Abstract
Diapause is an actively induced dormancy that has evolved in Metazoa to resist environmental stresses. In temperate regions, many diapausing insects overwinter at low temperatures by blocking embryonic, larval or adult development. Despite its Afro-tropical origin, Drosophila melanogaster migrated to temperate regions of Asia and Europe where females overwinter as adults by arresting gonadal development (reproductive diapause) at temperatures <13°C. Recent work in D. melanogaster has implicated the developmental hormones dILPs-2 and/or dILP3, and dILP5, homologues of vertebrate insulin/insulin-like growth factors (IGFs), in reproductive arrest. However, polymorphisms in timeless (tim) and couch potato (cpo) dramatically affect diapause inducibility and these dILP experiments could not exclude this common genetic variation contributing to the diapause phenotype. Here, we apply an extensive genetic dissection of the insulin signaling pathway which allows us to see both enhancements and reductions in egg development that are independent of tim and cpo variations. We show that a number of manipulations dramatically enhance diapause to ~100%. These include ablating, or reducing the excitability of the insulin-producing cells (IPCs) that express dILPs-2,3,5 employing the dilp2,3,5-/- triple mutant, desensitizing insulin signaling using a chico mutation, or inhibiting dILP2 and 5 in the hemolymph by over-expressing Imaginal Morphogenesis Protein-Late 2 (Imp-L2). In addition, triple mutant dilp2,3,5-/- females maintain high levels of diapause even when temperatures are raised in adulthood to 19°C. However at 22°C, these females all show egg development revealing that the effects are conditional on temperature and not a general female sterility. In contrast, over-expression of dilps-2/5 or enhancing IPC excitability, led to levels of ovarian arrest that approached zero, underscoring dILPs-2 and 5 as key antagonists of diapause.
Introduction
In temperate regions, several holometabolous insects overwinter in a state of diapause, an actively induced dormancy that blocks developmental growth at species-specific stages of their life cycles and enhances cold tolerance [1]. Drosophila melanogaster, despite its Afro-tropical origins, has evolved a diapause response in temperate regions where adult females induce reproductive dormancy by arresting gonadal growth in response to cold environmental conditions below 13°C in combination with photoperiodic stimuli [2–4]. A fine-tuned regulation of diapause timing is important for synchronizing Drosophila’s life-cycle with environmental changes and several genes have been implicated in reproductive arrest under colder temperatures and shorter photoperiods.
The s-tim/ls-tim natural polymorphism in the circadian clock gene timeless generates a substantial difference in diapause inducibility as revealed in several natural populations and confirmed with transgenic lines, where the recently derived ls-tim mutation enhances diapause at all photoperiods [2,5]. Two closely linked polymorphisms in couch potato (cpo), cpoA356V and cpoI462K (now reannotated as cpoA347V and as intronic polymorphism SNP 48034(A/T)–[6]) show latitudinal clines in North America, have also been suggested to modulate diapause induction [7] but no relationship between these polymorphisms and diapause is present in Australian populations [8]. cpo lies between the proximal and distal breakpoints of the cosmopolitan Payne inversion, In(3R)P, which shows a cline in frequency on both continents complicating interpretation of the cpo cline [9,10]. Nevertheless, complementation analysis reveals that these two natural cpo variants as well as artificially generated mutations of cpo, significantly affect diapause with higher levels of diapause inversely correlated with cpo expression levels [7].
In addition, natural variation in another gene that lies within In(3R)P and which encodes insulin-regulated phosphatidylinositol 3 kinase (PI3K), dp110, appears to affect diapause levels and may also contribute to a latitudinal cline in diapause induction that is observed in North America, but the molecular basis for the polymorphism remains obscure [11,12]. The insulin-like receptor (InR) gene also lies within In(3R)P and encodes considerable polymorphism, with one allele showing a similar latitudinal cline in frequency in both North America (standard chromosome only) and Australia (inverted chromosome only) [13]. Heteroallelic mutational combinations in InR have long been known to generate female sterility as the eggs remain previtellogenic at 25°C but whether these polymorphisms affect diapause in Drosophila has yet to be studied [14,15]. Thus, until recently, there was only circumstantial evidence that insulin signaling might be involved in conditional ovarian developmental arrest at low temperatures in D. melanogaster.
However, in a recent study of D. melanogaster diapause, females maintained in diapause for several weeks showed elevated gene expression for Drosophila insulin-like peptides, dILPs 2–6 [16]. If there exists a causal relationship between diapause and dilp levels, then mutations in some of these dilp genes might be expected to reduce levels of diapause. In contrast, mutations in dilp2-3 and dilp5 appeared to enhance the depth of diapause as measured by ovarian development [16], possibly caused by compensatory up-regulation of other dILPs [17]. One potential problem with this experiment, apart from the small numbers of female flies that were analysed, was that the wild-type and dilp2-3-/ -and dilp5-/- mutants were not assessed for either their cpo or tim genotypes which could conceivably have generated their different diapause profiles.
The Drosophila genome encodes a number of insulin/insulin-like protein genes (dilps) [18–21]. Two of them, dilps-2/5, are mainly expressed in a cluster of Median Neurosecretory Cells (MNCs), the insulin-producing cells (IPCs) [18,19]. dilps-2/5 are expressed independently in IPCs and differentially during normal development with dilp2 showing an earlier and stronger larval expression than dilp5 [18,22]. dilps-2/5 encode small peptide hormones of 137 aa and 107 aa, respectively, which are released into the haemolymph and signal through InR to inhibit the transcription factor Forkhead box-O (FoxO) in target organs. dILP-2/5 act redundantly to control a plethora of developmental and physiological functions such as larval growth rate, metamorphic timing, energy metabolism, fecundity and aging [17–26].
Given the available evidence that suggests insulin signaling involvement in the Drosophila overwintering response, we sought to further dissect the role of these dilps in diapause induction by using an extensive set of genetic manipulations on known tim and cpo genetic backgrounds to clarify the role of insulin signaling in this important seasonal adaptation.
Results/Discussion
We screened all our lines for the tim [2] and cpo [7] natural alleles (see Materials and Methods). Among our lines, the single dilp2-/-, dilp5-/-, dilp3-/-, chicoKG00032 mutants, UAS-dilp2 RNAi, UAS-dilp5RNAi, UAS-sNPF;UAS-sNPF, akh-Gal4 and Lk6DJ634-Gal4 carry the ls-tim allele so we used a corresponding ls-tim as a control. For cpo, the upstream C/T (cpoA347V) substitution showed some variation among strains whereas the downstream SNP 48034(A/T) substitution was represented as A in all strains except for UAS-hid,rpr which carried a T and is reported to promote reduced levels of diapause [6,7]. S1 Table carries the summary of all the diapause results and the background genotypes of all strains tested.
Manipulation of IPCs
In order to test whether neural dILPs-2,3,5 play a key role in diapause induction, we removed dILPs-2,3,5 signaling late in the larval stage (from mid-late third larval instar, L3) by ablating the IPCs, the main source of these dILPs [18,19], using the Gal4/UAS system. We used two different drivers, dilp2-Gal4 (dilp2>) and InsP3-Gal4 (InsP3>), which express Gal4 from mid-late L3 [17,27] and late L3/pupa [28], respectively, in order to drive the expression of two pro-apoptotic genes simultaneously, hid (head involution defective) and rpr (reaper) in IPCs. Because of their later larval expression, these drivers do not cause the lethality or severe developmental defects [27,28] that are induced by the early larval ablation of IPCs with the precocious dilp2(p)-Gal4 (dilp2(p)>) driver [18].
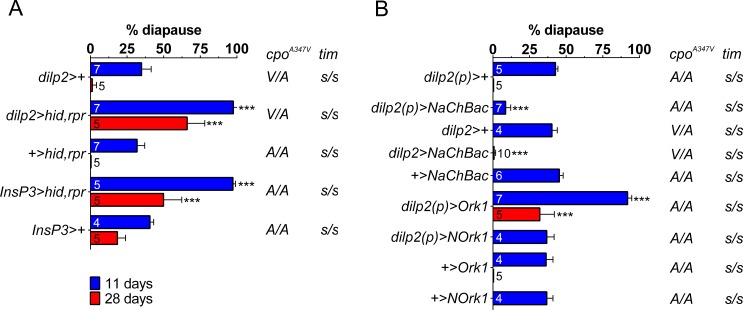
Strikingly, IPCs ablation (dilp2>hid,rpr and Insp3>hid,rpr) promotes a near complete diapause response (97.6 ± 2.9% and 97.3 ± 1.7%, respectively) compared to controls (Fig 1A).
Fig 1. Insulin-producing cells (IPCs) regulate reproductive dormancy in D. melanogaster.
(A) Ablation of IPCs with dilp2>hid,rpr and Insp3>hid,rpr significantly enhances diapause at both 11 and 28 days compared with controls even under a long summer photoperiod (LD16:8). (B) Hyperexcitability of IPCs decreases diapause frequency (NaChBac) whereas reducing excitability (Ork1) significantly enhances diapause also compared to the non-conducting control NOrk1. Blue 11 days, red 28 days. Numbers within bars represent replicates (see Methods). Mean ± SD, ***p<0.001. (ANOVA was performed using the arcsin transformation but the results are presented as percentages for simplicity).
This is not female sterility because at 23°C these females all produce late stage eggs (S2 Table and S1 Fig, also shown by others [29]). If the ablations were simply causing a slowing down in maturation of the gonads rather than an arrest, we should observe that after a longer period in diapause inducing conditions, the experimental genotypes should ‘catch up’ and give similar levels of diapause to controls. We therefore maintained our females for 28 days at 12°C and observed that diapause levels fell to 0–1% in dilp2>+ and UAS-hid,rpr controls, and 18% in InsP3>+ but were maintained at 66% and 50% in dilp2>hid,rpr and InsP3>hid,rpr respectively (Fig 1A). These results reveal a key role for neural IPCs and their functions in modulating and maintaining Drosophila diapause at colder temperatures.
To further understand the role of IPCs on reproductive dormancy, we disrupted the neuronal physiology of IPCs (and, in turn, the release of dILPs) by over-expressing a potassium (UAS-dOrkΔ-C, designated as UAS-Ork1) or a sodium channel (UAS-NaChBac) which reduce or enhance neuronal excitability, respectively [30,31]. Ork1ΔC over-expression from early larval life (dilp2(p)>Ork1) promotes very high levels of reproductive diapause in a similar manner to IPCs ablation (91.9 ± 2.8%) compared to its dilp2(p)>dORKΔ-NC (NOrk1) non-conducting control (36.8 ± 5.0%) whereas NaChBac over-expression (dilp2(p)>NaChBac) inhibits ovarian dormancy and induces gonadal growth in the cold (Fig 1B), reinforcing the notion that dILPs have a regulatory role on dormancy. Again Ork1ΔC over-expression females produced stage 14 eggs at 23°C and after 28 days, dilp2(p)>Ork1 females still showed >30% diapause compared to their corresponding driver and UAS controls that showed almost zero diapause (S1 Table and Fig 1B).
The higher levels of diapause in the experimental flies illustrated in Fig 1 are generated even though these females carry the low diapause s-tim allele and were maintained in the longer summer LD16:8 photoperiod. Furthermore, the most frequent cpo genotype in these lines was C/C A/A (cpo347Ala, cpo48034A, S1 Table). Zonato et al (submitted) reveals that the cpo48034A/T polymorphism does not play any role in diapause at either 12 or 28 days in the s-tim background. However the cpoA347V polymorphism was observed to have a significant effect with cpoVal showing higher levels of diapause than cpoAla. S1 Table shows the relevant cpoA347V genotypes for all the lines, and some are heterozygous, while the majority are homozygous for cpoAla. If we compare the control strains we observe at 11 and 28 days, diapause levels lie between 32–47% and 0–18% respectively, but there is no correlation with cpoA347V genotype, suggesting that the heterozygous combination of alleles does not enhance diapause compared with cpoAla homozygotes. Consequently, cpoA347V genotypes in the s-tim background do not appear to play any significant role in the large changes we observe in diapause induction compared to the dramatic effects we observe when we manipulate the IPCs.
The effects of altered expression of dilps on diapause induction
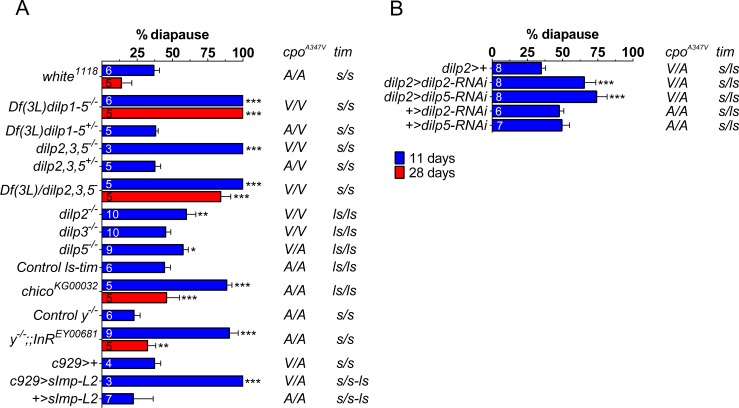
To investigate whether it was the dILPs within the IPCs that were generating these effects, we also examined whether a chromosomal deficiency uncovering five of the eight dilps, Df(3L)dilp1-5-/- [25], disrupted the wild-type response. Strikingly, Df(3L)dilp1-5-/- mutants induce 100% diapause at 12°C which is maintained at this level for 28 days whereas both the heterozygous mutant (Df(3L)dilp1-5+/-) and matched background controls (w1118) exhibited 38.1 ± 1.9% and 36.8 ± 4.0% diapause respectively (Fig 2A).
Fig 2. dilps-2/5 redundantly inhibit diapause.
(A) Combined dilps-2/5 null mutations promote diapause induction (Df(3L)dilp1-5-/- and dilp2,3,5-/-) at both 11 and 28 days. Among three dilps expressed in IPCs, only dilp2-/- and dilp5-/- single mutations modestly enhance diapause frequency. Hypomorphic mutations in chico and InR also significantly enhance diapause as does the over-expression of sImp-L2 using the neuropeptide cell driver c929. (B) Similarly RNAi driven by dilp2 promoter significantly enhances diapause induction. Mean ± SD, ANOVA on arcsin transformations, *p<0.05, **p<0.01, ***p<0.001.
Similarly, the two genotypes that were triply mutant, dilp2,3,5-/- [17] and Df(3L)dilp1-5/dilp2,3,5- induced maximum diapause which in the latter genotype was maintained for 28 days at 84% (Fig 2A).
We also investigated hypomorphic chico mutants (chicoKG00032) [23], that disrupt dILPs sensitivity, and observed that they too exhibited very high levels of diapause (89% falling to 46% after 28 days, Fig 2A) Similarly the weakly hypomorphic InREY00681 mutant [32] also showed very high levels of diapause (90.3 ± 6.2%) after 11 days, and over-expressing Imaginal Morphogenesis Protein—Late 2 (Imp-L2), using the neuroendocrine cell driver c929> [33,34], provoked 100% diapause, consistent with the function of Imp-L2 in inhibiting dILPs in the haemolymph [35]. All of these experiments were carried out at LD16:8 and all experimental genotypes carried the s-tim background that favors low diapause levels (except Imp-L2, where s/ls were segregating, Fig 2A). In contrast, none of the single null mutants (dilp2-/-, dilp3-/- or dilp5-/-) caused strong induction of diapause, compared to Df(3L)dilp1-5-/-, although individually, both dilp2-/- and dilp5-/- modestly enhanced the frequency of diapause (59.8 ± 7.0% and 57.5 ± 3.7%, respectively, Fig 2A) compared to controls sharing the same ls-tim backgrounds. As dilp3-/- was homozygous for the diapause promoting cpoVal allele, whereas dilp5-/- was polymorphic at this site, any enhancement of diapause in the latter compared to dilp3-/- is possibly underestimated. These results, coupled with the suggested autocrine regulatory role of dILP3 [17] lead us to suggest dILP2 and 5 as the key IPCs-released dILPs for diapause suppression. A similarly modest but significant enhancement was observed on knocking down dilp2 (65.5 ± 8.1%) or dilp5 (74.2 ± 7.4%) with RNAi using the dilp2 promoter, compared to the corresponding UAS-RNAi controls which both gave just under 50% diapause (Fig 2B). The knocked down flies were heterozygous for cpoVal whereas the controls were homozygous for cpoAla, which could possibly explain why these effects were a little more dramatic than the single mutants (S1 Table).
Both Df(3L)dilp1-5-/- and dilp2,3,5-/- nulls produce dwarf adults and developmentally delayed larvae [17,25], suggesting that dILPs-2/5 control of diapause is pleiotropically linked to larval growth. Yet although Df(3L)dilp1-5-/- and single null mutants (dilp2-/-, dilp3-/- or dilp5-/-) or late IPCs ablation (dilp2>hid, rpr) exhibit reduced fecundity [17,19,25], these females all lay viable embryos at 23°C whereas at 12°C oogenesis is blocked. In addition, the single dilp3-/- mutation causes fecundity defects [17], but it does not promote an enhanced diapause response revealing a decoupling of diapause from sterility. Furthermore, in our hands, the experimental genotype females are vitellogenic at 23°C (S2 Table and S1 Fig). Consequently dilps-2/5 genes appear to redundantly and specifically control a conditional temperature-dependent diapause/development switch.
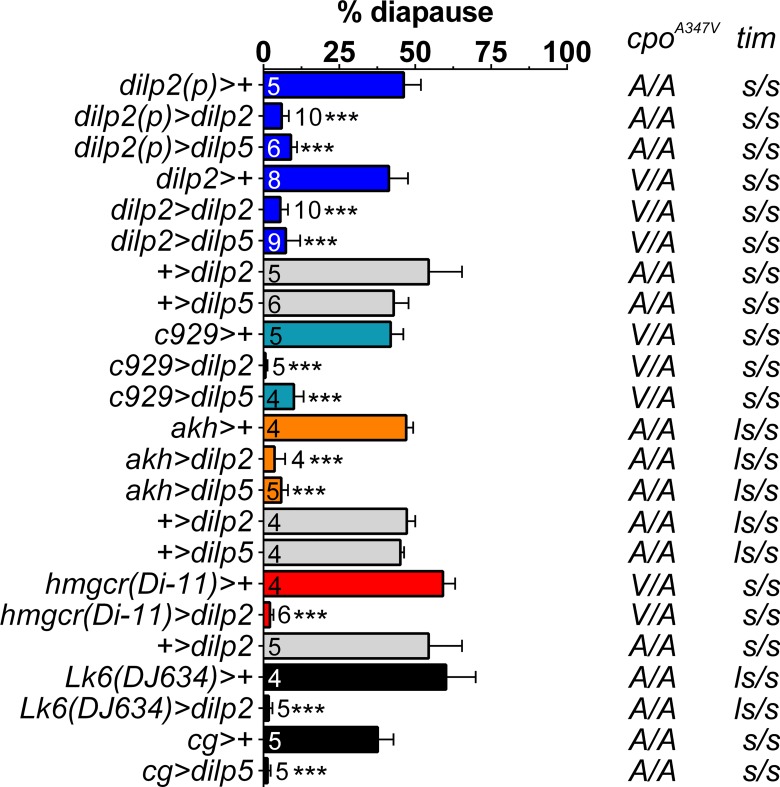
If combined dilps-2/5 loss, reducing sensitivity or inhibiting dILPs-2/5 promotes diapause under cold conditions, then conversely, dilps-2/5 over-expression might abolish the ability to induce diapause. We therefore over-expressed UAS-dilp5 or UAS-dilp2 transgenes from early L2 (dilp2(p)>dilp5 and dilp2(p)>dilp2) or mid-late L3 instar (dilp2>dilp5 and dilp2>dilp2). Strikingly, these manipulations caused almost complete inhibition of reproductive diapause at 12°C (even though these females were maintained in diapause-promoting winter LD8:16 photoperiods Fig 3).
Fig 3. Over-expression of dilp2 and dilp5 dramatically reduces diapause levels.
dilps-2/5 over-expression from early (dilp2(p)>dilp2 and dilp2(p)>dilp5) or late (dilp2>dilp2 and dilp2>dilp5) larval stages inhibit diapause at 12°C under short photoperiod (LD8:16). Mean ± SD, ANOVA on arcsin transformations, ***p<0.001. The same result is observed with ectopic drivers (see text for details).
Furthermore, we also induced ectopic dilps-2/5 over-expression with a number of promoters for neuroendocrine and endocrine cells (c929, [33,34]), larval (cg, [22]) or adult (Lk6DJ634, [36,37]), fat-body, corpus allatum (hmgcrDi-11 [38,39]) and corpus cardium (akh) [40]. All of these manipulations significantly reduced diapause inducibility in a similar manner to dILPs-2/5 over-expression in MNCs (Fig 3), suggesting that irrespective of the tissue from which dILPs are expressed and the timing of their release, it is their presence in the hemolymph that is the critical factor. Taken together, these results suggest that dILPs-2/5 are the key antagonists of diapause and that they lie at the core of the genetic mechanism underlying induction of ovarian dormancy.
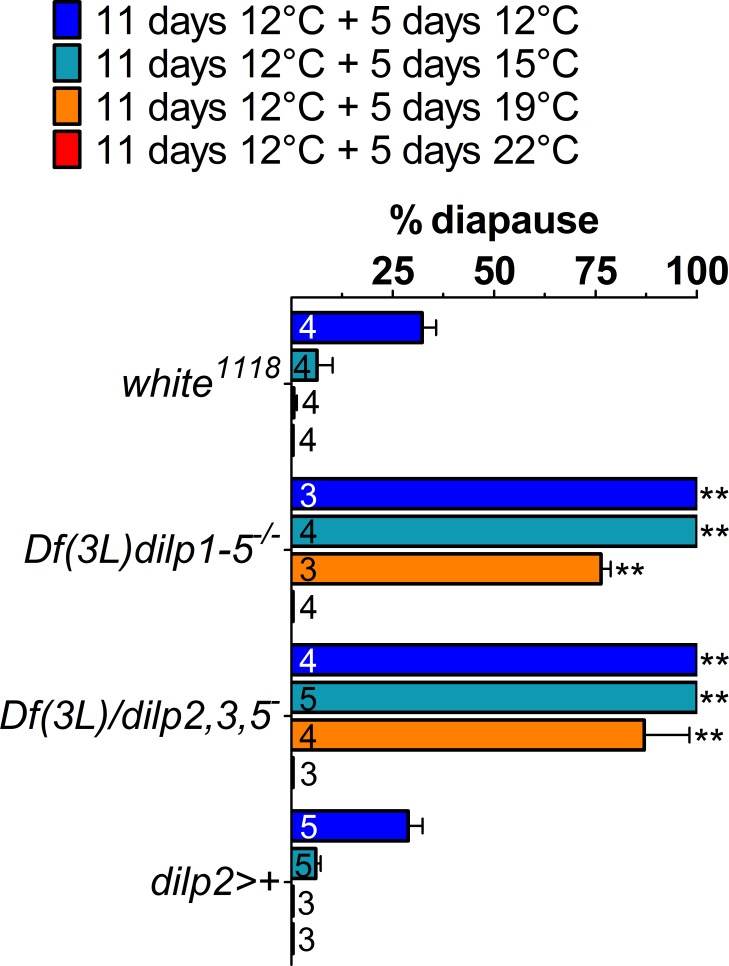
Wild-type diapausing flies can switch from diapause to full fecundity if they re-encounter a favorable environment [16]. If dILPs-2/5 levels are critical for modulating the diapause response, then we expect that Df(3L)dilps1-5-/- mutants should not transition out of diapause as quickly if they experience a shift to favorable conditions. We therefore exposed Df(3L)dilps1-5-/- and Df(3L)dilp1-5/dilp2,3,5- null mutants to diapause-inducing conditions (12°C) for 11 days before switching them for an additional 5 days to non-diapausing conditions (15°C, 19°C, or 22°C). We observed that even at 19°C, the null mutants maintained high frequencies of diapause compared to the controls (Time x Genotype F6,42, 18.2 p<0.01, Fig 4).
Fig 4. Combinations of dilp mutations maintain diapause under more favourable conditions.
Under warmer temperatures and longer photoperiods (LD16:8), conditions favourable to growth, Df(3L)dilp1-5-/- and Df(3L)dilp1-5/dilp2,3,5- mutants remain in diapause at high levels even at 19°C compared to controls, but ovaries mature at 23°C. Mean ± SD, ANOVA on arcsin transformations, **p<0.01.
Only at 23°C did the nulls start to mature their gonads, suggesting that dILPs are the limiting signals determining diapause in colder conditions. These results are important as they show clearly that these mutants are conditional sterile at low temperature and reinforce the view that we are examining diapause, a reversible phenomenon, not simply female sterility.
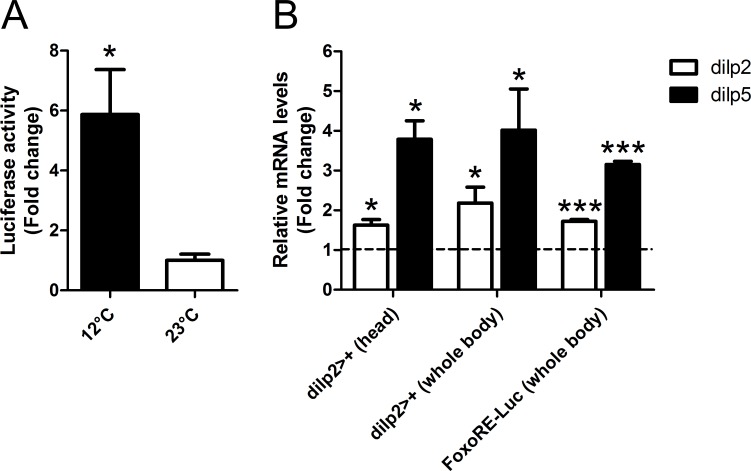
If our proposal is correct, then dILPs-2/5 signaling should be perturbed by environmental conditions that induce reproductive diapause. In particular, we predict that if dILPs-2/5 inhibit reproductive diapause, then dILPs-2/5 signaling should be repressed during dormancy induction. To address this issue, we used a FoxO response element-luciferase reporter (FoxO.RE-Luc) [41] to estimate the levels of insulin/FoxO signaling in diapausing flies (reared at 12°C, LD8:16) versus “non-diapausing” ones (reared at 23°C). Under conditions of reduced insulin signaling FoxO should enter the nucleus and up-regulate luciferase activity. Consistent with the notion that reduction in dILPs-2/5 signaling promotes reproductive dormancy, diapausing flies exhibited a significant (p<0.05) 6-fold up-regulation of FoxO activity in isolated abdomen (containing the gonads, direct downstream targets of brain dILPs [42]) (Fig 5A).
Fig 5. Regulation of FoxO and dilp2/5 in diapausing females.
(A) FoxO.RE-Luciferase reporter gene assay. Reporter activity in diapausing (12°C) versus non-diapausing flies (23°C) is shown (p = 0.032, t-test). Reporter activity is higher in flies abdomen at 12°C. Y-axis: Luciferase activity (fold change). (B) qPCR of dilps-2/5 mRNA levels in two different ‘wild-type’ genotypes, FoxO.RE.Luciferase used in A and dilp2>+. dilps-2/5 expression levels of diapausing (12°C) versus non-diapausing flies (23°C) are shown (ratio: diapausing/non-diapausing). dilps-2/5 are up-regulated in diapausing flies. Y-axis: mRNA levels (fold change). Dotted line indicates the expression levels in non-diapausing flies. Mean ± SE, *p<0.05, ***p<0.001 are based on t-test.
In other cases of insulin signaling down-regulation, there is often an accompanying compensatory enhancement of dilps-2/5 gene transcription in the IPCs. For example, increased mRNA levels of dilps-2/5 are observed in a SH2B-/- mutant (SH2B is an intracellular adaptor of InR) [23] or when Imp-L2 is over-expressed [43]. In dilp2-/- mutants there is up-regulation of the dilp3 and dilp5 transcripts, while in dilp5-/- mutants, dilp3 is up-regulated, with dilp2-3-/- double mutant also enhancing dilp5 expression [17]. As dilp3-/- mutants also reduce dilp2 and dilp5 expression, there is both positive and negative cross-regulation among these dilps [17]. Similar up-regulation of dilps-2/5 transcripts have also been reported upon Imp-L2 over-expression [43] and ablation of the germ line [44] which are direct targets of brain dILPs [42]. Thus, paradoxically, we predicted that diapausing flies might exhibit up-regulation of dilps-2/5 genes as a compensatory response. Strikingly, the expression levels of dilps-2/5 genes were both up-regulated in reproductively diapausing flies (reared at 12°C) versus non-diapausing flies (reared at 23°C), in both whole body and head-only samples of two different ‘wild-type’ strains, dilp2>+ and FoxO.RE-Luciferase (Fig 5B), both of which showed normal levels of diapause (40.3 ± 3.7% and 49.5 ± 4.8% in LD8:16 and 33.2 ± 4.6% and 36.2 ± 3.4% in LD16:8, respectively). A similar result was obtained with diapausing Canton-S flies by Kubrak et al [16]. Although this feedback mechanism remains to be clarified, these results support the notion that dILPs-2/5 signaling fails under the perturbing effects of an adverse environment and this is the key event for diapause induction.
We note that the up-regulation of dilps-2/5 mRNA levels is the opposite to that reported in the monarch butterfly [45] and mosquito [46] where diapause correlates with down-regulation of insulin/IGFs expression. This is a curious difference, but variation in hormonal regulation among insect species, especially with respect to diapause or metamorphosis regulation, is quite common (reviewed in [1]). For example, Juvenile hormone prevents metamorphosis by specifying larval molts in Lepidoptera but it does not in Drosophila, [47,48]). Independently of the feedback mechanism that regulates the compensatory phenotype, the key aspect of our results is that, in Drosophila, reproductively diapausing flies exhibit a disruption of the normal systemic dILPs-2/5 signaling, which is consistent with the shutdown of this hormonal signaling pathway during diapause of other insect species [1,45,46] and the role of insulin-like peptides as diapause antagonists.
Our findings provide direct genetic evidence that dILPs-2/5 signaling is a central regulator of reproductive diapause in Drosophila that is independent of tim and cpo genetic backgrounds and supports and significantly extends the work on dilp2-3-/- and dilp5-/- mutants of Kubrak et al [16]. Our primary data demonstrate that loss of dILPs-2/5 signaling promotes, whereas the over-expression of dilps-2/5 prevents ovarian dormancy. The level of change between these two genetic alternatives (~100%) far outstrips any changes in diapause levels that are determined by s-tim/ls-tim or by variants in cpo. We can now reconsider the results of Kubrak et al [16] who observed significantly reduced levels of ovarian development in dilp5-/- and dilp2-3-/- double mutants. The former single mutant is in a diapause promoting ls-tim background and has a polymorphic cpoA347V genotype so the effects may have been over-estimated by Kubrak et al [16], depending on whatever were the background genotypes of their controls. In a controlled genetic background, any effect on diapause of the single dilp5-/- or dilp2-/- mutants is modest in our hands while dilp3-/- has no effect. However, combining dilps2,3,5 mutations generates 100% diapause, demonstrating the epistatic nature of these mutations which will largely bypass the mRNA compensation phenotypes observed in each of the single mutants by Grönke et al [17].
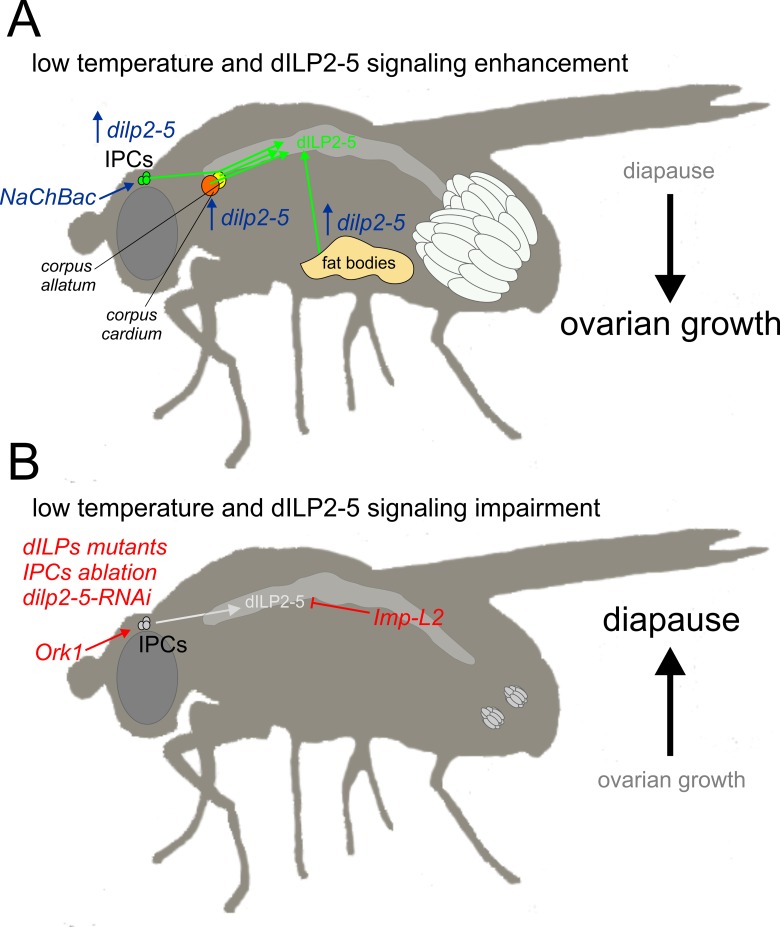
It is further worth pointing out that the changes we have observed by manipulating dILPs signaling are conditional on temperature, so, for example, dilps2-3,5-/- null mutants are not sterile, even though they remain in ovarian arrest at temperatures up to 19°C. Indeed all the variants we have used are fertile at 23°C, even the most severe, Df(3L)dilp1-5-/-, which as a homozygote has about 10% fertility and 50% viability at this temperature [25]. At 12°C this variant showed 100% diapause, so even partially fertile females show a temperature-sensitive phenotype. Furthermore, by extending some of our key observations to 28 days we avoid basing our interpretations solely on the first two weeks of diapause which can show some fluctuation in the dynamics of egg development [16]. We conclude that our extensive genetic manipulations of insulin-like signaling in D. melanogaster reveal dILPs-2/5 to be the key regulators and antagonists of seasonal diapause. Fig 6 shows a schematic summary of our major findings.
Fig 6. Schematic summary of dILP2-5 signaling effects on diapause.
(A) dILP2-5 signaling enhancement, obtained either through IPCs sensitization (NaChBac expression) or dilp2-5 over-expression (within IPCs or in other endocrine tissues, corpus allatum, corpus cardium and fat bodies) propels ovarian development even under diapause-inducing conditions (low temperature, 12°C, and short photoperiod). (B) Impairment of dILP2-5 signaling through Ork1 expression within IPCs, insulin signaling mutants (both dilp, InR and chico mutants), IPCs ablation, dilp2-5-RNAi, or over-expressing the dILPs inhibitor Imp-L2, consolidates diapause preventing further ovarian maturation.
Materials and Methods
Fly Stock and Maintenance
Flies were reared at 23°C under LD12:12 in cornmeal standard food. The following lines were used in this study: dilp2-Gal4, UAS-sImp-L2 and UAS-dilp2-RNAi were gifts from Linda Partridge; dilp2(p)-Gal4 (p, precocious) was a gift from Eric J. Rulifson; UAS-dilp2 and UAS-dilp5 were gifts from Ernst Hafen; InsP3-Gal4 was a gift from Michael J. Pankratz; Df(3L)dilp1-5-/- was a gift from Leslie Pick; FoxO.RE-Luciferase was a gift from Brian Staveley; c929-Gal4, UAS-NaChBac, UAS-dOrkΔ-C (designated as UAS-Ork1) and its negative control UAS-dOrkΔ-NC (designated as UAS-NOrk1) were gifts from Michael B. O’Connor; hmgcrDi-11-Gal4 was a gift from Jean-René Martin, UAS-hid,rpr was a gift from John R. Nambu; white1118 s-tim was a gift from Charlotte Helfrich-Förster. We used a (red-eyed) white mutant carrying (but not expressing) UAS-sNPF/+;UAS-sNPF/+ (parental line UAS-sNPF;UAS-sNPF was a gift from Kweon Yu) as the control for the single dilp mutants (also in a white background, but with red eyes). Df(3L)dilp2-3,dilp53 (30889), designated in this study as dilp2,3,5-/-; dilp51 (30884) designated as dilp5-/-; dilp21 (30881), designated as dilp2-/-; dilp31 (30882), designated as dilp3-/-; chicoKG00032 (14337); InREY00681 (15306); pdf-Gal4 (6900); akh-Gal4 (25684); Lk6DJ634-Gal4 (8614); cg-Gal4 (7011) were from Bloomington Drosophila Stock Center; UAS-dilp5-RNAi (v49520) was from VDRC; white ls from our lab.
Diapause
Larvae were reared in 12:12 Light/Dark cycles (LD12:12) at 23°C until pupal eclosion. Newly-eclosed adults (~ 60 females and 60 males) were collected within 5 hours of eclosion and exposed to 12°C (diapause-inducing temperature). Each of these samples provided a single ‘replicate’. After 11 days at least 50–60 surviving females were dissected (this number was reduced at 28 days) and scored as “diapausing” or not depending on the complete absence of developing vitellogenic oocytes in both gonads, in accordance with references [2, 7]. This provides a reliable and unambiguous all or none readout of the phenotype [7]. ANOVA (with Tukey post-hoc tests) were performed using R statistical software (2.15.1) on the arcsin transformed diapause percentages.
Genotyping of timeless locus
Amplification Refractory Mutation System (ARMS) PCRs [2] was performed to identify tim alleles. Genomic DNA was extracted independently from 10 males of each strain. Their homogenate was incubated at 37°C for 45 min in 50μL Solution A (Tris HCl pH8.2 10mM, EDTA 2mM, NaCl 25mM) plus 1μL Proteinase K (10mg/mL) and, then, 3 min at 100°C. Supernatant was processed via ARMS PCR as in [2]. Forward tim primers: 5’-tggaataatcagaactttga-3’ (ls-tim); 5’-tggaataatcagaactttat-3’ (s-tim). Reverse (common) primer: 5’-agattccacaagatcgtgtt-3’ (tim).
cpo sequencing
For cpo genotyping, the genomic DNA of several individuals from each strain used in the diapause assay was extracted, and the cpo region encompassing the nucleotide polymorphisms encoding the cpoA347V and cpo48034(A/T) was amplified via PCR. Primers used were: forward primer 5'-aacatccgttgctgctgtc-3’ reverse primer 5'-ccccaagctgtcacttttgt-3’ Templates were purified through minicolumns (Wizard® SV Gel and PCR Clean-Up System) and subsequently sequenced.
Luciferase Reporter Gene Assay
The FoxO.RE-Luciferase transgene contains a firefly luciferase reporter gene under the control of 8 consecutive FoxO-response elements (FRE) [41]. FoxO.RE-Luc larvae were reared as for the diapause assay. Females were dissected in dry ice and, then, frozen at -80°C until processing. Luciferase extraction was performed using the Promega Luciferase Assay System (Firefly Luciferase). 150μL lysis buffer (LB) was added to 40 abdomens (9 biological replicates). Samples were frozen in liquid nitrogen and thawed in a 37°C water bath three times and then centrifuged to remove debris. This process was repeated and the two resulting supernatants were combined and stored at -70°C. Luciferase activity of adult protein extracts was measured using a Berthold Technologies Luminometer microplate scintillation and counter. 100μL of Promega Luciferase Assay Reagent was added to 20μL of protein extract and light production was measured in relative light units (RLU) emitted over a 10 s time period. Final Luciferase values (Lv) were normalized to the protein concentration (RLU/μg of protein). Protein concentration was determined using the Pierce BCA Protein Assay Kit–Reducing Agent Compatible. Data from two temperatures (12 and 23°C) were compared using t-test.
qPCR and Gene expression
mRNA was extracted from 50 isolated heads (3 biological replicates) or 30 whole bodies (5 biological replicates) of adult females by using TRIzol (Invitrogen) and RNeasy Mini Kit and RNase-Free DNase Set (QIAGEN), respectively. The first-strand cDNA was synthesized by using the Invitrogen SuperScript II First-Strand Synthesis SuperMix. qPCR was performed in LightCycler DNA Master SYBR Green I (Roche) on LightCycler 480 System (Roche). Primers are following: dilp2 (heads), F: 5’-gtatggtgtgcgaggagtat, R: 5’-tgagtacacccccaagatag; dilp5 (heads), F: 5’-agttctcctgttcctgatcc, R: 5’-cagtgagttcatgtggtgag; rp49 (heads), F: 5’-agggtatcgacaacagagtg, R: 5’-caccaggaacttcttgaatc; dilp2 (body), F: 5’-acgaggtgctgagtatggtgtgcg, R: 5’-cacttcgcagcggttccgatatcg; dilp5 (body), F: 5’-tgttcgccaaacgaggcaccttgg, R: 5’-cacgatttgcggcaacaggagtcg; rpL23 (body), F: 5’-gacaacaccggagccaagaacc, R: 5’-gtttgcgctgccgaataaccac. For each dilps transcript, we normalized message levels relative to rpL23 (whole body) or rp49 (isolated heads) housekeeping genes by using the 2-ΔΔCT. Data were analyzed using t-test.
Supporting Information
Females from all highly diapausing lines used throughout the experiments (Df(3L)dilp1-5/dilp2,3,5-; chicoKG00032; dilp2>hid,rpr; InsP3>hid,rpr; dilp2(p)>Ork1 and c929>sImp-L2) exposed for 11 days at 23°C exhibited normal gonadal maturation comparable to controls (c929>+; InsP3>+; dilp2>+ and dilp2(p)>+). Therefore, at 23°C all ovaries were vitellogenic, indicating that the mutants listed above and genetically manipulated strains were fertile. Consequently, the non-vitellogenic phenotypes observed in diapausing conditions (12°C) were true diapause phenotypes. Bars = 0.2 mm.
(TIF)
(DOCX)
Females of high diapause strains dissected after 11 days at 23°C show no diapause. Flies were collected after 5 h post eclosion and exposed to 23°C LD12.12 for 11 days before dissection.
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
L.S. was supported by a doctoral fellowship from the University of Padova (Italy) and by NIH grant GM093301 (USA); R.C. was supported by grants from the European Community (the 6th Framework Project EUCLOCK no. 018741), Fondazione Cariparo (Progetti di Eccellenza 2011–2012), and the Ministero dell’Università e delle Ricerca (MIUR); C.P.K. by BBSRC grant BB/F014082/1. R.C. and C.P.K. are also supported by the INsecTIME Marie Curie Initial Training Network, grant PITN-GA-2012-316790. G.A. was supported by doctoral fellowships from the Fondazione CaRiPaRo (Italy). L.S. also thanks Fondazione Ing. “A. Gini” of University of Padova (Italy); M.B.O. is supported by NIH grant RO1GM093301 (USA).
References
- 1.Schiesari L, O’Connor MB. Diapause: delaying the developmental clock in response to a changing environment. Curr. Top. Dev. Biol. 2013;105: 213–246. 10.1016/B978-0-12-396968-2.00008-7 [DOI] [PubMed] [Google Scholar]
- 2.Tauber E, Zordan M, Sandrelli F, Pegoraro M, Osterwalder N, Breda C, et al. Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science. 2007;316: 1895–1898. 10.1126/science.1138412 [DOI] [PubMed] [Google Scholar]
- 3.Schimdt PS, Paaby AB. Reproductive diapause and life-history clines in north American populations of Drosophila melanogaster. Evolution. 2008;62: 1204–1215. 10.1111/j.1558-5646.2008.00351.x [DOI] [PubMed] [Google Scholar]
- 4.Schimdt PS, Matzkin L, Ippolito M, Eanes WF. Geographic variation in diapause incidence, life-history traits, and climatic adaptation in Drosophila melanogaster. Evolution. 2005;59: 1721–1732. 10.1111/j.0014-3820.2005.tb01821.x [DOI] [PubMed] [Google Scholar]
- 5.Sandrelli F, Tauber E, Pegoraro M, Mazzotta G, Cisotto P, Landskron J, et al. A molecular basis for natural selection at the timeless locus in Drosophila melanogaster. Science. 2007;316: 1898–1900. 10.1126/science.1138426 [DOI] [PubMed] [Google Scholar]
- 6.Cogni R, Kuczynski C, Koury S, Lavington E, Behrman EL, O'Brien KR, et al. The intensity of selection acting on the couch potato gene-spatial-temporal variation in a diapause cline. Evolution. 2014;68: 538–548. 10.1111/evo.12291 [DOI] [PubMed] [Google Scholar]
- 7.Schmidt PS, Zhu CT, Das J, Batavia M, Yang L, Eanes WF. An amino acid polymorphism in the couch potato gene forms the basis for climatic adaptation in Drosophila melanogaster. Proc Natl Acad Sci U.S.A. 2008;105: 16207–16211. 10.1073/pnas.0805485105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SF, Sgrò CM, Shirriffs J, Wee CW, Rako L, van Heerwaarden B, et al. Polymorphism in the couch potato gene clines in eastern Australia but is not associated with ovarian dormancy in Drosophila melanogaster. Mol Ecol. 2011;20 2973–2984. 10.1111/j.1365-294X.2011.05155.x [DOI] [PubMed] [Google Scholar]
- 9.Sezgin E, Duvernell DD, Matzkin LM, Duan Y, Zhu CT, Verrelli BC, et al. Single-locus latitudinal clines and their relationship to temperate adaptation in metabolic genes and derived alleles in Drosophila melanogaster. Genetics. 2004;168: 923–931. 10.1534/genetics.104.027649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson AR, Hoffmann AA, McKechnie SW, Umina PA, Weeks AR. The latitudinal cline in the In(3R)Payne inversion polymorphism has shifted in the last 20 years in Australian Drosophila melanogaster populations. Mol Ecol. 2005;14: 851–858. 10.1111/j.1365-294X.2005.02445.x [DOI] [PubMed] [Google Scholar]
- 11.Williams KD, Sokolowski MB. Diapause in Drosophila melanogaster females: a genetic analysis. Heredity. 1993;71: 312–317. 10.1038/hdy.1993.141 [DOI] [PubMed] [Google Scholar]
- 12.Williams KD, Busto M, Suster ML, So AK, Ben-Shahar Y, Leevers SJ, et al. Natural variation in Drosophila melanogaster diapause due to the insulin-regulated PI3-kinase. Proc Natl Acad Sci U.S.A 2006;103: 15911–15915. 10.1073/pnas.0604592103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paaby AB, Blacket MJ, Hoffmann AA, Schmidt PS. Identification of a candidate adaptive polymorphism for Drosophila life history by parallel independent clines on two continents. Mol Ecol. 2010;19: 760–774. 10.1111/j.1365-294X.2009.04508.x [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Jack J, Garofalo RS. The Drosophila insulin receptor is required for normal growth. Endocrinology. 1996;137: 846–856. 10.1210/endo.137.3.8603594 [DOI] [PubMed] [Google Scholar]
- 15.Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292: 107–110. 10.1126/science.1057987 [DOI] [PubMed] [Google Scholar]
- 16.Kubrak OI, Kučerová L, Theopold U, Nässel DR. The sleeping beauty: how reproductive diapause affects hormone signaling, metabolism, immune response and somatic maintenance in Drosophila melanogaster. PLoS One. 2014;9: e113051 10.1371/journal.pone.0113051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grönke S, Clarke DF, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genetics. 2010;6: e1000857 10.1371/journal.pgen.1000857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296: 1118–1120. 10.1126/science.1070058 [DOI] [PubMed] [Google Scholar]
- 19.Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 2002;12: 1293–1300. 10.1016/s0960-9822(02)01043-6 [DOI] [PubMed] [Google Scholar]
- 20.Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science. 2012;336: 579–582. 10.1126/science.1216735 [DOI] [PubMed] [Google Scholar]
- 21.Colombani J, Andersen DS, Leopold P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science. 2012;336: 582–585. 10.1126/science.1216689 [DOI] [PubMed] [Google Scholar]
- 22.Slaidina M, Delanoue R, Grönke S, Partridge L, Léopold P. A Drosophila insulin-like peptide promotes growth during non-feeding states. Dev. Cell. 2009;17: 874–884. 10.1016/j.devcel.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song W, Ren D, Li W, Jiang L, Cho KW, Huang P, et al. SH2B regulation of growth, metabolism, and longevity in both insects and mammals. Cell Metab. 2010;11: 427–437. 10.1016/j.cmet.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgar BA. How flies get their size: genetics meets physiology. Nature Genetics Review. 2006;7: 907–916. 10.1038/nrg1989 [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Liu J, Li CR, Momen B, Kohanski RA, Pick L. Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc. Natl. Acad. Sci. U.S.A. 2009;106: 19617–19622. 10.1073/pnas.0905083106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Géminard C, Rulifson EJ, Léopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10: 199–207. 10.1016/j.cmet.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 27.Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. U.S.A. 2005;102: 3105–3110. 10.1073/pnas.0405775102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab. 2008;7: 321–332. 10.1016/j.cmet.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 29.Broughton SJ, Slack C, Alic N, Metaxakis A, Bass TM, Driege Y, et al. DILP-producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell. 2010;9: 336–346. 10.1111/j.1474-9726.2010.00558.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109: 485–495. 10.1016/S0092-8674(02)00737-7 [DOI] [PubMed] [Google Scholar]
- 31.Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, et al. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26: 479–489. 10.1523/JNEUROSCI.3915-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167: 761–781. 10.1534/genetics.104.026427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taghert PH, Hewes RS, Park JH, O'Brien MA, Han M, Peck ME. Multiple amidated neuropeptides are required for normal circadian locomotor rhythms in Drosophila. J Neurosci. 2001;21: 6673–6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park D, Veenstra JA, Park JH, Taghert PH. Mapping peptidergic cells in Drosophila: where DIMM fits in. PLoS One. 2008;3:e1896 10.1371/journal.pone.0001896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto N, Nakamori R, Murai T, Yamauchi Y, Masuda A, Nishimura T. A secreted decoy of InR antagonizes insulin/IGF signaling to restrict body growth in Drosophila. Genes Dev. 2013;27: 87–97. 10.1101/gad.204479.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seroude L, Brummel T, Kapahi P, Benzer S. Spatio-temporal analysis of gene expression during aging in Drosophila melanogaster. Aging Cell. 2002;1: 47–56. 10.1046/j.1474-9728.2002.00007.x [DOI] [PubMed] [Google Scholar]
- 37.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004;14: 885–890. 10.1016/j.cub.2004.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belgacem YH, Martin JR. Hmgcr in the corpus allatum controls sexual dimorphism of locomotor activity and body size via the insulin pathway in Drosophila. PloS One. 2007;2: e187 10.1371/journal.pone.0000187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones D, Jones G, Teal P, Hammac C, Messmer L, Osborne K, et al. Suppressed production of methyl farnesoid hormones yields developmental defects and lethality in Drosophila larvae. Gen.Comp. Endo. 2010;165: 244–254. 10.1016/j.ygcen.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee G, Park JH. Haemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167: 311–323. 10.1534/genetics.167.1.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang HY, Smith-Caldas MS, Driscoll MV, Salhadar S, Shingleton AW. FOXO regulates organ-specific phenotypic plasticity in Drosophila. PLoS Genetics. 2011;7: e1002373 10.1371/journal.pgen.1002373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309: 1071–1073. 10.1126/science.1111410 [DOI] [PubMed] [Google Scholar]
- 43.Alic N, Hoddinott MP, Vinti G, Partridge L. Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumor suppressor. Aging Cell. 2011;10: 137–147. 10.1111/j.1474-9726.2010.00653.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flatt T, Min KJ, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, et al. Drosophila germ-line modulation of insulin signaling and lifespan. Proc. Natl. Acad. Sci. U.S.A. 2008;105: 6368–6373. 10.1073/pnas.0709128105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhan S, Merlin C, Boore JL, Reppert SM. The monarch butterfly genome yields insights into long-distance migration. Cell. 2011;147: 1171–1185. 10.1016/j.cell.2011.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sim C, Denlinger DL. A shut-down in expression of an insulin-like peptide, ILP-1, halts ovarian maturation during the overwintering diapause of the mosquito Culex pipiens. Insect Mol Biol. 2009;18: 325–332. 10.1111/j.1365-2583.2009.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daimon T, Kozaki T, Niwa R, Kobayashi I, Furuta K, Namiki T, et al. Precocious metamorphosis in the Juvenile Hormone–Deficient Mutant of the silkworm, Bombyx mori. PLoS Genetics. 2012;8: e1002486 10.1371/journal.pgen.1002486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riddiford LM, Truman JW, Mirth CK, Shen YC. A role for juvenile hormone in the pre-pupal development of Drosophila melanogaster. Development. 2010;137: 1117–1126. 10.1242/dev.037218 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Females from all highly diapausing lines used throughout the experiments (Df(3L)dilp1-5/dilp2,3,5-; chicoKG00032; dilp2>hid,rpr; InsP3>hid,rpr; dilp2(p)>Ork1 and c929>sImp-L2) exposed for 11 days at 23°C exhibited normal gonadal maturation comparable to controls (c929>+; InsP3>+; dilp2>+ and dilp2(p)>+). Therefore, at 23°C all ovaries were vitellogenic, indicating that the mutants listed above and genetically manipulated strains were fertile. Consequently, the non-vitellogenic phenotypes observed in diapausing conditions (12°C) were true diapause phenotypes. Bars = 0.2 mm.
(TIF)
(DOCX)
Females of high diapause strains dissected after 11 days at 23°C show no diapause. Flies were collected after 5 h post eclosion and exposed to 23°C LD12.12 for 11 days before dissection.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.