Abstract
Four Lactobacillus species, namely L. crispatus, L. iners, L. gasseri, and L. jensenii, commonly dominate the vaginal communities of most reproductive-age women. It is unclear why these particular species, and not others, are so prevalent. Historically, estrogen-induced glycogen production by the vaginal epithelium has been proffered as being key to supporting the proliferation of vaginal lactobacilli. However, the ‘fly in the ointment’ (that has been largely ignored) is that the species of Lactobacillus commonly found in the human vagina cannot directly metabolize glycogen. It would appear that this riddle has been solved as studies have demonstrated that vaginal lactobacilli can metabolize the products of glycogen depolymerization by α-amylase, and fortunately, amylase activity is found in vaginal secretions. These amylases are presumed to be host-derived, but we suggest that other bacterial populations in vaginal communities could also be sources of amylase in addition to (or instead of) the host. Here we briefly review what is known about human vaginal bacterial communities and discuss how glycogen-derived resources and resource competition might shape the composition and structure of these communities.
Keywords: vagina, microbiome, vaginal microbiome, microbial community, alpha-amylase, glycogen
Introduction
Previous studies have shown that while species of Lactobacillus tend to dominate the vaginal communities of most healthy reproductive-aged women [1-5], these communities are quite personalized in terms of species composition and temporal dynamics [1-3,6-9]. These lactobacilli are thought to have a protective role by maintaining an acidic environment through the production of lactic acid thereby restricting the growth of pathogenic organisms and evidence to support this has been extensively reviewed in the literature [10-15]. In a study of Black and White women done by Zhou et al., samples from 144 women were analyzed and eight major types of vaginal communities were found [2]. Eighty percent of these women had communities dominated by various Lactobacillus species, with L. iners being the most prevalent and found in 66 percent of the women. The other dominant Lactobacillus species included L. crispatus, L. gasseri, and L. jensenii. These same four lactobacilli were dominant in four of the five major groups of bacterial communities found in a later study that involved a larger cohort of 396 reproductive-age women [3]. These groups, which are referred to as community state types (CSTs), were determined by clustering vaginal bacterial communities based on bacterial composition and relative abundance. In that study L. crispatus was dominant in CST I, L. gasseri was dominant in CST II, while L. iners and L. jensenii were dominant in CST III and CST V, respectively (Figure 1) [3]. However, not all communities were dominated by lactobacilli [1,3] as the fifth CST (CST IV) lacked high proportions of Lactobacillus and instead was characterized by an increased relative abundance of strict anaerobes that included Gardnerella vaginalis, Prevotella, Atopobium, Megasphaera and others [3].
Figure 1.
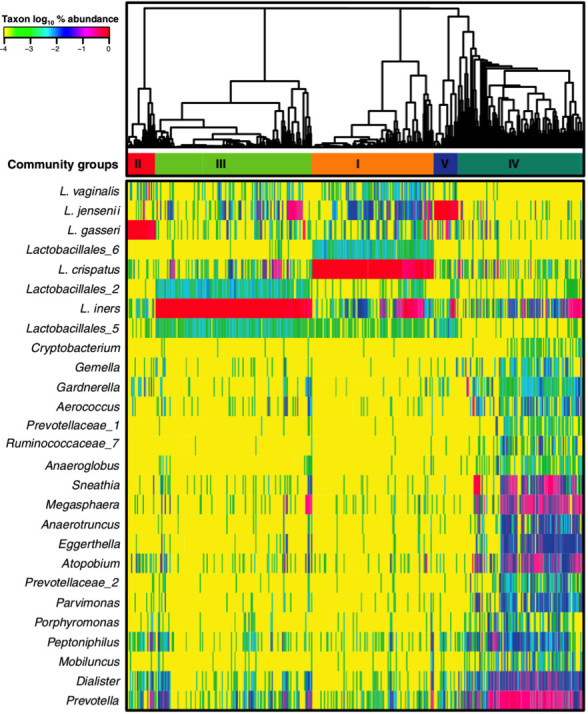
Composition and structure of vaginal bacterial communities found in 396 reproductive age women. The bacterial populations in each sample were classified based on partial 16S rRNA gene sequences and the communities were clustered based on the relative abundances of these bacterial populations. Major groups of communities were used to define community state types I to V. This heatmap shows the relative abundance (see color key) of bacterial taxa (listed on the left) in each community. (From [3]. Reprinted with permission from the Proceedings of the National Academy of Sciences USA.)
Both of these studies revealed significant differences in vaginal community composition between women of different ethnic groups. For example, Ravel et al. showed that Lactobacillus species were dominant in 80.2 percent and 89.7 percent of Asian and White women, but this was the case in only 59.6 percent and 61.9 percent of Black and Hispanic women. Similarly, Zhou et al. found Lactobacillus species to be dominant in 91 percent of White women but only 68 percent of Black women. These differences in vaginal community composition between women of different ethnicities were confirmed in subsequent studies done to characterize the vaginal communities of African American women and women of European ancestry [2,16]. Likewise, a more recent study found four kinds of vaginal communities in a population of South African women [17]. Of these women, only 37 percent had Lactobacillus dominated communities with L. iners being the most common species found.
Given that there are more than 130 species of Lactobacillus known it is unclear why just four of these species dominate most vaginal communities. At the very least this observation suggests that L. crispatus, L. gasseri, L. jensenii, and L. iners are especially well adapted to the vaginal environment and have specific traits that allow them to colonize this habitat. When surveyed for niche-specific traits, comparative genomic analysis of 25 Lactobacillus species showed that vaginal lactobacilli had significantly smaller genomes and lower %G+C content than non-vaginal species [18]. Efforts to identify a set of common traits that might account for their shared ability to successfully colonize the human vagina were unsuccessful. However, there were a number of species-specific traits identified, which hints at the possibility that each species has unique characteristics that enable them to effectively compete in the vaginal ecosystem. A more in-depth functional analysis of these traits will be necessary to define their relative importance in mediating interactions between lactobacilli and the host.
The stark differences in vaginal communities within and between women, especially those of different ethnicities, suggest that the host may have a prominent role in shaping the species composition of these communities. Some key questions include: 1) why do only certain species of Lactobacillus colonize the vagina; 2) why does the composition of vaginal bacterial communities differ between women; and 3) what drives communities to change in composition over time? In this review we explore what is known about how the host environment and competition for resources may influence the composition and stability of these communities.
Vaginal Community Composition Varies Over Time
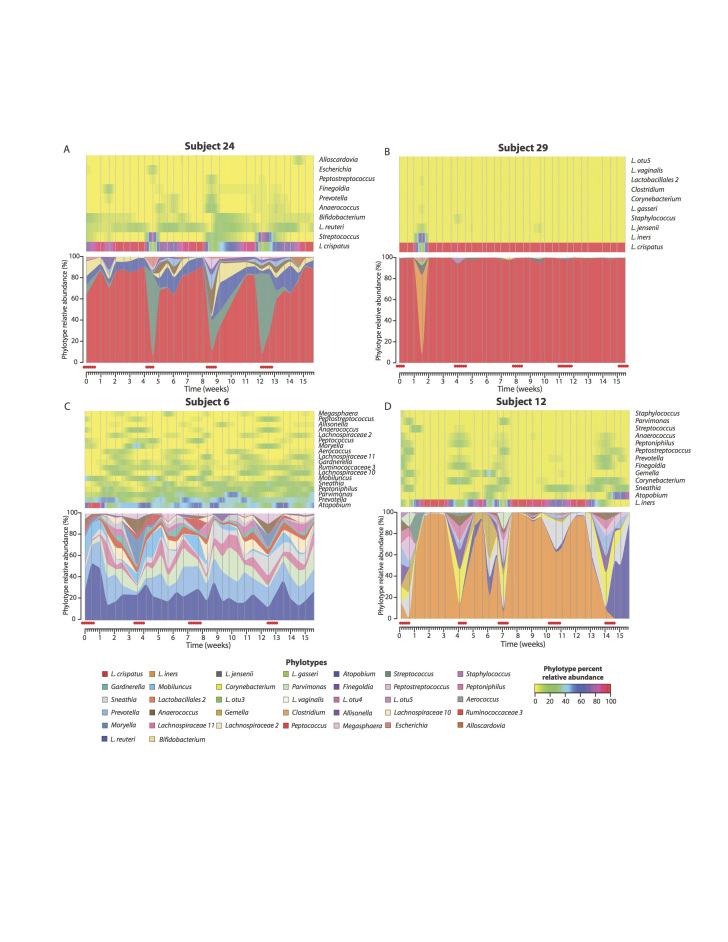
Studies on the longitudinal dynamics of the vaginal microbiome in healthy reproductive-age women have shown that these communities often change over relatively short periods of time [8,9,19-21]. Gajer et al. analyzed samples that were self-collected twice weekly over 16 weeks from 32 women [8]. The community composition in each sample from each time point was classified into CSTs, and the patterns of CSTs over time were clustered, demonstrating five temporal patterns. Of the Lactobacillus dominated CSTs, only CST I-III were found; probably because too few women were included in the study. Some communities changed in composition while others remained relatively stable over time (Figure 2). Moreover, not all transitions between CSTs were equally likely to occur. This study showed that community dynamics vary greatly between individuals. Unfortunately, the mechanisms that drive these dynamics in community composition are not well understood.
Figure 2.
Temporal dynamics of vaginal bacterial communities. Heat maps (top) and interpolated bar plots (bottom) depict changes in the relative abundances of bacterial taxa in the vaginal communities of four women over 16 weeks. The color code for each taxon is shown below the figure. (From [8]. Reprinted with permission from AAAS.)
Drivers of Community Composition and Change
All of the resources necessary to support vaginal bacterial communities are ultimately derived from the host. It follows that differences in host physiology that alter the kinds and abundances of resources present might influence which bacterial species will successfully colonize the vagina. Vaginal secretions and vaginal epithelial cells that are sloughed and subsequently lyse are thought to be the principle sources of nutrients that support bacterial growth. Vaginal secretions contain nutrients that include proteins, carbohydrates, amino acids, and cervicovaginal mucus, which is rich in mucins and glycoproteins [22,23]. The vaginal epithelium is rich in glycogen, which has long been thought to be a key nutrient for vaginal lactobacilli. However, this assertion is primarily based on the positive correlation that exists between the levels of free glycogen and the abundance of vaginal lactobacilli [24].
The availability of resources in the vagina seems to be driven by estrogen levels. Estrogen is known to increase the volume of vaginal secretions. Moreover, elevation of estrogen levels induces thickening of the vaginal epithelium and prompts the accumulation of glycogen. Together these are thought to create an environment that stimulates the proliferation of Lactobacillus [25]. Furthermore, changes in the relative abundance of vaginal lactobacilli is also associated with estrogen levels and glycogen content [26,27] over a woman’s life span. During reproductive years, estrogen levels are known to vary throughout the menstrual cycle. Estrogen levels are low during menses (< 50 pg/ml), peak before ovulation (200-250 pg/ml), decline shortly thereafter and peak again around day 21 (150 pg/ml) [8]. Changes in glycogen content have been shown to parallel these changes [28]. Variation in estrogen levels and glycogen content over the menstrual cycle might partly explain why there are differences in community composition within women over time.
In an effort to understand the effect of the menstrual cycle on changes in community composition, Gajer et al. modeled the rate of change in a community’s state as a function of the menstrual cycle. Based on their findings, community composition appears to be more stable when estrogen levels are highest [8]. This observation is also seen during pregnancy, a condition where estrogen levels are high. Estrogen levels increase dramatically during pregnancy due to additional estrogen produced by the placenta [29]. Studies evaluating the vaginal microbiome during pregnancy have shown that the vaginal communities of pregnant women are more stable and have higher relative abundances of lactobacilli than those of non-pregnant women [30,31]. Moreover, vaginal communities in pregnant women tend to be dominated by L. crispatus or L. iners, while CST IV (in which Lactobacillus is not dominant) is rarely observed [31,32]. Estrogen levels decrease post-partum and concomitant changes in communities are observed, with lactobacilli becoming less dominant [31]. These studies suggest a strong correlation between estrogen and community composition.
Glycogen per se is not a Key Nutrient for Vaginal Lactobacilli
While it is casually suggested that glycogen directly enriches for Lactobacillus, it should be noted that vaginal lactobacilli cannot directly metabolize glycogen. In 1964, Stewart-Tull isolated 36 strains of Lactobacillus from the vagina and cervix of pregnant women, and showed that none of the strains could metabolize glycogen [33]. Similarly, years later, Whylie and Henderson isolated 42 strains of Lactobacillus from the vaginas of pregnant women [34], of which 11 were strains of L. acidophilus. One of these could metabolize glycogen from oysters and two could metabolize glycogen isolated from the human vagina. Additional evidence that few or no vaginal lactobacilli are capable of directly using glycogen as a resource was obtained in a study by Martín et al. in which none of the L. crispatus, L. gasseri, and L. jensenii strains tested were able to metabolize glycogen [35]. Recently, Spear et al. confirmed these results with L. gasseri and L. jensenii [36].
While vaginal Lactobacillus species cannot metabolize glycogen, they have been shown to grow on smaller oligomers of glucose produced through the depolymerization of glycogen by α-amylase [36]. α-Amylase is an endoglycosidase that cleaves α-(1,4) glycosidic bonds [37] to produce maltose, maltotriose, as well as α-(1,4) and α-(1,6)-dextrins [38]. In humans, α-amylase is reportedly only found in saliva and the pancreas, but definitive evidence for its presence in vaginal secretions is lacking. Spear et al. showed that L. gasseri and L. jensenii could grow in media containing glycogen if saliva is first added [36]. The growth profiles in media containing glycogen and saliva were similar to those seen when these species were grown in media containing glucose alone, and likewise, both species were able to grow on maltose [36]. These investigators also showed that vaginal secretions exhibited amylase-like activity and could depolymerize glycogen. It is not clear whether the amylase (or multiple amylases) in vaginal secretions originated from the human host, from one or more bacterial populations in vaginal communities, or both.
With the premise that vaginal lactobacilli play a key role in the maintenance of health, and that α-amylase promotes the dominance of Lactobacillus species in the vagina, Nasioudis et al. conducted a study to evaluate α-amylase levels in women with bacterial vaginosis or vulvo-vaginal candidiasis [39]. Their results showed α-amylase levels were lowest in women with bacterial vaginosis and highest in healthy women without either vaginal disorder. This study did not analyze the composition of the vaginal communities; therefore, it is difficult to directly associate α-amylase levels with the dominance of Lactobacillus in the vaginal communities of these women. We can, however, suggest that high levels of α-amylase might be associated with healthy conditions in the vagina.
Concluding Remarks
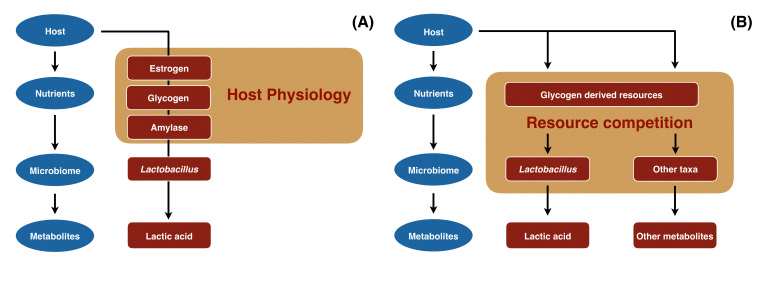
The evidence available can be used to paint a simple picture (Figure 3A). Glycogen levels in the vagina are driven by estrogen in the host and this resource is depolymerized by human α-amylase to produce simpler sugars. These carbohydrates are readily fermented by species of vaginal lactobacilli to produce lactic acid, which lowers the pH of the vagina creating an environment that putatively restricts the growth of non-indigenous organisms. The dominance of L. crispatus, L. gasseri, L. iners, and L. jensenii in the vaginal communities of most women suggests that these species may be particularly adept at competing for glycogen-derived resources. The key elements of this scenario – estrogen, glycogen and amylase – are all governed by the host and may vary over time due to physiological changes within an individual. Moreover, these elements might differ between individuals due to genetic or behavioral (e.g. dietary) differences between individuals and account for temporal variation in vaginal community composition.
Figure 3.
Schemes for the production and use of glycogen-derived resources by vaginal bacterial communities. Panel A depicts our current understanding of how glycogen-derived resources are produced and used by vaginal bacterial communities. Host estrogen stimulates the production and accumulation of glycogen, which is degraded by human α-amylase to produce simpler sugars that are consumed by vaginal lactobacilli and fermented to produce lactic acid. In Panel B we propose that bacterial populations in vaginal communities could also be sources of α-amylase in addition to (or instead of) the host. The resulting simpler sugars could serve as a ‘common good’ that is available to the entire bacterial community, thus setting the stage for interspecies competition for these resources. Both panels illustrate ecological networks that include species of Lactobacillus, various other bacterial populations and the host.
Here we suggest a few wrinkles that might be considered to refine our understanding. One concerns the source of amylases that mediate the depolymerization of glycogen. In humans, α-amylase has only been shown to be present in saliva and the pancreas, and we await confirmation that it is produced in the vagina. Aside from human α-amylase, it is plausible that various bacterial populations in vaginal communities also produce α-amylase (Figure 3B) since various anaerobic taxa are known to produce amylases and ferment the breakdown products of glycogen [40-44]. In theory, multiple sources of amylases would increase the redundancy of this function in vaginal communities and increase the probability that this critical function is maintained in the face of perturbations, thus rendering the community more stable. Secondly, there is no reason a priori to think that only lactobacilli use the glycogen-derived simpler sugars (Figure 3B). The hydrolysis of glycogen by these amylases almost certainly occurs in the extracellular environment, which produces resources (e.g. maltose) that are ‘common goods’ available to all members of the bacterial community. These glycogen-derived resources might be broadly shared and support the growth of other community members. While any community member capable of utilizing these resources can compete for them, perhaps vaginal lactobacilli more effectively compete for and sequester these resources. Within this conceptual framework, both glycogen levels and the functional redundancy of amylase production might be key to the maintenance of relatively stable bacterial communities that are dominated by Lactobacillus species.
Combining the simple picture and the wrinkles that we describe above there are at least four possibilities that may account for the dominance of lactobacilli and overall composition of bacterial communities in the human vagina. First, the numerical dominance of lactobacilli suggests that they simply out-compete other taxa for glycogen-derived resources. A second alternative is that other taxa use glycogen and/or glycogen-derived resources more efficiently than lactobacilli, but they in turn produce key nutrients (e.g. vitamins, amino acids) that spur the proliferation vaginal lactobacilli. This would imply that the prevalence of vaginal lactobacilli could be largely based on interactions with and metabolic dependence on other community members rather than direct interactions with the host. Third, while emphasizing the positive correlation between the levels of glycogen and the abundance of vaginal lactobacilli there is a tendency to gloss over the fact that lactobacilli cannot use glycogen directly. It could well be that high levels of glycogen develop precisely because vaginal lactobacilli cannot use it as a resource. Meanwhile, a high relative abundance of lactobacilli only allows for low proportions of other taxa that might metabolize glycogen. If the rate of glycogen metabolism does not exceed the rate at which it is produced this might explain why we continue to see high levels of glycogen in the presence of lactobacilli. Finally, the correlation between estrogen, glycogen and the selection of specific species of Lactobacillus could be a ‘red herring’ if there is no causal relationship between these factors. This tangle of possibilities will need to be sorted out in future research. Meanwhile researchers should avoid over simplifying what is most likely a complex ecological network that includes both the host and various bacterial species in these communities.
Acknowledgments
KLN and LJF wrote the manuscript and received funding from grants U19 AI084044 and R56 AI108775 from the National Institutes of Health. A Presidential Scholar Fellowship from the University of Idaho funds KLN.
Abbreviations
- CST
community state type
References
- Zhou X, Bent SJ, Schneider MG. et al. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004;150(Pt 8):2565–2573. doi: 10.1099/mic.0.26905-0. [DOI] [PubMed] [Google Scholar]
- Zhou X, Brown CJ, Abdo Z. et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. The ISME Journal. 2007;1(2):121–133. doi: 10.1038/ismej.2007.12. [DOI] [PubMed] [Google Scholar]
- Ravel J, Gajer P, Abdo Z. et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108(1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infec Dis. 1999;180(6):1950–1956. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- Burton JP, Cadieux PA, Reid G. et al. Improved understanding of the bacterial vaginal microbiota of women before and after probiotic instillation. Appl Environ Microbiol. 2003;69(1):97–101. doi: 10.1128/AEM.69.1.97-101.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman RW, Fukushima M, Diamond L. et al. Microbes on the human vaginal epithelium. Proc Natl Acad Sci USA. 2005;102(22):7952–7957. doi: 10.1073/pnas.0503236102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Hansmann MA, Davis CC. et al. The vaginal bacterial communities of Japanese women resemble those of women in other racial groups. FEMS Immunol Med Microbiol. 2010;58(2):169–181. doi: 10.1111/j.1574-695X.2009.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajer P, Brotman RM, Bai G. et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4(132):132ra52–132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman RM, Ravel J, Cone RA. et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86(4):297–302. doi: 10.1136/sti.2009.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Forney LJ, Ravel J. Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol. 2012;66(1):371–389. doi: 10.1146/annurev-micro-092611-150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey RJ, Zhou X, Pierson JD. et al. Understanding vaginal microbiome complexity from an ecological perspective. Transl Res. 2012;160(4):267–282. doi: 10.1016/j.trsl.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, Monif GR. Understanding the bacterial flora of the female genital tract. Clin Infect Dis. 2001;32(4):e69–e77. doi: 10.1086/318710. [DOI] [PubMed] [Google Scholar]
- Huang B, Fettweis JM, Brooks JP. et al. The changing landscape of the vaginal microbiome. Clin Lab Med. 2014;34(4):747–761. doi: 10.1016/j.cll.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wijgert JHHM, Borgdorff H, Verhelst R. et al. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One. 2014;9(8):e105998. doi: 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo-Lopez V, Cook RL, Sobel JD. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990;12(5):856–872. doi: 10.1093/clinids/12.5.856. [DOI] [PubMed] [Google Scholar]
- Fettweis JM, Brooks JP, Serrano MG. et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology. 2014;160(Pt 10):2272–2282. doi: 10.1099/mic.0.081034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anahtar MN, Byrne EH, Doherty KE. et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42(5):965–976. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Soares H, Suzuki H, Hickey RJ. et al. Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment. J Bacteriol. 2014;196(7):1458–1470. doi: 10.1128/JB.01439-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Liu C, Mitchell CM. et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5(4):e10197–e10198. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane FE, Ison CA, Taylor-Robinson D. et al. A longitudinal study of the vaginal flora over a menstrual cycle. Int J STD. 2008;8(8):1–6. doi: 10.1258/0956462971920631. [DOI] [PubMed] [Google Scholar]
- Schwebke JR, Richey CM, Weiss HL. et al. Correlation of behaviors with microbiological changes in vaginal flora. J Infect Dis. 1999;180(5):1–5. doi: 10.1086/315065. [DOI] [PubMed] [Google Scholar]
- Owen DH, Katz F. A vaginal fluid simulant. Contraception. 1999;59(2):91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- Geshnizgani AM, Onderdonk AB. Defined medium simulating genital tract secretions for growth of vaginal microflora. J Clin Microbiol. 1992;30(5):1323–1326. doi: 10.1128/jcm.30.5.1323-1326.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmonsef P, Hotton AL, Gilbert D. et al. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PLoS One. 2014;9(7):e102467. doi: 10.1371/journal.pone.0102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage M, Maibach H. Lifetime changes in the vulva and vagina. Arch Gynecol Obstet. 2006;273(4):195–202. doi: 10.1007/s00404-005-0079-x. [DOI] [PubMed] [Google Scholar]
- Cruickshank R, Sharman A. The biology of the vagina in the human subject. BJOG. 1934;41(2):208–226. [Google Scholar]
- Paavonen J. Physiology and ecology of the vagina. Scand J Infect Dis Suppl. 1983;40:31–35. [PubMed] [Google Scholar]
- Sjöberg I, Cajander S, Rylander E. Morphometric characteristics of the vaginal epithelium during the menstrual cycle. Gynecol Obstet Invest. 1988;26(2):136–144. doi: 10.1159/000293685. [DOI] [PubMed] [Google Scholar]
- Jones RERE. Human reproduction and sexual behavior. Englewood Cliffs, N.J.: Prentice-Hall; 1984. [Google Scholar]
- MacIntyre DA, Chandiramani M, Lee YS. et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep. 2015;5:8988–8989. doi: 10.1038/srep08988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Hassan SS, Gajer P. et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2(1):1–19. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther-António MRS, Jeraldo P, Berg Miller ME. et al. Pregnancy's stronghold on the vaginal microbiome. PLoS One. 2014;9(6):e98514. doi: 10.1371/journal.pone.0098514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Tull DE. Evidence that vaginal lactobacilli do not ferment glycogen. YMOB. 1964;88:676–679. doi: 10.1016/0002-9378(64)90898-1. [DOI] [PubMed] [Google Scholar]
- Wylie JG, Henderson A. Identity and glycogen-fermenting ability of lactobacilli isolated from the vagina of pregnant women. J Med Microbiol. 1969;2(3):363–366. doi: 10.1099/00222615-2-3-363. [DOI] [PubMed] [Google Scholar]
- Martín R, Soberón N, Vaneechoutte M. et al. Characterization of indigenous vaginal lactobacilli from healthy women as probiotic candidates. Int Microbiol. 2008;11(4):261–266. doi: 10.2436/20.1501.01.70. [DOI] [PubMed] [Google Scholar]
- Spear GT, French AL, Gilbert D. et al. Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis. 2014;210(7):1019–1028. doi: 10.1093/infdis/jiu231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayer GD, Luo Y, Withers SG. et al. The structure of human pancreatic alpha-amylase at 1.8 A resolution and comparisons with related enzymes. Protein Sci. 1995;4(9):1730–1742. doi: 10.1002/pro.5560040908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts PJ, Whelan WJ. The mechanism of carbohydrase action. 5. Action of human salivary alpha-amylase on amylopectin and glycogen. Biochem J. 1960;76:246–253. doi: 10.1042/bj0760246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasioudis D, Beghini J, Bongiovanni AM. et al. α-Amylase in vaginal fluid: association with conditions favorable to dominance of Lactobacillus. Reprod Sci. 2015;22(11):1393–1398. doi: 10.1177/1933719115581000. [DOI] [PubMed] [Google Scholar]
- Gupta R, Gigras P, Mohapatra H. et al. Microbial α-amylases: a biotechnological perspective. Process Biochem. 2003;38(11):1599–1616. [Google Scholar]
- Pandey A, Nigam P, Soccol CR. et al. Advances in microbial amylases. Biotechnol Appl Biochem. 2000;31(2):135. doi: 10.1042/ba19990073. [DOI] [PubMed] [Google Scholar]
- Reddy NS, Nimmagadda A, Samasiva Rao KRS. et al. An overview of the microbial α-amylase family. Afr J Biotechnol. 2003;2(12):645–648. [Google Scholar]
- MacGregor EA, Janecek S, Svensson B. et al. Relationship of sequence and structure to specificity in the alpha-amylase family of enzymes. Biochim Biophys Acta. 2001;1546(1):1–20. doi: 10.1016/s0167-4838(00)00302-2. [DOI] [PubMed] [Google Scholar]
- Elbein AD. Microbial Glycobiology. 1st ed. San Diego: Academic Press; 2010. Cytoplasmic carbohydrate molecules: trehalose and glycogen. pp. 185–201. [Google Scholar]