Abstract
Over the past decade, research has shown that diet and gut health affects symptoms expressed in stress related disorders, depression, and anxiety through changes in the gut microbiota. Psycho-behavioral function and somatic health interaction have often been ignored in health care with resulting deficits in treatment quality and outcomes. While mental health care requires the professional training in counseling, psychotherapy and psychiatry, complimentary therapeutic strategies, such as attention to a nutritional and diverse diet and supplementation of probiotic foods, may be integrated alongside psychotherapy treatment models. Development of these alternative strategies is predicated on experimental evidence and diligent research on the biology of stress, fear, anxiety-related behaviors, and the gut-brain connection. This article provides a brief overview on biological markers of anxiety and the expanding nutritional literature relating to brain health and mental disorders. A case study demonstrates an example of a biopsychosocial approach integrating cognitive psychotherapy, dietary changes, and mindfulness activities, in treating symptoms of anxiety. This case study shows a possible treatment protocol to explore the efficacy of targeting the gut-brain-axis that may be used as an impetus for future controlled studies.
Keywords: Nutritional psychiatry, gut brain axis, enteric nervous system, mental health, stress, anxiety, depression, microbiome, neurobiology, behavioral therapy, counseling
Introduction
In the Westernized world today, anxiety and depression are the most frequently diagnosed disorders [1]. Over time, the number and frequency of diagnoses have indeed grown due in part to greater awareness of manifestations of disease symptoms, but also due to the pace of modern life, inclusive of society, diet, and stress. From a human biological perspective, prevalence of somatic disorders that are characteristic of “diseases of affluence” (e.g. metabolic, immunologic, and endocrine) [2] is so great that the acquisition of disease is considered inevitable, or a natural outcome of aging. Collectively, the multi-modal and co-morbid disease epidemics depicted here share a common association with urbanization, industrialization, westernization, and resulting changes in agriculture and food processing. Considerable effort precedes us in the search for a catalyst to explain the sweep of health issues seen today, and has yielded some promising leads. This scrutiny draws attention to probable culprits such as: food production and refinement, overuse of xenotoxins (e.g. fertilizers, carbon emissions, and pesticides), vaccines, antibiotics, and various types of medications, particularly the third generation of antidepressants, commonly known as selective serotonin reuptake inhibitors, SSRIs. Furthermore, a growing consensus in psycho-behavioral research recognizes that disorders, specifically anxiety and depression, are often co-expressed with physiological symptoms, particularly digestive and bowel disorders. This hints at a shared communication or effector pathway between the emotional centers of the brain and the visceral organs of the gut [3].
Accumulating interest on the connection between gut health and psychological well-being has termed this the gut-brain-axis (GBA). The GBA is a bi-directional communicative and regulatory system involving (but not limited to) the brain and central nervous system (CNS) and the enteric environment of the gut, inclusive of human and microbial cells, metabolites, neuroactive chemicals, and energy substrates. The gut and the brain send and receive messages via the enteric nervous system (ENS) through neural pathways such as the efferent sympathetic system and the afferent vagal nerve, as well as through the bloodstream [4,5]. The ENS also innervates the GI tract, pancreas, and the gall bladder. Thus the gut and its microbiota affect immunity, endocrine function, and the nervous system, as well as regulation of behavior [6]. Therefore, stress and the accompanying arousal affect the gastro-intestinal function in top-down and bottom-up signals via the ENS [7].
The rise in the pathogenesis of anxiety and depression is concomitant with an epidemic of metabolic and auto-immune diseases, and may not be traceable to one or even a few etiologies. Instead, physiological and psychological comorbidities are likely reflective of a more insipid sweep of toxic changes permeating the lifestyles of people within urban-industrial societies [8]. If the shared axis of psycho-behavioral function and somatic health continues to be ignored, then the future ramifications could be rather daunting. Therefore, while mental health is necessarily the domain of psychotherapy and psychiatry, and requires the specialized care that is afforded by mental health professionals, complimentary therapeutic strategies should be coopted alongside standard models of care. Development of these alternative strategies is non-random. Instead, they are predicated on extensive experimental evidence and diligent research on the biology of stress, fear, anxiety-related behaviors, and the gut-brain connection.
This article provides an overview of the scientific and academic literature on biological markers of anxiety, briefly summarizing the expanding nutritional literature on brain health so as to connect the neuroendocrine routes and pathways of anxiety that can be manipulated or directly activated by the gut microbiome. The inclusion of a case study demonstrates an example that integrates therapy strategies in treating anxiety that target the gut-brain-axis. While the modes of stress, fear, and anxiety are chemical in nature, the exchange from stimulus to response moves through personal cognitive participation in emotional or visceral experiences. In fact, a modern paradigm of emotions is that they are experienced at three interrelated levels: mental, neurochemical or physiological, and behavioral [9]. Contrary to the notion of discrete purviews, physiology, neurochemistry, and psychology inscribe chain links along a continual bidirectional flow of human interaction with physical, emotional, and conceptual realities. Treatment for anxiety symptoms may selectively adjust any one link, but a more effective approach could incorporate the entire stream of psychosomatic processes through progressive and layered integration of counseling and therapeutic strategies. There exists already numerous compelling review and research articles that discuss and demonstrate the indelible link between gut and brain health, and these concepts are disseminating rapidly into frontier research in counseling, nutritional psychiatry [10] and psychoneuroimmunology. Therefore, the purpose of this paper is not to provide yet another review of topical material, but instead to focus on the neurological and visceral systems specific to symptoms of anxiety and to demonstrate how these can be modulated through lifestyle interventions to entrain anxiolytic behaviors and health. Furthermore, a proposal of a holistic therapeutic approach is largely considerate of gut and somatic health, and can be implemented safely and non-invasively alongside cognitive-behavioral therapy (CBT), mindfulness practice, and other counseling and therapeutic models. Finally, this proposed model applies to a case study patient (n = 1) receiving treatment for generalized anxiety with a report on the outcome to date.
Recognizing the Behavioral and Bodily Connections in Stress and Anxiety
The predictable consequence to traumatic and anxiety provoking incidents involves hyper-arousal in the nervous system, leading to the amygdala-mediated fight, flee, freeze, hypervigilance, or submission reactions. Freeze has been considered the more severe psychological price of earlier stressful or traumatic experiences and has been the major predictor of post-traumatic stress disorder (PTSD). This array of responses, especially freeze, compromises brain development and necessary neuro-endocrine functions [11]. Stress symptoms “can accumulate and increase from exposure to multiple events and reach a degree in which symptoms become a disorder” (p. 5) [12]. Thus, traumatized or stressed individuals may experience their free time as anxiety time and may be unable to be satisfied with the present and so need help to find calm and happiness within themselves [13]. Intense fear is staple of trauma provoking endocrine changes, compromised immune function, pervasive anxiety, and cognitive distortions leading to difficulty ignoring what is unimportant and selecting only what is most relevant [14].
Chronic hyperarousal depletes the body’s reserves and renders one vulnerable to a plethora of physical and mental health problems [14], as was demonstrated in the Adverse Childhood Experience (ACE) Study. The ACE study is an extensive investigation to assess associations between serious childhood stressors and later-life health and well-being. An ACE score assesses the total amount of stressors during childhood and as the score increases, the risk for health problems increases. Some of the ACE correlates include the following: early initiation of smoking, obesity, chronic obstructive pulmonary disease, liver disease, cancer, diabetes mellitus, ischemic heart disease, hypertension, hyperlipidemia, macular degeneration, psoriasis, depression, suicide attempts, alcohol and illicit drug abuse, and an overall compromised health-related quality of life [15,16].
So what are the underlying mechanisms that contribute the physical and psychological vulnerability of individuals who have had early trauma? Trauma fragments become subtle and generalize throughout body and behavior. These provoking triggers are recorded throughout body and brain, including the brain stem, as implicit or somatic memory. The more trauma fragments a person has the less neutral or pleasurable sensations this individual experiences in everyday activities [14,17]. The vulnerability manifests in behavior and interpersonal relationships that may persist into adulthood. This happens via the amygdala, an early warning system rather than a storage system, which is immune to the effects of stress hormones. The amygdala registers highly charged emotions with body sensations and emotion before the cerebral cortex gets the message. The body continues to alarm inappropriately and perpetuates alarm even after danger ceases [17]. Neural pathways encode emotions (via the amygdala), behaviors, perceptions, habits, and body memories and some contend that these memories are held in muscles or at the cellular level [18].
These memories are not accessible to the problem solving, analytical pre-frontal cortex. The active amygdala and unchecked adrenaline-suppressed hippocampus contribute to anxious thoughts and behaviors [12]. Triggers alert the brain stem, which floods the body with stress chemicals, e.g. cortisol and adrenalin. Thus, the body activates, but the muscles do not due to stress hormones disturbing the whole body and consequently contributing to passive coping in forms of emotional numbing or despondency [13]. Fortunately, the impact of hyper-arousal symptoms and intrusive thoughts is minimized when the memory of threat is processed and problem solving action can proceed.
Neurobiology of Anxiety and Fear
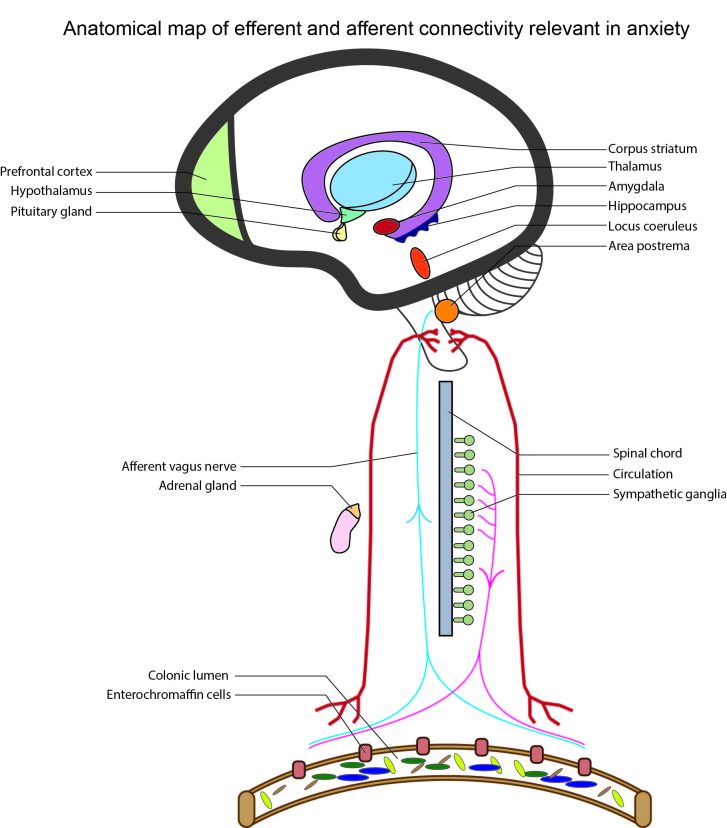
The biology of fear is well established in (namely murine) animal models, and a biological basis for core, putatively unlearned, emotions (e.g. fear, rage/anger, and love) is well supported and instructive [9]. However, among higher order mammals, particularly primates, it is often difficult to disentangle the etiological afferents given the multiple compounding effects of amygdalic and cortical mediation. Humans especially incorporate the activity of the prefrontal cortex (PFC) in managing and labeling emotional responses and integrating complex stimuli. In order to demonstrate a plausible link between the gut microbiota and neurobiological factors in anxiety, it is essential to first understand the major neuroendocrine activities that precipitate fear-based behavior, after which it is possible to evaluate the physical and chemical gateways for integration of activity arising from the enteric environment. This brief review rests heavily on a bottom-up approach to interpreting emotions, whereby visceral or sensory triggers detected by medullary and pons structures in the brain stem are relayed to the limbic system, including the thalamus, amygdala, hypothalamus, and extended subcortical regions. In effect, the working paradigm is an integrated James-Lange (bottom-up processing) and Cannon-Bard theory of emotion, with stimuli originating externally or viscerally, signal processing coordinated by the subcortical brain, and responses meted by the autonomic nervous system (ANS) [7,9]. The enteric environment, particularly of the colon, is home to trillions of microorganisms and comprises a dense meshwork of cells, nerves, molecules, and receptors. Due to its size and complexity, the ENS is considered a third arm of the ANS [5,19,20], and in recognition of this perspective, the executive centers of information collection and processing necessarily span from brain to gut (see Figure 1).
Figure 1.
Anatomical map of the efferent and afferent neurobiological connections that are relevant in fear and anxiety.
The human experience of anxiety may be an evolutionarily recycled system of elaborated fear that includes extenuating cognitive processing [21]. In this way, fear arousal, instigated by perceptible stimuli, is projected onto a more generalized, unknown, or uncertain threat [9]. For humans, the predilection towards heightened anxiety and the associated resulting behavior may be an evolutionarily adaptive trait that arose out of an enhanced need for future planning and threat prediction. Although all mammals display anxiety behavior, humans are specialists in associative labeling of emotional experience, or pattern finding cognitions [22], which results in an amplified ability to extrapolate threat scenarios from ordinary, everyday stimuli. This marries well with the notion that evolutionary complexity changes in degrees rather than in kind towards articulating the differences between human and non-human behavioral patterns. In modern urban societies where threats are more often of a distant or virtual nature, anxious behavior turns maladaptive and pathological, compounded by or resulting from everyday stress and emotional trauma [23].
Despite a large heterogeneity in human emotional reactivity [21], it is possible to interpolate the neural systems involved in the generation and expression of emotion and anxiety. According to John Watson, the father of behaviorism, emotions that trigger behavior are dominated by “visceral and glandular factors” [24]. The growing body of research in the past 15 years now largely substantiates this view. Anxiety behaviors and symptoms are predominantly activated through the ANS and the hypothalamus-pituitary-adrenal axis (HPA). The ANS has profound influence over the activity of the intestinal tract, not limited to merely activating smooth muscle contractions, but also in regulating pH, motility, mucus secretion, and mucosal immune response [19]. Both aspects of the ANS, sympathetic and parasympathetic, dispense cortical and subcortical directives, but the sympathetic system, using noradrenaline (NA) neurotransmitter, is the main promotor of characteristic anxiety symptoms such as alertness, arousal, sweating, hypertension, and tachycardia. The NA and HPA systems both function to mobilize the brain and body to confront or flee threatening situations. Hippocampal and amygdalic integration during anxiety permits conditional and emotional learning during a fearful situation, and is a core survival feature of the threat response. Dysregulation of the neuroendocrine responses to stress can result in exaggerated or aberrant behavior in the face of a perceived threat, and there appears to be a critical post-natal developmental window for inhibitory sensitization of the HPA circuit [25]. Furthermore, factors critical to learning and plasticity such as 5-hydroxytryptamine (5-HT) and brain derived neurotrophic factor (BDNF), along with regulated stimulation of the amygdala and hippocampus are necessary to train appropriate endocrine and behavioral reactivity to stressful situations [26]. Since inputs to the brain that initiate a stress response can originate from both external and visceral stimuli, and human emotion and association centers are primed for pattern learning, then anxiety producing situations where one feels a loss of control may rapidly accumulate from nonspecific or even crossed stimuli [27].
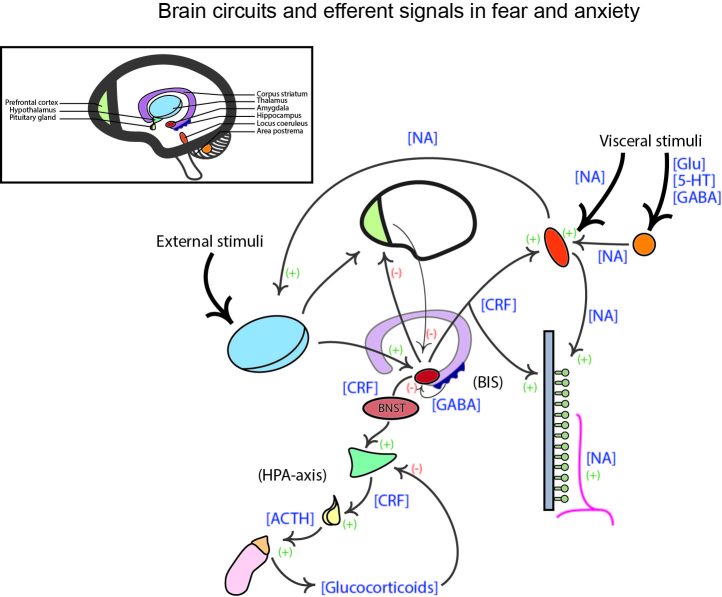
A diagram of the general brain and ANS signal routes that are activated in response to a serious or painful threat is shown in Figure 2. It is difficult to target one single central structure in the mediation of threatening stimuli, however, the key loci in this process are certainly the area postrema (AP), locus coeruleus (LC), amygdala/bed nucleus of the stria terminalis (BNST), and the hypothalamus. External and sensory information are relayed to the thalamus by the cortex, while visceral information encoded by hormones, neuropeptides, and cytokines in the circulation, and neurotransmitters in afferent nerves is detected by the AP and LC, which subsequently also innervate the thalamus. The thalamus integrates these signals and relays a response to the amygdala, which also receives contextual input from the hippocampus through the behavioral inhibition system (BIS). The BIS regulates the severity of emotional response through hippocampal inhibition on the amygdala. It compares the expected to the actual stimuli and assesses whether a proper adaptive action is possible, or whether loss of control has occurred. The amygdala, and its extension via the BNST, go on to innervate the LC and hypothalamus through corticotropin-releasing factor (CRF), and thus the cascade of stress induced response propagates throughout the ANS (via NA) and systemic adrenergic receptors. The amygdala is also responsible for inhibiting cortical processing, particularly in the PFC [28], and disrupts the ability to cognitively mitigate a threat, leading to anxiogenic feelings of uncertainty and heightened threat anticipation [27]. Through this lens, the expression of anxiety apparently hinges on amygdalic/BNST activity and innervation of extensive subcortical structures by CRF, but these are deep brain processing regions that are far downstream of the original threat signal. Their function is integral in determining the severity of the stress response, but our interest lies instead with the factors that provoke this response, in which the ENS and gut microbiome is heavily influential.
Figure 2.
Major brain circuits and efferent signals involved in transduction of fear and anxiety behavior. External and visceral stimuli hit the locus coeruleus (LC), and the area postrema (AP) in the brain stem, and the thalamus in the limbic brain. The LC is receptive to noradrenaline (NA) while the AP detects molecules in the circulation and vagal afferents. The LC and AP transmit input to the thalamus, which proceeds to innervate the amygdala. The amygdala also receives input about contextual and spatial information from the hippocampus, invoking the behavioral inhibition system (BIS). The amygdala sends inhibitory signals to the prefrontal cortex (PFC) and initiates sympathetic activity through positive innervation of the LC and peripheral NA systems via corticotropin-releasing factor (CRF). The extended amygdala, the bed nucleus of the stria terminalis (BNST), also sends excitatory signals to the hypothalamus, again via CRF, which initiates the hypothalamus-pituitary-adrenal axis (HPA) that release glucocorticoids, namely cortisol, throughout the body. Abbreviations in brackets indicate the transmitter of the signal, and positive and negative symbols indicate the excitatory or inhibitory signals respectively. Glu, glutamate; 5-HT, 5-hydroxytryptamine; GABA, γ-aminobutyric acid; ACTH, adrenocorticotropic hormone.
Connecting the Gut Microbiome to Circuits of Anxiety
The ENS is directly connected to the CNS through descending ANS nerves, ascending vagus nerve, and epithelial capillaries that carry hormones and other humoral signals [5,19,20]. Microbiota living in the colon comprise either an autochthonous (resident) or allochthonous (transient) population, both of which have important and influential functions for maintenance of the enteric ecosystem and for the host health [29,30]. Inflammation in the gut is operationally contributive to inflammation in the brain and somatic tissues. The role of microbiota in behavior was originally suggested by observations that a variety of mental illnesses, such as anxiety, depression, schizophrenia, and cognitive developmental disorders such as autism spectrum disorder (ASD) are co-expressed with gut dysfunction [31,32] and linked to autoimmune disorders and neuroinflammation via detection of serum cytokines such as IL-6 [8]. Therefore, inflammatory markers are implicit in the GBA signaling complex alongside mental illness, particularly of anxiety and depression, incorporating the activity of inflammasomes and innate host-mediated pathogen awareness in the gut-brain connection [33]. Gut microbiome remodeling through pro- or anti-biotics has also been shown to affect behavioral phenotypes in mice and in human patients [20,34].
Brain to gut signaling is transduced through chemical crosstalk engaged through a variety of way points: (1) Host enterocytes such as the enterochromaffin cells (ECC) and immunocytes such as dendritic and mast cells (DC and MC respectively) secrete hormones and cytokines directly into the lumen and in return, can receive signals from mucosal and luminal microbiota in the form of metabolites, neuropeptides, peptide hormones, and neurotransmitter-like molecules [5,19]; (2) Microbial molecules such as lipopolysaccharides and lipoproteins can leak into the host circulation through gaps in the epithelial tight junctions, termed “leaky gut”, stimulating a host immune response [19,20,35]; (3) Efferent adrenergic and NA signals from capillaries and nerve terminals within the gut wall “spill” into the lumen during stressful or traumatic events (including physical trauma) and disrupt enteric homeostasis [19]; and (4) Interkingdom signaling, particularly through adrenergic homologs that cross-activate bacterial and host adrenergic receptors [19]. Additionally, the gut is directly innervated by the ANS, with the sympathetic NA system exerting a restrictive signal on gut function and digestion. Through NA, the sympathetic system is able to halt intestinal motor function and fluid secretion, which restricts lumen motility and alters microbial activity such as gene expression and metabolite production. Therefore, anxiety and stress increase NA and glucocorticoid production in the body, which translates into a disturbed enteric environment that promotes pathogenicity through decreasing motility and shifting microbial composition in favor of opportunistic pathogens.
In turn, the gut reciprocates the brain with its own panoply of signals derived from microbial metabolites, which reach the brain through intermediary enterocytes and dendritic cells or directly through endocrine and neural signaling [5,19]. Candidate microbial metabolites that are implicated in GBA communication as well as behavioral modulation are an open area of investigation, with good evidence for the production and impact of short chain fatty acids (SCFAs), catecholamines (serotonin and dopamine), and neurotransmitters [34]. Microbial metabolites can influence enteric and systemic health in both a pro- and anti-inflammatory fashion. Microbial strains of Escheria, can disrupt gut motility by the release of peptides, which creates and imbalance in the population structure [19]. Pro-inflammatory members of Clostridiales such as Lachnospiraceae and Ruminococcaceae have also been associated with social avoidance behaviors in mice [36,37]. Pro-inflammatory and anxiogenic factors of microbial origin are often found in the bloodstream, suggestive of compromised epithelial function and intestinal permeability. Evidence for microbiome-related factors in mitigation, alleviation, or even prevention of disease are mainly limited to probiotic studies using just a few strains of Bifidobacteriaceae and Lactobacillaceae, and often in the presence of a pathogen-induced disease state [34,38,39]. Still, these results are critical to formulating a baseline of therapeutic approaches. Recent work demonstrates that butyrate and propionate SCFAs strongly affect monocyte-derived DC gene expression, as well as reduce pro-inflammatory chemokines and inhibit lipopolysaccharide-induced cytokines [40]. On the other hand, microbially-derived uremic toxins such as 4EPS and cresol are strongly associated with autism-like behaviors in mice [36], and recently, cresol was found to inhibit myelin gene expression in oligodendrocytes [37], implicating the microbiome in early developmental and long-term plasticity brain processes that directly affect stress tolerance and resilience.
Studies on probiotic supplementation in mice and in humans provides compelling evidence of microbial regulation over stress and anxiety induced neuroendocrine signaling. In particular, strains of Lactobacillus and Bifidobacterium are found to exert a profound anxiolytic influence through the production of γ-aminobutryic acid (GABA), 5-HT, and SCFAs, and by dampening HPA adrenergic reaction [26,34,38,41,42]. Moreover, the regression of anxiety after treatment in these studies was often associated with positive neurological changes such as increased BDNF, altered expression of PFC and hippocampal GABAA and GABAB receptors, increased circulating glutathione, and a reduction in inflammatory markers. Therefore, Lactobacillus and Bifidobacterium strains that are commonly found or enriched in foods that undergo lactic fermentation may be the critical factor in the potency of dietary probiotics towards supporting a reconfiguration of the enteric environment to supplant anxiogenic activity with anxiolytic regulation.
Interestingly, dysregulation of carbohydrate digestion and metabolism is also linked to clinical disorders such as depression, hyperactivity, and autism, though these associations require further empirical testing to clarify their mechanistic effect. Curiously though, there seems to exist a relationship between fructose malabsorption and circulating lipopolysaccharide (LPS), which could be abolished by administration of an antibiotic, thus implicating microbial activity [43]. Even further, fructose malabsorption is linked to depression and mood disorders [44] in which observable behaviors strongly implicate a dysregulation of the tryptophan and 5-HT production cycle that has direct impact on mood control in the brain. Even so, one can speculate upon the contextual factors that surround what, how, and when an individual manages food selection and ingestion. Consideration of environmental and social factors is often ignored in the medical treatment of physical, infectious, and affective disorders, and yet may be the focal point of disease etiology and prognosis for recovery, and shows promise for immediate application in psychotherapy.
In summary, the focus on the neurobiology of anxiety and the CNS connectivity in the gut has illuminated likely mechanisms where microbial interference can induce signals that govern emotions and behaviors through the fear and reward anticipation circuits. Importantly, medical and mental health professionals can and should use these insights to develop informed models of therapy for the treatment of anxiety symptoms in a professional setting. The NA and adrenergic systems seem to be the core activators of anxiety symptoms. In fact, the mammalian executive system of fear consists of the lateral and central nuclei of the amygdala, the ventral anterior and medial hypothalamus, and the mesencephalic periaqueductal gray (PAG) [9]. Therefore, their inhibition should be the goal of anxiolytic treatments, which is possible by dampening the HPA response to stress, improving immune function, and facilitating proper nutritional acquisition and absorption. The prescription is simple: rest, digest, exercise, practice mindful relaxation, and eat a nutritionally rich and varied diet. Through the gut microbiota, it is apparent that GABA and 5-HT are produced by specific bacterial strains [42], and that supplementation with these bacteria correspondingly attenuates presentation of anxious behaviors in animal models. Anxiolysis is also accomplished by reinstating normal gut function and community structure, suggesting that mitigating perception of anxiety is also possible by directly promoting gut health and treating dysbiosis through dietary modifications, specifically also adding a daily consumption of fermented foods. In this regard, diet, extenuating gut issues, and the contextual factors of eating behavior should be reviewed and treated before the application of psychotropic medication. This may also involve not only the treating medical and psychotherapy professional to educate oneself regarding gut issues, but also providing this information to the patient so as to increase cooperation in the dietary aspects of the treatment plan.
Nutritional Factors in Maintenance of CNS Health
Hypothetically, intuited understanding about the importance of nutrition is not new. Consider that Hippocrates said, “all health begins in the gut” or in other words, “we are what we absorb”. Since nutrients play a key role in preventing and alleviating mental disorders, then the medical and mental health systems need to radically shift away from reliance on prescribing psychotropic medications and move to providing dietary information and prioritizing dietary interventions as an important initial step in biopsychosocial treatment approaches. Thus, in recognizing the critical role of nutrition in mental and physical health, all healthcare should prioritize nutrition as an integral part of treatment, especially for those who are most vulnerable and at risk. It is prudent at this time to consider diverse combinations of nutrients from a variety of natural food sources, although some specific nutrients, such as omega-3 fatty acids or vitamin C, as treatment modalities [45,46].
A number of human studies and meta-analyses of dietary treatment approaches show connections in several aspects of mental health, mental disorders, and cognition: a relationship between diet and depression in children [47]; a connection between an unhealthy prenatal diet with later acting-out behavioral problems in pre-school children [48]; and an association between “junk food” and cognitive impairment [49]. The results of these studies must be weighed in considering the bidirectional aspects of diet and depression and other emotional problems. For example, people who are depressed or anxious are more likely to sacrifice on their diet quality due to symptoms such as lethargy, appetite issues, and cravings for comfort foods. Although it appears that diet does play a major role in mental as well as physical health for all ages, there is much yet to be studied about the complex interaction of diet, development, mood, exercise, trauma, among other variables [47]. Even so, in terms of mental health treatment, emotional and physical well-being can be managed through dietary life style interventions, unlike risk factors, some of which are uncontrollable, such as early trauma, natural disasters, poverty, and genetic vulnerabilities.
Gomez-Pinilla (2008) reviewed of the research literature on animal and human studies and identified select nutrients and their relationship to brain health and cognition (p. 26) [50]. Of the studies with human and animal subjects, Gomez-Pinilla discussed the effects of micro-nutrients on brain health, cognition, memory, and mental health. These nutrients include omega-3 fatty acids, vitamins B6, B12, C, D, and E, as well as minerals, choline, calcium, copper, and iron. Quirk, et al. (2013) conducted a meta-analysis of 25 studies from nine countries that met the criteria for evaluating various types of traditional diets and a connection to depression [47]. They found that there is “limited evidence to support an association between traditional diets (i.e. Mediterranean diet, Norwegian diet) and depression” (p. 19). In addition, they saw a “conflicting level of evidence for associations between (i) a traditional Japanese diet and depression, (ii) a healthy diet and depression, (iii) a Western diet and depression, and (iv) depression and the likelihood of eating a less healthy diet” (p. 19). Since depression is a major mental health concern across most parts of the world [1], this meta-analysis relating diet to depression is an important step in acknowledging the need for further investigation and to integrate dietary considerations in mental health treatment.
There are a variety of diets that tout their respective benefits, (e.g. for detoxification, high protein, anti-inflammatory, and high fiber) and many of these have limited research to really discern their efficacy. There is some consistent agreement though that diets should include a balance of organic fruits, vegetables, whole grains, fish rich in omega-3 fatty acids, and healthy oils like those from olives and nuts, (e.g. the Mediterranean diet) [31]. Critically diets that are diverse with regard to the types of polysaccharides (complex carbohydrates) are considered essential for feeding the microbiota, promoting long-term symbiosis with high diversity populations, and upregulating the metabolic output of both primary and secondary (i.e. crossfeeding or co-metabolizers) metabolism [51-53].
Much has been studied about diet and mental health in the past decade. In fact, at the May 2015 annual meeting of the American Psychiatric Association (APA), researchers presented studies showing the efficacy of using nutrients to treat symptoms of anxiety, stress, depression, attention deficit disorder (ADHD), aggression, mood, and even addictions. There are several challenges in demonstrating the effects of diet on mental health, particularly in controlling the confounding variables of other lifestyle and developmental factors, for example, trauma, community and social support networks, environmental effects of toxins, food supply, and culture. The fact that the APA is showing some recognition that mental health and physical health treatment reaches beyond psychotropic medication and other medications to include nutrition is a significant inroad in developing and funding rigorous studies on dietary associations and mental disorders. The National Institute of Mental Health (NIMH) has initiated a call to link mental illness to genetic and neural substrates. This initiation gives impetus to investigation and new treatment regimens with inclusion of dietary interventions and consideration of impact of gut microbiota on mental health. There has been recent support for the inclusion of mindfulness, hypnosis, stress management, and relaxation strategies to “regulate inflammatory activity” [54]. Thus, implementing dietary changes to minimizing leaky gut contributing factors to inflammation in gut brain interaction would be congruent with the NIMH initiative [55].
Case Study Report
Kevin1 is in his early thirties, educated, works in the art sector, and is married with cat. Athletic and outgoing, with only a touch of cynicism, his usual disposition meanders from curiosity to genuine satisfaction and enjoyment in life. Very rarely in the past did he feel that his emotional state was precarious or imbalanced, and never for very long. However, adult life and a beginning career has, as with many young adults, exacted a toll on his physical and mental well-being. Over time, with no particular warning or event, Kevin began to experience anxiety symptoms ranging in severity, and on occasion, a full-fledged panic attack. Eventually, he decided to seek counseling to address and help resolve the increasing concern about general anxiety he felt almost every day. After approximately four months of psychotherapy, Kevin was very motivated to explore other lifestyle changes that could help him accelerate the process of psychological and emotional growth and healing. Therefore, Kevin was recruited to participate as a case-study participant to demonstrate how one proposed model of treatment that integrated dietary lifestyle changes could be incorporated with minimal interference and invasiveness with mental health counseling. The implemented protocol and directional transcripts that were provided directly to the client are in the Supplementary Information.
This treatment protocol is a pilot trial, and represents only one of many iterations of integrated lifestyle therapies that can be developed. The basis behind the protocol designed for Kevin was to first examine the effect of his normal diet on blood glucose control, a significant and convenient health marker that is highly predictive of extenuating metabolic and gut health and personalized tolerance to different foods [56]. In addition to recording his diet and blood glucose response, he was asked to monitor his sleep, physical activity, and stress or anxiety symptom occurrences and record this information in the provided log-sheets (examples given in Supplementary Information). Importantly, within this baseline information collection week, Kevin was instructed to complete a Beck Anxiety Inventory (BAI) under the guidance of his mental health counselor. The BAI is a self-report inventory that assesses both physiological and cognitive symptoms of anxiety with norms on male and female outpatients. It is easy to score and discriminates anxiety symptom from depression [57]. Finally, in order to evaluate changes to the gut microbiome, Kevin was asked to collect one stool sample per week for the 3-week duration of the trial (1 baseline week and 2 test trial weeks). Trial weeks consisted of first a dietary intervention whereby the results of the blood glucose testing were evaluated along with his self-reported dietary record to create a new diet plan, and second an exercise to practice mindful self-exploration of emotional experiences in response to his diet. The new diet was written to mimic as much as possible Kevin’s standard diet, but with the addition of probiotic food choices, and omission of foods that caused long duration blood sugar spikes. Mindfulness exercise instructions are included in the full protocol provided in Supplementary Information.
Results
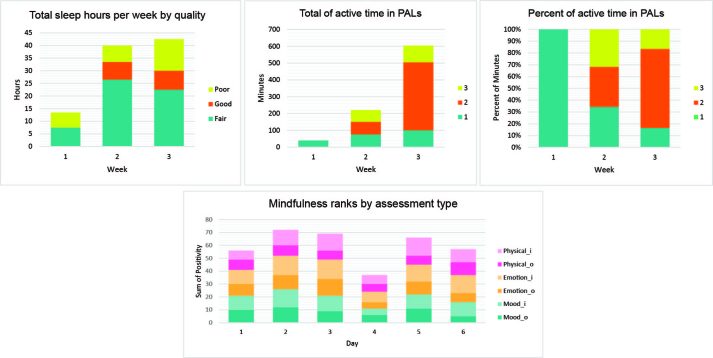
Records for sleep, physical activity, and mindfulness activities are summarized and reported in Figure 3 (note that for week 1, the sleep log contained only two days of information). Notably, total sleep hours and activity amount increased by the third week, which is important for reducing chronic stress. Mindfulness positivity ranks are reported on a scale of 1 to 5, with 5 being the most positive, 3 being neutral, and 1 being most negative. Across this limited spread of data, it is difficult to find any meaningful pattern, especially when other factors that may have contributed to emotional, physical, and mood states cannot be assessed. However, the BAI score (min = 0, max = 63) before the trial, during the baseline week was 21, which is right at the cusp of low to moderate anxiety (a sum exceeding 36 is regarded as high, and a significant cause for concern). After the two trial weeks, the BAI score plummeted from 21 to 5, a remarkable improvement.
Figure 3.
Bar charts summarize log records from the three-week case study (week 1 baseline; weeks 2 and 3 trial). PAL, physical activity level; 1 is light easy (light walking); 2 is moderate (load bearing or somewhat dynamic); 3 is vigorous (sprinting, heavy weight lifting).
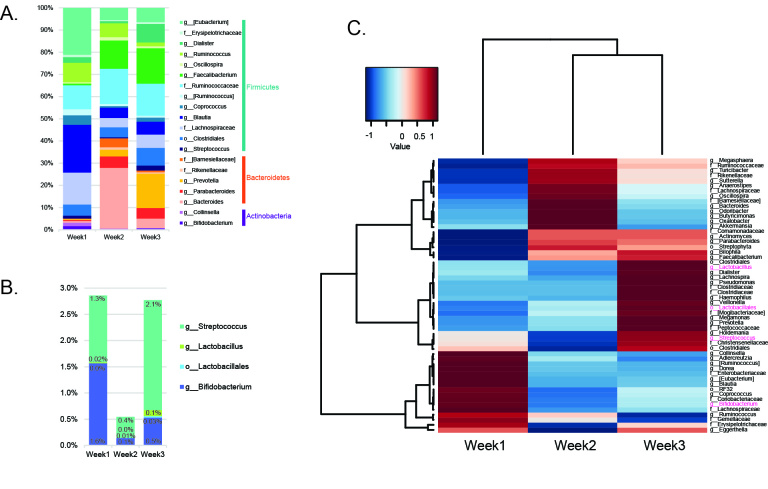
Bacterial composition of stool samples based on 16S rRNA gene sequencing is shown in Figure 4. Of note, a major turnover in abundant taxa occurred across the intervention weeks (weeks 2 and 3) compared to the baseline week (week 1), corresponding primarily to a relative increase in members of Lactobacillales and Bacteroidetes (Prevotella), and a reduction in Clostridiales members (Lachnospiraceae and Blautia) as well as a curious reduction in the Actinobacteria phylum, mainly from the loss of Collinsella. Bacterial diversity improved over the intervention weeks, both in phylogenetic diversity and number of operational taxonomic units (OTUs) (Supplementary Figure 1). These results suggest that the taxonomic arrangement of gut microbiota is reflective of probiotic supplementation, demonstrating a rearrangement and increase in taxa associated with beneficial effects in mitigating anxiety behaviors. Such rapid turnover offers encouraging prospects for long-term dietary interventions in reshaping the gut microbiome towards a more balanced and diverse community.
Figure 4.
Bacterial taxonomies show turnover across three week intervention. (a) Bar chart shows relative abundance profile of taxa with at least 1% abundance in one sample at the OTU level; (b) Bar chart shows Lactobacillaceae and Bifidobacteriaceae family abundance shifts; (c) Heatplot shows correlation of taxonomic abundance for each sample week. Taxa colored in magenta are implicated in mediating anxiety behaviors.
Summary Considerations for this Case Study
Kevin was very motivated in incorporating therapeutic interventions as well as the dietary changes in ameliorating his symptoms of anxiety, and found much relief and renewed vigor both physically and emotionally. Exploring with him what actions he found to be most helpful and ones that he would most likely integrate as part of his daily lifestyle habits is a necessary step in setting long-term health goals. The remaining and follow up counseling sessions could include Solution Focused Therapy (SFT) questions such as these: What are obstacles that would impede the progress that has been made? What are enhancements that contribute to his continued practice of the lifestyle, specifically dietary, change? On a scale from 1 to 10, with 10 being most confident, how confident are you that you will continue the progress and integration of the lifestyle changes and mental health practices? What would alert you that you might be slipping into former habits that contributed to your prior symptoms of anxiety? On a scale from 1 to 10, how likely do you think you would be to intervene to shift to the strategies that you learned and used during your counseling and dietary experience? [58]. Given this type of exploration, Kevin may provide himself with some insight about how he may sabotage the gains he has made and explore ways to mitigate those behaviors and cognitions that would contribute to a return to old habits. Additionally, this discussion with his counselor would allow him the opportunity to identify his success and strategies to maintain and enhance his progress.
Limitations
Psychiatry and psychodynamic psychotherapy has as its early foundation the use of case studies to support therapeutic interventions, e.g. the writings of Sigmund Freud, Carl Jung, and Alfred Adler. After more than a century, case study is now considered an initial starting point in exploring potential treatments. Thus, the inclusion of this case study is the impetus to explore a biopsychosocial treatment approach in this overview of the burgeoning research about the gut brain relationship to mental health. There are several limitations in attempting to extract conclusions and these are certainly warranted in deriving support for this integrated approach. Motivation is mentioned, although there is no objective assessment of the extent of the participant’s motivation. It seems that motivation to make significant lifestyle changes, especially in long standing dietary habits, certainly needs to be accounted for in any future research. There are also idiosyncratic characteristics of future sample participants that would need to be controlled, such as age, sex, developmental factors, culture, family of origin, dietary practices, current health status, among many other variables. A benefit to mental health and medical professionals of the information derived from this case is the reported improvement this participant experienced as a result of this integrated approach. Therefore, these protocols can serve as a template for future integrative models, and yet are highly adaptable to nuances presented with any individual patient.
Future Research
Presently, the connections and mechanisms between gut function and mental health are on a fast track to major paradigm shifts in the conceptualization of not only ‘self and other’, but also of environmental (or external) versus genetic (internal or deterministic) and behavioral (emotional and cognitive) governance over brain function. The concept of psychosomatic connections are not new, having been formally integrated into a theory of behavior well over 100 years ago [24]. Now, with the ability to interrogate micro-ecologies throughout the human body in astonishing detail, many open questions can be addressed. Importantly, personalized healthcare is far more feasible due to advances in machine learning and computation, allowing for accurate predictions about response variables given multidimensional parameters and highly diffuse metadata [56]. Also, there is a growing awareness of a need for individually tailored therapies. Therefore, in light of the presentation of material in this paper, future work can have two aims: (1) from the clinician side, an assessment of the ease of compliance of dietary, social, and environmental changes for clients across social and economic strata can help inform and implement a practical means of counseling through lifestyle transitions; and (2) at the research bench, investigators should focus on finding out if and to what degree aspects of modernization reshape the structure and functions of the gut microbiome longitudinally, which might help explain the observed rise in developmental and mental disabilities.
Conclusions
There are an increasing number of studies demonstrating this associative and mechanistic relationship between gut microbiota and mood and behavior. However, the translation of these significant findings to modes of therapeutic care remains limited in practice. Similar to nutritional therapy, beginning interventions to modulate gut-microbial communities in an attempt to improve gut health are inexpensive and non-invasive, and so there is little reason not to pursue such initial avenues of patient therapy in conjunction with standard care. Our demonstration of one style of layered dietary and cognitive interventions using a single-person case study example shows extremely promising results. Not only did the client report dramatic improvement in the BAI score, but also overall improvement in mood and a positive life outlook on the (uncertain) future. Importantly, compliance and satisfaction with the protocol suggests that it could have broad receptivity among other people who are similarly motivated to seek professional care.
Multiple vectors of psychological well-being and pathos exist. Early trauma may instate long-term anxiety, which modulates neuroendocrine signaling to and from the gut microbiota, effectively entrenching an anxiety phenotype. Models of physical and mental health treatment can use psychotherapy approaches such as CBT, Rational Emotive Behavior Therapy (REBT), Emotionally Focused Therapy (EFT), and Dialectical Behavioral Therapy (DBT), and mindfulness strategies to mitigate anxiety symptoms. These treatments prompt patients to identify and learn to manage the factors that contribute to anxiety and also teach patients to use relaxation and deep breathing to counteract the physical symptoms of anxiety. Indeed, these therapeutic strategies are designed to calm amygdalic activity and strengthen the cortical (cognitive) input and rationalization of emotion. However, it is no longer sufficient to use psychotherapeutic approaches alone, particularly where emotional trauma may play a role in the etiology of the presenting problem. With much evidence supporting the relation of systemic wellness in mental health, and in particular the status of the enteric ecosystem of the colon, health care must incorporate bottom-up as well as top down approaches towards treating anxiety disorders among other mental and physical health concerns. The search for the neurobiological substrates of fear and anxiety has had resounding success over decades of research, leaving no doubt that cognitive and emotional processes are merely one branch of an integrated systemic circuit, inclusive of the gut microbiota. Continued research and clinical application are essential to refine treatment strategies that will optimize enhancements in physical and mental health as well as engender long-term compliance for dietary and mental wellness related lifestyle changes.
Acknowledgments
We thank C. Hofman for lab support, K. Sankaranarayanan for informative discussions on data processing, and D. Geller for reagents and supplies.
Abbreviations
- SSRI
selective serotonin reuptake inhibitors
- GBA
gut-brain-axis
- CNS
central nervous system
- ENS
enteric nervous system
- CBT
cognitive-behavioral therapy
- PTSD
post-traumatic stress disorder
- ACE
Adverse Childhood Experience
- PFC
prefrontal cortex
- ANS
autonomic nervous system
- HPA
hypothalamus-pituitary-adrenal axis
- NA
noradrenaline
- 5-HT
5-hydroxytryptamine
- BDNF
brain derived neurotrophic factor
- AP
area postrema
- LC
locus coeruleus
- BNST
bed nucleus of the stria terminalis
- BIS
behavioral inhibition system
- CRF
corticotropin-releasing factor
- ASD
autism spectrum disorder
- ECC
enterochromaffin cells
- DC
dendritic cells
- MC
mast cells
- SCFA
short chain fatty acids
- GABA
γ-aminobutryic acid
- LPS
lipopolysaccharide
- PAG
mesencephalic periaqueductal gray
- APA
American Psychiatric Association
- ADD
attention deficit disorder
- NIMH
National Institute of Mental Health
- BAI
Beck Anxiety Inventory
- OTU
operational taxonomic units
- SFT
Solution Focused Therapy
- REBT
Rational Emotive Behavior Therapy
- EFT
Emotionally Focused Therapy
- DBT
Dialectical Behavioral Therapy
Supplementary Information. Integrative therapies in anxiety treatment with special emphasis on the gut microbiome
Author Contributions
SLS proposed the topic and contributions, SLS designed the case study report, SLS and HAB developed the case study protocols, SLS communicated and collected case study data, SLS prepared samples for sequencing, SLS conducted data analysis and reporting, SLS and HAB researched and wrote the manuscript. Sequence data are available upon request.
Footnotes
1Name has been changed to protect identity.
References
- Centers for Disease Control and Prevention. Burden of Mental Illness. Burden of Mental Illness. Mental health. 2013 [Google Scholar]
- Eaton SB. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med. 1985;312(5):283–289. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- Cryan JP, O’Mahoney S. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: Pathophysiology, clinical consequences, diagnostic approach, and treatment options. J Physiol Pharmacol. 2011;62(6):591–599. [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil. 2012;24(5):405–413. doi: 10.1111/j.1365-2982.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- Mayer E. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12(8):453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet MC, McQuaid RJ, Merali Z. et al. Cytokine variations and mood disorders: influence of social stressors and social support. Front Neurosci. 2014;8:416. doi: 10.3389/fnins.2014.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer T. The biology of fear- and anxiety-related behaviors. Dialogues Clin Neurosci. 2002;4(3):231–249. doi: 10.31887/DCNS.2002.4.3/tsteimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarris J, Logan AC, Akbaraly TN. et al. Nutritional medicine as mainstream in psychiatry. The Lancet Psychiatry. 2015;2(3):271–274. doi: 10.1016/S2215-0366(14)00051-0. [DOI] [PubMed] [Google Scholar]
- van der Hart O, Nijenhuis E, Steele K. The haunted self: Structural dissociation & the treatment of chronic traumatization. New York: Norton; 2006. [Google Scholar]
- Rothschild B. Applying the brakes: In trauma treatment, creating safety is essential. Psychother Networker. 2004;28(1):42–66. [Google Scholar]
- Cook A, Blaustein M, Spinazzola J. et al. Complex Trauma in Children & Adolescents. National Child Traumatic Stress Network. 2003 [Google Scholar]
- van der Kolk B. Frontiers of trauma treatment: The roles of attention, memory, arousal modulation and the therapeutic relationship. The essential connection: Mindfulness, brain & body in psychotherapy. 2008 [Google Scholar]
- Prevention C for DC&. Adverse childhood experience study: Prevalence of individual adverse childhood experiences. . 2005 [Google Scholar]
- Felitti V, Anda R, Nordenberg D. et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- van der Kolk B. The body keeps the score: Brain, mind, and body in the healing of trauma. New York; Viking Penguin: 2014. [Google Scholar]
- Pert C. Molecules of Emotion. 1st ed. New York: Simon & Schuster; 1999. [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer EA. et al. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(May):306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Mazmanian SK. Control of Brain Development, Function, and Behavior by the Microbiome. Cell Host Microbe. 2015;17(5):565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Well-being and affective style: neural substrates and biobehavioural correlates. Philos Trans R Soc. 2004;359:1395–1411. doi: 10.1098/rstb.2004.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. Superior pattern processing is the essence of the evolved human brain. Front Neurosci. 2014;8:1–17. doi: 10.3389/fnins.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105(2):325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Watson JB. Behaviorism. Revised ed. New York: University of Chicago Press; 1930. [Google Scholar]
- Sudo N, Chida Y, Aiba Y. et al. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol. 2004;558(1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N. Stress and gut microbiota: Does postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response? Psychosom Med. 2006;1287:350–354. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilgen J, Tejeda H, O’Donnell P. Amygdala inputs drive feedforward inhibition in the medial prefrontal cortex. Amygdala inputs drive feedforward inhibition in the medial prefrontal cortex. 2013;110(1):221–229. doi: 10.1152/jn.00531.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela M, Biagi E, Maccaferri S. et al. Intestinal microbiota is a plastic factor responding to environmental changes. Trends Microbiol. 2012;20(8):385–391. doi: 10.1016/j.tim.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Nava GM, Stappenbeck TS. Diversity of the autochthonous colonic microbiota. Gut Microbes. 2011;2(2):99–104. doi: 10.4161/gmic.2.2.15416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Addolorator G, Capristo E, Stefanini GF. et al. Inflammatory Bowel Disease: A Study of the Association between Anxiety and Depression, Physical Morbidity, and Nutritional Status. Gastroenterology. 1997;32(10):1013–1021. doi: 10.3109/00365529709011218. [DOI] [PubMed] [Google Scholar]
- Guo H, Callaway JB, Ting J-Y. et al. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson A. et al. Interactions Between the Microbiota and the Immune System. Science. 2012;33(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S. et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacias M, Gaspari S, Santos PG. et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. Elife. 2016:e13442. doi: 10.7554/eLife.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N. et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld K-A. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Nastasi C, Candela M, Bonefeld CM. et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep. 2015;5:1–10. doi: 10.1038/srep16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M. et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32(3):315–320. doi: 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Barrett E, Ross RP, Toole PWO. et al. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- Bergheim I, Weber S, Vos M. et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. Hepatology. 2008;48:983–992. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Bested AC, Logan AC, Selhub EM. Intestinal microbiota , probiotics and mental health: from Metchnikoff to modern advances: Part II – contemporary contextual research. Gut Pathog. 2013;5(3):1–14. doi: 10.1186/1757-4749-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MP, Hibbeln JR, Wisner KL. et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67(12):1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- Carr AC, Bozonet SM, Pullar JM. et al. Mood improvement in young adult males following supplementation with gold kiwifruit, a high-vitamin C food. J Nutr Sci. 2012;2:e24. doi: 10.1017/jns.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk S, Williams L, O’Neil A. et al. The association between diet quality, dietary patterns and depression in adults: A systematic review. BMC Psychiatry. 2013;13:175. doi: 10.1186/1471-244X-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka FN, Ystrom E, Brantsaeter AL. et al. Maternal and early postnatal nutrition and mental health of offspring by age 5 years: A prospective cohort study. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1038–1047. doi: 10.1016/j.jaac.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Jacka F, Kremer P, Berk M. et al. A prospective study of diet quality and mental health in adolescents. PLoS One. 2011;6(9):e24805. doi: 10.1371/journal.pone.0024805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9(7):568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg ED, Sonnenburg JL. Perspective Starving our Microbial Self : The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates. Cell Metab. 2014;20(5):779–786. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg ED, Smits SA, Tikhonov M. et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg JL, Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nat Rev. 2016:535. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, LK J, Tyson L. et al. The inflammatory hypothesis of depression: Implications of diagnosis and practice. J Ment Heal Couns. 2016;38(2):124–138. [Google Scholar]
- Korn L. Nutrition essentials for mental health: A complete guide to the food-mood connection. New York: W. W. Norton & Co; 2016. [Google Scholar]
- Zeevi D, Korem T, Zmora N. et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell. 2015;163:1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G. et al. An inventory for measuring clinical anxiety: Psychometric properties. J Counsulting Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Guterman J. Mastering the art of solution-focused counseling. 2nd ed. Alexandria, VA: American Counseling Association; 2013. [Google Scholar]