |
| Smolen et al2 | TCZ 4.00 mg/kg | 27.0 | 7.3 | P = 0.0063 (>MCID) | 31.5 | 40.1 | 9.7 | P < 0.0010 | 5.7 | P = 0.0394 |
| TCZ 8.00 mg/kg | 27.7 | 8.6 | P < 0.0001 (>MCID) | 32.1 | 40.9 | 9.5 | P < 0.0010 | 7.3 | P = 0.0012 | |
| Control | 26.7 | 4.0 | NA | 32.3 | 39.1 | 5.0 | NA | 2.7 | NA | |
Abbreviations: SF-36, Short Form 36; FACIT, Functional Assessment of Chronic Illness Therapy; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; TCZ, tocilizumab; MTX, methotrexate; DMARD, disease-modifying antirheumatic drug; MCID, minimal clinically important difference.
Fatigue is very common among RA patients. The prevalence rates for fatigue in RA range from 42% to 80%. By using the Checklist of Individual Strength (CIS), persistent severe fatigue is found in 40% of RA patients (according to the FDA). Table 4 also shows the results regarding fatigue in the two double-blind RCTs in which it was measured.1,2 As for QoL, the patients’ feelings of fatigue improved substantially more than the MCID, when compared to the control patients. The open-label studies support the conclusions stemming from the randomized trials, with 7 and 9 units of improvement.22,23 Overall, this important aspect of fatigue associated with RA seems to change favorably when TCZ is used.
Rheumatoid arthritis also affects patients’ quality of sleep. This may be due to pain, mood changes, and/or disease activity. There was only one published study, an open-label study, that reported the effects of TCZ on sleep.22 This study used the PSQI and ESS instruments. It showed that patients reported better quality of sleep after taking just two doses of TCZ. No prior sleeping medications were used in this study, so the results are ascribable to the use of TCZ alone. Using the ESS, sleep improved from 7.7 (3.1) to 3.4 (2.2); using the PSQI, it improved from 8.7 (3.3) to 6.7 (4.3). Both changes are more than the MCID’s. Nonetheless, because these data originate from an open-label study, these results should be interpreted with caution.
Conclusion
Based on a systematic review supplemented by the RCTs and open-label studies, TCZ improves function and quality of life, and decreases fatigue, in patients with RA; all are probably as important to patients as are joint swelling counts and so forth. It must be noted that sleep, too, may be favorably affected. This supports the general efficacy of TCZ in RA, as demonstrated through conventional measures such as joint tenderness and swelling, and through combined indices such as the ACR response criteria or the 28 joint counts.1,2,12–16
Acknowledgements and disclosure
Dr Furst has received consultant fees, speaking fees, and/or honoraria (usually less than $US5000 each and rarely $US5000–<$US10,000) from Abbott, Actelion, Amgen, BMS, Biogen Idec, Centocor, Genentech, Gilead, GSK, NI, Nitec, Novartis, Pfizer, Roche, and UCB; serves on the advisory boards for Abbott, Amgen, BMS, Centocor, Genentech, Biogen Idec, Roche, and UCB; and serves as Director of Publications for the Consortium of Rheumatology Researchers of North America (CORRONA).
References
- 1.Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–2980. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatic arthritis (OPTION study): a double-blind, placebo-controlled, randomized trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 3.Bruce B, Fries JF. The Health Assessment Questionnaire (HAQ) Clin Exp Rheumatol. 2005;23:S14–S18. [PubMed] [Google Scholar]
- 4.Talamo J, Frater A, Gallivan S, Young A. Use of the short form 36 (SF36) for the health status measurement in rheumatoid arthritis. Br J Rheumatol. 1997;36:463–469. doi: 10.1093/rheumatology/36.4.463. [DOI] [PubMed] [Google Scholar]
- 5.Luo N, Chew LH, Fong KY, Koh DR, Ng SC, Yoon KH. Validity and reliability of the EQ-5D self-report questionnaire in Chinese-speaking patients with rheumatic diseases in Singapore. Ann Acad Med Singapore. 2003;32:685–690. [PubMed] [Google Scholar]
- 6.Calculating the US population-based EQ-5D Index Score. Agency for Healthcare Research and Quality; Rockville, MD: Aug, 2005. [Accessed April 17, 2011]. http://www.ahrq.gov/rice/EQ5Dscore.htm. [Google Scholar]
- 7.Webster K, Cella D, Yost K. The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandran V, Bhella S, Schentag C, Gladman DD. Functional assessment of chronic illness therapy-fatigue scale is valid in patients with psoriatic arthritis. Ann Rheum Dis. 2007;66:936–939. doi: 10.1136/ard.2006.065763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FACIT.org [homepage on the Internet] [Accessed April 17, 2011]. Available from: http://www.facit.org/FACITOrg/Questionnaires.
- 10.Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Laisze Lee. Relationships between the Pittsburgh sleep quality index (PSQI), Epworth sleepiness scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4:563–571. [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimoto N, Miyasaka N, Kamamoto K, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19:12–19. doi: 10.1007/s10165-008-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maini RN, Taylor PC, Scechinski J, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–2829. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- 13.Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biological: results from a 24-week multicenter randomized placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomized controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–1167. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimoto N, Yosizaki K, Miyasaka N, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1716–1769. doi: 10.1002/art.20303. [DOI] [PubMed] [Google Scholar]
- 17.Choy EH, Isenberg DA, Garrood T, et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interlukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 2002;46(12):3143–3150. doi: 10.1002/art.10623. [DOI] [PubMed] [Google Scholar]
- 18.Khraishi M, Alten R, Gomez-Reino JJ, et al. Long-term efficacy of tocilizumab (TCZ) in patients with rheumatoid arthritis (RA) treated up to 3.7 years [abstract] ACR. 2010:S760–S761. [Google Scholar]
- 19.Takeuchi T, Tanaka Y, Amano K, et al. Clinical, structural and functional remission in the treatment of rheumatoid arthritis with tocilizumab in daily clinical practice-REACTION-2 study [abstract] ACR. 2010:S747. [Google Scholar]
- 20.Kremer JM, Furst DE, Burgos-Vargas R, et al. LITHE: Tocilizumab (TCZ) inhibits radiographic progression and improves physical function in rheumatoid arthritis (RA) patients (pts) at 3 years with maintenance of clinical efficacy over time [abstract] Arthritis Rheum. 2011;63(3):609–621. [Google Scholar]
- 21.Jones G, Sebba A, Calvo A, et al. Efficacy of tocilizumab in patients with rheumatoid arthritis who had never been exposed to or had never failed methotrexate: analysis of up to 3 years of treatment in a long-term extension study [abstract] Ann Rheum Dis. 2010;69:386. [Google Scholar]
- 22.Fragiadaki K, Sfikakis PP. EULAR. Effect of the first and second infusion of tocilizumab on sleep and daytime sleepiness in patients with active rheumatoid arthritis [abstract] Ann Rheum Dis. 2010;69:683. [Google Scholar]
- 23.Burmester G, Feist E, Kellner H, Braun J, Iking-Konert C, Rubbert-Roth A. Extended report: Effectiveness and safety of the interleukin 6-receptor antagonist tocilizumab after 4 and 24 weeks in patients with active rheumatoid arthritis: the first phase IIIb real-life study (TAMARA) Ann Rhem Dis. 2011;70:755–759. doi: 10.1136/ard.2010.139725. [DOI] [PMC free article] [PubMed] [Google Scholar]