Abstract
Background
The goal of this study was to investigate the relationship between cognitive functions and the level of endogenous estradiol in postmenopausal women, according to which estrogen receptor α (ERα) polymorphism the woman carries.
Material/Methods
The study group consisted of 210 women. The inclusion criteria were: minimum 2 years after the last menstruation, FSH concentration 30 U/ml, and no dementia signs on Montreal Cognitive Assessment (MoCA). A computerized battery of Central Nervous System Vital Signs (CNS VS) test was used to diagnose cognitive functions. Genotyping of the ERα polymorphism was performed using a polymerase chain reaction and restriction enzymes (PCR-RFLP). Blood plasma was tested for FSH and estradiol (E2). Statistical analysis was performed using STATISTICA software.
Results
A relationship was confirmed between standard scores for 3 cognitive functions: general memory, verbal memory, and processing speed, and the XbaI polymorphism in the women in the study. In the group of women with genotype TT PvuII, significant positive relationships were observed between the concentration of E2 and the standard scores of 3 cognitive functions: general memory, verbal memory, and processing speed. In the group of women with genotype TC PvuII, significant negative correlations were found between the concentration of E2 and the standard scores of 4 cognitive functions: NCI, general memory, verbal memory, and processing speed.
Conclusions
ERα polymorphism exerted an effect on the interaction between the concentration of estradiol and the results for cognitive functions. The concentration of estradiol did not depend on Xba1 and PvuII polymorphisms. The results for cognitive functions depended on which Xba1 polymorphism the woman carried.
MeSH Keywords: Cognitive Science, Estrogen Receptor alpha, Menopause
Background
Postmenopausal women have benefitted from progress in medical science, but many are struggling with new health and social problems. Statistical data show that one-third of the life of a woman falls in the menopausal period. It is considered that menopause-related decrease in the level of estrogens is responsible for the occurrence of cognitive and psychological disorders during this period of life. However, the results of studies concerning the effect of menopause estrogen therapy on cognitive functions are contradictory. Thus, the essence of the problem is more complicated than merely the deficit of hormones. A new research trend is the testing of the hypothesis that estrogen receptor alpha (ERα) polymorphisms may determine various effects of estrogens on cognitive functions.
Estrogens determine the normal functioning of the nervous system. These hormones exert a strong effect on synaptogenesis, protect against oxidative stress, affect inflammatory responses, induce the production of growth factors, improve blood supply to the CNS, and affect hypothalamic volume [1–3]. They show a neuroprotective effect with respect to: neurotoxicity related with beta amyloid, and the risk of development of strokes and Parkinson’s disease [4,5]. By exerting a strong effect on neurotransmitters (e.g., acetylcholine, serotonin, and dopamine norepinephrine), estrogens affect mood and cognitive functions [6,7]. These hormones act through 2 steroid receptors – ERα and ERβ – which differ with respect to the structure and functions encoded by various genes, with different tissue localization. The ERα gene is located on the long arm of chromosome 6 (6q24–27), while the ERβ gene is located on the long arm of chromosome 14 (14q22–24) [8]. The presence of ER in the CNS indicates many relationships between the nervous and endocrine systems [2,9]. ERα and β are located, in the pituitary gland, cerebellum, hippocampus, amygdala, locus coeruleus, raphe nuclei, and other structures associated with emotions and cognitive functions [10].
Genes encoding ERα have many polymorphic forms, among which 2 types of single-nucleotide polymorphisms (SNP) are distinguished – Xba1 and PvuII. The Xba1 polymorphism (also termed IVS1-351) is the result of A/G (A/G rs9340799) transition, located in intron 1 of the ER α gene 351 bp 5′ end upstream of exon 2. The Xba1 is located approximately 50 bp away from PvuII polymorphism site (T/C, rs2234693), known as IVS1-397T/C. It is caused by T/C transition in intron 1, 397 bp 5′ end upstream of exon 2 [11].
ERα polymorphisms may play an important role in the functioning of the body and are associated with the development of many pathologies, such as breast cancer, endometriosis, and osteoporosis [12,13]. In the present study explores the effect of ERα polymorphisms on the concentration of estradiol and cognitive functions of women after menopause.
Cognitive functions are the basis of the functioning of an individual by appropriate use of previously acquired knowledge in daily life. The basic cognitive functions are perception, attention, and memory, while the complex cognitive functions include thinking, imagination, executive functions, linguistic functions, and cognitive control [14].
It is commonly considered that cognitive functions deteriorate with age. This may result from changes taking place in the CNS (decreased blood flow through the brain and the slowing of neuronal metabolism and conducting nerve impulses), as well as the effect of factors such as sex, education, occupation, physical and mental activity, diet, state of health, concomitant diseases, and social and economic factors [2,15]. It is important to discover whether menopause is an additional factor impairing cognitive functions.
The goal of this study was to investigate the relationship between cognitive functions and the level of endogenous estradiol in postmenopausal women, according to which estrogen receptor α (ERα) polymorphism a woman carries.
Material and Methods
The study was conducted in 2013 and 2014 at the Institute of Rural Health in Lublin, Poland. The study group were women from south-eastern Poland. The inclusion criteria were age 50–65 years and completed at least elementary education level. The women were qualified into the study group also based on clinical symptoms (minimum 2 years from the last menstrual period) and based on the criterion of FSH level (FSH >30 mlU/ml). The exclusion criteria were: chronic diseases within the last 5 years, including diseases of the thyroid gland; active cancerous disease within the 5 years after recruitment; medical history of mental diseases; addiction to drugs and/or alcohol; diagnosed disease entity with the symptoms of dementia; current or past use of HRT; and severe menopausal symptoms according to the Kupperman scale. At the stage of qualification to the study, a brief MoCA test was performed in order to include into the study the women who did not show the features of dementia. Women who obtained scores of at least 26 were included into the study.
Cognitive functions were assessed by means of the diagnostic instrument – Central Nervous System-Vital Signs (CNS-VS) (Polish version), with CNS software (CNS Vital Signs, Chapel Hill, NC). The CNS-VS includes the following tests: Verbal Memory Test-VBM, Finger Tapping Test-FTT, Symbol Digit Modalities Test-SDMT, Stroop Test-ST, Shifting Attention Test-SAT, and Continuous Performance Test. The CNS-VS assesses 9 cognitive functions: memory, verbal memory, visual memory, processing speed, executive functioning, psychomotor speed, reaction time, complex attention, and cognitive flexibility. Based on 5 of these functions – memory, psychomotor speed, reaction time, complex attention, and cognitive flexibility – the Neurocognition Index (NCI) is calculated. The computer report from the CNS-VS test provides subject scores, standard scores, percentiles, and assessments according to a 5-degree scale for each of the 9 cognitive functions examined, and the Neurocognition Index. These assessments are as follows: above average (more than 109 standard scores), average (90–109), low average (80–89), low (70–79), and very low (less than 70).
Blood was collected for determination of estradiol (E2) and follicle-stimulating hormone (FSH). Blood samples were supplied to SYNEVO, an accredited laboratory.
Genome DNA was isolated from the whole blood of patients using commercial kits for the isolation of DNA from blood (Qiagen). Genotypes of ERα polymorphism were determined using polymerase chain reaction and restriction enzymes (RFLP-PCR). Amplification products were detected in agarose gels after performing electrophoresis, and subsequently, by means of restriction enzymes, PvuII and Xba1 polymorphisms were determined. The alleles of Xba1 polymorphism were determined as A and G: heterozygotes AG, wild-type GG, and homozygotes AA. The alleles of PvuII polymorphism were determined as T and C: TC heterozygotes, TT homozygotes, and wild-type CC. The studies of ERα polymorphism were conducted by a technician from the Department of Zoonoses at the Institute of Rural Health in Lublin.
Women qualified for the study completed a questionnaire which consisted of a section describing social and demographic characteristics, and the health section based on a gynecological interview.
Statistical analysis
Statistical analysis of the collected data was performed using the STATISTICA computer statistical package. In the characteristics of the sample, concentration of estradiol and cognitive functions (standard scores) arithmetic means were estimated (M) ± standard deviations (SD) for quantitative characteristics and/or absolute numbers (n) and relative numbers (%) for qualitative characteristics. The F test for analysis of variance was used in order to analyze relationships between polymorphism Xba1, PvuII, and age, estradiol concentration, age at last menstruation, and cognitive functions, as well as between educational level and cognitive functions. The χ2 test for stochastic independence was used to analyze the relationship between educational level and polymorphism Xba1 and PvuII. Pearson’s correlation coefficient was used to analyze the relationship between cognitive functions and age, age at last menstruation, and estradiol concentration. The correlation between cognitive functions and estradiol concentration were investigated in all examined women, as well as in groups according to XbaI and PvuII polymorphism. In statistical tests, the significance level was set at 0.05.
Results
The study included 210 women, aged 50–65 years, and a mean age of 56.5±3.5 years. Nearly a half of the examined women (47.62%) had secondary school education, followed by 40.00% with university education, 9.05% with primary vocational education, and 3.33% with only primary education.
The last menstruation among the women in the study occurred at the age of 42–56 years. Mean age at the last menstruation was 50.3±2.7 years. The concentration of estradiol in blood serum of the examined women was up to 100 pg/ml, and was 23.5±20.5 pg/ml on average.
In the examined group, 71 women (33.81%) possessed the genotype ERα Xba1 AA, 104 (49.52%) were carriers of ERα Xba1 AG, and 35 (16.67%) had genotype ERα Xba1 GG. With respect to polymorphism ERα PvuII, 57 (27.14%) respondents had genotype TT, 97 (46.19%) had genotype TC, and 56 (26.67%) had genotype CC.
Possession of polymorphism ERα Xba1 did not significantly relate to age of the examined women (F=0.658, p=0.519), level of education (χ2=8.254, p=0.220), age at last menstruation (F=0.853, p=0.428), or level of estradiol (F=1.262, p=0.285). Possession of polymorphism ERα PvuII did not significantly relate to age of the examined women (F=0.822, p=0.441), level of education (χ2=4.766, p=0.574), age at last menstruation (F=0.492, p=0.612), or level of estradiol (F=0.006, p=0.994).
No linear correlation was observed between standard scores of cognitive functions and age during the considered age interval of menopause (50–65), as well as age at occurrence of last menstruation. Nevertheless, a relationship was confirmed between 6 of the 10 cognitive functions (standard scores) and the level of education of the examined women. Women with university education obtained higher results with respect to the NCI, general and verbal memory, processing speed, and reaction time (Table 1).
Table 1.
Relationships between cognitive functions (standard scores) and age, age at last menstruation and educational level in the total group of the examined women.
| Domain (standard scores) | Age (years) | Age at last menstruation (years) | Educational level | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary or basic vocational | Secondary | Tertiary | Significance of differences | |||||||||
| r | p | r | p | M | SD | M | SD | M | SD | F | p | |
| NCI | −0.063 | 0.366 | −0.001 | 0.994 | 75.69 | 18.70 | 82.26 | 17.05 | 87.69 | 15.17 | 5.869 | 0.003 |
| Memory | −0.064 | 0.359 | −0.040 | 0.565 | 81.81 | 19.99 | 87.45 | 16.69 | 91.98 | 14.61 | 4.285 | 0.015 |
| Verbal memory | −0.080 | 0.247 | −0.080 | 0.250 | 80.15 | 20.68 | 89.40 | 19.63 | 93.92 | 15.20 | 5.851 | 0.003 |
| Visual memory | −0.012 | 0.865 | 0.073 | 0.293 | 89.50 | 20.82 | 91.56 | 14.78 | 93.88 | 14.68 | 0.961 | 0.384 |
| Processing speed | −0.006 | 0.927 | 0.030 | 0.671 | 71.23 | 15.03 | 78.20 | 14.11 | 82.52 | 14.78 | 6.369 | 0.002 |
| Executive functioning | −0.018 | 0.799 | 0.025 | 0.718 | 74.50 | 23.36 | 77.90 | 26.26 | 82.31 | 24.70 | 1.214 | 0.299 |
| Psychomotor speed | −0.103 | 0.135 | 0.034 | 0.624 | 72.27 | 23.11 | 82.29 | 17.46 | 87.45 | 14.47 | 7.994 | 0.000 |
| Reaction time | −0.073 | 0.292 | −0.016 | 0.821 | 78.50 | 15.89 | 85.76 | 15.42 | 92.11 | 15.22 | 8.839 | 0.000 |
| Complex attention | 0.001 | 0.999 | 0.026 | 0.710 | 74.23 | 31.94 | 80.36 | 28.26 | 85.74 | 26.36 | 1.913 | 0.150 |
| Cognitive flexibility | −0.026 | 0.713 | 0.012 | 0.866 | 72.88 | 25.15 | 76.28 | 27.60 | 81.63 | 25.40 | 1.489 | 0.228 |
The mean score on the Neurocognition Index was 83.62, reflecting low average level of cognitive functions. The lowest results concerned cognitive flexibility (mean score 78.00), processing speed (mean score 79.07), and executive functioning (mean score 79.24), reflecting a low level of these cognitive functions. The best results with respect to cognitive functions were in visual, verbal, and general memory (mean scores close to 90), reflecting an average to low average level. Moderate results were obtained by on complex attention, psychomotor speed and reaction time (mean values 81–87 scores), reflecting a low average level of these cognitive functions (Table 2).
Table 2.
Cognitive functions (standard scores) in total group and according to XbaI and PvuII polymorphism in the examined women.
| Domain (standard scores) | Total | XbaI | PvuII | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | Significance of differences | TT | TC | CC | Significance of differences | |||||||||||
| M | SD | M | SD | M | SD | M | SD | F | p | M | SD | M | SD | M | SD | F | p | |
| NCI | 83.62 | 16.92 | 84.58 | 16.77 | 84.30 | 17.03 | 79.66 | 16.83 | 1.159 | 0.316 | 81.28 | 18.33 | 85.05 | 16.37 | 83.52 | 16.40 | 0.892 | 0.411 |
| Memory | 88.56 | 16.60 | 90.75 | 17.60 | 89.67 | 15.28 | 80.83 | 16.50 | 4.817 | 0.009 | 88.81 | 18.14 | 90.19 | 15.24 | 85.50 | 17.09 | 1.429 | 0.242 |
| Verbal memory | 90.06 | 18.54 | 91.04 | 18.05 | 91.98 | 18.33 | 82.37 | 18.74 | 3.764 | 0.025 | 88.77 | 17.25 | 92.32 | 18.99 | 87.46 | 18.88 | 1.412 | 0.246 |
| Visual memory | 92.23 | 15.59 | 94.35 | 17.91 | 92.15 | 14.21 | 88.17 | 14.00 | 1.860 | 0.158 | 93.56 | 18.99 | 92.65 | 14.66 | 90.16 | 13.23 | 0.734 | 0.481 |
| Processing speed | 79.07 | 14.86 | 80.23 | 14.15 | 80.67 | 13.60 | 71.94 | 17.95 | 5.032 | 0.007 | 80.26 | 13.26 | 80.60 | 14.35 | 75.20 | 16.72 | 2.640 | 0.074 |
| Executive functioning | 79.24 | 25.33 | 77.55 | 24.24 | 81.30 | 26.77 | 76.57 | 23.17 | 0.694 | 0.501 | 73.77 | 27.13 | 81.87 | 24.84 | 80.27 | 23.84 | 1.912 | 0.150 |
| Psychomotor speed | 83.11 | 17.72 | 84.01 | 17.54 | 83.25 | 17.24 | 80.89 | 19.74 | 0.369 | 0.692 | 81.88 | 16.99 | 83.20 | 17.96 | 84.23 | 18.27 | 0.249 | 0.779 |
| Reaction time | 87.40 | 15.96 | 88.69 | 14.61 | 86.99 | 16.03 | 86.00 | 18.50 | 0.398 | 0.672 | 87.44 | 16.32 | 86.35 | 15.27 | 89.18 | 16.87 | 0.555 | 0.575 |
| Complex attention | 81.75 | 28.12 | 80.96 | 27.40 | 83.88 | 28.13 | 77.03 | 29.65 | 0.820 | 0.442 | 76.47 | 30.07 | 85.33 | 27.04 | 80.93 | 27.49 | 1.828 | 0.163 |
| Cognitive flexibility | 78.00 | 26.51 | 76.49 | 25.49 | 80.02 | 27.88 | 75.06 | 24.52 | 0.630 | 0.534 | 72.47 | 28.26 | 80.88 | 26.24 | 78.64 | 24.67 | 1.841 | 0.161 |
A relationship was confirmed between standard scores for 3 cognitive functions (general memory, verbal memory, and processing speed), and the XbaI polymorphism in the women in the study. Women with the genotype GG obtained significantly lower results with respect to the above-mentioned cognitive functions, compared to those with genotypes AA and AG. However, no significant differences in standard scores for these functions were noted between the group of women with genotype AA and those with genotype AG. No relationships were found between the standard scores of cognitive functions and the PvuII polymorphism (Table 2).
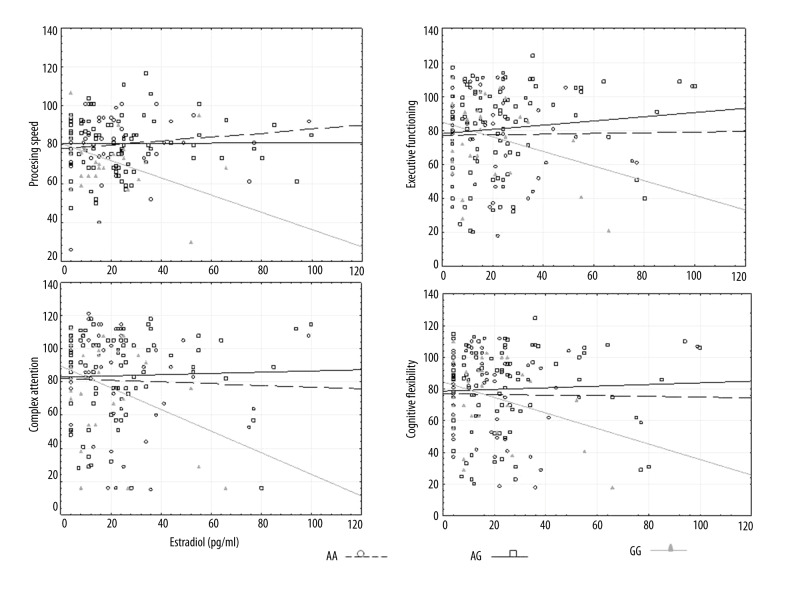
No linear correlation was observed between standard scores of cognitive functions and the concentration of estradiol in the total group of the examined women. However, in the group of women with genotype GG, significant negative relationships were found between the concentration of estradiol (pg/ml) and the standard scores for 4 cognitive functions: processing speed, executive functioning, complex attention, and cognitive flexibility, showing that higher levels of estradiol were associated with lower scores for these cognitive functions. In the groups of women with genotypes AA and AG, no linear relationships were observed between the concentration of estradiol and the standard scores for cognitive functions (Table 3, Figure 1).
Table 3.
Correlation coefficients between concentration of estradiol (pg/ml) and cognitive functions (standard scores) in total group and according to XbaI and PvuII.
| Domain (standard scores) | Total | XbaI | PvuII | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | TT | TC | CC | |||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| NCI | −0.049 | 0.485 | 0.005 | 0.970 | −0.054 | 0.587 | −0.243 | 0.159 | 0.138 | 0.306 | −0.176 | 0.050 | −0.043 | 0.752 |
| Memory | 0.031 | 0.657 | 0.153 | 0.202 | −0.141 | 0.153 | 0.187 | 0.282 | 0.279 | 0.036 | −0.224 | 0.027 | 0.168 | 0.217 |
| Verbal memory | 0.025 | 0.714 | 0.113 | 0.349 | −0.094 | 0.343 | 0.128 | 0.464 | 0.325 | 0.014 | −0.196 | 0.050 | 0.140 | 0.303 |
| Visual memory | 0.033 | 0.636 | 0.126 | 0.296 | −0.088 | 0.375 | 0.148 | 0.396 | 0.128 | 0.344 | −0.085 | 0.409 | 0.129 | 0.343 |
| Processing speed | 0.006 | 0.935 | 0.146 | 0.225 | 0.003 | 0.978 | −0.406 | 0.016 | 0.231 | 0.050 | −0.058 | 0.571 | −0.092 | 0.502 |
| Executive functioning | 0.031 | 0.658 | 0.017 | 0.889 | 0.099 | 0.320 | −0.305 | 0.050 | 0.131 | 0.331 | −0.034 | 0.738 | 0.030 | 0.826 |
| Psychomotor speed | −0.109 | 0.115 | −0.016 | 0.897 | −0.174 | 0.077 | −0.143 | 0.414 | 0.051 | 0.707 | −0.222 | 0.029 | −0.066 | 0.627 |
| Reaction time | −0.015 | 0.826 | 0.136 | 0.260 | −0.137 | 0.165 | 0.082 | 0.641 | 0.144 | 0.285 | −0.085 | 0.410 | −0.067 | 0.625 |
| Complex attention | −0.039 | 0.576 | −0.040 | 0.740 | 0.028 | 0.777 | −0.361 | 0.033 | 0.095 | 0.482 | −0.110 | 0.283 | −0.075 | 0.585 |
| Cognitive flexibility | −0.015 | 0.824 | −0.016 | 0.893 | 0.040 | 0.689 | −0.328 | 0.050 | 0.093 | 0.493 | −0.091 | 0.377 | −0.006 | 0.966 |
Figure 1.
Correlations between concentration of estradiol (pg/ml) and cognitive functions (standard scores) according to XbaI.
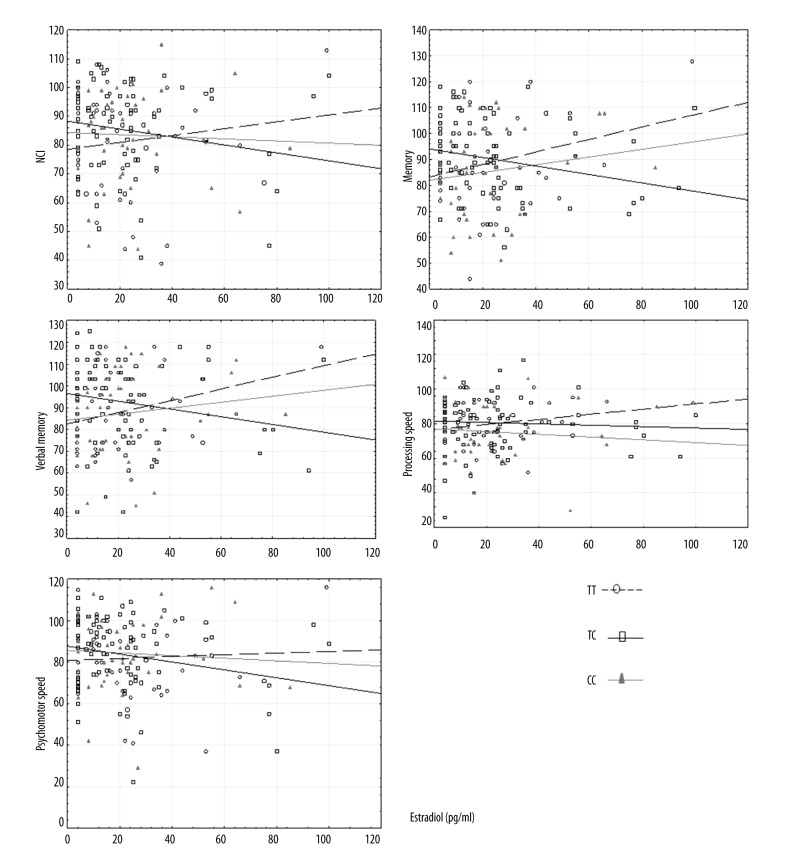
In the group of women with genotype TT, significant positive relationships were observed between the concentration of estradiol (pg/ml) and the standard scores of 3 cognitive functions – general memory, verbal memory, and processing speed – showing that higher levels of estradiol were associated with higher scores for these cognitive functions. In the group of women with genotype TC, significant negative correlations were found between the concentration of estradiol (pg/ml) and the standard scores of 4 cognitive functions – NCI, general memory, verbal memory, and processing speed – showing that higher concentrations of estradiol were associated with lower scores for these cognitive functions. In the group of women with genotype CC, no linear relationship was found between the concentration of estradiol and the standard scores for cognitive functions (Table 3, Figure 2).
Figure 2.
Correlations between concentration of estradiol (pg/ml) and cognitive functions (standard scores) according to PvuII.
Discussion
Many researchers consider older age as an independent risk factor for mild cognitive disorders and dementia [16]. Others, with respect to women, report that from the age of approximately 50 years, cognitive functions gradually weaken, and the majority of women examined reported the deterioration of verbal memory, which was the highest within 12 months after the last menstruation [17].
In the present study, we found a correlation between the level of education and numerical results of the NCI, general and verbal memory, processing and psychomotor speed, and reaction time, indicating the protective effect of university education on cognitive functions, which has been confirmed by many studies [17]. University education is considered to be related with psychological activity, which is also understood as ‘cognitive activity’, and educated older people tend to have greater ‘cognitive reserve’ and less severe symptoms of dementia, compared to those with less education and less cognitively activity [18,19].
The largest number of the menopausal women examined were carriers of AG Xba1 and TC PvuII. The prevalence of Xba1 and PvuII polymorphisms indicates certain differences, according to scientific reports, and the studies mainly concern PvuII polymorphism. According to Mysliwska, 20% of the population of white women are homozygous CC, slightly more than 20% are homozygous TT, and most are heterozygous TC [8]. Similarly, Koch and Shearman reported that genotype TC was the most common, and the genotype CC PvuII is the least common [20,21]. According to Schuit, the genotype TT is the least common in the population, whereas the genotype TC PvuII is the most common [22]. Lamon-Fava, in a study conducted among postmenopausal white women, observed that among women with the PvuII polymorphism, the most common was genotype was TC, while the least common was CC. With respect to Xba1 polymorphism, the largest number of the examined women had the genotype AG, whereas the fewest had GG [23]. Lian reported that among Europeans, genotypes AG Xba1 and TC PvuII are most prevalent, while Dai found that genotypes AA Xba1 and TC PvuII were most common in Asians. It is interesting that both researchers report that both among whites and Asians, the least common genotypes are GG Xba1 and CC PvuII [24–26].
In the present study, we found no correlation between the numerical results of cognitive functions and the concentration of endogenous estradiol. Other researchers also indicated the lack of a relationship between the level of endogenous estrogens and cognitive functions. Henderson states that he found no relationship between the concentration of estradiol and the development of cognitive disorders among postmenopausal women [27]. Weber confirmed the lack of a significant correlation between the concentration of estradiol, and the results of verbal memory and verbal fluency. An exception were motor skills, where a higher concentration of endogenous estradiol was associated with better results [28]. However, other researchers support the thesis that the decreasing levels of estradiol after menopause are related with the deterioration of cognitive functions, both directly and indirectly, by exerting an effect on the development of hypertension and hypercholesterolemia, which are independent risk factors for the development of cognitive disorders [29–31]. Here, it is worth quoting the results of a large study, the ‘Kronos Early Estrogen Prevention Cognitive and Affective Study’ (KEEPS-Cog), in which the negative correlation was emphasized between estrogens deficiency and the results for cognitive functions, through effects on cardiovascular system parameters of arterial hypertension and blood lipid levels [29]. Tuomisto also indicates the deterioration of results for cognitive functions after menopause, compared to peri-menopausal women who have a higher concentration of estradiol [32]. Ryan observed correlation between estrogens deficiency and the deterioration of visual memory results and verbal fluency in the later period of life. He also adds that a premature menopause by 30% increases the risk of deterioration of psychomotor speed and the NCI within 7 years after menopause [33].
The results of the Study of Osteoporotic Fractures show that women with a high level of estradiol had a lower probability of developing cognitive disorders 6 years after menopause, compared to those with a low level of this hormone [34], suggesting the hypothesis that high concentrations of endogenous estrogens favor the maintenance of normal results for cognitive functions in older women. However, Laughlin indicated the fact that only 1 large cross-sectional study has confirmed this hypothesis; the research studies 402 women in Holland and showed that those with a high level of estradiol or estrone had 50% lower risk of cognitive disorders (MMSE <27) in later life compared to women who had a lower concentration of these hormones [35,36]. Pourganji reported on the protective effect of high concentrations of endogenous estradiol on memory function and prevention of AD, via inhibition of damage to the cerebral tissue by free oxygen species, which are activated in the course of AD by lipopolysaccharides [37]. Newhouse called attention to the beneficial effect of estradiol on cognitive functions, especially complex attention, as well as verbal and visual memory. This effect is considered to take place through interaction with the cholinergic system, which also modulates CNS functioning [38]. The results of a study by Laughlin are noteworthy, in which women with higher levels of estrone and estradiol in blood showed significantly higher deficits in verbal fluency developing within 4 years, compared to those with lower concentrations of these hormones. In women who had the highest concentrations of estradiol and estrone, the loss of verbal fluency in the Fluency Category was twice as high [35].
It is probable that ERα polymorphisms exert an effect on cognitive functions through the determination of the concentration of sex hormones [39]. However, our results show that the concentration of estradiol in the examined women did not depend on ERα Xba1 or PvuII polymorphisms. This view is supported by Yaffe, among others [40]. Sowers et al., based on the results obtained in 1538 women participating in the Study of Women’s Health Across the Nation (SWAN), similarly found that the concentration of endogenous estradiol does not significantly depend on which ERα polymorphism a woman carries. These results were important because they were obtained in a sample of women of various races: African American, Chinese, Japanese, and white [41]. Schuit observed that after menopause, the concentration of endogenous estradiol is lowest in carriers of the alleles T PvuII and A Xba1 and highest in homozygous CC PvuII carriers [42]. Sundermann found that a higher concentration of endogenous estradiol in postmenopausal women is typical of the carriers of the genotype GC [43], whereas according to Lorentzon, the presence of higher concentrations of estradiol is related with the genotype GG Xba1 [43–45]. He conducted a clinical follow-up examination of 478 women with POF and 918 healthy Asian women, and reported that all genotypes TT, CT, and TT PvuII polymorphism are related with low concentration of estradiol, increased risk of POF, and the development of early menopause, but this relationship was not found for carriers of Xba1 polymorphism [45].
An important problem in the present study was the investigation of the relationship between Xba1 and/or PvuII ERα polymorphisms, and the results for cognitive functions. Such a relationship was confirmed only for GG Xba1 polymorphism, where the carriers of GG had significantly lower numerical results for general memory, verbal memory, and processing speed.
Other researchers also agree concerning the effect of ERα polymorphisms on cognitive functions. A study by Bousmann, which included women aged 56–67, showed that the carriers of TT PvuII obtained the worst results for logical memory, compared to those with CC PvuII, who obtained the best results. The researcher postulates the necessity for further studies in order to prove the hypothesis that the T PvuII allele is the genetic marker of memory disorders [46]. Yaffe found a significant relationship between A Xbal and/or T PvuII polymorphisms, and lower results for cognitive functions in the Modified Mini-Mental Status Examination (3MS). The researcher found more rapid deterioration of cognitive functions within 4 years in the carriers of AA Xba1 and/or TT PvuII, who were also at a 3 times higher risk of the development of AD or other forms of dementia, compared to the women with GG Xbal or CC PvuII [40]. Corbo combines the presence of GG Xba1 and/or PvuII polymorphisms with worse results for cognitive functions in the MMSE, their quicker deterioration, and the development of AD [47]. Olsen, in the Orientation-Memory-Concentration test, found a relationship between GG Xba1 polymorphism and the deterioration of cognitive functions in Dutch postmenopausal women [48].
Many studies have addressed the problem of the effect of ERα Xba1 and PvuII polymorphisms on the development of dementia on the AD background. Lee, in meta-analysis of studies involving approximately 6000 people (males and females), investigated this effect, reporting a relationship between CC and CT PvuII polymorphism and the development of AD in males, without any effect on the development of AD in females [49].
Ethnic differences seem to be a very important issue, and many researchers focus on this problem. ERα polymorphisms may differ based on the expression of DNA genes typical of a given race. Cheng performed a meta-analysis of 18 studies which covered 13 192 participants; the material was collected from PubMed, Embase, Web of Science, Science Direct, SpringerLink, and the Chinese National Knowledge Infrastructure databases. The result of the analysis was confirmation of the relationship between ERα PvuII polymorphism and the risk of AD in whites (CC+CT vs. TT), while no such a relationship was found in the Asian population. Also, no relationship was observed between Xbal polymorphism, and an increase in the risk of AD among white and Asian populations [50]. Wang observed a positive correlation between the development of AD in the European population, and the presence of Xba1 polymorphism, and a negative correlation between morbidity due to AD in southern Europe, and the PvuII (TT vs. CC+CT) polymorphism [51].
However, other researchers found no relationship between Xba1 and PvuII polymorphisms, and the results for cognitive functions and the development of AD [52–54]. Ryan did not discover any relationship between the presence of Xba1 and/or PvuII ERα polymorphisms, and found worse results for cognitive functions, including visual memory, psychomotor speed, executive functions, global functions, and verbal fluency [55].
Based on the results of the above-described studies, in the present study we tried to determine whether the effect of endogenous estradiol concentration on the results for cognitive functions is related with ERα Xba1 and PvuII polymorphisms. In the women examined in this study, ERα Xba1 and PvuII polymorphisms exerted an effect on the correlation between the concentration of estradiol and the results for cognitive functions. The many studies on the effect of ERα polymorphism on the concentration of estradiol and the results of cognitive functions seem to confirm the presence of a relationship between the concentration of estradiol and the results of cognitive functions according to the possessed ERα polymorphism in postmenopausal women. We hope the present study starts a discussion on the possibilities of using studies of ERα Xba1 and PvuII polymorphisms in the designation of women who would benefit from estrogen therapy with respect to cognitive functions. However, considering the unequivocal views of researchers concerning the effect on cognitive functions of factors such as HTM, estradiol concentration, environmental factors, and ERα Xba1 and PvuII polymorphisms, further studies are necessary to achieve a consensus on this. The results of these studies may be helpful in undertaking proper preventive actions to improve the quality of life of women after menopause, and could be a basis for further efforts on new methods of therapy which, in a longer perspective, would bring about many health, economic, and social benefits.
Conclusions
ERα polymorphism exerted an effect on the interaction between the concentration of estradiol and the results for cognitive functions.
The concentration of estradiol did not depend on Xba1 and PvuII polymorphisms.
The results for cognitive functions depended on the Xba1 polymorphism carried.
Footnotes
Source of support: This study was sponsored by the Institute of Rural Health in Lublin, Poland
References
- 1.Bielawska-Batorowicz E, Cieślik I, Cwalina E. [The role of gender and age in creating the image of women in menopause]. Prz Menopauz. 2003;6:68–73. [in Polish] [Google Scholar]
- 2.Bojar I, Gujski M, Raczkiewicz D, Rothenberg KG. Cognitive functions, apolipoprotein E genotype and hormonal replacement therapy of postmenopausal women. Neuro Endocrinol Lett. 2013;34(7):635–42. [PubMed] [Google Scholar]
- 3.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll JC, Rosario ER. The potential use of hormone-based therapeutics for the treatment of Alzheimer’s disease. Curr Alzheimer Res. 2012;9:18–34. doi: 10.2174/156720512799015109. [DOI] [PubMed] [Google Scholar]
- 5.Deroo B, Korach K. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–70. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Compton J, Murphy D. Imaging the brain in healthy postmenopausal users and non-users of hormone replacement therapy. Climacteric. 2003;6:180–83. [PubMed] [Google Scholar]
- 7.Osterlund MK. Underlying mechanisms mediating the antidepressant effects of estrogens. Biochim Biophys Acta. 2010;1800(10):1136–44. doi: 10.1016/j.bbagen.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Myśliwska J. [Hormone replacement therapy and cardio-vascular diseases in women. A step forward]. Family Medicine Forum. 2009;3(1):1–9. [in Polish] [Google Scholar]
- 9.Mannella P, Simoncini T. Sex steroids and their receptors: Molecular action on brain cells. Gynecol Endocrinol. 2012;28(1):2–4. doi: 10.3109/09513590.2012.651934. [DOI] [PubMed] [Google Scholar]
- 10.Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann NY Acad Sci. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- 11.Mansur AP, Nogueira CCM, Strunz CMC, et al. Genetic polymorphisms of estrogen receptors in patients with premature coronary artery disease. Arch Med Res. 2005;36(5):511–17. doi: 10.1016/j.arcmed.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Choi YM, Jun JK, et al. Estrogen receptor dinucleotide repeatpolymorphism is associated with minimal or mild endometriosis. Fertil Steril. 2005;84(3):774–77. doi: 10.1016/j.fertnstert.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 13.Shin A, Kang D, Nishio H, et al. Estrogen receptor alpha gene polymorphisms and breast cancer risk. Breast Cancer Res Treat. 2003;80(1):127–31. doi: 10.1023/a:1024439202528. [DOI] [PubMed] [Google Scholar]
- 14.Kolan M. [Cognitive impairment and ischemic brain]. Annales Academiae Medicae Stetinensis. 2011:94–105. [in Polish] [Google Scholar]
- 15.Kołodziejczyk I. [In a healthy body, healthy mind? The impact of physical activity on cognitive functioning in old age]. Cosmos. 2007;56(3–4):361–69. [in Polish] [Google Scholar]
- 16.van Rossum ME, Koek HL. Predictors of functional disability in mild cognitive impairment and dementia. Maturitas. 2016;90:31–36. doi: 10.1016/j.maturitas.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Santoro N, Epperson CN, Mathews SB. Menopausal Symtoms and the management. Endocrinol Metab Clin North Am. 2015;44(3):497–515. doi: 10.1016/j.ecl.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(3) Suppl 2:69–74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 19.Hugo J, Ganguli M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin Geriatr Med. 2014;30(3):421–42. doi: 10.1016/j.cger.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch W, Hoppmann P, Pfeufer A, et al. No replication of association between estrogen receptor α gene polymorphisms and susceptibility to myocardial infarction in a large sample of patients of European descent. Circulation. 2005;112(14):2138–42. doi: 10.1161/CIRCULATIONAHA.105.545913. [DOI] [PubMed] [Google Scholar]
- 21.Shearman AM, Cupples LA, Demissie S. Association between estrogen receptor α gene variation and cardiovascular disease. JAMA. 2003;290(17):2263–70. doi: 10.1001/jama.290.17.2263. [DOI] [PubMed] [Google Scholar]
- 22.Schuit SCE, Oei HH, Witteman JCM, et al. Estrogen receptor α gene polymorphisms and risk of myocardial infarction. JAMA. 2004;291(24):2969–77. doi: 10.1001/jama.291.24.2969. [DOI] [PubMed] [Google Scholar]
- 23.Lamon-Fava S, Asztalos BF, Howard TD, et al. Association of polymorphisms in genes involved in lipoprotein metabolism with plasma concentrations of remnant lipoproteins and HDL subpopulations before and after hormone therapy in postmenopausal women. Clin Endocrinol (Oxf) 2010;72(2):169–75. doi: 10.1111/j.1365-2265.2009.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lian K, Lui L, Zmuda JM, et al. Estrogen receptor alpha genotype is associated with a reduced prevalence of radiographic hip osteoarthritis in elderly Caucasian women. Osteoarthr Cartil. 2007;15:972. doi: 10.1016/j.joca.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai X, Wang C, Dai J, et al. Association of single nucleotide polymorphisms in estrogen receptor alpha gene with susceptibility to knee osteoarthritis: A case-control study in a Chinese Han population. Biomed Res Int. 2014;2014:151457. doi: 10.1155/2014/151457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren Y, Tan B, Yan P, et al. Association between polymorphisms in the estrogen receptor alpha gene and osteoarthritis susceptibility: A meta-analysis. BMC Musculoskelet Disord. 2015;16:44. doi: 10.1186/s12891-015-0506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson VW, St John JA, Hodis HN, et al. Cognition, mood, and physiological concentrations of sex hormones in the early and late postmenopause. Proc Natl Acad Sci USA. 2013;110(50):20290–95. doi: 10.1073/pnas.1312353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: A systematic review and meta-analysis. J Steroid Bioch Mol Biol. 2014;142:90–98. doi: 10.1016/j.jsbmb.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wharton W, Gleason CE, Dowling NM, et al. The KEEPS-Cognitive and Affective Study: Baseline associations between vascular risk factors and cognition. J Alzheimers Dis. 2014;40(2):331–41. doi: 10.3233/JAD-130245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maric-Bilkan C, Manigrasso MB. Sex differences in hypertension: Contribution of the Renin- Angiotensin system. Gend Med. 2012;9(4):287–91. doi: 10.1016/j.genm.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Berent-Spillson A, Persad CC, Love T, et al. Hormonal environment affects cognition independent of age during the menopause transition. J Clin Endocrinol Metab. 2012;97(9):1686–94. doi: 10.1210/jc.2012-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tumisto H, Salo P, Saarinen R, et al. The association of serum oestradiol level, age, and education with cognitive performance in peri- and late postmenopausal women. Maturitas. 2012;71(2):173–79. doi: 10.1016/j.maturitas.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 33.Ryan J, Scali J, Carrière I, et al. Impact of a premature menopause on cognitive function in later life. BJOG. 2014;121(13):1729–39. doi: 10.1111/1471-0528.12828. [DOI] [PubMed] [Google Scholar]
- 34.Yaffe K, Lui LY, Grady D, et al. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. Lancet. 2000;356:708–12. doi: 10.1016/S0140-6736(00)02628-3. [DOI] [PubMed] [Google Scholar]
- 35.Laughlin GA, Kritz-Silverstein D, Barrett-Connor E. Endogenous oestrogens predict 4-year decline in verbal fluency in postmenopausal women: The Rancho Bernardo Study. Clin Endocrinol (Oxf) 2010;72(1):99–106. doi: 10.1111/j.1365-2265.2009.03599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebrun CE, van der Schouw YT, de Jong FH, et al. Endogenous oestrogens are related to cognition in healthy elderly women. Clin Endocrinol (Oxf) 2005;63(1):50–55. doi: 10.1111/j.1365-2265.2005.02297.x. [DOI] [PubMed] [Google Scholar]
- 37.Pourganji M, Hosseini M, Soukhtanloo M, et al. Protective role of endogenous ovarian hormones against learning and memory impairments and brain tissues oxidative damage induced by lipopolysaccharide. Iran Red Crescent Med J. 2014;16(3):e13954. doi: 10.5812/ircmj.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newhouse P, Dumas J. Estrogen-cholinergic interactions: Implications for cognitive aging. Horm Behav. 2015;74:173–85. doi: 10.1016/j.yhbeh.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding J, Xu H, Yin X, et al. Estrogen receptor α gene PvuII polymorphism and coronary artery disease: A meta-analysis of 21 studies. Univ-Sci B (Biomed & Biotechnol) 2014;15(3):243–55. doi: 10.1631/jzus.B1300220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaffe K, Lindquist K, Sen S, et al. Estrogen receptor genotype and risk of cognitive impairment in elders: findings from the Health ABC study. Neurobiol Aging. 2009;30(4):607–14. doi: 10.1016/j.neurobiolaging.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sowers MR, Symons JP, Jannausch ML, et al. Sex steroid hormone polymorphisms, high-density lipoprotein cholesterol, and lipoprotein A-1 from the Study of Women’s Health Across the Nation (SWAN) Am J Med. 2006;119(9 Suppl 1):61–68. doi: 10.1016/j.amjmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Schuit SCE, de Jong FH, Stolk L, et al. Estrogen receptor alpha gene polymorphisms are associated with estradiol levels in postmenopausal women. Eur J Endocrinol. 2005;153:327–34. doi: 10.1530/eje.1.01973. [DOI] [PubMed] [Google Scholar]
- 43.Sunderman EE, Maki PM, Bishop JR. A review of estrogen receptor alpha gene (ESR1) polymorphisms, mood, and cognition. Menopause. 2010;17(4):874–86. doi: 10.1097/gme.0b013e3181df4a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorentzon M, Lorentzon R, Bäckström T, Nordström P. Estrogen receptor gene polymorphism, but not estradiol levels, is related to bone density in healthy adolescent boys: A cross-sectional and longitudinal study. J Clin Endocrinol Metab. 1999;84(12):4597–601. doi: 10.1210/jcem.84.12.6238. [DOI] [PubMed] [Google Scholar]
- 45.He M, Shu J, Huang X, Tang H. Association between estrogen receptora gene (ESR1) PvuII (T/C) and XbaI (A/G) polymorphisms and premature ovarian failure risk: Evidence from a meta-analysis. J Assist Reprod Genet. 2015;32(2):297–304. doi: 10.1007/s10815-014-0393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bousman CA, Szoeke C, Chen K, et al. Oestrogen alpha-receptor variants and two-year memory decline in midlife Australian women. Neuropsychobiology. 2012;66(4):259–65. doi: 10.1159/000341879. [DOI] [PubMed] [Google Scholar]
- 47.Corbo RM, Gambina G, Ruggeri M, Scacchi R. Association of estrogen receptor alpha (ESR1) PvuII and XbaI polymorphisms with sporadic Alzheimer’s disease and their effect on apolipoprotein E concentrations. Dement Geriatr Cogn Disord. 2006;22(1):67–72. doi: 10.1159/000093315. [DOI] [PubMed] [Google Scholar]
- 48.Olsen L, Rasmussen HB, Hanset T, et al. Estrogen receptor alpha and risk for cognitive impairment in postmenopausal women. Psychiatr Genet. 2006;16(2):85–88. doi: 10.1097/01.ypg.0000194445.27555.71. [DOI] [PubMed] [Google Scholar]
- 49.Lee YH, Song GG. Estrogen receptor 1 PvuII and XbaI polymorphisms and susceptibility to Alzheimer’s disease: A meta-analysis. Genet Mol Res. 2015;14(3):9361–69. doi: 10.4238/2015.August.10.17. [DOI] [PubMed] [Google Scholar]
- 50.Cheng D, Liang B, Hao Y, Zhou W. Estrogen receptor alpha gene polymorphism and risk of Alzheimer’s disease: Evidence from a meta-analysis. Clin Interv Aging. 2014;9:1031–38. doi: 10.2147/CIA.S65921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang T. Meta-analysis of PvuII, XbaI variants in ESR1 gene and the risk of Alzheimer’s disease: the regional European difference. Neurosci Lett. 2014;574:41–46. doi: 10.1016/j.neulet.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 52.Maruyama H, Toji H, Harrington CR, et al. Lack of an association of estrogen receptor alpha gene polymorphisms and transcriptional activity with Alzheimer disease. Arch Neurol. 2000;57(2):236–40. doi: 10.1001/archneur.57.2.236. [DOI] [PubMed] [Google Scholar]
- 53.Usui C, Shibata N, Ohnuma T, et al. No genetic association between the myeloperoxidase gene –463 polymorphism and estrogen receptor-alpha gene polymorphisms and Japanese sporadic Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21(5–6):296–99. doi: 10.1159/000091437. [DOI] [PubMed] [Google Scholar]
- 54.Monastero R, Cefalù AB, Camarda C, et al. A case control study. J Alzheimers Dis. 2006;9(3):273–78. doi: 10.3233/jad-2006-9306. [DOI] [PubMed] [Google Scholar]
- 55.Ryan J, Carriere I, Amieva H, et al. Prospective analysis of the association between estrogen receptor gene variants and the risk of cognitive decline elderly women. Eur Neuropsychopharmacol. 2013;23(12):1763–68. doi: 10.1016/j.euroneuro.2013.06.003. [DOI] [PubMed] [Google Scholar]