Abstract
The human ocular surface, consisting of the cornea and conjunctiva, is colonized by an expansive, diverse microbial community. Molecular-based methods, such as 16S rRNA sequencing, has allowed for more comprehensive and precise identification of the species composition of the ocular surface microbiota compared to traditional culture-based methods. Evidence suggests that the normal microbiota plays a protective immunological role in preventing the proliferation of pathogenic species and thus, alterations in the homeostatic microbiome may be linked to ophthalmic pathologies. Further investigation of the ocular surface microbiome, as well as the microbiome of other areas of the body such as the oral mucosa and gut, and their role in the pathophysiology of diseases is a significant, emerging field of research, and may someday enable the development of novel probiotic approaches for the treatment and prevention of ophthalmic diseases.
Keywords: microbiota, microbiome, ophthalmic disease, ocular surface, genomics, infection, ophthalmology
Introduction
The Human Microbiome Project, launched in 2008 by the National Institutes of Health, has revealed a remarkably abundant and diverse community of microbial species that inhabit the human body [1]. Understanding the role of the trillions of bacteria, viruses, and fungi that comprise the human microbiota is critical to enhance our understanding of a variety of human diseases and their pathophysiologies. Advances in the technology of next-generation sequencing and bioinformatics tools have facilitated the characterization of the human microbiome [2]. Discovery of various aspects of the human microbiota and its role in physiology and pathogenesis has revolutionized our approach in studying certain diseases and developing novel treatment modalities. The intestinal microbiota has been implicated in a variety of autoimmune and inflammatory diseases, including inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, and type 1 diabetes [3-5].
While the Human Microbiome Project initially studied five main body areas – the gastrointestinal tract, the skin, the urogenital tract, the oral mucosa, and the nasal mucosa [6,11] – an emerging area of research is focusing on the eye and the microbiota of the ocular surface [7,14]. The fundamental question to explore is whether the ocular surface harbors a resident microbiota and if so, what species of micoorganisms comprise it. Subsequently, we must examine how the microbial community is influenced by host and environmental factors. Finally, we can provide further clinical significance by investigating the potential associations between alterations of the microbiome and the pathogenesis of ophthalmic diseases. This study aims to systematically review and summarize evidence regarding the most up-to-date understanding of microbiota colonizing the ocular surface, as well as investigate the potential role of the human microbiota in ophthalmic disease.
The Eye and the Ocular Surface Microbiota
Structurally, the eye is composed of an internal compartment – which consists of the anterior and posterior chambers, the iris, the lens, the vitreous cavity, the retina, the ciliary body, the choiroid, and intrinsic ocular muscles – and an external compartment – which consists of the conjunctiva, the cornea, the sclera, and the tear film (Figure 1). The internal compartment of the eye maintains a sterile environment, and is physically separated from the immune system by the blood-retinal barrier. In contrast, the external compartment of the eye is exposed to microorganisms in the environment [8].
Figure 1.
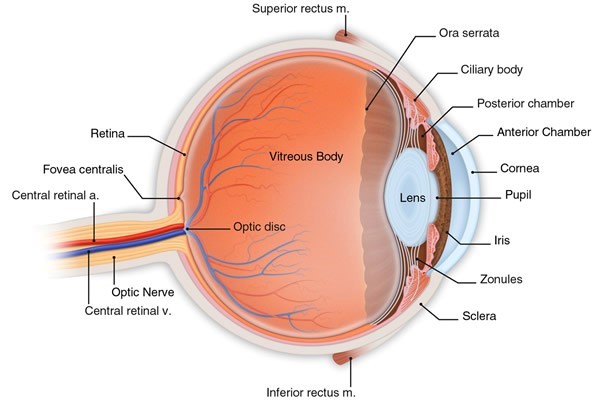
Anatomy of the eye.(© 2016 American Academy of Ophthalmology)
The ocular surface is comprised of the cornea and its overlying tissue, the conjunctiva. Thus, the ocular surface microbiota refers to the resident microorganisms that colonize the conjunctiva and the cornea and importantly, excludes the eyelid (microbes found in the eyelid are considered part of the skin microbiota, which is included in the five main research areas in the Human Microbiome Project). Direct exposure to the external environment means that the ocular surface is susceptible to a gamut of antigens and pathogens. In addition to serving as a physical barrier against the external environment, the ocular surface has a critical role in innate immunity [9]. The mucosal immune system protects the conjunctival and corneal epithelia via innate and adaptive defense mechanisms present in the tissue and tear film [10]. While the ocular surface epithelium is constantly in contact with commensal bacteria, the epithelial cells of the cornea and conjunctiva in a healthy individual do not undergo an inflammatory response. Studies have shown that ocular surface epithelial cells recognize and selectively respond to microbial components of ocular pathogenic bacteria by producing pro-inflammatory cytokines [9]. The lack of an inflammatory response to non-pathogenic bacteria suggests a unique innate immune response of the ocular surface epithelium that supports the colonization of a resident microbiota.
While a substantial amount of evidence strongly supports the existence of an ocular surface microbiota, it is worthwhile to note that analysis of the ocular microbiome is currently in its early stages. Willcox et. al. argues that determination of the ocular microbiome with molecular techniques has lagged in relation to analysis of the microbiome of other areas of the body, and a greater number of rigorous cross-sectional and longitudinal studies examining changes in the microbiota are necessary to maintain current hypotheses [7]. Turnbaugh et al., in 2007, questioned the existence of a stable core microbiome, proposing that there exist only transiently present organisms on the ocular surface, as opposed to a resident microbiota [11]. In recent years however, investigation of the ocular microbiome using molecular techniques such as 16S rRNA gene analysis have identified, with greater precision, a wider range of homeostatic species that colonize the ocular surface, reinforcing the concept of a stable, unique ocular surface microbiota.
Characterization of the Ocular Microbiota
Previous analyses of the ocular surface microbiota, performed using microbiological culture techniques, reported a significantly different and less diverse profile than what has most recently been discovered using molecular techniques. The characterization of the ocular surface microbiota using culture-based methods was purported to be dominated by Gram-positive species, especially Staphylococcus, Streptococcus, Corynebacterium, and Propionibacterium [12]. In addition, some Gram negative species, such as Haemophilus and Neisseria, as well as fungal isolates were cultured from the ocular surface of healthy human subjects [12,13]. A major disadvantage of culture-based techniques that may account for its inaccuracy in microbiome characterization is that species detection is significantly biased towards fast-growing microorganisms that can successfully be cultivated on standard media [14-16].
Genomics-based detection and identification of microbial species has exposed a significantly expanded diversity of ocular surface microbiota than what had been previously uncovered by culture-based methods. The first gene sequencing-based survey of the bacterial species found on the ocular surface, using 16S rRNA PCR, was conducted in 2011 by researchers at the Bascom Palmer Eye Institute. Deep sequencing of conjunctival DNA revealed an average of 221 species of bacteria per subject [14]. The bacteria were classified into five phyla and 59 distinct genera, with twelve genera being ubiquitous among all subjects in the analyzed cohort (Table 1). Of the five bacterial phyla, Proteobacteria, Actinobacteria, and Firmicutes accounted for more than 87 percent of all sequences; the remaining two phyla, Cyanobacteria and Bacteroides, were present in contamination-level quantities and were excluded from further analysis. The twelve common genera – Pseudomonas, Propionibacterium, Bradyrhizobium, Corynebacterium, Acinetobacter, Brevundimonas, Staphylococci, Aquabacterium, Sphingomonas, Streptococcus, Streptophyta, and Methylobacterium – comprised more than 96 percent of the classified microbiome [14]. The five most abundant genera out of the twelve ubiquitous genera identified by Dong et al. were Pseudomonas, Bradyrhizobium, Propionibacterium, Acinetobacter, and Corynebacterium, followed by Brevundimonas, Staphylococcus, Aquabacterium, Sphyngomonas, and Streptococcus. As these twelve genera accounted for more than 96 percent of the total classified bacterial DNA sequences, the data strongly suggests that the healthy conjunctiva is colonized by a resident homeostatic microbiota. To date, the findings from this study remain the most comprehensive DNA sequencing-based characterization of bacterial diversity at the ocular surface.
Table 1. Composition of the ocular surface microbiota by phylum and genus, determined according to relative abundance of classified 16S rRNA gene reads [14].
| Percentage of all sequencesa (%) | ||
| Phylum | Proteobacteria | 64% |
| Acetinobacteria | 19.6% | |
| Firmicutes | 3.9% | |
| Unclassifiedb | 12.5% | |
| Genus | Pseudomonas | 18% |
| Bradyrhizobium | 12% | |
| Propionibacterium | 11% | |
| Acinetobacter | 9% | |
| Corynebacterium | 8% | |
| Brevundimonas | 4% | |
| Staphylococcus | 2% | |
| Aquabacterium | 2% | |
| Sphyngomonas | 0.5% | |
| Streptococcus | 0.5% | |
| Other | 2% | |
| Unclassifiedb | 31% |
aDong et al. analyzed 115,003 sequences in total. bThe Ribosomal Database Project-II software was unable to classify 12.5% and 31% of sequences to the phyum and genus level, respectively. Unclassified bacteria are designated as novel phylotypes.
Consistent with findings that genus composition of the microbiota varies at different layers in other areas of the body such as the human epidermis, the microbiota of the ocular surface appears to have vertical stratification of species composition [17]. Swabbing the ocular surface with light pressure yielded sequences of opportunistic and environmental species, such as Rothia, Herbaspirillum, Leptothrichia, and Rhizobium. These bacteria captured from the superficial layer likely represent transient species on the ocular surface. In contrast, using a “deep” swab, in which dry cotton was applied with greater pressure, yielded an abundance of Staphylococci, Cornyebacteriae, and Proteobacteria species, which localize to the mucosal layer and conjunctival epithelium [14]. Deep swabbing is thus necessary to obtain an accurate and comprehensive characterization of the diversity of the ocular surface microbiota.
Host and Environmental Factors That May Alter the Ocular Surface Microbiota
The ocular surface microbiota can become altered by host factors, environmental insults, and disease states [18]. Disruption of the ocular surface may breach the innate immune system at the corneal and conjunctival epithelia and allow microbial ligands to trigger ocular inflammation [12,19]. Alterations in the microbiota of the ocular surface have been associated with conditions such as dry eye syndrome, contact lens wear, keratoprosthesis, antibiotics, and infection [12].
The tear film, which lubricates the ocular surface epithelia, contains antimicrobial compounds such as lysozyme, lactoferrin, immunoglobulin A (IgA), lipocalin, and complement [41]. Given the immunological role of tears in the defense against potential pathogens, it is reasonable to conjecture that certain situations that affect the tear film, such as dry eye syndrome and contact lens wear, may alter the ocular surface microbiota.
Dry eye syndrome has consistently been associated with inflammatory ocular surface conditions such as anterior blepharitis, keratitis, and ocular rosacea [20]. Graham et al. used both conventional culture and 16S rDNA PCR to compare the bacterial population of the ocular surface of normal and dry eye subjects, collecting conjunctival swab specimens from a cohort of patients with dry eyes as well as healthy control subjects over a three-month period. They found that certain bacteria species were found in samples from dry eye subjects only, including Bacillus sp. and Klebsiella oxytoca, in addition to an association between elevated bacterial count (CFU/swab) and the incidence of blepharitis [21]. Increasing bacterial count was correlated with a decrease in goblet cells among the subgroup of 27 subjects, consistent with previous studies that have demonstrated a depletion of goblet cells in other areas of the body after colonization by bacteria [22]. A reduction in goblet cells, which produce the mucins found on the ocular surface, results in a thinned tear film and diminishes the barrier to infiltration and colonization of the ocular surface by external pathogens [22]. The production of mucins on the ocular surface may pose resemblance to the production of fucosylated and sialylated glycoproteins in the intestinal tract [40]. Perhaps the human host secretes glycans and polysaccharides to promote the growth of certain microbial species in the ocular surface in an analogous manner to findings in the intestinal tract.
Alterations of the ocular surface microbiota in contact lens wear has also been studied. Larkin et al. examined the microbial colonization of the conjunctiva in contact lens wearers and compared them to control subjects. Their results included the finding that the ocular surface of contact lens wearers yielded higher bacterial counts than that of control subjects. However, the authors found no qualitative variation in the species of bacteria identified between the lens-wearing and control groups [23].
Studies of the bacterial microbiota colonizing the ocular surface of patients with Boston type 1 keratoprostheses (K-Pros) have found no quantitative difference in positive cultures from conjunctival swabs of K-Pro and control patients [24,25]. Qualitatively however, researchers found that samples from K-Pro patients grew not only coagulase-negative Staphylococcus, which was the singular species of bacteria to grow in the control group samples, but also a variety of other Gram-positive bacteria [24].
Ophthalmic antibiotics are used to treat and prevent a variety of infectious and inflammatory ocular conditions. The Antibiotic Resistance of Conjunctiva and Nasopharynx Evaluation (ARCANE) study, which aimed to determine the effects of repeated exposure of topical antibiotics on resistance patterns of the conjunctival microbiota, concluded that the repeated use of macrolide and fluoroquinolone ophthalmic antibiotics leads to a significant increase in Gram-positive species, particularly Staphylococcus epidermidis, isolated from culture [26]. Fontes et al. investigated the effect of orally administered trimethoprim-sulfamethoxazole (TMP-SMX) administration on the conjunctival microbiota in patients with HIV infection. Chronic administration of TMP-SMX was found to be associated with an altered conjunctival microbiota that contained a significantly greater percentage of antibiotic-resistant Staphylococcus species [27]. Yin et al. also discovered a shift in the conjunctival flora as a result of antibiotic use. Repeated use of a topical antibiotic, such as moxifloxacin, after intravitreal (IVT) injection was correlated with increased antibiotic resistance of the ocular surface microbiota [28].
Infectious pathologies have also been linked to shifts in the homeostatic ocular microbiome. An analysis of the microbial composition of the ocular surface of healthy eyes and eyes with bacterial keratitis, utilizing DNA sequencing, found that bacterial keratitis was associated with the depletion of homeostatic ocular microorganisms and the emergence of a pathological microbiome dominated by Pseudomonas aeruginosa [29]. Investigation of the conjunctival flora in patients with human immunodeficiency virus (HIV) infection compared to HIV-negative patients, however, concluded that there was no significant difference between the types and proportions of microbial organisms isolated from HIV-positive and HIV-negative eyes [30]. Analysis of the conjunctival flora of HIV patients who received antibiotic treatment with systemic clarithromycin demonstrated a significant decrease in the conjunctival flora [30].
Role of the Microbiome in Ocular Disease
Many diseases involving the ocular surface, such as dry eye syndrome, chronic follicular conjunctivitis, and various inflammatory eye diseases, appear to have idiopathic etiologies. Evidence indicating the existence of a resident ocular surface microbiota suggests that the normal microbiota plays a protective role in preventing proliferation of pathogenic species, and that ophthalmic pathologies are linked to alterations in the homeostatic microbiome.
Ocular Microbiota and Ophthalmic Diseases
Recent studies have investigated the link between alterations in the ocular surface microbiota and ophthalmic disease. Sankaridurg et al. conducted a study exploring the microbial colonization of soft contact lenses as a risk factor associated with corneal infiltrative events (CIE) and found that colonization of lenses with pathogenic bacteria, especially Gram-negative bacteria such as Serratia marcescens and Haemophilus influenzae, was significantly associated with CIE [31]. Lee et al. conducted DNA sequencing analysis of ocular surface samples from blepharitis patients and health controls and concluded that blepharitis may be induced by a change in the microbial composition – namely, greater quantities of Streptophyta, Corynebacterium, and Enhydrobacter species [20].
As suggested by Dong et al., a number of potentially pathogenic bacteria may reside at the ocular surface [14]. Post-operative infectious endophthalmitis is a serious, vision-threatening complication of ocular surgery that involves inflammation of the ocular surface, the anterior and posterior compartments of the eye, as well as adjacent structures. Acute, post-operative endophthalmitis presumably occurs when the patient’s own periocular bacteria enter the sterile intraocular compartments of the eye during surgery and cause diffuse infection and inflammation. Thus, pre-operative, intra-operative, and post-operative antibiotic prophylaxis are used to prevent acute endophthalmitis [32]. Consideration of the ocular surface microbiota may have two-fold significance in acute endophthalmitis. First, alterations in the microbiota can influence the species of microorganisms that colonize the ocular surface and heighten the risk for intraocular infection by pathogenic bacteria. Second, knowledge of the composition of both the core and transient microbiota of the ocular surface can aid in the determination of the most effective antibiotic prophylaxis to prevent post-operative acute endophthalmitis in patients [33].
Miller et al. evaluated the concept that infectious microorganisms play a role, perhaps as a cofactor to genetic and environmental risk factors, in ocular adnexal neoplasms. Several microorganisms may have a pathogenic role in ocular malignancies, including human papilloma virus in conjunctival papilloma and squamous cell carcinoma, HIV in conjunctival squamous cell carcinoma, Kaposi sarcoma-associated herpes virus in conjunctival Kaposi sarcoma, and Helicobacter pylori, Chlamydia, and hepatitis C virus in ocular adnexal mucosa-associated lymphoid tissue lymphomas [12]. Depletion or alteration in the commensal microbiota, due to antibiotics or other factors, may allow for colonization of the ocular surface by opportunistic pathogens, producing a greater risk for infection-associated ocular adnexal neoplasms [34].
Non-ocular Microbiota and Ophthalmic Diseases
Finally, we must consider the potential role of the general, non-ocular microbiome in ophthalmic disease. The pathophysiology of glaucoma involves local inflammatory responses. A study comparing the oral microbiome of patients with glaucoma and healthy controls found that patients with glaucoma had a higher quantity of bacterial organisms compared to controls [35]. The researchers of the study proposed that an increased bacterial count can lead to neurodegeneration of the optic nerve via activation of microglia in the retina and optic nerve; the altered commensal microbiome induces changes in cytokine signaling and complement activation [35]. Additionally, other glaucoma researchers have implicated the role of the human microbiome in modulating levels of brain-derived neurotrophic factor (BDNF), which has been shown to have an effect on the survival of retinal ganglion cells in an animal model [36].
A number of recent studies have proposed a link between the intestinal microbiome and uveitis [37]. Uveitis, or intraocular inflammation, has various etiologies which include infectious pathologies as well as a number of immune-mediated conditions. Animal models of experimental autoimmune uveitis have demonstrated that administration of oral antibiotics that altered the intestinal microbiota resulted in a significant attenuation of the uveitis [38]. Presumably, an altered commensal flora led to an increase in regulatory T lymphocytes in lymphoid tissues as well as in the eye, leading to decreased inflammation. Another animal model study of experimental autoimmune uveitis found that oral antibiotics or a germ-free state significantly decreased the severity of uveitis [39].
Conclusion
Advances in next-generation sequencing and bioinformatics tools have revealed an expansive, diverse microbial community inhabiting the human cornea and conjunctiva. The most abundant genera in the microbiota of the ocular surface, identified using 16S rRNA sequencing, were Pseudomonas, Bradyrhizobium, Propionibacterium, Acinetobacter, and Corynebacterium. The ocular surface microbiota can be altered by a variety of host factors, environmental influences, and pathological states, including dry eye syndrome, contact lens wear, keratoprosthesis, antibiotics, and infection. Evidence strongly suggests that the homeostatic microbiome plays a protective role in preventing colonization of pathogenic species. Thus, disruption of the normal ocular surface microbiota may play a significant role as a cofactor in the pathogenesis of ophthalmic diseases, such as contact lens-associated corneal infiltrative events, blepharitis, and post-operative infectious endophthalmitis. Futhermore, recent studies suggest that the microbiome of other areas of the body are involved in the pathophysiology of certain ophthalmic diseases, such as the oral microbiome and glaucoma, as well as the intestinal microbiome and uveitis. Continued investigation of the ocular surface microbiome is necessary to enhance our understanding of the role of homeostatic microorganisms in ophthalmic diseases and inspire the development of novel, probiotic-based therapies for the prevention and treatment of ocular disease.
Glossary
- rRNA
Ribosomal RNA
- PCR
Polymerase chain reaction
- DNA
Deoxyribonucleic acid
- IgA
Immunoglobulin A
- CFU
Colony-forming unit
- IVT
intravitreal
- TMP-SMX
trimethoprim-sulfamethoxazole
- CIE
corneal infiltrative events
- BDNF
brain-derived neurotrophic factor
References
- Gevers KR, Petrosino JF, Huang K. et al. The Human Microbiome Project--A Community Resource for the Healthy Human Microbiome. PLoS Biology. 2012;10(8):e1001377. doi: 10.1371/journal.pbio.1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano NC, Millan P, Paez MC. Non-HLA associations with autoimmune diseases. Autoimmun Rev. 2006;5:209–214. doi: 10.1016/j.autrev.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Scher JU, Abramson SB. he microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:569–578. doi: 10.1038/nrrheum.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JR, Davis SL, Chaykosky DM. et al. Probiotics and Fecal Microbiota Transplant for Primary and Secondary Prevention of Clostridium difficile Infection. . Pharmacotherapy. 2015;35:1016–1025. doi: 10.1002/phar.1644. [DOI] [PubMed] [Google Scholar]
- Willcox M. Characterization of the normal microbiota of the ocular surface. Experimental Eye Research. 2013;117:99–105. doi: 10.1016/j.exer.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Caspi R. In this issue: Immunology of the eye--inside and out. Int Rev Immunol. 2013;32:1–3. doi: 10.3109/08830185.2012.750138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta M, Kinoshita S. Innate immunity of the ocular surface. Brain Res Bull. 2010;81:219–228. doi: 10.1016/j.brainresbull.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Knop KM. Anatomy and immuology of the ocular surface. Chemical Immunology and Allergy. 2007;92:36–49. doi: 10.1159/000099252. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ. et al. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D, Iovieno A. The role of microbial flora on the ocular surface. Curr Opin Allergy Clin Immunol. 2009;9:466–470. doi: 10.1097/ACI.0b013e3283303e1b. [DOI] [PubMed] [Google Scholar]
- Wu TG. et al. Molecular analysis of the pediatric ocular surface for fungi. Current Eye Research. 2009;26:33–36. doi: 10.1076/ceyr.26.1.33.14253. [DOI] [PubMed] [Google Scholar]
- Dong BJ, Iovieno A, Bates B. et al. Diversity of Bacteria at Healthy Human Conjunctiva. Invest Ophthalmol Vis Sci. 2011;52(8):5408–5413. doi: 10.1167/iovs.10-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo PC, Lau SK, Teng JL. et al. Then and now: use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin Microbiol Infect. 2008;14:908–934. doi: 10.1111/j.1469-0691.2008.02070.x. [DOI] [PubMed] [Google Scholar]
- Burns DG, Camakaris HM, Janssen PH. et al. Combined use of cultivation-dependent and cultivation-independent methods indicates that members of most haloarchaeal groups in an Australian crystallizer pond are cultivable. Appl Environ Microbiol. 2004;70:5258–5265. doi: 10.1128/AEM.70.9.5258-5265.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Renaud G. et al. A diversity profile of the human skin microbiota. Genome Res. 2008;18:1043–1050. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegans ME, Van Gelder RN. Considerations in understanding the ocular surface microbiome. Am J Ophthalmol. 2014;158:420–422. doi: 10.1016/j.ajo.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto C, Shi G, Gery I. Microbial products trigger autoimmune ocular inflammation. Ophthalmic Res. 2008;40:193–199. doi: 10.1159/000119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Jung JY, Kim JC. Comparative Ocular Microbial Communities in Humans with and without Blepharitis. Invest Ophthalmol Vis Sci. 2012;53(9):5585–5593. doi: 10.1167/iovs.12-9922. [DOI] [PubMed] [Google Scholar]
- Graham JE. et al. Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Invest Ophthalmol Vis Sci. 2007;8:5616–5623. doi: 10.1167/iovs.07-0588. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Sasaki I, Ogawa H. et al. Colonisation of microflora in mice: mucosal defence against luminal bacteria. J Gastroenterol. 1999;34:54–60. doi: 10.1007/s005350050216. [DOI] [PubMed] [Google Scholar]
- Larkin LJ. Quantitative Alterations of the Commensal Eye Bacteria in Contact Lens Wear. Eye (Lond). 1991;5(Pt 1):70–74. doi: 10.1038/eye.1991.14. [DOI] [PubMed] [Google Scholar]
- Jassim SH. Bacteria Colonizing the Ocular Surface in Eyes With Boston Type 1 Keratoprosthesis: Analysis of Biofilm-Forming Capability and Vancomycin Tolerance. Invest Ophthalmol Vis Sci. 2015;56:4689–4696. doi: 10.1167/iovs.15-17101. [DOI] [PubMed] [Google Scholar]
- Robert MC, Eid EP, Saint-Antoine P. et al. Microbial colonization and antibacterial resistance patterns after Boston type 1 keratoprosthesis. Ophthalmology. 2013;120:1521–1528. doi: 10.1016/j.ophtha.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Dave SB, Toma HS, Kim SJ. Changes in ocular flora in eyes exposed to ophthalmic antibiotics. Ophthalmology. 2013;120:937–941. doi: 10.1016/j.ophtha.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Fontes BM. et al. Effect of chronic systemic use of trimethoprim-sulfamethoxazole in the conjunctival bacterial flora of patients with HIV infection. Am J Ophthalmol. 2004;138:678–679. doi: 10.1016/j.ajo.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Yin VT. et al. Antibiotic resistance of ocular surface flora with repeated use of a topical antibiotic after intravitreal injection. JAMA Ophthalmol. 2013;131:456–461. doi: 10.1001/jamaophthalmol.2013.2379. [DOI] [PubMed] [Google Scholar]
- Tuzhikov A, Panchin A, Thanathanee O. et al. Keratitis-induced changes to the homeostatic microbiome at the human cornea. Invest Ophthalmol Vis Sci. 2013;54:2891. [Google Scholar]
- Yamauchi Y. et al. Conjunctival flora in patients with human immunodeficiency virus infection. Ocul Immunol Inflamm. 2005;13:301–304. doi: 10.1080/09273940590951106. [DOI] [PubMed] [Google Scholar]
- Sankaridurg PR, Willcox M, Naduvilath TJ. et al. Bacterial Colonization of Disposable Soft Contact Lenses Is Greater during Corneal Infiltrative Events than during Asymptomatic Extended Lens Wear. J Clin Microbiol. 2000;38(12):4420–4424. doi: 10.1128/jcm.38.12.4420-4424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower EW, Lindsley K, Nanji AA. et al. Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database Syst Rev. 2013;7:CD006364. doi: 10.1002/14651858.CD006364.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barría von-B F, Moreno R, Ortiz R. et al. Microbial flora isolated from patient’s conjunctiva previous to cataract surgery. Revista chilena de infectología. 2015;32(2):150–157. doi: 10.4067/S0716-10182015000300003. [DOI] [PubMed] [Google Scholar]
- Verma V, Shen D, Sieving PC. et al. The role of infectious agents in the etiology of ocular adnexal neoplasia. Surv Ophthalmol. 2008;53:312–331. doi: 10.1016/j.survophthal.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafurov K, Ren L, Dong CQ. et al. Oral Microbiome Link to Neurodegeneration in Glaucoma. PloS One. 2014;9(9):e104416. doi: 10.1371/journal.pone.0104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KRG. et al. Gene Therapy with Brain-Derived Neurotrophic Factor As a Protection: Retinal Ganglion Cells in a Rat Glaucoma Model. Invest Ophthalmol Vis Sci. 2003;44(10):4357–4365. doi: 10.1167/iovs.02-1332. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JT, Lin P, Asquith M. The microbiome, HLA, and the pathogenesis of uveitis. Jpn J Ophthalmol. 2016;60:1–6. doi: 10.1007/s10384-015-0416-y. [DOI] [PubMed] [Google Scholar]
- Lin P, Asquith M, Gruner H. et al. The role of the gut microbiota in immune-mediated uveitis. Invest Ophthalmol Vis Sci. 2015;56:870. [Google Scholar]
- Horai R. et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity. 2015;43:343–353. doi: 10.1016/j.immuni.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatkin NM. et al. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10(5):323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AM. Antimicrobial compounds in tears. Exp Eye Res. 2013;117:53–61. doi: 10.1016/j.exer.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


