Abstract
Staphylococcus aureus is a major human pathogen associated with high mortality. The emergence of antibiotic resistance and the inability of antibiotics to counteract bacterial cytotoxins involved in the pathogenesis of S. aureus call for novel therapeutic approaches, such as passive immunization with monoclonal antibodies (mAbs). The complexity of staphylococcal pathogenesis and past failures with single mAb products represent considerable barriers for antibody-based therapeutics. Over the past few years, efforts have focused on neutralizing α-hemolysin. Recent findings suggest that the concerted actions of several cytotoxins, including the bi-component leukocidins play important roles in staphylococcal pathogenesis. Therefore, we aimed to isolate mAbs that bind to multiple cytolysins by employing high diversity human IgG1 libraries presented on the surface of yeast cells. Here we describe cross-reactive antibodies with picomolar affinity for α-hemolysin and 4 different bi-component leukocidins that share only ∼26% overall amino acid sequence identity. The molecular basis of cross-reactivity is the recognition of a conformational epitope shared by α-hemolysin and F-components of gamma-hemolysin (HlgAB and HlgCB), LukED and LukSF (Panton-Valentine Leukocidin). The amino acids predicted to form the epitope are conserved and known to be important for cytotoxic activity. We found that a single cross-reactive antibody prevented lysis of human phagocytes, epithelial and red blood cells induced by α-hemolysin and leukocidins in vitro, and therefore had superior effectiveness compared to α-hemolysin specific antibodies to protect from the combined cytolytic effect of secreted S. aureus toxins. Such mAb afforded high levels of protection in murine models of pneumonia and sepsis.
Keywords: monoclonal antibody, toxin neutralization, engineered cross-reactivity, exotoxins, Staphylococcus aureus, in vitro potency, in vivo efficacy
Abbreviations
- mAb
monoclonal antibody
- Hla
α-hemolysin
- LukSF
leukocidin SF
- HlgAB and HlgCB
gamma-hemolysins
- LukED
leukocidin ED
- PMN
polymorphonuclear cells
- RBC
red blood cell
- IC50
inhibitory concentration
- EC50
effective concentration
- BLI
biolayer interferometry
Introduction
Staphylococcus aureus is the most common cause of healthcare-associated infections associated with high mortality among patients who develop pneumonia or sepsis. The spread of antibiotic resistant clones (hospital and community associated methicillin-resistant S. aureus; HA- and CA-MRSA) is an additional concern, emphasizing the need for novel therapeutic approaches.1 Several attempts have been made to induce protective immunity by vaccination or passive immunization against S. aureus infections. All these approaches aimed at enhancing opsonophagocytic uptake and killing by phagocytic cells, and all have fallen short of demonstrating efficacy in the clinic.2,3
An increased understanding of the major contribution of cytotoxins to the pathogenesis of S. aureus infections has led to new immune approaches.4,5 Alpha-hemolysin (Hla), a major virulence factor that damages several types of human cells, has shown promise as a vaccine antigen and monoclonal antibody (mAb) target in animal models of S. aureus disease.6-11 Hla is currently being evaluated in human trials in both active and passive immunization settings.
Members of the bi-component cytotoxin family, gamma-hemolysins (HlgAB and HlgCB), Panton-Valentine Leukocidin (PVL or LukSF), LukED and LukGH (also known as LukAB) can lyse and activate human phagocytic cells and are therefore implicated to play a role in evasion of the innate immune response, a hallmark of S. aureus pathogenesis.12-15 In addition, HlgAB is a potent toxin for human red blood cells12 and LukED has recently been reported to target human T cells.16 PVL/LukSF and LukGH are species specific and have very low or no lytic activity toward murine cells.5,12-14,17
The vast majority of S. aureus clinical isolates express Hla, HlgABC, and LukGH, and approximately 50–75% of them also carry LukED. The LukSF/PVL gene, encoded by prophages, is present in 5–10% of strains and implicated in the manifestation of more severe disease.12
Seroepidemiology studies suggest a correlation between higher serum levels of toxin-specific antibodies and favorable clinical outcome.18,19 Therefore, supplementing the antibody repertoire with toxin-neutralizing mAbs is expected to be beneficial for patients with low endogenous levels of such antibodies.
The subunits of leukocidins, the S- and F-components – secreted individually in inactive forms – are highly related structurally and share up to 80% amino acid identity, apart from LukGH (<40%). The bi-component toxin monomers form barrel-like oligomeric pores that resemble those built by Hla monomers in spite of low amino acid sequence conservation (∼25–27%).20-24
Given the complex pathogenesis of S. aureus, mAbs that are able to neutralize several virulence factors implicated in severe disease are likely to be highly beneficial. Here we report the discovery of human mAbs that cross-neutralize Hla and several leukocidins and provide improved potency compared to Hla-specific antibodies in vitro and elicit high levels of protection in murine models of S. aureus pneumonia and sepsis.
Results
Selection of Hla and leukocidin cross-reactive human mAbs
Based on the amino acid conservation among the bi-component leukocidins and their structural homology with Hla, we hypothesized that mAbs could be identified that would bind to more than one toxin and hence cross-neutralize them (Fig. 1).
Figure 1.
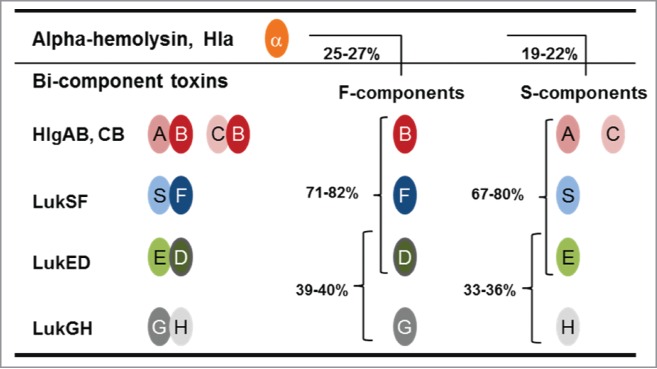
Sequence homology among staphylococcal cytotoxins. The cartoon depicts the cognate pairs of S- and F-components of bi-component leukocidins. Numbers represent the percent of amino acid identity among S- and F-components and between these components and α-hemolysin (Hla).
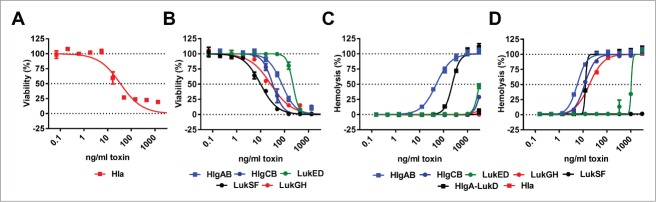
Ten recombinant toxin molecules – Hla, 5 S-components (HlgA, HlgC, LukS, LukE and LukH) and 4 F-components (HlgB, LukF, LukD and LukG) – were generated based on the genome sequence of the USA300 CA-MRSA strain TCH1516. To ensure high quality and functionality of protein baits used for antibody discovery, toxins were tested for cytolytic activity in in vitro assays. Due to the known species specificity of some of the staphylococcal cytotoxins, in vitro assays employed human cells, in addition to the rabbit red blood cells (RBCs) widely used for testing Hla. Hla activity was determined in assays measuring lysis of human alveolar epithelial cells (A549 cell line) or rabbit RBCs (Fig. 2A, D), while potency of the leukocidins was tested with freshly isolated human polymorphonuclear cells (PMNs) and human or rabbit RBCs (Fig. 2B–D). We observed that the S- and F-components of LukSF, LukED and HlgABC also formed active leukocidins with non-cognate pairing in all 8 possible permutations (data not shown). HlgAB and HlgA-LukD were also highly potent in lysing human RBCs, unlike Hla that is known to have low potency with this cell type (Fig. 2C). Rabbit RBCs were efficiently killed by almost all toxins, except LukSF and LukGH (Fig. 2D).
Figure 2.
In vitro potency of recombinant S. aureus toxins. Cytolytic activity of indicated toxins was measured using (A): A549 cells, (B): human PMNs, (C): human RBCs or (D): rabbit RBCs in the indicated concentration range. Error bars indicate mean +/− SEM, n = 2.
Three toxins - Hla, HlgAB and HlgA-LukD - proved to be lethal when administered to mice intravenously in the 0.125–1 μg/mouse dose range. Hla also killed the animals when applied intranasally with a minimal lethal dose of 0.25 μg/animal as a result of massive lung damage (Fig. S1).
Due to the well-established dominant role of Hla in S. aureus virulence, we initially focused on the identification of highly potent anti-Hla antibodies without regard to their cross-reactivity with S- or F-components. Antibody discovery efforts were based on a library of full-length human IgG1s presented on the surface of yeast cells, according to previously published methods.25-29 MAbs selected with biotinylated Hla from IgG1 libraries and displaying neutralization activity were further optimized for higher affinity in 2 successive selection cycles by introducing diversity into the antibody genes and using decreasing concentrations of Hla. These mAbs reached KD values in the single-digit picomolar range and high neutralization potency (IC50 of ∼0.2 mAb:toxin ratio) (Fig. 3).
Figure 3.
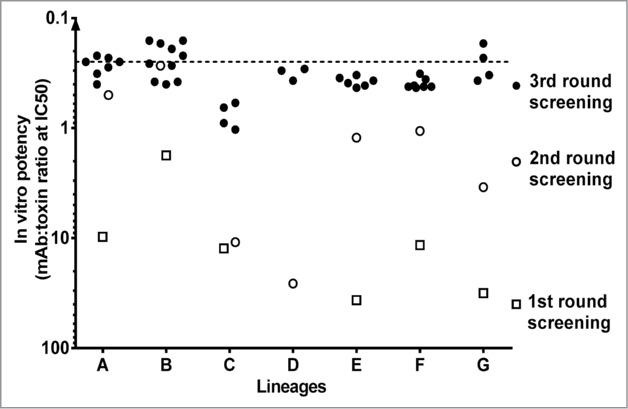
Selection of Hla neutralizing antibodies from human IgG1 libraries expressed by yeast. IgGs obtained in 3 rounds of selection and purified from yeast clones were tested for Hla neutralizing activity in cytolysis assay with human lung epithelial cells (A549). Antibody potency is expressed as mAb:toxin ratio at half maximal inhibition of cell lysis (IC50).
To determine whether these Hla-reactive mAbs interacted with bi-component toxins, binding to individual S- and F-components was measured by biolayer interferometry (BLI, fortéBio). A small number of mAbs (mainly in lineage G, Fig. 3) were identified that exhibited cross-reactivity to HlgB, LukD or LukF; however, no binding was detected with S-components or LukGH subunits (data not shown).
Separate selections were also performed using naïve IgG1 libraries with either the S- or the F-components both individually and in an alternating fashion in successive selection rounds. These experiments resulted in numerous mono-specific, double, triple and (in the case of S- components) quadruple cross-reactive S- and F-component specific mAbs. None of the cross-reactive mAbs displayed binding to LukH or LukG. Hla was then introduced in the selections with sub-libraries generated from heavy chains of the cross-reactive clones with new light chain diversity. This resulted in a low number of antibodies with cross-reactivity to HlgB, LukD, LukF and Hla, but not with LukG or any of the S-components.
In an effort to improve the affinity of the cross-reactive mAbs identified by the 2 approaches, complementarity-determining region (CDR)-focused mutagenesis libraries were created and subjected to further rounds of alternating selections with F-components. As a result, several mAbs with improved affinity toward one, 2 or all 3 F-components were generated. Most importantly, these antibodies retained their very high affinity for Hla (KD = <2–37 pM), while exhibiting high affinity binding to HlgB (KD = <2–160 pM), LukF (KD = <3–120 pM), and LukD (KD = 86 pM −2.2 nM) (Table 1).
Table 1.
Binding affinity (Fab KD) measured by Meso Scale Discovery (MSD) and in vitro neutralization potency of mAbs used in the study
|
In vitro neutralization potency |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Affinity (MSD Fab KD, pM) |
IC50 expressed as mAb: toxin ratio |
||||||||||||
| Hla | HlgB | LukF | LukD | Hla |
Hla |
HlgCB |
HlgAB |
LukSF |
LukED |
HlgA-LukD |
Hla LukSF HlgCB HlgAB LukED |
Hla LukSF HlgCB HlgAB LukED |
|
| mAbs | rRBC | A549 | hPMN | hRBC | hPMN | hPMN | hRBC | hPMN | hRBC | ||||
| Hla-F#1 | 2.9a | 5.1 | 120 | 2200 | <0.25 | <0.5 | 1.2 | 13.5 | 7.8 | 4.9 | 8.2 | 3.5 | 2.9 |
| Hla-F#2 | 37a | 18 | < 3 | 1700 | <0.25 | 0.6 | 0.7 | 6.5 | 0.5 | 4.8 | 7.3 | 1.1 | 1.6 |
| Hla-F#3 | 23a | 140 | nd | 86 | <0.25 | <0.5 | 147.6 | nd | nd | 0.3 | 1.2 | nd | nd |
| Hla-F#4 | 30a | 160 | nd | 160 | <0.25 | <0.5 | 180.7 | nd | nd | 0.4 | 1.4 | nd | nd |
| Hla-F#5 | <2a, 2.1b | < 3 | < 3 | 400 | < 0.25 | < 0.5 | 0.7 | 5.6 | 0.7 | 1.0 | 3.0 | 0.5 | 1.1 |
| Hla-F#6 | < 2a | < 2 | 50 | 290 | < 0.25 | < 0.5 | 0.8 | 2.8 | 2.2 | 0.9 | 2.3 | 0.8 | 0.6 |
| Hla-F#7 | < 2a | < 2 | 73 | 780 | < 0.25 | < 0.5 | 0.3 | 4.2 | 2.7 | 2.2 | 4.8 | 1.2 | 0.9 |
| Hla | 40b | nd | nd | nd | < 0.25 | < 0.5 | nd | nd | nd | nd | nd | nd | nd |
| HlgB | nd | 4.2 | nd | nd | nd | nd | 1.6 | 1.6 | nd | nd | nd | nd | nd |
| LukD | nd | nd | nd | 7.0 | nd | nd | nd | nd | nd | 0.2 | 1.2 | nd | nd |
| LukF | nd | nd | 9000 | nd | nd | nd | nd | nd | 93.4 | nd | nd | nd | nd |
| 243-4 | 13b | nd | nd | nd | < 0.25 | < 0.5 | nd | nd | nd | nd | nd | nd | nd |
| LC10 | 13b | nd | nd | nd | < 0.25 | < 0.5 | nd | nd | nd | nd | nd | nd | nd |
| LTM14 | 4.5b | nd | nd | nd | < 0.25 | < 0.5 | nd | nd | nd | nd | nd | nd | nd |
nd: not detectable
a KD values of yeast produced mAbs measured in the same assay (fitted antigen concentrations between 20 and 30 nM were used to calculate lower KD limits (10% of the antigen concentration); b KD values of mammalian cell produced mAbs measured in the same assay at 20 pM antigen concentration (KD limit ∼ 2 pM). 95% confidence intervals of the predicted KDs are typically within two fold or less from the fitted KD values reported here.
Neutralization of recombinant toxins by Hla cross-reactive mAbs
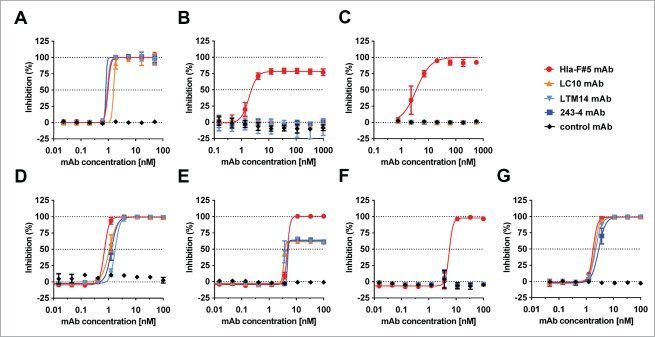
Several Hla-LukF-LukD-HlgB quadruple cross-reactive antibodies were generated that were highly efficient to neutralize Hla in rabbit RBC or human lung epithelial cell lysis assays, to prevent lysis of human phagocytic cells by LukSF, LukED, HlgCB and that of human RBCs by HlgAB and HlgA-LukD (Table 1). The potency of these mAbs was not restricted to the 4 cognate toxin pairs but was equally evident against the 8 leukocidins formed by non-cognate pairing (example shown for HlgA-LukD in Table 1). Importantly, these mAbs did not display off-target binding demonstrated by lack of polyreactivity or neutralization of an unrelated cytolysin. We found that binding affinity toward the different toxins was highly predictive for toxin neutralizing potency of mAbs (Fig. 4A, Table 1).
Figure 4.
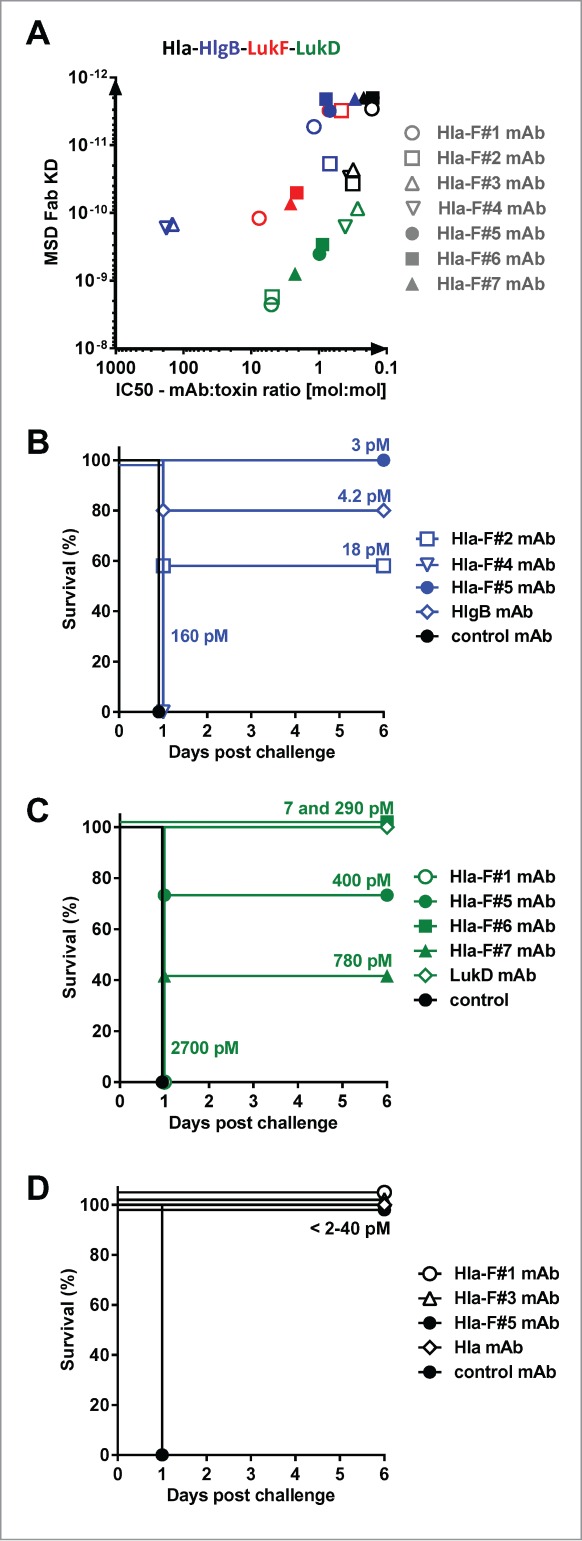
Correlation between affinity and potency of toxin cross-neutralizing mAbs. (A): Neutralization potency of toxin cross-reactive mAbs Hla-F#1-7 was determined with rabbit RBCs for Hla, and with freshly isolated human PMNs for HlgCB, LukED and LukSF and expressed as mAb:toxin ratio at half maximal inhibition of cell lysis (IC50). For all toxins the correlation between in vitro potency and affinity was significant based on Pearson's correlation coefficient analysis (r > 0.9, P < 0.005). In vivo potency of mAbs was determined in intravenous challenge of mice with HlgAB (B) and HlgA-LukD (C) or with intranasal challenge with Hla (D). Groups of 5 mice were given 100 μg of indicated mAbs intraperitoneally before challenge with 100% lethal dose of toxins. Fab affinities (KD) measured by MSD are indicated.
Next, we investigated the relationship between toxin binding affinity/in vitro neutralization potency and in vivo efficacy in murine toxin challenge models. In these experiments, mAbs were administered intraperitoneally at 100 μg/mouse dose (∼5 mg/kg) 24 h prior to a lethal toxin challenge with HlgAB or HlgA-LukD given intravenously and Hla intranasally. We detected a broad range of protection levels with the Hla - F-component cross-reactive mAbs that correlated well with their affinity and neutralization potency toward HlgB and LukD. MAbs with the best affinity were highly potent in preventing death as efficiently as high affinity HlgB and LukD monospecific antibodies derived from the yeast libraries (Fig. 4B, C). High protection levels were observed in the intranasal lethal Hla challenge model with both the cross-reactive mAbs and a Hla-specific mAb selected from yeast libraries, with all mAbs displaying high affinity for Hla (Fab KD <2–40 pM) (Fig. 4D). Based on these experiments, we concluded that cross-reactivity of antibodies toward the different toxin molecules was not associated with reduced potency against the individual components, provided that sufficiently high binding affinity was achieved.
The importance of affinity toward HlgB, LukD and LukF was further demonstrated in in vitro assays when cells were intoxicated with a mixture of recombinant leukocidins, added at concentrations able to cause >80% cell lysis on their own. We observed that antibodies that lacked neutralization activity even against only one of the toxins failed to provide protection against lysis of human PMNs or RBCs, exemplified by mAbs Hla-F#3 and #4 that did not bind to LukF and had low affinity toward HlgB (Table 1). Three mAbs with the highest overall affinity for F-components, Hla-F#5, 6 and 7, were found to be the most effective in these assays.
In vitro potency of toxin cross-reactive and Hla-specific mAbs
Three human anti-Hla mAbs have been reported in the literature to be potent in neutralizing Hla and to confer protection against S. aureus infections in murine models: 243-4,30 LC1010,31 and LTM14.11,32 These mAbs, which were discovered using human B cells, transgenic mouse technology and phage display libraries, respectively, were expressed based on published sequence information to further investigate if the multi-specific anti-toxin mAbs were compromised in their Hla neutralization potency.30-32 All mAbs used in these comparative studies were expressed in mammalian cells, and were comparable based on biochemical assays testing for purity, solubility and stability. The binding affinities of the LC10, LTM14 and 243-4 mAbs for Hla were in the low picomolar range (KD = 4.5–13 pM), which were comparable to the KD values measured with Hla–leukocidin cross-reactive mAbs (KD = <2–37 pM). The KD values measured with the LTM14 and LC10 mAbs expressed in our laboratory are in good agreement with those published by others (1.7 and 10 pM, respectively).11,33 None of the 3 anti-Hla mAbs displayed any detectable binding to F- or S-components and consequently had no neutralizing activity against the recombinant leukocidins (Table 1). The Hla neutralization potencies of the 2 types of antibodies were very comparable as measured either using purified Hla with rabbit RBCs or A549 cells (Table 1) or bacterial culture supernatants of the S. aureus TCH1516 strain, with the latter cell type being sensitive only to Hla but not to leukocidins (Fig. 5A). Lysis of human RBCs and human PMNs induced by bacterial culture supernatants could only be prevented by Hla-leukocidin cross-reactive mAbs (Fig. 5B, C). Superior potency of cross-reactive mAbs was also observed when rabbit RBCs - sensitive to HlgAB, HlgCB, LukED, HlgA-LukD in addition to Hla - were intoxicated with different concentrations of bacterial culture supernatant of the TCH1516 strain. At low concentrations, all antibodies were equally effective, while at higher concentrations the Hla-specific mAbs were only partially or not protective, due to the increasing amounts of bi-component toxins (Fig. 5D–F). The major contribution of the bi-component toxins to cell lysis in these assays, as well as the comparable Hla-neutralizing efficacy of the different antibodies were further demonstrated by using an isogenic TCH1516 mutant strain that lacks expression of all bi-component toxins due to targeted gene deletions (Fig. 5G).
Figure 5.
Inhibition of native cytolysins with toxin cross-reactive and Hla-specific mAbs. Target cells were intoxicated with sterile filtered bacterial culture supernatants (CS) after pre-incubation with Hla-F#5 cross-reactive and indicated Hla-specific mAbs. (A): Human lung epithelial cells (A549) with CS from TCH1516 strain; (B): human RBCs with CS from Newman strain; (C): human PMNs with CS from TCH1516ΔlukGH strain. (D–G): rabbit RBCs with CS from TCH1516 strain at 17.5×, 7.5× and 2.5× dilutions (D, E and F, respectively) and with CS from the TCH1516ΔhlgABCΔlukEDΔlukSFΔlukGH strain at 4× dilution. Inhibition of supernatant cytotoxicity by mAbs was measured by determining cell viability or hemolysis in case of RBCs. Error bars represent SD, n = 3.
Toxin binding site of cross-reactive mAbs
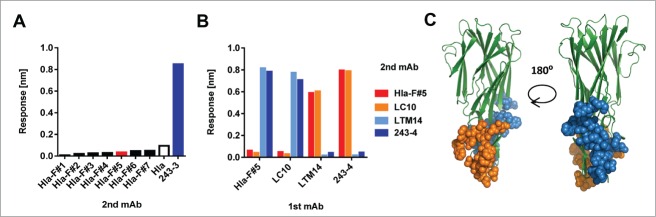
Epitope binning performed with BLI revealed that all Hla - F-component cross-reactive mAbs competed with each other, suggesting that they bind to the same region of Hla (Fig. 6A). A representative toxin cross-reactive antibody, Hla-F#5 competed very well with the LC10 Hla-specific mAb, but not with the LTM14 and 243-4 Hla mAbs (Fig. 6B). The latter 2 competed with each other for Hla binding. Based on these results there are 2 distinct binding regions of the Hla-reactive antibodies analyzed in this study. For LC10 and LTM14, the X-ray crystal structure of the Fab-Hla complexes have been determined and revealed that both mAbs recognize the rim domain of Hla involved in cell binding, but in distinct regions without amino acid overlap in the contact residues (recapitulated in Fig. 6C).12,31-33 This is consistent with our finding that these 2 mAbs did not compete with each other.
Figure 6.
Delineating toxin binding sites of mAbs by competition studies. Binning of antibodies was performed by BLI/fortéBio by coating anti-human capture sensors with “1st mAbs," followed by addition of Hla; antibody competition was assessed by detecting binding of indicated “2nd mAbs” (A, B). (A): 1st mAb: Hla-F#5, 2nd mAbs as indicated. (B): 1st and 2nd mAbs as indicated. (C): The epitopes of LC10 and LTM14 mAbs are shown in orange and blue, respectively, based on published data in the Hla structural model, shown in green.11,31,32
To further delineate the binding epitope of the cross-reactive mAbs to the F- components, we analyzed sequence conservation among the cytotoxins. 68% of the amino acid residues of LukF are identical in LukD and HlgB, many of these are surface exposed based on the X-ray crystal structure of LukF, but only 1/3 of these are conserved in Hla (Fig. 7A).23,34 The largest patch of surface-located amino acid residues conserved among Hla, LukF, LukD and HlgB, but not with LukG or the S-components, of the TCH1516 strain can be found in the rim domain of Hla in the LC10 epitope region. Analysis of the toxin sequences available in public data bases revealed that none of the - otherwise few - amino acid polymorphisms detected in more than 200 S. aureus genomes was located in this conserved surface patch (Fig. S2).
Figure 7.
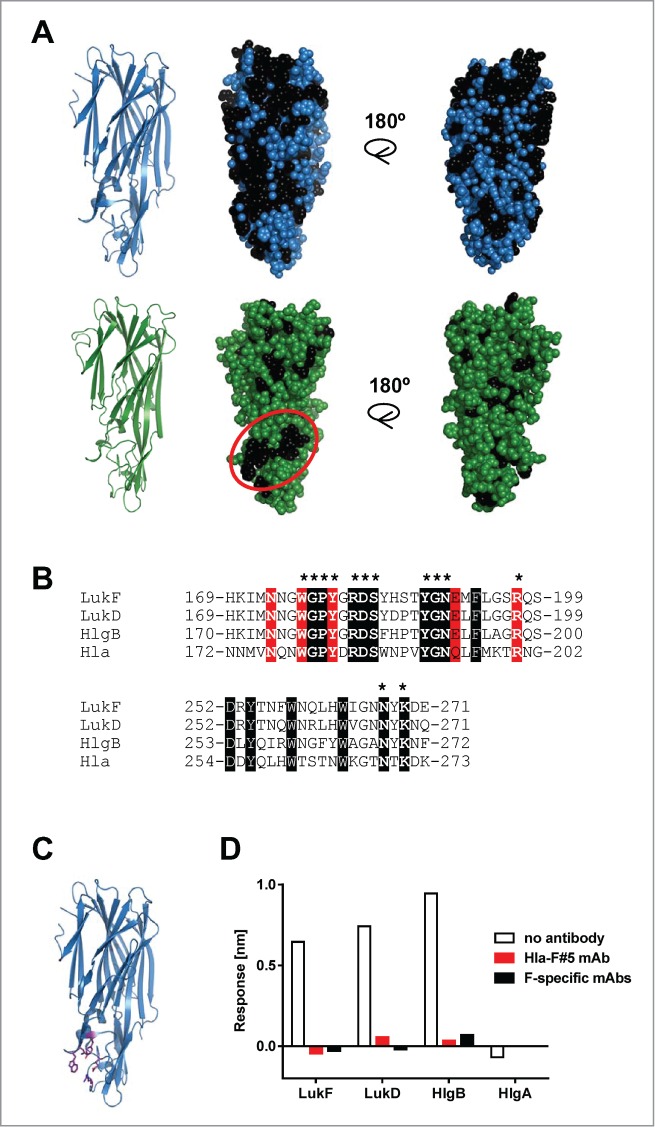
Binding epitope of Hla – F-component cross-reactive mAbs. (A): Conserved amino acids among LukF, HlgB and LukD are indicated in black on the LukF crystal structure (PDB entry: 1PVL) shown in blue (upper panel). Amino acids conserved between the 3 F-components and Hla are indicated in black on the structural model of Hla monomer, shown in green (lower panel). (B): Sequence alignment of Hla and the F-components for the region of the rim domain showing the highest homology; identical amino acids in white, phosphocholine (PC)-binding residues in red; amino acids of the circled area of lower panel in A are shown in bold and starred. (C): Amino acids forming the PC-binding pocket are shown in purple in the LukF structure. (D): PC-binding to F-components was measured by BLI in the presence or absence of Hla-F#5 and HlgB-, LukD- or LukF-specific mAbs. HlgA was used as negative control for PC-binding.
This conserved region encompasses the phosphocholine (PC)-binding pocket that was shown to be essential for binding and functional pore formation by Hla and F-components (Fig. 7B, C).20,34-38 In a BLI-based assay, we detected binding of PC to F-components, but not to HlgA that lacks PC-binding activity, that was inhibited in the presence of Hla-F#5 (Fig. 7D). Interestingly, the LukF, HlgB and LukD specific mAbs also interfered with binding to PC. These data suggest that the PC-binding region of the cell binding domain of cytotoxins is a hot-spot for neutralizing antibodies.
Based on the localization of the epitope, we hypothesized that the mode of action of the neutralizing antibodies is inhibition of toxin binding to target cells. We tested this by measuring cell binding of biotinylated Hla to A549 cells or HlgAB, HlgCB, LukED and LukSF to human PMNs using flow cytometry. For the bi-component toxins we used an equimolar mixture of unlabeled S-components and biotinylated F-components. Target cell binding was detected using fluorescently labeled Streptavidin. All the Hla-specific mAbs and the cross-reactive Hla-F#5 mAb were able to block Hla binding to A549 cells. In line with the antigen binding and neutralization data, the Hla-F#5 mAb could also inhibit the binding of the bi-component toxins to PMNs (Fig. S3).
In vivo efficacy of Hla-F component cross-reactive mAb
Protective efficacy of the Hla-F#5 mAb was evaluated in murine models of S. aureus pneumonia and bacteremia/sepsis. Infections were induced by intranasal or intravenous challenge with lethal doses of the USA300 CA-MRSA TCH1516 strain 24 hours following passive immunization with 100 μg of mAb (∼5 mg/kg) via the intraperitoneal route. In the stringent pneumonia model, most control mice died within 24 hours following challenge, while in the bacteremia/sepsis model death was not immediate and mainly occurred between day 2 and 5. Animals treated prophylactically with Hla-F#5 mostly survived the lethal challenge in both models (P < 0.0001) (Fig. 8A, B).
Figure 8.
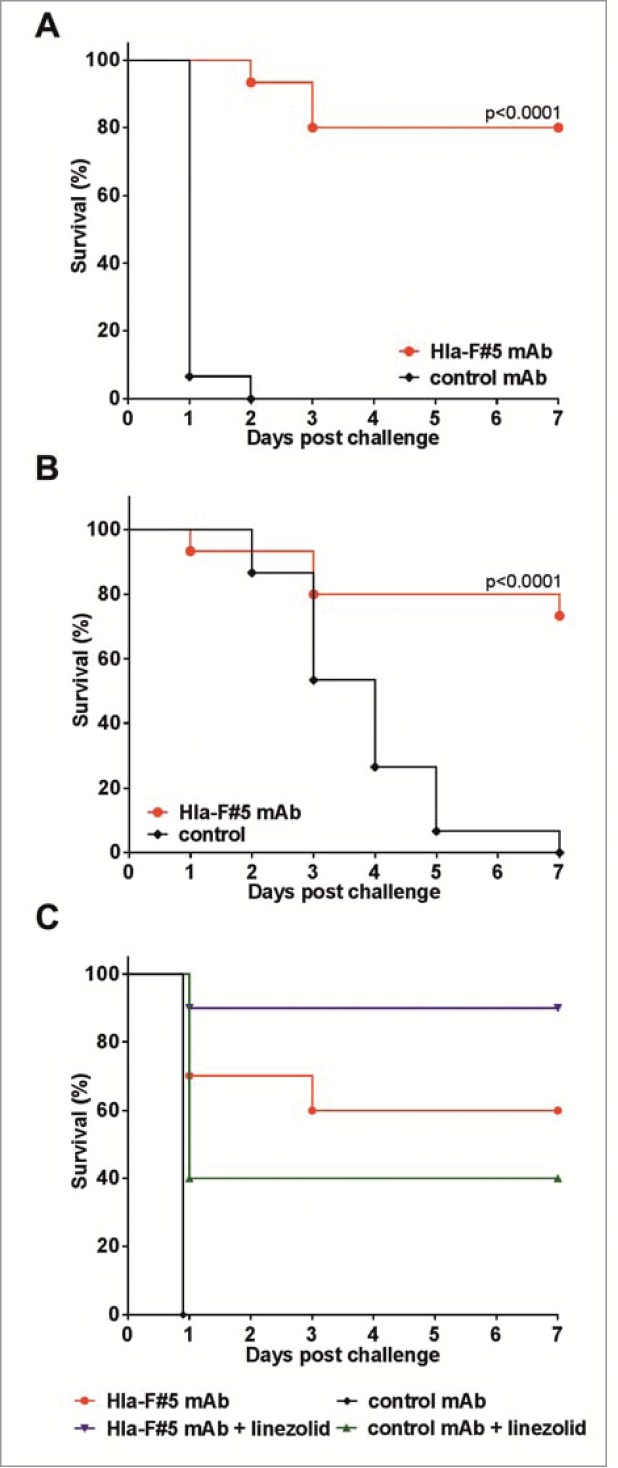
Efficacy of an Hla bi-component toxin cross-neutralizing mAb in murine bacterial challenge models. (A, B): Mice were treated with 100 μg of Hla-F#5 mAb intraperitonally 24 h prior to bacterial challenge. Animals were challenged with the TCH1516 USA300 CA-MRSA strain with 6×108 cfu dose intranasally (A) or 5 × 107 cfu intravenously (B). (C): Mice were treated with 50 μg (∼2.5 mg/kg) Hla-F#5 and/or 20 μg (∼1 mg/kg) linezolid or both 2 hours post i.n. challenge with 6×108 cfu TCH1516. Control mice received isotype-matched irrelevant mAb or vehicle (PBS). Data are derived from 3, 3 and 2 independent experiments for A, B, and C, respectively, each with 5 mice/group. Survival curves were statistically compared by the Log-rank (Mantel-Cox) test. (C): All groups were statistically significant compared to the control mAb: Hla-F#5 mAb + linezolid, P < 0.0001; Hla-F#5 mAb alone, p = 0.0014; control mAb + linezolid, p = 0.0293. The combination of Hla-F#15 mAb + linezolid was also statistically significant versus control mAb + linezolid treatment, p = 0.0223.
Next, we tested this antibody for its ability to enhance therapeutic efficacy of an antibiotic that is clinically relevant in the treatment of MRSA infections. In preliminary experiments, we determined the approximately 50% protective dose of linezolid (20 μg per animal, ∼1 mg/kg; single dose) and Hla-F#5 (50 μg, ∼2.5 mg/kg) when given 2 hours after the intranasal lethal bacterial challenge (data not shown). We found that combined treatment with Hla-F#5 and linezolid was significantly more effective than linezolid alone (Fig. 8C).
Discussion
In this study, using yeast surface displayed IgG1 libraries we generated unique human mAbs that bind 4 different toxin molecules of S. aureus with high affinity: α-hemolysin and 3 F-components of the bi-component leukocidins HlgABC, LukED and LukSF. It is a rather surprising finding given the low amino acid homology between Hla and the bi-component toxins. Importantly, this multi-specific binding leads to inhibition of cytotoxic activity of the corresponding toxin molecules, with potencies that are not inferior to those observed with monospecific antibodies. Binding affinities for the individual toxins were highly predictive for neutralization potency and efficacy, similarly to other toxin neutralizing antibodies; one of the best examples is the anthrax toxin neutralizing mAbs described by Maynard et al.39
Antigen binding competition studies with anti-Hla mAbs with defined epitope specificity suggested that the Hla – F-component cross-reactive mAbs bind to the part of the cell binding (rim) domain of Hla that encompasses a micro-domain that represents the most conserved region between Hla and F-components and is also fully conserved among different S. aureus strains. This micro-domain binds PC present in cell membranes and is crucial for toxin function.20,34-38 The loss of PC binding to the F-component toxins in the presence of Hla-F-component cross-reactive mAb confirmed that the epitope is located in this region. The lack of cross-reactivity of the Hla-specific LC10 mAb with any F-component toxins, despite sharing the binding region with Hla-F#5, suggests that the respective epitopes are distinct. Based on the recently published study that delineated the exact contact sites of LC10 Fab with Hla33 and sequence alignment of Hla with the F-component toxin monomers, 8 of 15 contact residues between LC10 and Hla are not conserved between Hla and HlgB, LukD and LukF, including the strong ionic interaction between Lys266 in Hla and Asp93 from LC10 light chain. X-ray crystal structure analysis to determine the exact epitope of Hla-F#5 is ongoing.
We determined that the Hla cross-reactive mAbs inhibited the binding of both Hla and bi-component toxins to their target cells. Moreover, all Hla-specific mAbs we tested had the same inhibitory effect on Hla binding to human alveolar epithelial cells. The same finding was reported with LTM14.11 Previously, it was shown that LC10 did not prevent binding of Hla to rabbit RBC ghosts;40 however, in a recent study with LC10, binding inhibition was seen when human target cells were used.33
The key to the generation of broadly cross-reactive antibodies was the combination of employing high diversity human IgG libraries, specifically looking for cross-reactive binders, successive screening of diversified sub-libraries with highly functional, correctly folded native antigens and evaluation of antibody function in a series of predictive in vitro assays. Quadruple toxin cross-reactive antibodies were reported before, generated against 4 different serotypes of botulinum toxin that share as low as 36% overall amino acid identity.41 These antibodies were derived from B cells of immunized humans and mice and optimized for high affinity and broad binding specificity with yeast-displayed scFv libraries. Similarly to the antibodies described in this study, the botulinum toxin cross-neutralizing antibodies also targeted an epitope that is well conserved among the different toxin types and important for toxin function.41
The functional consequence of multi-specific binding of the Hla – bi-component toxin cross-reactive antibodies is the simultaneous inhibition of several virulence mechanisms of S. aureus. We found that a single Hla-LukF-LukD-HlgB cross-reactive mAb was able to inhibit lysis of epithelial cells by α-hemolysin, destruction of phagocytic cells by the bi-component cytolysins and lysis of human red blood cells by HlgAB and HlgA-LukD in in vitro assays (summarized in Fig. 9). Due to the complementary and redundant role of cytotoxins in the complex pathogenesis of S. aureus, inactivation of the major cytolysins is likely to be required for efficacious intervention by antibodies.4,5,12,13
Figure 9.
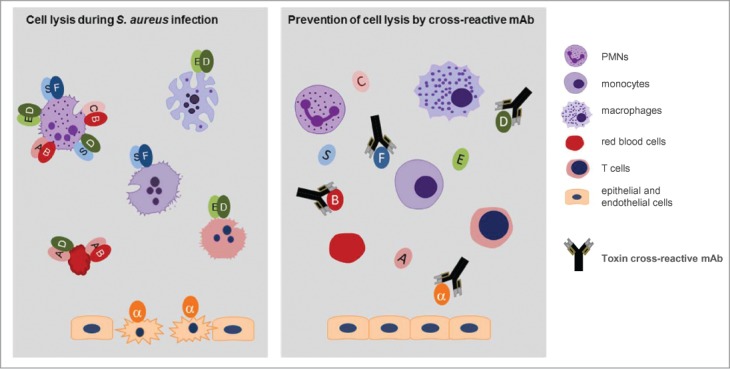
A single toxin cross-reactive mAb inactivates multiple S. aureus virulence mechanisms. The drawing depicts the major finding of this work. A single monoclonal antibody that binds to 4 different toxin molecules can prevent lysis of multiple human cells targeted by α-hemolysin (α) and bi-component leukocidins formed by cognate and non-cognate pairing with HlgA (A), HlgC (C), HlgB (B), LukS (S), LukF (F), LukE (E) and LukD (D).
We demonstrated that passive immunization with Hla-F-component toxin cross-reactive antibody used at a relatively low dose (∼5 mg/kg) was able to prevent lethal pneumonia and sepsis induced by a USA300 CA-MRSA strain. The mAb also showed therapeutic effect when administered 2 hours post intranasal challenge, and synergized with an anti-MRSA antibiotic, linezolid. Others have also reported the therapeutic efficacy of α-hemolysin antibodies in mice infected with MRSA strains, alone or in combination with antibiotics.10,11 We did not, however, observe consistent and significant effect on bacterial load (tested so far only in the pneumonia model) with mAb treatment using BALB/c mice and the TCH1516 strain (unpublished observation). Therefore, we conclude that, at least in the pneumonia model, the protective effect seems to be the prevention of tissue damage. Other groups observed moderate reduction in bacterial load (∼1 log) in the lung with Hla-specific mAbs, polyclonal immune sera or vaccination with Hla. Interestingly, all these experiments were performed with the C57BL/6J mouse strain, suggesting that genetic background or susceptibility to S. aureus infections might explain this discrepancy.7,10,42
Pathogenesis of S. aureus infections in mice is mainly driven by Hla, since hla deficient strains are non-lethal or require significantly higher doses than wild type strains to cause lethality. The role of bi-component toxins in murine models is less clear. Deletion of the hlgABC operon in the USA300 CA-MRSA LAC strain was shown to have a moderate and transient effect on bacterial load upon intravenous challenge, but no influence on lethality.43 However, hlgABC and lukED deletion mutant Newman strains displayed greatly reduced virulence in lethal bacteremia/sepsis models.16,44 It is an interesting observation in this context that the MSSA Newman strain produces less Hla and more bi-component toxins in vitro compared to the TCH1516 strain based on comparative immunoblot studies (unpublished observation). Therefore, it is necessary to test different S. aureus strains to assess the involvement of HlgABC and LukED and the potential beneficial effects of neutralizing multiple cytotoxins. LukSF has no lytic activity toward murine cells.12-14 However, in the rabbit necrotizing pneumonia model, LukSF, similarly to Hla, was shown to greatly contribute to S. aureus pathogenesis.45 Therefore, it is a suitable model to evaluate the effect of simultaneous inhibition of S. aureus cytotoxins.
It is highly relevant for S. aureus as a major nosocomial pathogen that several antibiotics have been shown to increase toxin production in vitro and in vivo; therefore, toxin neutralizing antibodies may prove useful as adjunct therapy to antibiotics.46-48
S. aureus is well-known for its complex pathomechanism, which necessitates a multi-target approach for prophylaxis or therapy. Although the contribution of Hla and in particular the bi-component toxins to human S. aureus infections has not yet been elucidated, based on the potent cytolytic effects toward human cells, it is reasonable to assume that a multi-potency antibody deactivating 5 potent cytolysins might be beneficial in a clinical setting.
Materials and Methods
Bacterial strains, culture supernatants
The TCH1516 USA300 CA-MRSA strain (ATCC® BAA-1717TM) was obtained from ATCC. The Newman MSSA strain was kindly provided by Claire Poyart (Cochin Hospital, Paris). The isogenic mutant strains lacking lukGH or hlgABC, lukED, lukSF and lukGH were generated in the TCH1516 background with homologous recombination based on previously published methods using gene specific primer pairs and the pKFT gene-deletion vector.49,50
Generation of recombinant toxins
The genes for Hla and the S- and F-components were derived from the TCH1516 strain, expressed in E. coli and purified to > 95% purity (details in the Supplemental Material).
In vitro assays to measure toxin mediated cell lysis
Hla or equimolar mixtures of the F- and S-components were used for intoxication of cells. Toxin potency was assessed by measuring cellular ATP levels with Cell Titer-Glo® Luminescent Cell Viability Assay Kit (Promega) or by determining hemolysis activity with red blood cells. For Hla, 2 different in vitro assays were performed using either the human alveolar epithelial cell line A549 or rabbit red blood cells. A549 cells were seeded 12–16 h before the cytotoxicity assays were performed, at a density of 2×104 cells per well in F12K medium (Gibco, USA) supplemented with 10% FCS, in 96-well plates. Cells were intoxicated for 6 hours at 37°C. Red blood cells were purified from rabbit EDTA-whole blood (New Zealand White Rabbits) or heparinized human blood (from the Austrian Red Cross) diluted 1:1 with PBS without Ca2+/Mg2+. Hemolysis assays were performed with 5×107 RBCs for 1 h at 37°C. To measure leukocidal activity of bi-component toxins, PMNs were isolated from heparinized fresh human blood, obtained from the Austrian Red Cross using a Percoll gradient. 2.5×104 cells/well re-suspended in RPMI 1640 supplemented with 10% FCS, 2 mM L-Glutamine and penicillin/streptomycin were used for intoxication with toxin for 4 h at 37°C. Culture supernatants were used at concentrations that caused complete cell lysis in the absence of antibodies.
Selection of monoclonal antibodies
Toxin-specific antibodies were isolated from a full-length human IgG1 antibody library using an in vitro yeast selection system and associated methods.25-29 Toxin-binding mAbs were enriched by incubating biotin labeled Hla or leukocidin monomers with antibody expressing yeast cells at different concentrations followed by magnetic bead selection (Miltenyi, Biotec) and fluorescence-activated cell sorting on a FACSAria II cell sorter (BD Biosciences) employing streptavidin secondary reagents in several successive selection rounds. After the last round of enrichment, yeast cells were sorted and plated onto agar plates, clones were analyzed by DNA sequencing and used for IgG production. Optimization of antibodies for higher affinity was performed in successive cycles of selection rounds using lower concentrations of toxin baits with sub-libraries generated by light chain shuffling, targeted mutagenesis of CDR1 and CDR2 of heavy chains and ePCR of the variable region of the heavy or light chain. Cross-reactive antibodies were also selected by alternating the different toxin molecules for successive yeast cell selection rounds.
Generation of purified antibodies
Antibodies derived from the yeast library were produced either by selected yeast clones, or by FreeStyle™ 293F (Invitrogen/Life Technologies) or CHO-3E7 (Biotechnology Research Institute, Canada) cells transiently transfected with vector plasmids encoding human antibody (IgG1) heavy and light chains using mammalian expression vectors pTT5 (Biotechnology Research Institute, Canada) using PEI MAX™ transfection reagent (Polysciences). Yeast-produced antibodies were used in screening experiments and for the in vivo toxin challenge studies. The LC10, LTM14 and 243-4 mAbs were expressed in the mammalian cell expression systems. Supernatants were harvested 8 days after transfection. IgGs were purified by Protein A affinity chromatography and eluted under the same conditions. Based on a series of biochemical characterization, such as size exclusion chromatography to determine solubility (monomeric content, > 95%), SDS-PAGE to determine purity and integrity, all mAbs produced in mammalian cells were comparable. Antibody concentrations were determined from the absorption at 280 nm.
Determining cross-reactivity and binding affinity of mAbs
Binding of IgGs to the different toxins was determined by BLI measurements using a fortèBio Octet Red96 instrument (Pall Life Sciences). The biotinylated antigen or the antibody was immobilized on the sensor (streptavidin or AHC [anti-human capture], respectively, Pall Life Sciences) and the association and dissociation of the antibody or of the antigen, respectively, were measured, and data were analyzed using the ForteBio Data Analysis Software 7. Fab KD values were measured by MSD (Meso Scale Discovery) method using a Sector Imager 2400 instrument as described previously.51
Determining toxin neutralizing activity of antibodies
MAbs were serially diluted in assay medium and mixed with toxins or bacterial culture supernatants at fixed concentrations, determined to result in at least 80% cell lysis. MAbs and antibodies were pre-incubated for 30 min prior to addition to the target cells. % inhibition of toxin activity was calculated using the following formula: % inhibition = [(normal activity - inhibited activity) / (normal activity)] × 100. A human IgG1 control mAb generated against an irrelevant antigen and expressed by yeast or mammalian cells was included in all assays.
Epitope binning and binding inhibition by phosphocholine
The structural models of the Hla and LukD monomers were generated with SWIS-MODEL using 1PVL as template.52 Competition between the Hla mAbs was studied by BLI, using the fortèBio Octet instrument. The first antibody was loaded onto AHC sensors and the un-occupied Fc binding sites on the sensors were blocked with human IgG. Then the sensors were exposed to toxin molecules (300 nM in PBS with 1% BSA), followed by addition of the second antibody (67 nM); the responses for the second antibody binding were measured. Binding of PC to the toxins in presence and absence of antibody was quantified by fortéBio by immobilizing biotinylated toxins on streptavidin sensors, followed by exposing the sensors either to the mAb (300 nM) or to buffer, and subsequently to a PC-BSA conjugate (1 μM, PC4-BSA, Biosearch Technologies) diluted in PBS with 1% BSA.
Inhibition of toxin binding
Biotinylated Hla or biotinylated F-components mixed with non-biotinylated S-components (60 nM), were pre-incubated with a 5-fold molar excess of mAbs for 30 min at RT prior to incubation with 1×106 A549 cells or PMNs, respectively for 30 min on ice. After washing the cells in HBSS, cell-bound toxins were detected with Alexa488 labeled Streptavidin (Molecular Probes), and analyzed by flow cytometry. Toxin binding is expressed as median fluorescent intensity. Samples stained with secondary reagent only (staining control) and cells incubated with toxin and negative control antibody were included in all experiments.
Animal experiments
All animal experiments were performed according to Austrian Law (BGB1 Nr. 501/1989, approved by MA58, Vienna). In all experiments, female 6–8 week old BALB/cJRj mice were used (Janvier), 5 mice/group in each experiment. Animals were anesthetized prior to intranasal challenge with intraperitoneal injection of ketamine and xylazine. Statistical analysis was performed by analysis of survival curves by the Logrank (Mantel-Cox) test using GraphPad Prism 5.04 Software. Toxins were applied in 100 μl for intravenous challenge and in 40 μl for intranasal challenge. Minimal lethal doses were determined in experiments using serial dilutions of toxins in the 0.125 to 2 μg range.
For bacterial challenge experiments S. aureus TCH1516 (USA300 CA-MRSA) strain was grown to mid log phase (OD600 of 0.5) in tryptic soy broth and diluted to 5×108 cfu/ml for intravenous injection (100 μl, 5 × 107 cfu challenge dose) or to 1.5×1010 cfu/ml for intranasal application (40 μl, 6×108 cfu challenge dose).
Prophylactic immunization was performed via intraperitoneal injection of 100 μg (∼5 mg/kg) of mAb diluted in 500 μl PBS 24 h prior to the lethal challenge by toxins or bacteria. In the therapeutic setting, passive immunization and antibiotic treatment was performed 2 hours post challenge. MAbs (50 μg in 500 μl PBS) were applied intraperitoneally, whereas linezolid treatment (1 mg/kg in 100 μl) was given subcutaneously. Linezolid was dissolved in hydroxypropyl-β-cyclodextrin to a concentration of 1 mg/ml. Control groups received either PBS or isotype matched (IgG1) irrelevant mAb.
Disclosure of Potential Conflicts of Interest
The authors declare a potential conflict of interest being employees of the 2 biotechnology companies involved in this research work.
Ethical statement
The animal experiments were conducted according to ethical and legal requirements according to Austrian law and were approved by the relevant authority.
HR and AB contributed equally to this work.
Acknowledgments
We thank S Zettl and F Leslie for the critical reading of the manuscript, S Bhakdi for providing purified α-hemolysin, G Nauman, R Rondeau and J Torrey for technical assistance in the antibody discovery. Yeast and HEK293 cell expressed IgG material was generated and analyzed by the Molecular Core, High Throughput Expression and Analytical Group of Adimab.
Funding
This work was supported by the FFG “Basisprogramm” grants (No: 832915, 837128 and 841918) from the Austrian Research Promotion Agency, awarded to Arsanis Biosciences.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 2009; 7:629-641; PMID:19680247; http://dx.doi.org/ 10.1038/nrmicro2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jansen KU, Girgenti DQ, Scully IL, Anderson AS. Vaccine review: “Staphyloccocus aureus vaccines: problems and prospects”. Vaccine 2013; 31:2723-2730; PMID:23624095; http://dx.doi.org/ 10.1016/j.vaccine.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 3. Oleksiewicz MB, Nagy G, Nagy E. Anti-bacterial monoclonal antibodies: back to the future? Arch Biochem Biophys 2012; 526:124-131; PMID:22705202; http://dx.doi.org/ 10.1016/j.abb.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 4. Cheung GY, Otto M. The potential use of toxin antibodies as a strategy for controlling acute Staphylococcus aureus infections. Expert Opin Ther Targets 2012; 16:601-612; PMID:22530584; http://dx.doi.org/ 10.1517/14728222.2012.682573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aman MJ, Adhikari RP. Staphylococcal bicomponent pore-forming toxins: targets for prophylaxis and immunotherapy. Toxins (Basel) 2014; 6: 950-972; PMID:24599233; http://dx.doi.org/ 10.3390/toxins6030950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 2013; 5:1140-1166; PMID:23888516; http://dx.doi.org/ 10.3390/toxins5061140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 2008; 205:287-294; PMID:18268041; http://dx.doi.org/ 10.1084/jem.20072208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 2010; 202:1050-1058; PMID:20726702; http://dx.doi.org/ 10.1086/656043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adhikari RP, Karauzum H, Sarwar J, Abaandou L, Mahmoudieh M, Boroun AR, Vu H, Nguyen T, Devi VS, Shulenin S, et al. Novel structurally designed vaccine for S. aureus alpha-hemolysin: protection against bacteremia and pneumonia. PLoS One 2012; 7:e38567; PMID:22701668; http://dx.doi.org/ 10.1371/journal.pone.0038567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R, et al. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 2014; 58:1108-1117; PMID:24295977; http://dx.doi.org/ 10.1128/AAC.02190-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foletti D, Strop P, Shaughnessy L, Hasa-Moreno A, Casas MG, Russell M, Bee C, Wu S, Pham A, Zeng Z, et al. Mechanism of action and in vivo efficacy of a human-derived antibody against Staphylococcus aureus alpha-hemolysin. J Mol Biol 2013; 425:1641-1654; PMID:23416200; http://dx.doi.org/ 10.1016/j.jmb.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 12. Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol 2012; 2:12; PMID:22919604; http://dx.doi.org/ 10.3389/fcimb.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alonzo F, 3rd, Torres VJ. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol Mol Biol Rev 2014; 78: 199-230; PMID:24847020; http://dx.doi.org/ 10.1128/MMBR.00055-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DuMont AL, Torres VJ. Cell targeting by the Staphylococcus aureus pore-forming toxins: it's not just about lipids. Trends Microbiol 2014; 22:21-27; PMID:24231517; http://dx.doi.org/ 10.1016/j.tim.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin North Am 2009; 23:17-34; PMID:19135914; http://dx.doi.org/ 10.1016/j.idc.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alonzo F, III, Kozhaya L, Rawlings SA, Reyes-Robles T, DuMont AL, Myszka DG, Landau NR, Unutmaz D, Torres VJ. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 2013; 493:51-55; PMID:23235831; http://dx.doi.org/ 10.1038/nature11724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malachowa N, Kobayashi SD, Braughton KR, Whitney AR, Parnell MJ, Gardner DJ, Deleo FR. Staphylococcus aureus leukotoxin GH promotes inflammation. J Infect Dis 2012; 206:1185-1193; PMID:22872735; http://dx.doi.org/ 10.1093/infdis/jis495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adhikari RP, Ajao AO, Aman MJ, Karauzum H, Sarwar J, Lydecker AD, Johnson JK, Nguyen C, Chen WH, Roghmann MC. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis 2012; 206:915-923; PMID:22807524; http://dx.doi.org/ 10.1093/infdis/jis462 [DOI] [PubMed] [Google Scholar]
- 19. Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, Bubeck Wardenburg J, Hunstad DA. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 2013; 56:1554-1561; PMID:23446627; http://dx.doi.org/ 10.1093/cid/cit123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 1996; 274:1859-1866; PMID:8943190; http://dx.doi.org/ 10.1126/science.274.5294.1859 [DOI] [PubMed] [Google Scholar]
- 21. Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev 1991; 55:733-751; PMID:1779933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaneko J, Kamio Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci Biotechnol Biochem 2004; 68:981-1003; PMID:15170101; http://dx.doi.org/ 10.1271/bbb.68.981 [DOI] [PubMed] [Google Scholar]
- 23. Guillet V, Roblin P, Werner S, Coraiola M, Menestrina G, Monteil H, Prevost G, Mourey L. Crystal structure of leucotoxin S component: new insight into the staphylococcal beta-barrel pore-forming toxins. J Biol Chem 2004; 279:41028-41037; PMID:15262988; http://dx.doi.org/ 10.1074/jbc.M406904200 [DOI] [PubMed] [Google Scholar]
- 24. Yamashita K, Kawai Y, Tanaka Y, Hirano N, Kaneko J, Tomita N, Ohta M, Kamio Y, Yao M, Tanaka I. Crystal structure of the octameric pore of staphylococcal gamma-hemolysin reveals the beta-barrel pore formation mechanism by two components. Proc Natl Acad Sci USA 2011; 108:17314-17319; http://dx.doi.org/ 10.1073/pnas.1110402108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boder ET, Wittrup KD. Yeast surface display for screening combinatorial polypeptide libraries. Nat Biotechnol 1997; 15:553-557; PMID:9181578; http://dx.doi.org/ 10.1038/nbt0697-553 [DOI] [PubMed] [Google Scholar]
- 26. Blaise L, Wehnert A, Steukers MP, van den Beucken T, Hoogenboom HR, Hufton SE. Construction and diversification of yeast cell surface displayed libraries by yeast mating: application to the affinity maturation of Fab antibody fragments. Gene 2004; 342:211-218; PMID:15527980; http://dx.doi.org/ 10.1016/j.gene.2004.08.014 [DOI] [PubMed] [Google Scholar]
- 27. Rakestraw JA, Aird D, Aha PM, Baynes BM, Lipovsek D. Secretion-and-capture cell-surface display for selection of target-binding proteins. Protein Eng Des Sel 2011; 24:525-530; PMID:21402751; http://dx.doi.org/ 10.1093/protein/gzr008 [DOI] [PubMed] [Google Scholar]
- 28. Vasquez M, Feldhaus M, Gerngross TU, Wittrup KD, inventors; Adimab Inc, assignee Rationally designed, synthetic antibody libraries and uses therefor. World patent WO2009036379 2009. March 19. [Google Scholar]
- 29. Xu Y, Roach W, Sun T, Jain T, Prinz B, Yu TY, Torrey J, Thomas J, Bobrowicz P, Vasquez M, et al. Addressing polyspecificity of antibodies selected from an in vitro yeast presentation system: a FACS-based, high-throughput selection and analytical tool. Protein Eng Des Sel 2013; 26:663-670; PMID:24046438; http://dx.doi.org/ 10.1093/protein/gzt047 [DOI] [PubMed] [Google Scholar]
- 30. Rudolf M, Koch H, inventors; Kenta Biotech AG, assignee Human monoclonal antibody against S. aureus derived alpha-toxin and its use in treating or preventing abscess formation patent. World patent WO2011018208 2011. February 17. [Google Scholar]
- 31. Sellman B, Tkaczyk C, Hua L, Chowdhury P, Varkey R, Damschroder M, Peng L, Oganesyan V, Hilliard JJ, inventors; MedImmune LLC, assignee Antibodies that specifically bind Staphylococcus aureus alpha toxin and methods of use patent. World patent WO2012109285 2012. August 16. [Google Scholar]
- 32. Foletti DL, Chapparo Riggers JF, Glanville JEG, Shaughnessy LMB, Shelton DL, Strop P, Zhai W, inventors; Rinat Neuroscience Corp., assignee Staphylococcus aureus specific antibodies and uses thereof. US patent US20130164308 2013. June 27. [Google Scholar]
- 33. Oganesyan V, Peng L, Damschroder MM, Cheng L, Sadowska A, Tkaczyk C, Sellman BR, Wu H, Dall’Acqua WF. Mechanisms of neutralization of a human anti-alpha toxin antibody. J Biol Chem 2014; 289:29874-29880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olson R, Nariya H, Yokota K, Kamio Y, Gouaux E. Crystal structure of staphylococcal LukF delineates conformational changes accompanying formation of a transmembrane channel. Nat Struct Biol 1999; 6:134-140; PMID:10048924; http://dx.doi.org/ 10.1038/5821 [DOI] [PubMed] [Google Scholar]
- 35. Pedelacq JD, Maveyraud L, Prevost G, Baba-Moussa L, Gonzalez A, Courcelle E, Shepard W, Monteil H, Samama JP, Mourey L. The structure of a Staphylococcus aureus leucocidin component (LukF-PV) reveals the fold of the water-soluble species of a family of transmembrane pore-forming toxins. Structure 1999; 7:277-287; PMID:10368297; http://dx.doi.org/ 10.1016/S0969-2126(99)80038-0 [DOI] [PubMed] [Google Scholar]
- 36. Valeva A, Hellmann N, Walev I, Strand D, Plate M, Boukhallouk F, Brack A, Hanada K, Decker H, Bhakdi S. Evidence that clustered phosphocholine head groups serve as sites for binding and assembly of an oligomeric protein pore. J Biol Chem 2006; 281:26014-26021; PMID:16829693; http://dx.doi.org/ 10.1074/jbc.M601960200 [DOI] [PubMed] [Google Scholar]
- 37. Potrich C, Bastiani H, Colin DA, Huck S, Prevost G, Dalla Serra M. The influence of membrane lipids in Staphylococcus aureus gamma-hemolysins pore formation. J Membr Biol 2009; 227:13-24; PMID:19067025; http://dx.doi.org/ 10.1007/s00232-008-9140-6 [DOI] [PubMed] [Google Scholar]
- 38. Monma N, Nguyen VT, Kaneko J, Higuchi H, Kamio Y. Essential residues, W177 and R198, of LukF for phosphatidylcholine-binding and pore-formation by staphylococcal gamma-hemolysin on human erythrocyte membranes. J Biochem 2004; 136:427-431; PMID:15625310; http://dx.doi.org/ 10.1093/jb/mvh140 [DOI] [PubMed] [Google Scholar]
- 39. Maynard JA, Maassen CBM, Leppla SH, Brasky K, Patterson JL, Iverson BL, Georgiou G. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat Biotechnol 2002; 20:597-601; PMID:12042864; http://dx.doi.org/ 10.1038/nbt0602-597 [DOI] [PubMed] [Google Scholar]
- 40. Tkaczyk C, Hua L, Varkey R, Shi Y, Dettinger L, Woods R, Barnes A, MacGill RS, Wilson S, Chowdhury P, et al. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin Vaccine Immunol 2012; 19:377-385; PMID:22237895; http://dx.doi.org/ 10.1128/CVI.05589-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garcia-Rodriguez C, Geren IN, Lou J, Conrad F, Forsyth C, Wen W, Chakraborti S, Zao H, Manzanarez G, Smith TJ, Brown J, et al. Neutralizing human monoclonal antibodies binding multiple serotypes of botulinum neurotoxin. Protein Eng Des Sel 2011; 24:321-331; PMID:21149386; http://dx.doi.org/ 10.1093/protein/gzq111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ragle BE, Bubeck Wardenburg J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun 2009; 77:2712-2718; PMID:19380475; http://dx.doi.org/ 10.1128/IAI.00115-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malachowa N, Whitney AR, Kobayashi SD, Sturdevant DE, Kennedy AD, Braughton KR, Shabb DW, Diep BA, Chambers HF, Otto M, DeLeo FR. Global changes in Staphylococcus aureus gene expression in human blood. PLoS One 2011; 6:e18617; PMID:21525981; http://dx.doi.org/ 10.1371/journal.pone.0018617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ishii K, Adachi T, Yasukawa J, Suzuki Y, Hamamoto H, Sekimizu K. Induction of virulence gene expression in Staphylococcus aureus by pulmonary surfactant. Infect Immun 2014; 82:1500-10; PMID:24452679; http://dx.doi.org/ 10.1128/IAI.01635-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, Mai TT, Marbach H, Braughton KR, Whitney AR, et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. USA 2010; 107:5587-5592; http://dx.doi.org/ 10.1073/pnas.0912403107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen HY, Chen CC, Fang CS, Hsieh YT, Lin MH, Shu JC. Vancomycin activates sigma(B) in vancomycin-resistant Staphylococcus aureus resulting in the enhancement of cytotoxicity. PLoS One 2011; 6:e24472; PMID:21912698; http://dx.doi.org/ 10.1371/journal.pone.0024472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pichereau S, Pantrangi M, Couet W, Badiou C, Lina G, Shukla SK, Rose WE. Simulated antibiotic exposures in an in vitro hollow-fiber infection model influence toxin gene expression and production in community-associated methicillin-resistant Staphylococcus aureus strain MW2. Antimicrob Agents Chemother 2012; 56:140-147; PMID:22064533; http://dx.doi.org/ 10.1128/AAC.05113-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rudkin JK, Laabei M, Edwards AM, Joo HS, Otto M, Lennon KL, O’Gara JP, Waterfield NR, Massey RC. Oxacillin alters the toxin expression profile of community-associated methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2014; 58:1100-1107; PMID:24295979; http://dx.doi.org/ 10.1128/AAC.01618-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McNamara PJ, Lindsay JA, editor. Staphylococcus: Molecular Genetics, 1st ed., Genetic Manipulation of Staphylococcus aureus. Norfolk, UK: Caister Academic Press; 2008. 100-12 p. [Google Scholar]
- 50. Kato F, Sugai M. A simple method of markerless gene deletion in Staphylococcus aureus. J Microbiol Methods 2011; 87:76-81; PMID:21801759; http://dx.doi.org/ 10.1016/j.mimet.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 51. Estep P, Reid F, Nauman C, Liu Y, Sun T, Sun J, Xu Y. High throughput solution-based measurement of antibody-antigen affinity and epitope binning. Mabs 2013; 5:270-278; PMID:23575269; http://dx.doi.org/ 10.4161/mabs.23049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 2006; 22:195-201; PMID:16301204; http://dx.doi.org/ 10.1093/bioinformatics/bti770 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.