Abstract
Background
We conducted a systematic review of mathematical models of transmission dynamic of Clostridium difficile infection (CDI) in healthcare settings, to provide an overview of existing models and their assessment of different CDI control strategies.
Methods
We searched MEDLINE, EMBASE and Web of Science up to February 3, 2016 for transmission-dynamic models of Clostridium difficile in healthcare settings. The models were compared based on their natural history representation of Clostridium difficile, which could include health states (S-E-A-I-R-D: Susceptible-Exposed-Asymptomatic-Infectious-Resistant-Deceased) and the possibility to include healthcare workers and visitors (vectors of transmission). Effectiveness of interventions was compared using the relative reduction (compared to no intervention or current practice) in outcomes such as incidence of colonization, CDI, CDI recurrence, CDI mortality, and length of stay.
Results
Nine studies describing six different models met the inclusion criteria. Over time, the models have generally increased in complexity in terms of natural history and transmission dynamics and number/complexity of interventions/bundles of interventions examined. The models were categorized into four groups with respect to their natural history representation: S-A-I-R, S-E-A-I, S-A-I, and S-E-A-I-R-D. Seven studies examined the impact of CDI control strategies. Interventions aimed at controlling the transmission, lowering CDI vulnerability and reducing the risk of recurrence/mortality were predicted to reduce CDI incidence by 3–49%, 5–43% and 5–29%, respectively. Bundles of interventions were predicted to reduce CDI incidence by 14–84%.
Conclusions
Although CDI is a major public health problem, there are very few published transmission-dynamic models of Clostridium difficile. Published models vary substantially in the interventions examined, the outcome measures used and the representation of the natural history of Clostridium difficile, which make it difficult to synthesize results and provide a clear picture of optimal intervention strategies. Future modeling efforts should pay specific attention to calibration, structural uncertainties, and transparent reporting practices.
Introduction
Clostridium difficile (C. difficile) is a major public health problem that directly affects patient safety and disrupts hospital operations, causing significant health and economic consequences to the healthcare system [1–3]. C. difficile is an endospore-forming bacterium, which is spread mainly through the fecal-oral route. C. difficile can colonize the small intestine and the colon following the disturbance of the gut flora, typically caused by the exposure to antimicrobials. Proliferation of C. difficile can cause a broad range of clinical manifestations, varying from asymptomatic carriage, to diarrhea (C. difficile infection (CDI)), to pseudomembranous colitis and, in some cases, death. The greatest incidence of CDI is among individuals exposed to antimicrobial therapy, proton-pumps inhibitors and the elderly with a past medical history of hospitalization [4]. In hospital settings, three main pathways of C. difficile transmission have been documented: 1) contacts with infectious patients (asymptomatically colonized and symptomatic) [5, 6]; 2) exposure following contacts with healthcare workers (HCW) [7]; and 3) exposure caused by the environmental contamination [8, 9].
Over the past two decades, the incidence and severity of CDI and its related health care costs have increased dramatically in high income countries, mainly because of the emergence of a more virulent strain (BI/NAP1/027) [10]. Fortunately, new technologies and control strategies (e.g., rapid diagnostic tests [11], vaccines [12], introduction of antimicrobial stewardship programs [13–15] and enhanced infection control practices [16]) offer tremendous promise for the reduction of hospital-associated CDI. However, randomized clinical trials examining the optimal use and the combinations of these strategies are lacking because of: 1) their prohibitive costs; 2) challenges in comparing and isolating the benefit of multiple interventions within one study; 3) ethical issues (e.g., randomizing patients into experimental groups during outbreaks); and 4) limited generalizability because of differences in patient populations, hospital characteristics, and antimicrobial use can have an important impact on study findings. Hence, evidence on the efficacy of individual interventions or groups of interventions (bundles) are based mostly on observational studies [17] and remain very limited.
Mathematical models of infectious disease transmission dynamics have proven extremely valuable to address questions that are unfeasible or unethical in a clinical trial setting [18–20]. For healthcare-associated CDI, modeling can provide a formal framework to test and compare the effectiveness and cost-effectiveness of a large number of prevention and control strategies considering different hospital/patient characteristics and to address many possible assumptions about the natural history of C. difficile. However, predicting the impact of interventions against communicable diseases is particularly challenging since prevention, treatment or contact precautions in an individual can indirectly protect others by reducing transmission. The non-linear dynamics produced by this indirect protection (e.g., herd immunity) has played an important role in the success of vaccines against infectious diseases (e.g., eradication of smallpox [21]) and screening for sexually transmitted diseases (e.g., reduction of HIV by screening sex workers) [22]. It is particularly important to use transmission dynamic models (which inherently capture indirect effects), when examining the effectiveness and the cost-effectiveness of C. difficile prevention and control strategies, such as screening and vaccination, as an anticipated major benefit of such strategies is the reduction of C. difficile transmission within hospitals.
We conducted a systematic review of mathematical models of transmission dynamics of healthcare-associated C. difficile infection and colonization in order to provide an overview of current models and their predictions of the effectiveness of CDI control strategies. Specific aims were to describe and compare: 1) the models’ structure and assumptions about C. difficile natural history and transmission dynamics; and 2) C. difficile prevention and control strategies investigated by the models and their predictions of effectiveness.
Methods
Search strategy and selection criteria
We performed a systematic review of the literature, which we report according to PRISMA guidelines [23] (see S1 Table). The studies were eligible for inclusion in the systematic review if they included a mathematical model of transmission dynamics for hospital-acquired C. difficile infection or colonization. We searched MEDLINE (PubMed), EMBASE and Web of Science databases, with no restriction on the language of the articles, document type or year of publication. The last search was performed on February 3, 2016. We developed the search strategy by ensuring equivalent design and terminology in each database (with the use of Medical Subject Heading (MeSH) or Emtree terms used in PubMed and EMBASE respectively). The search strategy was divided into four groups of keywords concerning: #1) Clostridium difficile; AND #2) mathematical modeling; AND #3) hospitals and healthcare-associated infection; NOT #4) animals (see S1 Appendix for a detailed list of keywords). GG and MHG independently 1) identified eligible studies through review of titles and abstracts; 2) retrieved the full-text studies; and 3) assessed the eligibility of studies. To identify additional studies, we also reviewed the references of the selected articles.
Data extraction for description of models
We extracted the data for the description of the models using an extraction form containing the following components: 1) model objectives and specifications; 2) natural history assumptions; 3) transmission pathways; 4) parameterization methods, calibration and uncertainty/sensitivity analysis; 5) C. difficile prevention and control strategies examined; and 6) model output/outcomes. Data extraction was performed independently by two reviewers (GG and MHG) using the predefined form. We contacted the authors of the studies to request further specific clarifications when information was lacking or unclear.
Assessment of the quality of reporting
To our knowledge, no checklist or formal assessment tools are available to evaluate the quality of reporting for transmission-dynamic models. However, guidelines have been published concerning best modeling research practices. To assess the quality of the reporting of the models included in this systematic review, we developed a questionnaire-based grid, considering the recommendations from various modeling practice guidelines [24–41]. The questionnaire consisted of the following categories: 1) research question; 2) natural history representation and the transmission dynamics; 3) parameter estimates and data sources; 4) modeling approaches and mathematical methods; 5) outcomes of models; 6) uncertainty and sensitivity analyses; 7) validation and quality of documentation (see S2 Appendix for details). For all studies, every question was assessed in order to establish if the items defined in the question were fully/partially/not addressed by the study or if the items were not applicable. The quality of the reporting was assessed independently by two reviewers (GG and MHG).
Representation of natural history and transmission dynamics
Natural history refers to the progression of the disease between health states in an individual over time. It can be represented schematically by a set of health state compartments linked by arrows indicating the possible transitions (progression/regression) between the health states. To compare the natural history representation of the models, we developed an epidemic model template that divides the natural history representations of C. difficile into six possible health states (S-E-A-I-R-D: Susceptible-Exposed-Asymptomatic-Infectious-Resistant-Deceased) (see S3 Appendix for details). For each model, the template allowed us to: 1) stratify every health state, according to modeled population characteristics; 2) clearly identify every health state transition defined in the natural history representation of the models; and 3) identify each transmission pathway.
Effectiveness of C. difficile control strategies
For each primary health outcome defined, we extracted the effect of interventions and we reported its effectiveness. The outcomes of interest were the relative reduction in: 1) C. difficile colonization (incidence or ratio of colonized patients discharged to the colonized patients admitted); 2) CDI incidence; 3) incidence of recurrence of CDI; 4) CDI mortality; and 5) length of stay. We extracted the relative reduction in each outcome either directly from the studys’ text and tables, by using a graphical approach or by retrieving the simulation data from an author’s website [42]. In a second step, we categorized each of these interventions into one of the three groups according to their principal aim: 1) reducing the transmission; 2) reducing vulnerability to CDI; 3) reducing the risk of CDI recurrence or CDI mortality. Also, we reported the effectiveness of modeled bundles of interventions which can comprise any combination of the previous items.
Results
Study Selection
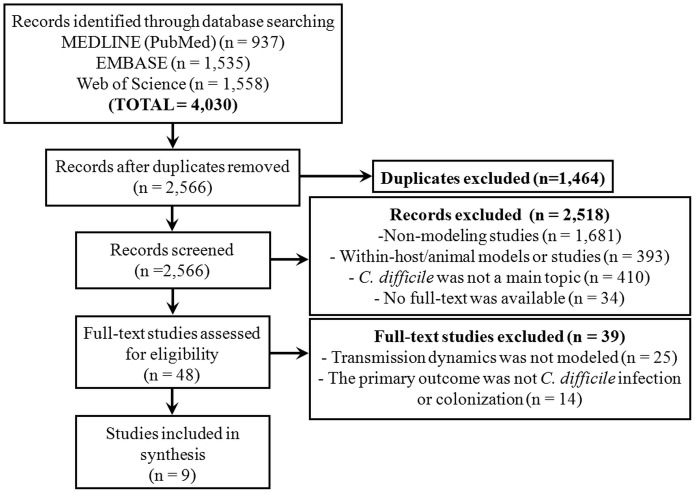
We identified 2,566 records (articles and abstracts) after duplicates were removed. After screening of titles and abstract, 47 studies were selected for a full-text review (Fig 1). Nine studies met the inclusion criteria and were included in the systematic review. Within these nine studies, we identified six independent models (three studies were based on adaptations of previously published models). The studies were published between 2001 and 2015 and originate from three countries (the US, the UK, and Australia) (Table 1).
Fig 1. Flowchart of study selection.
Table 1. Characteristics of studies included in the systematic review.
| First author (year) | Starr, JM (2001) [43] | Starr, JM (2009) [44] | Lanzas, C (2011) [45] | Yakob, L (2013) [46] | Rubin, MA (2013) [47] | Lofgren, ET (2014) [48] | Yakob, L (2014) [49] | Lanzas, C (2014) [50] | Codella, J (2015) [51] |
|---|---|---|---|---|---|---|---|---|---|
| GENERAL INFORMATION | |||||||||
| Country | United Kingdom | United Kingdom | United States of America | Australia | United States of America | United States of America | Australia | United States of America | United States of America |
| Model adapted from another model included in this review | No | Yes. Adapted from Starr et al. (2001) | No | No | No | No | Yes. Adapted from Yakob et al. (2013) | Yes. Adapted from Lanzas et al. (2011) | No |
| Objectives | Increase knowledge of natural history | Evaluate the impact of interventions | Increase knowledge of natural history | Evaluate the impact of interventions | Evaluate the impact of interventions | Evaluate the impact of interventions | Evaluate the impact of interventions | Evaluate the impact of interventions | Evaluate the impact of interventions |
| Aggregate or individual-based | Not specified | Not specified | Aggregate and individual-based | Aggregate | Agent-based | Aggregate | Aggregate | Agent-based | Agent-based |
| HOSPITAL SETTINGS | |||||||||
| Settings | 1 hospital ward for the elderly | 2 hospital wards for the elderly | 6 wards at a tertiary hospital | 1 large hospital | 11 hospital wards | 1 intensive care unit (ICU) | 1 large hospital | 6 wards at a tertiary hospital | 1 mid-sized hospital |
| Rooms/beds layout | 4 rooms with 6 beds | Each ward has 4 separate rooms with 6 beds and 6 rooms with 1 bed | 2 wards with 26 beds, 1 wards with 29 beds, and 3 wards with 30 beds | Not specified | 9 acute care units (ACU) with 30 rooms, and 2 intensive care units (ICU) with 15 rooms (all private rooms) | 12 rooms (1 bed per room) | Not specified | 2 wards with 26 beds, 1 wards with 29 beds, and 3 wards with 30 beds | 10 wards with 10 rooms (2 beds per room) |
| Number of beds | 24 beds | 60 beds | 171 beds | 1000 beds | 300 beds | 12 beds | 1000 beds | 171 beds | 200 beds |
| NATURAL HISTORY AND TRANSMISSION | |||||||||
| Health statesa | S-A-I-R | S-A-I-R | S-A-I-R | S-E-A-I | S-A-I | S-A-I | S-E-A-I | S-A-I-R | S-E-A-I-R-D |
| Hospital wards, rooms and beds layout taken into account in transmission (spatial heterogeneity) | Not specified | Yes. Transmission occurs between 1) patients in the same room, and 2) patients in different rooms | No. Transmission occurs homogeneously between patients at ward level | No. Transmission occurs homogeneously between patients at hospital level | Yes. Transmission occurs through HCWs within wards (nurses, doctors), across wards (doctors) and according to the patient location | No. Transmission occurs homogeneously between patients at ward level | No. Transmission occurs homogeneously between patients at hospital level | No. Transmission occurs homogeneously between patients at ward level | Yes. Transmission occurs between patients and HCW through movements within and between wards (for HCWs) |
| Transmission pathways | Patient-patient, environment-patient | Patient-patient, environment-patient | Patient-patient | Patient-patient | Environment-patient, HCW-patient | HCW-patient | Patient-patient | Patient-patient | Patient-patient, environment-patient, HCW/visitor-patient |
| C. difficile strain included in model | Single epidemic strain | Single epidemic strain | Not specified | Not specified | Toxigenic and non-toxigenic strains | Not specified | Not specified | Epidemic (ribotype 027) and other strains | Single epidemic strain |
| MODEL UNCERTAINTY AND CALIBRATION | |||||||||
| Sensitivity analyses carried out | Not specified | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Model calibration | Not specified | Yes | Not specified | Not specified | Not specified | Not specified | Not specified | Not specified | Yes |
| HEALTH OUTCOMES | |||||||||
| CD colonization | — | — | Secondary cases generated by colonized or disease patients (R0) | Ratio of colonized discharged to colonized admitted | Cases per 10,000 patient-days | — | Ratio of colonized discharged to colonized admitted | Cases per 1,000 admissions | Cases per year and %c |
| CDI | Prevalence in the ward (number of cases) | Cases per year | Cases per 1,000 admissions | Cases per 1,000 hospital bed-days | Cases per 10,000 patient-days (actual and reportedb) | Cases per year | Cases per 1,000 hospital bed-days | Cases per 1,000 admissions (CO and HO) | Cases per year and %c |
| CDI recurrence incidence | — | — | — | — | — | Cases per year | — | — | Cases per year and %c |
| CDI mortality | — | — | — | — | — | — | — | — | Cases per year and %c |
| Length of stay | — | — | — | — | — | — | — | — | Days |
| Measure of effect of interventions | Not applicable | Absolute reduction compared to the standard care scenario | Not applicable | Absolute reduction compared to the standard care scenario | Absolute reduction and percentage reduction compared to the standard care scenario | Absolute reduction compared to no intervention | Absolute reduction compared to the standard care scenario | Absolute reduction compared to no intervention, number needed to treat (NNT) | Absolute reduction compared to no intervention |
| BASELINE EPIDEMIOLOGY | |||||||||
| CD colonization | — | — | R0 from 0.52 to 1.99 (mean of 1.07 and median of 1.04) | 7.5 (ratio of colonized patients discharged to colonized patients admitted) | 152.6 cases per 10,000 patient-days | — | 7.5 (ratio of colonized patients discharged to colonized patients admitted) | 100 cases per 1,000 admissions | 961 cases per year |
| CDI | Not specified | 21.19 cases per year | 17.85 cases per 1,000 admissions | 2.8 cases per 1,000 hospital bed-days | 14.3 cases per 10,000 patient-days (8.3 cases per 10,000 patient-days)b | Median(IQR): 0(0-1) cases per year, (0.81 cases per year) | 2.8 cases per 1,000 hospital bed-days | 14.5 cases (HO) per 1,000 admissions | 601 cases per year |
| CDI recurrence incidence | — | — | — | — | — | Median(IQR): 2(0-6) cases per year, (4.35 cases per year) | — | — | 142 cases per year |
| CDI mortality | — | — | — | — | — | — | — | — | 122 cases per year |
| Length of stay | — | — | — | — | — | — | — | — | 5.41 days |
CD: Clostridium difficile; CDI: Clostridium difficile infection; CO: community-onset; HCW: healthcare worker; HO: hospital-onset; IQR: interquartile range.
a Health states are categorized into four groups of models according to our epidemic model template that divides the natural history representations of C. difficile into six possible health states (S-E-A-I-R-D: Susceptible-Exposed-Asymptomatic-Infectious-Resistant-Deceased) (see S3 Appendix).
b Reported CDI incidence is based on symptom recognition and positive results of C. difficile testing (enzyme immunoassay (EIA) test (sensitivity: 0.70; specificity: 0.97; turnaround time: 2 hours)).
c Percentages of the whole hospital population for one year (the total number of patients admitted was not specified).
Study & model characteristics
Settings
The hospital setting was very variable between the modeling studies. The models either included one or more hospital wards (n = 5) or an entire hospital (n = 4) (Table 1).
Natural history representation and transmission dynamics
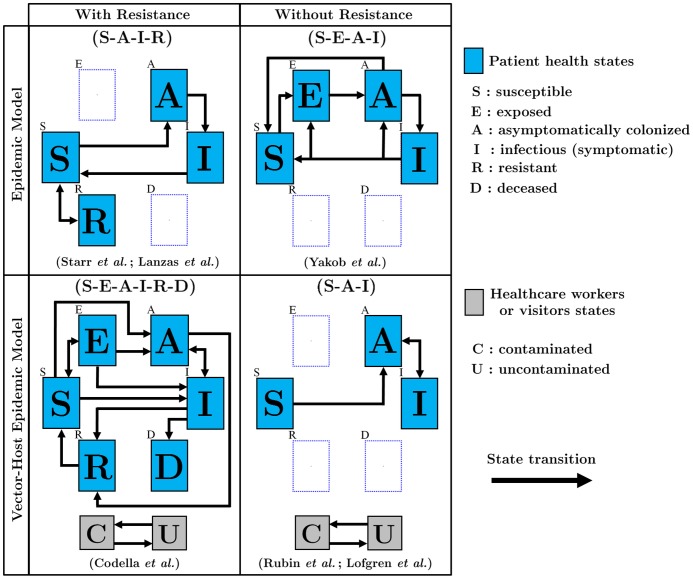
Natural history representations were categorized into four groups of models (see Fig 2 and Table 1 and S3 Appendix for a detailed representation of the natural history of each model).
Fig 2. Natural history representations.
Only the principal transitions are presented and stratifications were omitted from this figure for simplicity (e.g., heterogeneities related to the exposure to antibiotics). Patients can only be in one of the mutually exclusive health states at a given time (S-E-A-I-R-D: Susceptible-Exposed-Asymptomatic-Infectious-Resistant-Deceased). Two compartments (C and U) are included for interactions between patients and HCWs/visitors. See S3 Appendix for the full description of the natural history assumptions and transmission pathways included in each model.
All models included Susceptible (S), Asymptomatically colonized (A) and Infectious symptomatic (I) health states. Two studies included at least two C. difficile strain types in their framework. Key differences in the natural history between models were whether or not they included latent Exposed (E) and/or Resistant/Immune (R) health states. The Exposed (E) health state refers to the acquisition of the pathogen through the ingestion of C. difficile spores and it represents a latent period of non-infectivity where the spores germinate into vegetative cells. For the five models that include resistance states (R), type and duration of resistance varied dramatically. Studies either assumed temporary resistance to colonization (related to the barrier protection provided by a healthy gut microbiota, n = 4) or natural immunity following clearance of C. difficile colonization/infection (n = 1).
Transmission
The majority of studies (n = 7) included transmission between patients (exposure to infectious patients (symptomatic and colonized)) (Table 1). However, a subgroup of studies (n = 5) also included indirect transmission pathways (exposure from a contaminated HCW/visitor/environment). Environment as a source of transmission was incorporated explicitly in four studies, three of which also included the layout of the healthcare services (wards, rooms, beds) when modeling transmission dynamics. For the other studies, the transmission was independent of the location of C. difficile infected patients in the hospital. All studies used stochastic mathematical methods for transmission dynamics. Two approaches were used to model transmission: 1) homogeneous mixing between individuals of the population (the force of infection is based upon the mass-action assumption) (n = 6); or 2) contact patterns/networks between individuals/agents (n = 3). In the first case, the force of infection is determined by: 1) the prevalence of asymptomatically colonized and/or symptomatic patients, and/or contaminated HCW; 2) the effective contact rates between infectious individuals (patients/HCW); and/or 3) a static background of environmental contamination. The second method relies on properties of agent-based modeling, which accounts for contacts between individuals or between individuals and the environment. This requires modeling: 1) patient spatial heterogeneities; 2) mobility of agents (patient, HCW/visitor); and/or 3) contact patterns or contact rates.
Parameter estimation/calibration and uncertainty analyses
Only two modeling studies clearly stated that they used calibration methods, identifying parameter values by fitting their models to empirical data (Table 1). For the other studies, most parameters were directly inferred from the literature. The majority of studies reported using parametric sensitivity analysis. However, no study reported examining structural uncertainties related to the natural history representation, transmission pathways or contact patterns/networks.
Quality assessment of models reporting
Generally, the studies provided a clear description of: 1) research question; 2) model assumptions about natural history and transmission; and 3) modeling approaches and mathematical methods (S2 Appendix). On the other hand, the main limitations of study reporting were in the description of: 1) model outcomes, including their comparators (e.g., absence or partial reporting of the baseline values of health outcomes) or the denominators used (e.g., unknown time horizon or patient population considered); and 2) the uncertainty and sensitivity analyses. Indeed, for the great majority of studies, uncertainty analysis was limited to a few parameters without assessment of structural uncertainty. Finally, two studies reported model validation.
Study results
Two main objectives were addressed in the modeling studies: 1) to increase the understanding of C. difficile natural history and transmission dynamics (n = 2); and 2) to assess the impact of interventions (n = 7) (Table 1).
Understanding Clostridium difficile natural history
Both studies examining C. difficile natural history and transmission dynamics suggested that transmission between hospitalized patients is a key determinant of CDI incidence. Starr et al. [43], highlighted that hospital outbreaks are driven by transmission between patients, and are not caused by an environmental reservoir. Lanzas et al. [45] suggested that antibiotics play a smaller role on hospital acquired CDI compared to C. difficile transmission from colonized patients and the length of stay of patients (which increases their risk of exposure to C. difficile during their hospitalization).
Interventions for the prevention and control of CDI
The predictions of models regarding the impacts of CDI prevention and control strategies are presented in Table 2. The measure of the impact of interventions is assessed using a comparison to current practices (n = 3) or no intervention (n = 2). Five studies examined targeted approaches to reduce C. difficile transmission within hospitals including: 1) reduction of average length of stay (n = 2); 2) screening on admission followed by isolation of colonized patients (n = 2); 3) hand hygiene with soap and water (n = 1); 4) enhancement of the environmental decontamination (n = 1); and 5) improvement of adherence to contact precautions in isolation rooms (n = 1). These interventions showed a reduction of CDI incidence ranging from 3% to 49%. Four studies analyzed interventions aimed at reducing patients’ vulnerability to CDI: 1) antimicrobial stewardship (n = 3); 2) administration of probiotics (n = 2); and 3) prophylactic use of fecal microbiota transplantation (FMT) (n = 2). The models predicted that these interventions could reduce the incidence of CDI by 5% to 43%. Two studies investigated interventions designed to reduce the risk of recurrence or mortality: 1) oral vancomycin as an initial treatment for CDI (n = 1); and 2) use of FMT for all patients with CDI (n = 1). For these interventions, the predicted reductions in CDI recurrence were 42% and 90% respectively. Moreover, the vancomycin therapy provided up to 69% reduction in CDI-related mortality and the greatest reduction in length of stay (22%). Finally, five studies evaluated the impact of bundles of interventions. These multi-pronged interventions strategies predicted the largest reductions in CDI incidence (between 14% and 84%) as well as in new colonizations by C. difficile (42% to 89%), CDI-related mortality (93%) and length of stay (22%).
Table 2. Relative reduction (%) in CDI outcomes resulting from interventions of CDI prevention and/or control.
| Relative reduction (%) in C. difficile health outcomes | |||||
|---|---|---|---|---|---|
| INTERVENTIONS OF CDI PREVENTION AND/OR CONTROL | CD colonization | CDI incidence | CDI recurrence incidence | CDI mortality | Length of stay |
| INTERVENTIONS AIMED AT REDUCING THE TRANSMISSION | |||||
| • Reduction of the transmission rate | |||||
| Starr et al., (2009): (halving transmission from infected patients in the same room) | — | 3 | — | — | — |
| Starr et al., (2009): (halving transmission from infected patients in other rooms) | — | 9 | — | — | — |
| Starr et al., (2009): (halving all transmission sources (from all infected patients within same/other rooms and from the environmental contamination)) | — | 15 | — | — | — |
| Yakob et al., (2013,2014): (improved hand hygiene and sanitation, (no transmission)) | 77 | 49 | — | — | — |
| • Reduction of patients length of stay | |||||
| Yakob et al., (2013,2014): (length of stay reduced from 6 days to 3 days) | 66 | 38 | — | — | — |
| • Screening on admission and isolation of colonized patients | |||||
| Yakob et al., (2013): (screening with a sensitivity of 100%) | 11 | 7 | — | — | — |
| Lanzas et al., (2014): (PCR with sensitivity of 90% and turnaround time of 1 day) | 41–43 | 14–25 | — | — | — |
| Lanzas et al., (2014): (PCR with sensitivity of 95% and turnaround time of 0.5 day) | 52 | 25 | — | — | — |
| • Improved use of soap and water for hand hygiene | |||||
| Codella et al., (2015): (mean adherence of 48%) | 10 | 20 | 18 | 19 | 5 |
| • Improved environmental decontamination (routine (24h) bleach of patient rooms) | |||||
| Codella et al., (2015): (100% adherence) | 22 | 43 | 42 | 43 | 9 |
| • Improved adherence/performing contact precautions in isolation rooms | |||||
| Codella et al., (2015): (mean adherence of 62%) | 12 | 23 | 24 | 24 | 5 |
| INTERVENTIONS AIMED AT REDUCING VULNERABILITY TO CDI | |||||
| • Reduction of the rate of becoming vulnerable to CDI | |||||
| Starr et al., (2009): (halving the rates of resistant patients becoming susceptible) | — | 43 | — | — | — |
| Yakob et al., (2013,2014): (reduction of antibiotic prescription (0 per day)) | 44 | 13 | — | — | — |
| • Prophylactic use of FMT: (proportion of patients treated from 20% to 100%) | |||||
| Lofgren et al., (2014): (among patients exposed to high risk antimicrobials) | — | 5–28 | 0–9 | — | — |
| Lofgren et al., (2014): (among patients exposed to high risk antimicrobials and PPI) | — | 7–27 | 0-1 | — | — |
| • Use of probiotics/FMT in susceptible patients to expedite gut microbiota recovery | |||||
| Yakob et al., (2013,2014): (gut microbiota recovery time varied from 90 days to 10 days) | 26 | 11 | — | — | — |
| INTERVENTIONS AIMED AT REDUCING THE RISK OF CDI RECURRENCE OR CDI MORTALITY | |||||
| • Use of FMT after CDI: (proportion of patients treated from 20% to 100%) | |||||
| Lofgren et al., (2014): (discharged patients (cleared of CDI or in process of recovering)) | — | 5–16 | 28–90 | — | — |
| • Vancomycin as initial treatment | |||||
| Codella et al., (2015): (oral vancomycin (2g/day), CDI diagnostic accuracy of 100%) | 15 | 29 | 42 | 69 | 22 |
| BUNDLES OF INTERVENTIONS | |||||
| Starr et al., (2009): 1 bundle of 2 interventions | |||||
| • Halving all rates of becoming susceptible and halving all transmission rates | — | 54 | — | — | — |
| Rubin et al., (2013): 2 types of bundles (typical/optimal) including 6 interventions each: 1) improved adherence with hand hygiene; 2) improved hand hygiene with soap and water during contact with CDI patients; 3) improved contact precautions during contact with CDI patients; 4) improved environmental decontamination; 5) aggressive/early testing; and 6) empiric isolation and treatment of suspected cases. | |||||
| • Typical bundle of interventions: improvement over the base-case values expected from a hospital focusing on improving adherence practices. | |||||
| ∘ Model predictions | 61–67 | 68–74 | — | — | — |
| ∘ Reported CDI incidence (positive results of C. difficile tests (EIA test)) | — | 44–57 | — | — | — |
| • Optimal bundle of interventions: maximum effects anticipated from enhanced adherence practices and an aggressive campaign to reduce the pathogen transmission. | |||||
| ∘ Model predictions | 74–77 | 80–83 | — | — | — |
| ∘ Reported CDI incidence (positive results of C. difficile tests (EIA test)) | — | 57–63 | — | — | — |
| Lofgren et al., (2014): 1 bundle of 2 interventions | |||||
| • Prophylactic use of FMT among patients exposed to high risk antimicrobials and PPI and use of FMT after CDI. (The proportion of patients treated is from 20% to 100%.) | — | 14–25 | 16–89 | — | — |
| Yakob et al., (2014): 6 bundles of 2 interventions | |||||
| • Reduction of antibiotic prescriptions and use of probiotics | 69 | 30 | — | — | — |
| • Reduction of antibiotic prescriptions and reduction of length of stay | 75 | 59 | — | — | — |
| • Use of probiotics and reduction of length of stay | 65 | 64 | — | — | — |
| • Improved hand hygiene/sanitation and reduction of antibiotic prescriptions | 89 | 65 | — | — | — |
| • Improved hand hygiene/sanitation and use of probiotics | 89 | 66 | — | — | — |
| • Improved hand hygiene/sanitation and reduction of length of stay | 86 | 68 | — | — | — |
| Codella et al., (2015): 1 bundle of 4 interventions | |||||
| • Combination of 4 interventions: 1) vancomycin as initial treatment; 2) improved adherence when performing contact precautions in isolation rooms; 3) improved hand hygiene with soap and water; and 4) improved environmental decontamination (routine bleach of patient rooms). | 42 | 84 | 86 | 93 | 22 |
CD: Clostridium difficile; CDI: Clostridium difficile infection; EIA: enzyme immunoassay; FMT: fecal microbiota transplantation; PCR: polymerase chain reaction; PPI: proton-pumps inhibitors.
Discussion
To our knowledge, this is the first systematic review of transmission-dynamic mathematical models of C. difficile infection and colonization in healthcare settings. Only six distinct models were identified in the literature, despite: 1) the important health and economic burden of C. difficile infections on healthcare systems; and 2) the extensive use of such models to predict effectiveness and cost-effectiveness of prevention and control strategies for other infectious diseases to inform policy decisions [19, 52–54]. The models differed substantially in their natural history representations, healthcare settings modeled, outcome measures and interventions examined, which made it impossible to draw specific conclusions about optimal CDI prevention and control strategies. However, generally, the models suggested that individual or groups of interventions aimed at reducing C. difficile transmission within healthcare settings were more effective than interventions aimed at reducing CDI vulnerability, CDI recurrence or CDI mortality. Finally, the more recent models tended to be more complex, by integrating greater patient heterogeneity (e.g., local contamination levels depending on the spatial location of patients in the hospital or differential risks of colonization/CDI by level of antibiotic exposure), and more elaborate contact patterns/networks and interventions.
According to good modeling practice guidelines [25–27, 32, 35], model validation should include a between-model corroboration (convergent validity) and, when possible, external and predictive validation. Our systematic review shows that there is important variability in the predictions of the effectiveness of CDI control and prevention strategies which are due to the significant heterogeneity of interventions assessed through various natural history representation structures. C. difficile transmission-dynamic models examined three main categories of interventions for which we can consider external validity: 1) measures aimed at cutting transmission paths (mostly by environmental decontamination); 2) practices to prevent colonization/infection (e.g., antimicrobial stewardship programs); and 3) bundles of interventions. Firstly, the predicted effectiveness of interventions aimed at reducing CDI incidence through transmission control were generally in line with results from epidemiological studies. For example, these studies have shown that enhancing environmental decontamination (daily/terminal cleaning of hospital rooms) can reduce CDI incidence by 38–85% [55–61], which is similar to the model predictions of the effectiveness of environmental decontamination (43% compared to no intervention [51]) and hand hygiene and sanitation (49% compared to standard care [46]). Secondly, only two models investigated the impact of antimicrobial stewardship as an intervention. When examined, antimicrobial stewardship practices were indirectly incorporated into the models through an overall rate of antibiotic prescription without taking into account the differential risks of CDI according to antimicrobial classes. Both models predicted moderate (43% [44]) or little impact (11% [46]) of antibiotic use on CDI incidence, which contrasts with the moderate to high empirical effectiveness of antimicrobial stewardship programs at reducing CDI incidence (by 36–79% [14, 15, 62–68]). Thirdly, the models predicted that the effectiveness of bundles of interventions in preventing CDI incidence is 15–84%, which is similar to reductions reported by observational and intervention studies (31–71%) [69–73]. However, given the wide intervals and variability in the interventions included in the bundles, it is very difficult to conclude that the models are accurately predicting empirical outcomes.
There are three main uncertainties about the natural history, transmission and overall epidemiology of C. difficile in healthcare settings, which make it very difficult to model CDI control and prevention strategies. Firstly, the role of asymptomatically colonized patients in the transmission dynamics of C. difficile remains unclear (e.g., their contributions to new infections/colonizations and their period of incubation have not been precisely measured). However, evidence strongly suggests that asymptomatically colonized patients play an important role in C. difficile transmission within healthcare settings. Asymptomatically colonized patients are a source of importation of toxigenic C. difficile strains [74] and form a large reservoir for horizontal transmission [5, 75]. Furthermore, routine screening and isolation/cohorting of asymptomatic patients has been shown to be effective in decreasing CDI incidence [76]. Secondly, empirical data on the role/contribution of HCWs on overall transmission of C. difficile, especially due to their contact patterns/networks with patients and others HCWs, are lacking. The third key knowledge gap is the level of immunity/resistance to C. difficile colonization and development of disease. The basic resistance to C. difficile relies primarily on the presence of commensal bacteria, which confer protection against colonization. As depicted in the majority of C. difficile models, the use of antibiotics can disrupt these protective bacterial populations, which can lead to colonization of the intestinal tract by C. difficile and a higher risk of CDI. However, other forms of immunity/resistance have not been explored by the models, such as, for example, the effect of an anamnestic immune response after clearance of infection from a first episode on the risk of CDI recurrences.
We identified four modeling gaps. Firstly, antimicrobial stewardship programs modeled should include more mechanistic elements in order to examine the impact of various stewardship strategies, which would be informative for decision makers. In particular, future models could include differential risks of CDI associated with antibiotic classes or could examine how CDI health outcomes are affected by treatment duration and antimicrobial consumption. Secondly, none of the transmission-dynamic models have examined the cost-effectiveness of CDI interventions, although such analyses are currently a crucial element in policy decisions. Thirdly, structural uncertainty analysis should be included and reported more systematically, and the model should be cross-validated with observational studies. The variability in model predictions of effectiveness found in this review could be explained by the wide variety of model structures and interventions examined. Examining the impact of structural assumptions would provide insights on which assumptions have the greatest impact on model predictions, and allow better assessment of the validity of the models. For example, it would be important to examine whether specific assumptions about transmission, due to the focus on transmission dynamics, could explain why interventions aimed at reducing transmission were more effective than antibiotic stewardship (which does not seem to be consistent with the results from epidemiological studies). Fourthly, the emergence of more virulent strains has changed the epidemiology of C. difficile during the last decade. However, although significantly higher risk of colonization/CDI/recurrence has been linked with some specific hypervirulent C. difficile strains (e.g., BI/NAP1/027 [4, 77] or BK/NAP7/078 [78]), this has not systematically been included in published C. difficile transmission-dynamic models. Including strain-specific transition rates would allow models to capture the differential risk of CDI morbidity (e.g., severe infection, complications, ICU admission, treatment failure due to antimicrobial resistance) and mortality between historic and hypervirulent strains [79–81]. Finally, the review highlights key features that modelers should consider including in their models to increase their usefulness and validity. Models should preferably be agent-based, allowing an easier integration of environmental heterogeneity of the healthcare settings and consequently of the transmission pathways between patients and other agents such as healthcare workers (e.g., Rubin et al. [47] and Codella et al. [51] use such models). More importantly, models should be calibrated to epidemiological data and model fit should be shown/described.
Conclusions
Mathematical models of transmission dynamics of Clostridium difficile in healthcare settings are scarce considering the significant burden generated by this pathogen on healthcare systems. Current models vary substantially in their natural history assumptions, outcome measures presented and interventions examined, which lead to an unclear picture of the optimal intervention strategies to control and prevent C. difficile infections. Future modeling efforts should pay specific attention to calibration, structural uncertainties, cost-effectiveness and good transparency practices.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Canada Research Chairs program (http://www.chairs-chaires.gc.ca/), (support for MB), an operating grant from the Canadian Institutes of Health Research (http://www.cihr-irsc.gc.ca/), (grant no. MOP-136830), and a foundation scheme grant from the Canadian Institutes of Health Research (grant no. FDN-143283). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Scott, RD 2nd. The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention. Division of Healthcare Quality Promotion National Center for Preparedness, Detection, and Control of Infectious Diseases; 2009 [cited 2016 Apr 11]. Available from: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf.
- 2. Ghantoji SS, Sail K, Lairson DR, DuPont HL, Garey KW. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010;74(4):309–318. 10.1016/j.jhin.2009.10.016 [DOI] [PubMed] [Google Scholar]
- 3. Levy AR, Szabo SM, Lozano-Ortega G, Lloyd-Smith E, Leung V, Lawrence R, et al. Incidence and Costs of Clostridium difficile Infections in Canada. Open Forum Infect Dis. 2015;2(3):ofv076 PubMed Central PMCID: PMC4503917. 10.1093/ofid/ofv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, et al. Host and Pathogen Factors for Clostridium difficile Infection and Colonization. N Engl J Med. 2011;365(18):1693–1703. 10.1056/NEJMoa1012413 [DOI] [PubMed] [Google Scholar]
- 5. Clabots CR, Johnson S, Olson MM, Peterson LR, Gerding DN. Acquisition of Clostridium difficile by Hospitalized Patients: Evidence for Colonized New Admissions as a Source of Infection. J Infect Dis. 1992;166(3):561–7. 10.1093/infdis/166.3.561 [DOI] [PubMed] [Google Scholar]
- 6. Eyre DW, Cule ML, Wilson DJ, Griffiths D, Vaughan A, O’Connor L, et al. Diverse Sources of C. difficile Infection Identified on Whole-Genome Sequencing. N Engl J Med. 2013;369(13):1195–205. PubMed Central PMCID: PMC3868928. 10.1056/NEJMoa1216064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Landelle C, Verachten M, Legrand P, Girou E, Barbut F, Brun-Buisson C. Contamination of Healthcare Workers’ Hands with Clostridium difficile Spores after Caring for Patients with C. difficile Infection. Infect Control Hosp Epidemiol. 2014;35(1):10–5. 10.1086/674396 [DOI] [PubMed] [Google Scholar]
- 8. Fekety R, Kim KH, Brown D, Batts DH, Cudmore M, Silva J Jr. Epidemiology of Antibiotic-Associated Colitis: Isolation of Clostridium Difficile from the Hospital Environment. Am J Med. 1981;70(4):906–908. 10.1016/0002-9343(81)90553-2 [DOI] [PubMed] [Google Scholar]
- 9. Weber DJ, Rutala WA. The Role of the Environment in Transmission of Clostridium difficile Infection in Healthcare Facilities. Infect Control Hosp Epidemiol. 2011;32(3):207–9. 10.1086/658670 [DOI] [PubMed] [Google Scholar]
- 10. Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366(9491):1079–84. 10.1016/S0140-6736(05)67420-X [DOI] [PubMed] [Google Scholar]
- 11. Catanzaro M, Cirone J. Real-time polymerase chain reaction testing for Clostridium difficile reduces isolation time and improves patient management in a small community hospital. Am J Infect Control. 2012;40:663–666. 10.1016/j.ajic.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health. Study of a Candidate Clostridium Difficile Toxoid Vaccine (Cdiffense) in Subjects at Risk for C. Difficile Infection. 2014 [cited 2016 Apr 11];Available from: http://clinicaltrials.gov/ct2/show/NCT01887912?term=clostridium+difficile&rank=5.
- 13. Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4). 10.1002/14651858.CD003543.pub3 [DOI] [PubMed] [Google Scholar]
- 14. Wenisch JM, Equiluz-Bruck S, Fudel M, Reiter I, Schmid A, Singer E, et al. Decreasing Clostridium difficile Infections by an Antimicrobial Stewardship Program That Reduces Moxifloxacin Use. Antimicrob Agents Chemother. 2014;58(9):5079–5083. PubMed Central PMCID: PMC4135825. 10.1128/AAC.03006-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sarma JB, Marshall B, Cleeve V, Tate D, Oswald T, Woolfrey S. Effects of fluoroquinolone restriction (from 2007 to 2012) on Clostridium difficile infections: interrupted time-series analysis. J Hosp Infect. 2015;91(1):74–80. 10.1016/j.jhin.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 16. Hughes GJ, Nickerson E, Enoch DA, Ahluwalia J, Wilkinson C, Ayers R, et al. Impact of cleaning and other interventions on the reduction of hospital-acquired Clostridium difficile infections in two hospitals in England assessed using a breakpoint model. Infect Control Hosp Epidemiol. 2013;84(3):227–234. 10.1016/j.jhin.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 17. Khanafer N, Voirin N, Barbut F, Kuijper E, Vanhems P. Hospital management of Clostridium difficile infection: a review of the literature. J Hosp Infect. 2015;90(2):91–101. 10.1016/j.jhin.2015.02.015 [DOI] [PubMed] [Google Scholar]
- 18. Boily MC, Abu-Raddad L, Desai K, Masse B, Self S, Anderson R. Measuring the public-health impact of candidate HIV vaccines as part of the licensing process. Lancet Infect Dis. 2008;8(3):200–7. 10.1016/S1473-3099(07)70292-X [DOI] [PubMed] [Google Scholar]
- 19. Heesterbeek H, Anderson RM, Andreasen V, Bansal S, De Angelis D, Dye C, et al. Modeling infectious disease dynamics in the complex landscape of global health. Science. 2015;347 (6227). PubMed Central PMCID: PMC4445966. 10.1126/science.aaa4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kiskowski M, Chowell G. Modeling household and community transmission of Ebola virus disease: epidemic growth, spatial dynamics and insights for epidemic control. Virulence. 2015;p. 1–11. 10.1080/21505594.2015.1076613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henderson DA. Lessons from the eradication campaigns. Vaccine. 1999;17, Supplement 3:S53–S55. 10.1016/S0264-410X(99)00293-5 [DOI] [PubMed] [Google Scholar]
- 22. Steen R, Dallabetta G. Sexually Transmitted Infection Control with Sex Workers: Regular Screening and Presumptive Treatment Augment Efforts to Reduce Risk and Vulnerability. Reprod Health Matters. 2003;11(22):74–90. 10.1016/S0968-8080(03)02295-X [DOI] [PubMed] [Google Scholar]
- 23. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ. 2009;339 PubMed Central PMCID: PMC2714672. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Environmental Protection Agency, Science Policy Council. White Paper on the Nature and Scope of Issues on Adoption of Model Use Acceptability Guidance; 1999 [cited 2016 Mar 18]. Available from: http://www.epa.gov/sites/production/files/2015-02/documents/whitepaper_1999.pdf.
- 25. Weinstein MC, Toy EL, Sandberg EA, Neumann PJ, Evans JS, Kuntz KM, et al. Modeling for Health Care and Other Policy Decisions: Uses, Roles, and Validity. Value Health. 2001;4(5):348–361. 10.1046/j.1524-4733.2001.45061.x [DOI] [PubMed] [Google Scholar]
- 26. Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. Principles of Good Practice for Decision Analytic Modeling in Health-Care Evaluation: Report of the ISPOR Task Force on Good Research Practices–Modeling Studies. Value Health. 2003;6(1):9–17. 10.1046/j.1524-4733.2003.00234.x [DOI] [PubMed] [Google Scholar]
- 27. Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess. 2004;8(36):172 10.3310/hta8360 [DOI] [PubMed] [Google Scholar]
- 28. Thacker BH, Doebling SW, Hemez FM, Anderson MC, Pepin JE, Rodriguez EA. Concepts of Model Verification and Validation. Los Alamos National Laboratory; 2004. [cited 2016 Mar 17]. Available from: http://www.ltas-vis.ulg.ac.be/cmsms/uploads/File/LosAlamos_VerificationValidation.pdf. [Google Scholar]
- 29. Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good Practice Guidelines for Decision-Analytic Modelling in Health Technology Assessment. Pharmacoeconomics. 2006;24(4):355–371. 10.2165/00019053-200624040-00006 [DOI] [PubMed] [Google Scholar]
- 30.Committee on Models in the Regulatory Decision Process, Board on Environmental Studies and Toxicology, Division on Earth and Life Studies, National Research Council. Models in Environmental Regulatory Decision Making. National Academies Press; 2007 [cited 2016 Mar 15]. Availaible from: http://www2.lbl.gov/today/2007/Jun/22-Fri/Models-report.pdf.
- 31.The US Environmental Protection Agency, Council of Regulatory Environmental Modeling, Office of the Science Advisor. Guidance on the Development, Evaluation, and Application of Environmental Models; 2009 [cited 2015 Mar 15]. Available from: http://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P1003E4R.PDF.
- 32. Kopec J, Fines P, Manuel D, Buckeridge D, Flanagan W, Oderkirk J, et al. Validation of population-based disease simulation models: a review of concepts and methods. BMC Public Health. 2010;10(1):710 PubMed Central PMCID: PMC3001435. 10.1186/1471-2458-10-710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD. Model Parameter Estimation and Uncertainty: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-6. Value Health. 2012;15(6):835–842. 10.1016/j.jval.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 34. Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling Good Research Practices-Overview: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Value Health. 2012;15(6):796–803. 10.1016/j.jval.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 35. Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model Transparency and Validation: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32(5):733–743. 10.1177/0272989X12454579 [DOI] [PubMed] [Google Scholar]
- 36. Pitman R, Fisman D, Zaric GS, Postma M, Kretzschmar M, Edmunds J, et al. Dynamic Transmission Modeling: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-5. Value Health. 2012;15(6):828–834. 10.1016/j.jval.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M. Conceptualizing a Model: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-2. Value Health. 2012;15(6):804–811. PubMed Central PMCID: PMC4207095. 10.1016/j.jval.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)-Explanation and Elaboration: A Report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250. 10.1016/j.jval.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 39. Kuntz K, Sainfort F, Butler M, Taylor B, Kulasingam S, Gregory S, et al. Decision and Simulation Modeling in Systematic Reviews. Agency for Healthcare Research and Quality; (US: ); 2013. [cite 2016 Mar 15]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK127482/. [PubMed] [Google Scholar]
- 40. Jaime Caro J, Eddy DM, Kan H, Kaltz C, Patel B, Eldessouki R, et al. Questionnaire to Assess Relevance and Credibility of Modeling Studies for Informing Health Care Decision Making: An ISPOR-AMCP-NPC Good Practice Task Force Report. Value Health. 2014;17(2):174–182. 10.1016/j.jval.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 41. Peñaloza Ramos MC, Barton P, Jowett S, Sutton AJ. A Systematic Review of Research Guidelines in Decision-Analytic Modeling. Value Health. 2015;18(4):512–529. 10.1016/j.jval.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 42.Lofgren ET. Fecal Transplant—Simulation and analysis code for stochastic compartmental models of fecal transplant for the prevention of Clostridium difficile in intensive care units.; 2014 [cited 2016 Mar 31]. Available from: https://github.com/elofgren/FecalTransplant.
- 43. Starr JM, Campbell A. Mathematical modeling of Clostridium difficile infection. Clinical Microbiol and Infect. 2001;7(8):432–437. 10.1046/j.1198-743x.2001.00291.x [DOI] [PubMed] [Google Scholar]
- 44. Starr JM, Campbell A, Renshaw E, Poxton IR, Gibson GJ. Spatio-temporal stochastic modelling of Clostridium difficile. J Hosp Infect. 2009;71(1):49–56. 10.1016/j.jhin.2008.09.013 [DOI] [PubMed] [Google Scholar]
- 45. Lanzas C, Dubberke ER, Lu Z, Reske KA, Gröhn YT. Epidemiological Model for Clostridium difficile Transmission in Healthcare Settings. Infect Control Hosp Epidemiol. 2011;32(6):553–561. PubMed Central PMCID: PMC3645005. 10.1086/660013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yakob L, Riley T, Paterson D, Clements A. Clostridium difficile exposure as an insidious source of infection in healthcare settings: an epidemiological model. BMC Infect Dis. 2013;13(1):376 PubMed Central PMCID: PMC3751620. 10.1186/1471-2334-13-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rubin MA, Jones M, Leecaster M, Khader K, Ray W, Huttner A, et al. A Simulation-Based Assessment of Strategies to Control Clostridium Difficile Transmission and Infection. PLoS One;8(11):e80671 PubMed Central PMCID: PMC3836736. 10.1371/journal.pone.0080671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lofgren ET, Moehring RW, Anderson DJ, Weber DJ, Fefferman NH. A Mathematical Model to Evaluate the Routine Use of Fecal Microbiota Transplantation to Prevent Incident and Recurrent Clostridium difficile Infection. Infect Control Hosp Epidemiol. 2014;35(1):18–27. PubMed Central PMCID: PMC3977703. 10.1086/674394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yakob L, Riley TV, Paterson DL, Marquess J, Clements ACA. Assessing control bundles for Clostridium difficile: a review and mathematical model. Emerg Microbes Infect. 2014;3:e43 PubMed Central PMCID: PMC4078791. 10.1038/emi.2014.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lanzas C, Dubberke ER. Effectiveness of Screening Hospital Admissions to Detect Asymptomatic Carriers of Clostridium difficile: A Modeling Evaluation. Infect Control Hosp Epidemiol. 2014;35(08):1043–1050. 10.1086/677162 [DOI] [PubMed] [Google Scholar]
- 51. Codella J, Safdar N, Heffernan R, Alagoz O. An Agent-based Simulation Model for Clostridium difficile Infection Control. Med Decis Making. 2015;35(2):211–29. PubMed Central PMCID: PMC4311776. 10.1177/0272989X14545788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jit M, Brisson M. Modelling the Epidemiology of Infectious Diseases for Decision Analysis. Pharmacoeconomics. 2012;29(5):371–386. 10.2165/11539960-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van Kleef E, Robotham J, Jit M, Deeny S, Edmunds W. Modelling the transmission of healthcare associated infections: a systematic review. BMC Infect Dis. 2013;13(1):294 PubMed Central PMCID: PMC3701468. 10.1186/1471-2334-13-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Watson CH, Edmunds WJ. A review of typhoid fever transmission dynamic models and economic evaluations of vaccination. Vaccine. 2015;33, Suppl 3:C42–C54. PubMed Central PMCID: PMC4504000. 10.1016/j.vaccine.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Struelens MJ, Maas A, Nonhoff C, Deplano A, Rost F, Serruys E, et al. Control of Nosocomial Transmission of Clostridium difficile Based on Sporadic Case Surveillance. Am J Med. 1991;91(3, Supplement 2):S138–S144. 10.1016/0002-9343(91)90359-6 [DOI] [PubMed] [Google Scholar]
- 56. Mayfield JL, Leet T, Miller J, Mundy LM. Environmental Control to Reduce Transmission of Clostridium difficile. Clin Infect Dis. 2000;31(4):995–1000. 10.1086/318149 [DOI] [PubMed] [Google Scholar]
- 57. Boyce JM, Havill NL, Otter JA, McDonald LC, Adams NMT, Cooper T, et al. Impact of Hydrogen Peroxide Vapor Room Decontamination on Clostridium difficile Environmental Contamination and Transmission in a Healthcare Setting. Infect Control Hosp Epidemiol. 2008;29(8):723–729. 10.1086/589906 [DOI] [PubMed] [Google Scholar]
- 58. Hacek DM, Ogle AM, Fisher A, Robicsek A, Peterson LR. Significant impact of terminal room cleaning with bleach on reducing nosocomial Clostridium difficile. Am J Infect Control. 2010;38(5):350–353. 10.1016/j.ajic.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 59. Orenstein R, Aronhalt KC, McManus JE, Fedraw LA. A Targeted Strategy to Wipe Out Clostridium difficile. Infect Control Hosp Epidemiol. 2011;32(11):1137–1139. 10.1086/662586 [DOI] [PubMed] [Google Scholar]
- 60. Manian FA, Griesnauer S, Bryant A. Implementation of hospital-wide enhanced terminal cleaning of targeted patient rooms and its impact on endemic Clostridium difficile infection rates. Am J Infect Control. 2013;41(6):537–541. 10.1016/j.ajic.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 61. Best EL, Parnell P, Thirkell G, Verity P, Copland M, Else P, et al. Effectiveness of deep cleaning followed by hydrogen peroxide decontamination during high Clostridium difficile infection incidence. J Hosp Infect. 2014;87(1):25–33. 10.1016/j.jhin.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 62. Carling P, Fung T, Killion A, Terrin N, Barza M. Favorable Impact of a Multidisciplinary Antibiotic Management Program Conducted During 7 Years. Infect Control Hosp Epidemiol. 2003;24(9):699–706. 10.1086/502278 [DOI] [PubMed] [Google Scholar]
- 63. Fowler S, Webber A, Cooper BS, Phimister A, Price K, Carter Y, et al. Successful use of feedback to improve antibiotic prescribing and reduce Clostridium difficile infection: a controlled interrupted time series. J Antimicrob Chemother. 2007;59(5):990–995. 10.1093/jac/dkm014 [DOI] [PubMed] [Google Scholar]
- 64. Valiquette L, Cossette B, Garant MP, Diab H, Pepin J. Impact of a Reduction in the Use of High-Risk Antibiotics on the Course of an Epidemic of Clostridium difficile -Associated Disease Caused by the Hypervirulent NAP1/027 Strain. Clin Infect Dis. 2007;45 Suppl 2:S112–21. 10.1086/519258 [DOI] [PubMed] [Google Scholar]
- 65. Price J, Cheek E, Lippett S, Cubbon M, Gerding DN, Sambol SP, et al. Impact of an intervention to control Clostridium difficile infection on hospital- and community-onset disease; an interrupted time series analysis. Clin Microbiol Infect. 2010;16(8):1297–1302. 10.1111/j.1469-0691.2009.03077.x [DOI] [PubMed] [Google Scholar]
- 66. Talpaert MJ, Gopal Rao G, Cooper BS, Wade P. Impact of guidelines and enhanced antibiotic stewardship on reducing broad-spectrum antibiotic usage and its effect on incidence of Clostridium difficile infection. J Antimicrob Chemother. 2011;66(9):2168–2174. 10.1093/jac/dkr253 [DOI] [PubMed] [Google Scholar]
- 67. Dancer SJ, Kirkpatrick P, Corcoran DS, Christison F, Farmer D, Robertson C. Approaching zero: temporal effects of a restrictive antibiotic policy on hospital-acquired Clostridium difficile, extended-spectrum β-lactamase-producing coliforms and meticillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2013;41(2):137–142. 10.1016/j.ijantimicag.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 68. Beaulac K, Corcione S, Epstein L, Davidson LE, Doron S. Antimicrobial Stewardship in a Long-Term Acute Care Hospital Using Offsite Electronic Medical Record Audit. Infect Control Hosp Epidemiol. 2016;37(04):433–439. 10.1017/ice.2015.319 [DOI] [PubMed] [Google Scholar]
- 69. Muto CA, Blank MK, Marsh JW, Vergis EN, O’Leary MM, Shutt KA, et al. Control of an Outbreak of Infection with the Hypervirulent Clostridium difficile BI Strain in a University Hospital Using a Comprehensive “Bundle” Approach. Clin Infect Dis. 2007;45(10):1266–1273. 10.1086/522654 [DOI] [PubMed] [Google Scholar]
- 70. Salgado CD, Mauldin PD, Fogle PJ, Bosso JA. Analysis of an outbreak of Clostridium difficile infection controlled with enhanced infection control measures. Am J Infect Control. 2009;37(6):458–464. 10.1016/j.ajic.2008.11.010 [DOI] [PubMed] [Google Scholar]
- 71. Weiss K, Boisvert A, Chagnon M, Duchesne C, Habash S, Lepage Y, et al. Multipronged Intervention Strategy to Control an Outbreak of Clostridium difficile Infection (CDI) and Its Impact on the Rates of CDI from 2002 to 2007. Infect Control Hosp Epidemiol. 2009;30(2):156–162. 10.1086/593955 [DOI] [PubMed] [Google Scholar]
- 72. Bishop J, Parry MF, Hall T. Decreasing Clostridium difficile Infections in Surgery: Impact of a Practice Bundle Incorporating a Resident Rounding Protocol. Conn Med. 2013. [cited 2016 Apr 13];77(2):69–75. Available from: MEDLINE with Full Text. [PubMed] [Google Scholar]
- 73. You E, Song H, Cho J, Lee J. Reduction in the incidence of hospital-acquired Clostridium difficile infection through infection control interventions other than the restriction of antimicrobial use. Int J Infect Dis. 2014;22:9–10. 10.1016/j.ijid.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 74. Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization With Toxinogenic C. difficile Upon Hospital Admission, and Risk of Infection: A Systematic Review and Meta-Analysis. Am J Gastroenterol;110(3):381–390. 10.1038/ajg.2015.22 [DOI] [PubMed] [Google Scholar]
- 75. Curry SR, Muto CA, Schlackman JL, Pasculle AW, Shutt KA, Marsh JW, et al. Use of Multilocus Variable Number of Tandem Repeats Analysis Genotyping to Determine the Role of Asymptomatic Carriers in Clostridium difficile Transmission. Clin Infect Dis. 2013;57(8):1094–1102. PubMed Central PMCID: PMC3783061. 10.1093/cid/cit475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Longtin Y, Paquet-Bolduc B, Gilca R, Garenc C, Fortin E, Longtin J, et al. Effect of Detecting and Isolating Clostridium difficile Carriers at Hospital Admission on the Incidence of C difficile Infections: A Quasi-Experimental Controlled Study. JAMA Intern Med. 2016;. 10.1001/jamainternmed.2016.0177 [DOI] [PubMed] [Google Scholar]
- 77. Vardakas KZ, Konstantelias AA, Loizidis G, Rafailidis PI, Falagas ME. Risk factors for development of Clostridium difficile infection due to BI/NAP1/027 strain: a meta-analysis. Int J Infect Dis. 2012;16(11):e768–73. 10.1016/j.ijid.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 78. Goorhuis A, Bakker D, Corver J, Debast SB, Harmanus C, Notermans DW, et al. Emergence of Clostridium difficile Infection Due to a New Hypervirulent Strain, Polymerase Chain Reaction Ribotype 078. Clin Infect Dis. 2008;47(9):1162–1170. 10.1086/592257 [DOI] [PubMed] [Google Scholar]
- 79. Miller M, Gravel D, Mulvey M, Taylor G, Boyd D, Simor A, et al. Health Care-Associated Clostridium difficile Infection in Canada: Patient Age and Infecting Strain Type Are Highly Predictive of Severe Outcome and Mortality. Clin Infect Dis. 2010;50(2):194–201. 10.1086/649213 [DOI] [PubMed] [Google Scholar]
- 80. See I, Mu Y, Cohen J, Beldavs ZG, Winston LG, Dumyati G, et al. NAP1 Strain Type Predicts Outcomes From Clostridium difficile Infection. Clin Infect Dis. 2014;58(10):1394–1400. PubMed Central PMCID: PMC4697926. 10.1093/cid/ciu125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rao K, Micic D, Natarajan M, Winters S, Kiel MJ, Walk ST, et al. Clostridium difficile Ribotype 027: Relationship to Age, Detectability of Toxins A or B in Stool With Rapid Testing, Severe Infection, and Mortality. Clin Infect Dis. 2015;61(2):233–241. PubMed Central PMCID: PMC4565993. 10.1093/cid/civ254 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.