Abstract
The significance of the gut microbiota as a determinant of drug pharmacokinetics and accordingly therapeutic response is of increasing importance with the advent of modern medicines characterised by low solubility and/or permeability, or modified-release. These physicochemical properties and release kinetics prolong drug residence times within the gastrointestinal tract, wherein biotransformation by commensal microbes can occur. As the evidence base in support of this supplementary metabolic “organ” expands, novel opportunities to engineer the microbiota for clinical benefit have emerged. This review provides an overview of microbe-mediated alteration of drug pharmacokinetics, with particular emphasis on studies demonstrating proof of concept in vivo. Additionally, recent advances in modulating the microbiota to improve clinical response to therapeutics are explored.
Keywords: microbiota, microbiome, drug metabolism, pharmacokinetics, gastrointestinal
Introduction
The human gut microbiome, comprising up to 100 trillion microbes (microbiota) and their genomes [1,2], functions symbiotically with the host superorganism it inhabits. These unique populations of bacteria, viruses and fungi are crucial not only for the innate maintenance of health, but also in processing exogenous compounds (medicines) intended to rectify homeostatic imbalances. The realisation of this latter action of the microbiota has altered the concept of pharmaceutical-microbiota interactions, shifting the influencer role from medicines to an appreciation of microbiome-medicine interplay. The microbiota, and in particular microbiome-encoded enzymes, now represent plausible intermediate targets to alter drug pharmacokinetics (absorption, distribution, metabolism and elimination) to consequently enhance clinical response.
The gut microbiota is not static, but rather is subject to dynamic transformations as a consequence of; pharmacological interventions (most notably antibiotic therapy), pathology (gastroenteric and systemic infection), nutritional status, circadian rhythm and environmental influences [3,4]. Inter-individual or intra-individual temporal microbiota diversity or dysbiosis are thus of potential clinical significance, since microbe-mediated bioactivation of prodrug formulations may vary. Supplementation with “good” bacteria, that is, probiotics, may accordingly unify or augment patient responses. Conversely, microbial biotransformation may also generate bioinactive or toxic metabolites, such that strategies to nullify the microbiome may be transiently beneficial. Herein, this intriguing prospect of manipulating the microbiome to improve clinical patient outcomes is discussed with reference to the current literature.
Impact of the Microbiome on Drug Pharmacokinetics and Therapeutic Outcomes
Oral delivery of pharmaceuticals presents a multitude of challenges, including an assurance of drug stability in the gastrointestinal lumen. Implementation of formulation strategies, such as enteric coating, have successfully reduced the degradative effect of upper gastrointestinal pH. However, the contribution of the distally increasing population of microbes to drug stability is frequently unappreciated. Previous reports suggested that the relevance of the microbiota in dictating the pharmacokinetic profile of a drug is determined by its presentation to the distal gut [5]. Therefore, traditionally, it has been accepted that the majority of drugs, which are absorbed from the small intestine, will have little interaction with the microbiota unless they are candidates for sustained-release or enterohepatic circulation. However, recent insights into the impact of the small intestinal flora on mammalian hosts [6] may lead to a paradigm shift in the considered gastrointestinal sites of microbial biotransformation. Newly emerging drug candidates have a tendency toward low solubility and/or permeability, properties which result in prolonged gastrointestinal residence times and therefore a greater probability of microbial interactions. It has thus become increasingly necessary to consider the numerous metabolic processes coordinated by the microbiome, a selection of which are examined below and in Figure 1 and Table 1.
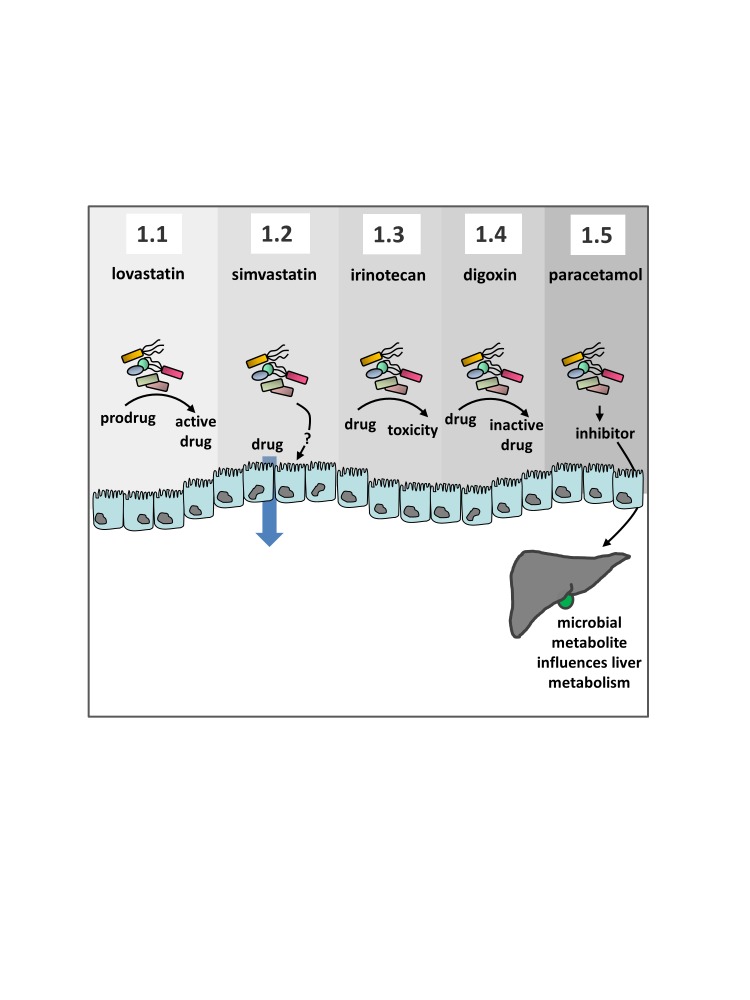
Figure 1.
A summary of selected mechanisms by which the microbiota influences drug pharmacokinetics. Individual panels correspond to the article text. 1.1 Agents including lovastatin or sulfasalazine are directly activated by the gut microbiota. 1.2 The availability and uptake of drugs including simvastatin and amiodarone is influenced by the microbiota or by co-administration of probiotics through unknown mechanisms. 1.3 Toxicity of irinotecan is elevated by microbial β-glucuronidase activity and can be selectively inhibited by antibiotics or specific microbial β-glucuronidase inhibitors. 1.4 Digoxin is inactivated in the gut by specific enzymatic activity associated with specific strains of Eggerthella lenta (cgr+). 1.5 Paracetamol detoxification in the liver is competitively inhibited by the gut microbial metabolite p-Cresol.
Table 1. Impact of the intestinal microflora on drug pharmacokinetics.
| Drug | Pharmacotherapeutic classification | Effect of the gut microbiota on drug pharmacokinetics | Implicated microbe or microbial enzyme (if known) | Postulated effect of the gut microbiota on drug bioavailability (F)/activity/toxicity | Ref |
| Amiodarone | Class III antiarrhythmic | ↑ absorption | Escherichia coli Nissle 1917 | ↑ F | [13] |
| Calcitonin | Calciotropic hormone | ↑ metabolism (proteolysis) | ↓ F and activity | [42] | |
| Diclofenac | Non-steroidal antiinflammatory drug | ↑ metabolism (deglucuronidation) and delayed excretion | β-glucuronidase enzymes | ↑ toxicity (enterohepatic circulation) | [16] |
| Digoxin | Cardiac glycoside | ↑ metabolism (reduction) | Eggerthella lenta | ↓ F and cardiac response | [18][19][20] |
| Indomethacin | Non-steroidal anti-inflammatory drug | ↑ metabolism (deglucuronidation) and delayed excretion | β-glucuronidase enzymes | ↑ toxicity (enterohepatic circulation) | [16] |
| Insulin | Anti-diabetic drug | ↑ metabolism (proteolysis) | Protease enzymes | ↓ F and activity | [42] |
| Irinotecan | Topoisomerase I inhibitor | ↑ metabolism (deglucuronidation) and delayed excretion | β-glucuronidase enzymes produced by bacteria, including Escherichia coli, Bacteroides vulgatus and Clostridium ramosum | ↑ toxicity (regeneration of active SN-38 within the intestinal lumen) | [14] |
| Ketoprofen | Non-steroidal antiinflammatory drug | ↑ metabolism (deglucuronidation) and delayed excretion | β-glucuronidase enzymes | ↑ toxicity (enterohepatic circulation) | [16] |
| Levodopa | Anti-parkinson | 1. ↓ absorption 2. ↑ metabolism (dehydroxylation) | 1. Helicobacter pylori | ↓ F and activity | 1.[36][37][38][39] 2.[35] |
| Loperamide oxide | Anti-propulsive | ↑ metabolism (reduction) | ↑ activity (prodrug activation) | [43] | |
| Lovastatin | HMG-CoA-reductase inhibitor | ↑ metabolism (hydrolysis) | ↑ F of active β-hydroxy acid metabolite, therefore, potentially ↑ pharmacological effect | [12] | |
| Metronidazole | Anti-protozoal and antibacterial | ↑ metabolism (reduction) | ↑ toxicity | [44] | |
| Nitrazepam | Benzodiazepine | ↑ metabolism (nitroreduction) | Nitroreductase enzymes | ↑ toxicity (postulated association with nitrazepam-induced teratogenicity in rats) | [45] |
| Nizatidine | H2-receptor antagonist | ↑ metabolism (cleavage of N-oxide bond) | ↓ systemic F | [46] | |
| Olsalazine | Aminosalicylate | ↑ metabolism (reduction) | Azoreductase enzymes | ↑ activity (prodrug activation) | [47] |
| Paracetamol | Analgesic and antipyretic | ↓ metabolism (p-Cresol-mediated competitive sulfonation) | Clostridium difficile and others | ↑ risk of hepatotoxicity | [21] |
| Prontosil | Sulfa drug | ↑ metabolism (reduction) | Azoreductase enzymes | ↑ activity (prodrug activation) | [8] |
| Ranitidine | H2-receptor antagonist | ↑ metabolism (cleavage of N-oxide bond) | ↓ systemic F | [46] | |
| Risperidone | Antipsychotic | ↑ metabolism (scission of the isoxazole ring) | [48] | ||
| Sulfasalazine | Aminosalicylate | ↑ metabolism (reduction) | Azoreductase enzymes | ↑ activity due to liberation of active 5-aminosalicyclic acid. Also, potentially ↑ toxicity due to enhanced generation of sulfapyridine, which can be systemically absorbed | [10] |
| Zonisamide | Antiepileptic | ↑ metabolism (reduction) | Clostridium sporogenes, Bifidobacterium bifidum, Bacteroides vulgatus, Escherichia coli, Salmonella typhimurium, Pseudomonas fluorescens, Lactobacillus rhamnosus, Streptococcus faecalis | [49] |
Microbe-Mediated Prodrug Activation
The impact of the commensal (indigenous) microbiota on therapeutic drug efficacy has long been recognised; for example, in the late 1930s the sulfa antibiotic class were identified as substrates for microbial transformation [7]. The liberation of the active sulfanilamide metabolite by gut bacteria, revealed that two genomes, human and microbial, were implicated in the pharmacological response [7]. Preclinical studies in rats illustrated that antibiotic administration could decrease the conversion of the oral prodrug Prontosil to sulfanilamide [8] . On this premise, it can be hypothesised that an interference in human microbial metabolism, for instance with a course of antibiotics, may diminish the therapeutic efficacy of certain co-administered medicines. Similarly, inter-individual variability in the composition of the gut microbiome could potentially dictate the effectiveness of prodrug conversion.
Sulfasalazine, indicated in ulcerative colitis, similarly exploits azo-reductase enzymes secreted by colonic bacteria to generate sulfapyridine and 5-aminosalicylic acid, an active anti-inflammatory moiety [9,10]. Conceivably, changes in the intestinal microflora may therefore have implications for sulfasalazine activation and ultimately response. For example, Mikov et al. reported that probiotic treatment significantly increased sulfasalazine reduction in rat colon contents [11]. The gut microbiota can also be favourably exploited to achieve site-specific drug release. Recently, the co-administration of probiotics with a polysaccharide-based colon targeted formulation has been shown, in rodent models of colitis, to ameliorate sulfasalazine efficacy [9].
Lovastatin, a lactone prodrug, has been shown to be hydrolysed to its lipid-lowering β-hydroxy acid metabolite when incubated with human and rat fecalase (enzyme fraction of feces), indicating microbe-directed metabolism [12]. Further to this, antibiotic treatment reduced systemic exposure to β-hydroxy acid by 35 to 50 percent following oral lovastatin administration to rats. Chronic antibiotic usage in the clinic may thus put patients at risk of therapeutic inefficacy should this effect prove translational.
Microbe-Mediated Alteration of Drug Absorption
Concomitant administration of the probiotic bacterium Escherichia coli Nissle 1917 has been demonstrated to increase the bioavailability of amiodarone, a Vaughan-Williams class III antiarrhythmic, in rats [13]. Matuskova et al. propose that the observed 43 percent increased bioavailability may be due to a reduction in intestinal pH, facilitating enhanced ionisation of the molecule and consequently mucosal transit. Alternatively, it is theorised that the heighted uptake could be attributed to upregulated expression of the influx transporter OATP2B1 [13].
Microbe-Mediated Deconjugation: Enterohepatic Circulation of Drugs
Microbiota-mediated xenobiotic metabolism can also adversely affect host outcomes, limiting the clinical applicability of drug candidates. Identification of the microbial mechanisms that are responsible has the potential to lead to precise interventions to eliminate the activity and improve drug tolerability.
Irinotecan, an intravenous prodrug formulation of the antineoplastic topoisomerase I inhibitor SN-38, is associated with delayed diarrhea. This dose-limiting gastrointestinal toxicity arises through the intra-luminal regeneration of SN-38 from its hepatic-derived, non-toxic glucuronide metabolite SN-38G. The deglucuronidation activity and resultant toxicity is attributed to microbial β-glucuronidases [14]. Interventions to reduce toxic side effects have thus focused on suppressing β-glucuronidase, initially, through broad-spectrum antibiotic therapy and, more recently, with selective microbial β-glucuronidase inhibitors [14,15]. Mitigation of SN-38-mediated toxicity is the epitome of advantageous modulation of the microbiome to advance patient outcomes, and interestingly extends the scope of this therapeutic strategy to intravenous, as well as oral medicines.
The extended applicability of small molecule inhibitors of microbial β-glucuronidase to improve pharmaceutical tolerability has been examined with non-steroidal anti-inflammatory drugs (NSAIDs). The enteropathic adverse reactions associated with this commonly prescribed class are, like SN-38, related to the hepatobiliary transit of glucuronides, followed by enterohepatic circulation of microbial recovered aglycones [16]. Murine models have demonstrated that small intestinal insults arising from protracted exposure to carboxylic acid-bearing NSAIDs (diclofenac, indomethacin, ketoprofen) can be prevented by selective blockade of microbial β-glucuronidase activity [16]. A subsequent study by Liang et al. advances this evidence base with pharmacokinetic confirmation that perturbation of the microbiota can influence indomethacin metabolism and correspondingly enteropathy. Antibiotic-driven β-glucuronidase depletion resulted in reduced reabsorption of indomethacin, as exemplified by accelerated elimination and a shortened half-life in mice [17], which may explain the diminished enteropathy reported by Saitta et al. [16]. It was also shown that indomethacin reciprocally alters the microbiota, which could have ramifications for patient health [17]. Whilst there is presently a paucity of data to support the translation of these findings to the clinical setting, this research provides further impetus to alter the microbiota in the clinic.
Microbe-Mediated Drug Inactivation
Digoxin, a cardiac glycoside used in the treatment of congestive heart failure and atrial fibrillation, has a narrow therapeutic index, meaning that modest changes in bioavailability can induce toxicity. In the 1980s, researchers discovered that Eggerthella lenta, an anaerobic colonic bacterium, possessed the capacity to inactivate digoxin [18]. Decades later, it was shown that whilst colonisation with E. lenta is fundamental to digoxin inactivation, only a proportion of individuals harboring the bacterium will inactivate the drug. This disparity in microbial metabolic activity was determined to be a strain-specific effect with only some strains of E. lenta possessing the “cardiac glycoside reductase” (cgr) operon, which is responsible for inactivation of digoxin [19]. In patients that carry cgr+ E. lenta, a routine antibiotic regimen may temporarily abolish the generation of dihydrodigoxin, the inactive metabolite, and concomitantly increase digoxin serum levels compared to pre-antibiotic baseline [20]. In the absence of antibiotic intervention these patients may be at risk of exhibiting reduced drug efficacy. Dietary modulation also represents a strategy to suppress cgr operon expression and consequently digoxin inactivation [19].
Microbe-Mediated Alteration of Host Drug Metabolism
The gut microbiota is additionally indirectly implicated in drug metabolism. Inter-individual variability in the processing of paracetamol (acetaminophen), an analgesic and antipyretic, has been shown to be correlated with the endogenous microbial metabolite p-Cresol [21]. Paracetamol is predominantly metabolised in the liver via two phase II pathways, glucuronidation and sulfation. p-Cresol and paracetamol are both substrates for hepatic sulfotransferases, and therefore high baseline p-Cresol levels are postulated to decrease paracetamol metabolism through competitive sulfonation. Patients harbouring an abundance of p-Cresol are thus expected to be at elevated risk of hepatotoxicity [21].
Emerging Insights into the Complex Role of Bacteria in Influencing Clinical Outcomes
Antineoplastic Medicines
Recently, the impact of the intestinal microflora on the immunomodulatory effect of chemotherapeutics has been uncovered. Sivan et al. observed improved tumor control in mice with a combination of Bifidobacterium and anti-PD-L1 monoclonal antibody therapy compared to the immunotherapeutic intervention alone [22]. On this basis, the authors concluded that the commensal microbiota could be regulated for clinical benefit, and postulated that the approach could be extended to other cancer immunotherapies.
Cyclophosphamide, an alkylating agent, provokes immunogenic apoptosis of cancer cells, overturning immunosuppressive T cells and promoting TH1 and TH17 cellular responses [23]. Viaud et al. established that cyclophosphamide changes the composition of the murine small intestinal microbiota and elicits the translocation of specific Gram-positive bacterial species to peripheral lymphoid organs, wherein a “pathogenic” subset of T helper 17 (pTH17) cells and memory TH1 cells are resultantly generated [23]. Tumors of germ-free or antibiotic-treated mice were resistant to cyclophosphamide, an effect attributed to reduced pTH17 responses [23].
Vétizou et al. similarly demonstrated that the tumor response to ipilimumab, a human monoclonal antibody targeting CTLA-4 (a negative regulator of T cell activation), is reliant on specific Bacteroides species [24]. Germ-free and antibiotic-treated mice were non-responsive to ipilimumab, an adverse outcome that was overturned by B. fragilis gavage, inoculation with B. fragilis polysaccharides, or by adoptive immunotherapy with B. fragilis-specific murine T cells [24]. Transplantation of Bacteroides-containing feces from patients with metastatic melanoma likewise restored murine CTLA-4 blockade.
Lehouritis et al. hypothesise that tumor-associated bacteria may influence chemotherapeutic drug efficacy, resistance or off-target toxicity on the basis of in vitro and murine data [25]. Thirty chemotherapeutic agents were subjected to an in vitro MTS cell cytotoxicity assay, which identified 10 drugs with reduced efficacy and six drugs with enhanced efficacy in combination with bacteria. For instance, the cytotoxicity of gemcitabine was negated by bacteria, a finding that translated in vivo. Conversely, CB1954 was shown to be activated by bacteria both in vitro and in vivo. Bacterial co-localisation with a tumor due to gut translocation or infection could therefore result in the off-target bioactivation and toxicity of certain chemotherapeutics. However, specific manipulation of intra-tumoral bacteria could enhance the cytotoxicity of these same agents by modulating biotransformation in situ.
These pre-clinical studies, if translational, highlight the potentially serious implications of microbial disturbance, inflicted by antibiotic co-prescribing, bacterial translocation or infection, on certain cancer therapies. Furthermore, this data underscores the potential therapeutic benefit of microbiome-modulating interventions for enhancing chemotherapeutic efficacy.
Cardiovascular Medicines
Statins, 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitors, are commonly prescribed for dyslipidemia, lowering low-density lipoprotein cholesterol (LDL-C) levels by up to 55 percent [26,27]. Despite the acknowledged effectiveness of statin therapy in the prophylaxis and management of cardiovascular disease, inter-individual variability in response exists [27,28]. Kaddurah-Daouk et al. noted, through metabolomics, that increased simvastatin plasma concentrations correlated with higher pre-treatment levels of microbially synthesised secondary bile acids [29]. This implies that the microbiota are possibly involved in determining simvastatin bioavailability. Furthermore, this research elucidated a link between higher baseline levels of coprostanol, a reduced bacterial metabolite of endogenous cholesterol, and simvastatin response. Interestingly, administration of Lactobacillae bacterial strains capable of converting cholesterol to coprostanol have been proposed as a probiotic lipid-lowering approach [29,30]. As previously discussed, Yoo et al. recently concluded that the gut microbiota influences lovastatin pharmacokinetics [12]. These findings suggest that patients receiving long term antibiotic therapy may fail to respond adequately to statins, posing an increased risk of serious cardiovascular sequelae.
Central Nervous System Active Medicines
Olanzapine, an atypical antipsychotic, is associated with a myriad of adverse metabolic effects including weight gain and insulin tolerance [31]. Clinically significant increases in body mass are observed in approximately one-third of olanzapine treated patients, a predisposing factor for the development of metabolic syndrome, comorbid diseases such as type 2 diabetes mellitus, and poor compliance with medication regimens [31,32]. Considering the established link between human obesity and the gut microflora [33], as well as preclinical studies suggestive of olanzapine-induced fecal microbiota alteration [34], Davey et al. investigated the hypothesis that the gut microbiota may be responsible for some of olanzapine’s metabolic effects. It was determined that decimation of the rat microbiota with broad-spectrum antibiotics diminished olanzapine-induced metabolic dysfunction [32]. Harnessing probiotics or prebiotics to prevent microflora shifts may thus constitute a novel approach to successively enhance olanzapine’s side effect profile, medication adherence rate and therapeutic response.
Levodopa (L-dopa), a dopamine precursor used in the treatment of Parkinson’s disease, must partition the blood-brain barrier and undergo decarboxylation within the central nervous system (CNS) in order to exert its dopaminergic therapeutic effect. Incubation of levodopa with rat cecal contents revealed a gut microbial dehydroxylation process yielding m-tyramine and m-hydroxyphenylacetic acid metabolites [35] , which could translate as decreased bioavailable levodopa. Infection with the gastric pathogen Helicobacter pylori is more prevalent amongst patients with Parkinson’s disease compared to healthy controls. Several studies have illustrated a causal relationship between H. pylori infection and dampened levodopa responses, proposing decreased absorption as the underpinning effect [36-39]. H. pylori screening and eradication regimens (antibiotics and proton-pump inhibitors) thus represent a potential strategy to optimize levodopa doses and clinical effect.
Immunosuppressant Medicines
Tacrolimus, a calcineurin inhibitor used in the prophylaxis of transplant rejection, has a narrow therapeutic index [40]. Elucidating the mechanisms influencing drug concentrations at the extremes of the therapeutic range is hence of interest from the perspectives of treatment response and patient safety. Lee et al. hypothesised that the gut microbiota dictate tacrolimus dosing requirements since post-transplant diarrhea and enterocolitis, as well as antibiotic use have previously been correlated with altered trough levels of the drug [41]. In a pilot study of 19 transplant recipients, the levels of fecal carriage of Faecalibacterium prausnitzii in the week immediately after transplantation, was greater in patients subsequently requiring a 50 percent dosage escalation in the first month of treatment [41]. Whilst the precise manner in which F. prausnitzii impacts tacrolimus pharmacokinetics was not examined, this study may serve to explain inter-patient variability in therapeutic tacrolimus dosages.
Conclusion
In addition to the metabolic capability of enterocytes and hepatocytes, it is now increasingly accepted that the gut microbiota influences drug pharmacokinetics and correspondingly bioavailability, efficacy or adverse effects. Modulating the microbiome, either through exogenous replacement (probiotics) or curtailing interventions, such as antibiotics or specific inhibitors, affords exciting opportunities to improve healthcare outcomes and advance personalised medicine. The contribution of the gut microbiome to drug pharmacokinetic determination should therefore now be reflected in the drug development process so that more efficacious and tolerable medicines ensue.
Acknowledgments
Elaine Enright is a recipient of a Government of Ireland Postgraduate Scholarship from the Irish Research Council (grant number GOIPG/2015/3261). The authors acknowledge the funding of the APC Microbiome Institute by the Science Foundation of Ireland Centres for Science, Engineering and Technology (CSET) programme (Grant Number SFI/12/RC/2273).
Abbreviations
- cgr
Cardiac Glycoside Reductase
- CNS
Central Nervous System
- CTLA-4
Cytotoxic T-Lymphocyte-Associated protein 4
- HMG-Co A
3-Hydroxy-3-Methyl-Glutaryl-CoA
- LDL-C
Low Density Lipoprotein Cholesterol
- L-dopa
Levodopa
- NSAID
Non-Steroidal Anti-Inflammatory Drug
References
- Bäckhed F, Ley RE, Sonnenburg JL. et al. Host-Bacterial Mutualism in the Human Intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome. 2015;3:31. doi: 10.1186/s40168-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpellini E, Ianiro G, Attili F. et al. The human gut microbiota and virome: Potential therapeutic implications. Digestive and Liver Disease. 2015;47(12):1007–1012. doi: 10.1016/j.dld.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt RM, Forsyth CB, Green SJ. et al. Circadian Disorganization Alters Intestinal Microbiota. PloS One. 2014;9(5):e97500. doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa T, Paterson R, Moore V. et al. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm. 2008;363(1-2):1–25. doi: 10.1016/j.ijpharm.2008.07.009. [DOI] [PubMed] [Google Scholar]
- El Aidy S, van den Bogert B, Kleerebezem M. The small intestine microbiota, nutritional modulation and relevance for health. Curr Opin Biotechnol. 2015;32:14–20. doi: 10.1016/j.copbio.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Mani S, Boelsterli UA, Redinbo MR. Understanding and Modulating Mammalian-Microbial Communication for Improved Human Health. Annu Rev Pharmacol Toxicol. 2014;54:559–580. doi: 10.1146/annurev-pharmtox-011613-140007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingell R, Bridges JW, Williams RT. The Role of the Gut Flora in the Metabolism of Prontosil and Neoprontosil in the Rat. Xenobiotica. 1971;1(2):143–156. doi: 10.3109/00498257109044386. [DOI] [PubMed] [Google Scholar]
- Prudhviraj G, Vaidya Y, Singh SK. et al. Effect of co-administration of probiotics with polysaccharide based colon targeted delivery systems to optimize site specific drug release. European Journal of Pharmaceutics and Biopharmaceutics. 2015;97(Pt A):164–172. doi: 10.1016/j.ejpb.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Peppercorn MA, Goldman P. The role of intestinal bacteria in the metabolism of salicylazosulfapyridine. J Pharmacol Exp Ther. 1972;181(3):555–562. [PubMed] [Google Scholar]
- Mikov M, Lee HJ, Fawcett JP. The influence of probiotic treatment on sulfasalazine metabolism in rat gut contents. Asian J Pharmacokinet Pharmacodynam. 2006;6:337–342. [Google Scholar]
- Yoo DH, Kim IS, Van Le TK. et al. Gut microbiota-mediated drug interactions between lovastatin and antibiotics. Drug Metab Dispos. 2014;42(9):1508–1513. doi: 10.1124/dmd.114.058354. [DOI] [PubMed] [Google Scholar]
- Matuskova Z, Anzenbacherova E, Vecera R. et al. Administration of a probiotic can change drug pharmacokinetics: effect of E. coli Nissle 1917 on amidarone absorption in rats. PloS One. 2014;9(2):e87150. doi: 10.1371/journal.pone.0087150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BD, Wang H, Lane KT. et al. Alleviating Cancer Drug Toxicity by Inhibiting a Bacterial Enzyme. Science. 2010;330(6005):831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrer DFS, Sparreboom A, Verweij J. et al. Modulation of Irinotecan-induced Diarrhea by Cotreatment with Neomycin in Cancer Patients. Clin Cancer Res. 2001;7(5):1136–1141. [PubMed] [Google Scholar]
- Saitta KS, Zhang C, Lee KK. et al. Bacterial β-glucuronidase inhibition protects mice against enteropathy induced by indomethacin, ketoprofen or diclofenac: mode of action and pharmacokinetics. Xenobiotica. 2014;44(1):28–35. doi: 10.3109/00498254.2013.811314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Bittinger K, Li X. et al. Bidirectional interactions between indomethacin and the murine intestinal microbiota. Elife. 2015;4:e08973. doi: 10.7554/eLife.08973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha JR, Butler V, Neu H. et al. Digoxin-inactivating bacteria: identification in human gut flora. Science. 1983;220(4594):325–327. doi: 10.1126/science.6836275. [DOI] [PubMed] [Google Scholar]
- Haiser HJ, Gootenberg DB, Chatman K. et al. Predicting and Manipulating Cardiac Drug Inactivation by the Human Gut Bacterium Eggerthella lenta. Science. 2013;341(6143):295–298. doi: 10.1126/science.1235872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbaum J, Rund DG, Butler VPJ. et al. Inactivation of Digoxin by the Gut Flora: Reversal by Antibiotic Therapy. N Engl J Med. 1981;305(14):789–794. doi: 10.1056/NEJM198110013051403. [DOI] [PubMed] [Google Scholar]
- Clayton TA, Baker D, Lindon JC. et al. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc Natl Acad Sci U S A. 2009;106(34):14728–14733. doi: 10.1073/pnas.0904489106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan A, Corrales L, Hubert N. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S, Saccheri F, Mignot G. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vétizou M, Pitt JM, Daillère R. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehouritis P, Cummins J, Stanton M. et al. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep. 2015;5:14554. doi: 10.1038/srep14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MH, Toth PP. Comparative effects of lipid-lowering therapies. Prog Cardiovasc Dis. 2004;47(2):73–104. doi: 10.1016/j.pcad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Postmus I, Trompet S, Deshmukh HA. et al. Pharmacogenetic meta-analysis of genome-wide association studies of LDL cholesterol response to statins. Nat Commun. 2014:5. doi: 10.1038/ncomms6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangravite LM, Thorn CF, Krauss RM. Clinical implications of pharmacogenomics of statin treatment. Pharmacogenomics J. 2006;6(6):360–374. doi: 10.1038/sj.tpj.6500384. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, Baillie RA, Zhu H. et al. Enteric microbiome metabolites correlate with response to simvastatin treatment. PloS One. 2011;6(10):e25482. doi: 10.1371/journal.pone.0025482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye HS, Rusul G, Liong MT. Removal of cholesterol by lactobacilli via incorporation and conversion to coprostanol. J Dairy Sci. 2010;93(4):1383–1392. doi: 10.3168/jds.2009-2574. [DOI] [PubMed] [Google Scholar]
- Albaugh VL, Judson JG, She P. et al. Olanzapine promotes fat accumulation in male rats by decreasing physical activity, repartitioning energy and increasing adipose tissue lipogenesis while impairing lipolysis. Mol Psychiatry. 2011;16(5):569–581. doi: 10.1038/mp.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey KJ, Cotter PD, O'Sullivan O. et al. Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry. 2013;3:e309. doi: 10.1038/tp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T. et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey KJ, O'Mahony SM, Schellekens H. et al. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology. 2012;221(1):155–169. doi: 10.1007/s00213-011-2555-2. [DOI] [PubMed] [Google Scholar]
- Goldin BR, Peppercorn MA, Goldman P. Contributions of host and intestinal microflora in the metabolism of L-dopa by the rat. J Pharmacol Exp Ther. 1973;186(1):160–166. [PubMed] [Google Scholar]
- Hashim H, Azmin S, Razlan H. et al. Eradication of Helicobacter pylori infection improves levodopa action, clinical symptoms and quality of life in patients with Parkinson's disease. PloS One. 2014;9(11):e112330. doi: 10.1371/journal.pone.0112330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierantozzi M, Pietroiusti A, Galante A. et al. Helicobacter pylori-induced reduction of acute levodopa absorption in parkinson's disease patients. Ann Neurol. 2001;50(5):686–687. doi: 10.1002/ana.1267. [DOI] [PubMed] [Google Scholar]
- Pierantozzi M, Pietroiusti A, Brusa L. et al. Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology. 2006;66(12):1824–1829. doi: 10.1212/01.wnl.0000221672.01272.ba. [DOI] [PubMed] [Google Scholar]
- Lee WY, Yoon WT, Shin HY. et al. Helicobacter pylori infection and motor fluctuations in patients with Parkinson's disease. Mov Disord. 2008;23(12):1696–1700. doi: 10.1002/mds.22190. [DOI] [PubMed] [Google Scholar]
- Venkataramanan R, Swaminathan A, Prasad T. et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29(6):404–430. doi: 10.2165/00003088-199529060-00003. [DOI] [PubMed] [Google Scholar]
- Lee JR, Muthukumar T, Dadhania D. et al. Gut Microbiota and Tacrolimus Dosing in Kidney Transplantation. PloS One. 2015;10(3):e012239. doi: 10.1371/journal.pone.0122399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozaki H, Emi Y, Horisaka E. et al. Degradation of insulin and calcitonin and their protection by various protease inhibitors in rat caecal contents: implications in peptide delivery to the colon. J Pharm Pharmacol. 1997;49(2):164–168. doi: 10.1111/j.2042-7158.1997.tb06773.x. [DOI] [PubMed] [Google Scholar]
- Lavrijsen K, van Dyck D, van Houdt J. et al. Reduction of the prodrug loperamide oxide to its active drug loperamide in the gut of rats, dogs, and humans. Drug Metab Dispos. 1995;23(3):354–362. [PubMed] [Google Scholar]
- Koch RL, Goldman P. The anaerobic metabolism of metronidazole forms N-(2-hydroxyethyl)-oxamic acid. J Pharmacol Exp Ther. 1979;208(3):406–410. [PubMed] [Google Scholar]
- Takeno S, Sakai T. Involvement of the intestinal microflora in nitrazepam-induced teratogenicity in rats and its relationship to nitroreduction. Teratology. 1991;44(2):209–214. doi: 10.1002/tera.1420440209. [DOI] [PubMed] [Google Scholar]
- Basit AW, Newton JM, Lacey LF. Susceptibility of the H2-receptor antagonists cimetidine, famotidine and nizatidine, to metabolism by the gastrointestinal microflora. Int J Pharm. 2002;237(1-2):23–33. doi: 10.1016/s0378-5173(02)00018-2. [DOI] [PubMed] [Google Scholar]
- Wadworth AN, Fitton A. Olsalazine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in inflammatory bowel disease. Drugs. 1991;41(4):647–664. doi: 10.2165/00003495-199141040-00009. [DOI] [PubMed] [Google Scholar]
- Meuldermans W, Hendrickx J, Mannens G. et al. The metabolism and excretion of risperidone after oral administration in rats and dogs. Drug Metab Dispos. 1994;22(1):129–138. [PubMed] [Google Scholar]
- Kitamura S, Sugihara K, Kuwasako M. et al. The role of mammalian intestinal bacteria in the reductive metabolism of zonisamide. J Pharm Pharmacol. 1997;49(3):253–256. doi: 10.1111/j.2042-7158.1997.tb06790.x. [DOI] [PubMed] [Google Scholar]