Abstract
Host responses to pathogens include defenses that reduce infection burden (i.e., resistance) and traits that reduce the fitness consequences of an infection (i.e., tolerance). Resistance and tolerance are affected by an organism's physiological status. Corticosterone (“CORT”) is a hormone that is associated with the regulation of many physiological processes, including metabolism and reproduction. Because of its role in the stress response, CORT is also considered the primary vertebrate stress hormone. When secreted at high levels, CORT is generally thought to be immunosuppressive. Despite the known association between stress and disease resistance in domesticated organisms, it is unclear whether these associations are ecologically and evolutionary relevant in wildlife species. We conducted a 3x3 fully crossed experiment in which we exposed American toads (Anaxyrus [Bufo] americanus) to one of three levels of exogenous CORT (no CORT, low CORT, or high CORT) and then to either low or high doses of the pathogenic chytrid fungus Batrachochytrium dendrobatidis (“Bd”) or a sham exposure treatment. We assessed Bd infection levels and tested how CORT and Bd affected toad resistance, tolerance, and mortality. Exposure to the high CORT treatment significantly elevated CORT release in toads; however, there was no difference between toads given no CORT or low CORT. Exposure to CORT and Bd each increased toad mortality, but they did not interact to affect mortality. Toads that were exposed to CORT had higher Bd resistance than toads exposed to ethanol controls/low CORT, a pattern opposite that of most studies on domesticated animals. Exposure to CORT did not affect toad tolerance to Bd. Collectively, these results show that physiological stressors can alter a host’s response to a pathogen, but that the outcome might not be straightforward. Future studies that inhibit CORT secretion are needed to better our understanding of the relationship between stress physiology and disease resistance and tolerance in wild vertebrates.
Introduction
Hosts responses to pathogens can be broken into two strategies: those that prevent pathogen colonization or reduce infection burden (i.e., resistance) and those that reduce the fitness consequences of an infection (i.e., [1, 2]). Resistance reduces host infection burden and can thereby reduce disease prevalence within populations of hosts. In doing so, host resistance imposes negative selection pressures on pathogens, which can potentially increase their virulence within a population [3]. In contrast, tolerance responses mitigate the fitness consequences association with an infection (e.g., reducing self-damage from immune responses). Tolerance is measured as the slope of the relationship between the change in fitness and change in parasite burden [1, 2]. The fitness of tolerant host genotypes are relatively unaffected by an increasing pathogen burden [2]. Thus, in contrast to resistance responses, tolerance responses do not reduce or eliminate pathogen infection burdens and thus are hypothesized to increase pathogen prevalence within a host population while having neutral or positive selection pressures on pathogen virulence (but see [3, 4]). Although host genotypes vary naturally in their resistance and tolerance to infection, these responses are often also mediated by environmental conditions in which hosts and pathogens interact (reviewed in [5]). In addition, host traits such as sex [6], age/developmental stage [7], nutritional condition [8], and physiological state [9] are known to affect host immunocompetence and thus their resistance and tolerance to pathogens.
These context dependent effects on host immunity, coupled with a growing interest in differentiating between host resistance and tolerance in animal-pathogen systems, has generated considerable interest in the relationship between host condition and disease. Stress hormones, such as glucocorticoids, are associated with host condition because they are released during periods of stress and can have profound effects on the physiological state of vertebrates. For instance, corticosterone (hereafter “CORT”; the primary amphibian stress hormone) mobilizes energy reserves, which are often needed during the stress response [10]. In addition, CORT can alter the strength, distribution, and character of host immune defenses [11] and can mediate their responses to pathogens [12].
When CORT is chronically elevated, it typically reduces host resistance to infection [12] by limiting immunological defenses (glucocorticoids can also enhance immune functions in certain contexts [13]). For instance, inflammatory responses promote host resistance because they prevent the establishment and persistence of pathogens, even those for which a host does not have any immune memory. Inflammation, however, is dampened when CORT remains chronically high [11] and can thus reduce host resistance. Some evidence suggests that CORT can promote anti-inflammatory cytokines that reduce host damage associated with inflammation [14], which is thought to promote tolerance to pathogens [15]. Because innate immune responses begin with inflammatory responses and inflammation is an effective response to a diversity of pathogens, CORT is particularly relevant to ecological and evolutionary research questions targeted at host-pathogen interactions.
Despite knowing the effects of CORT on physiological and immunological responses of domesticated animals, few studies have manipulated this stress hormone to test how CORT mediates host-pathogen interactions using wild vertebrates (but see [16, 17–19]). Here, we present the results of a laboratory experiment in which we examined how CORT affects amphibians’ resistance and tolerance to Batrachochytrium dendrobatidis (Bd). Bd is a fungal disease of amphibians that causes the disease chytridiomycosis. Amphibians that develop chytridiomycosis generally undergo hyperkeratosis (the thickening of the stratum corneum), which disrupts cutaneous function and can lead to cardiac arrest [20]. Bd has been implicated in the declines of hundreds of amphibian species worldwide [21]. Bd generally causes high mortality in many species of amphibians but some individuals and species are known to be resistant or tolerant to infection [22–24]. In addition, amphibian resistance to Bd can be dependent on host condition [25, 26] and there is an association between Bd infection CORT release in amphibians [27–30], suggesting that host physiological condition might mediate resistance or tolerance to Bd.
We utilized a 3 x 3 fully factorial laboratory experiment in which we exposed American toads (Anaxyrus [Bufo] americanus) to one of three doses of CORT (high, low, and none) and then challenged them with Bd (high, low, and none) and measured how changes in their survival, resistance, and tolerance. We predicted that toads exposed to high doses of Bd would have higher Bd infections and mortality compared to toads given low doses (i.e., decreased resistance). We did not hypothesize that Bd dose would interact with CORT dose and thus we predicted and that the effects of Bd dose would occur independent of their exposure to CORT. If CORT is immunosuppressive the doses we used in our experiment, we predicted that exposure to CORT would reduce toad resistance to Bd (measured by infection abundance) and that Bd-induced mortality would increase as a function of increasing CORT dose. Because CORT is thought to promote tolerance to pathogens, we predicted that toads in the high CORT treatment would have higher tolerance to Bd than toads given low or no CORT (i.e., the slope of the relationship between change in fitness and Bd abundance would be negative for toads in the low or no CORT but flat for toads in the high CORT treatment).
Methods
Animal collection and husbandry
We collected recently hatched American toad (Anaxyrus [Bufo] americanus) tadpoles (stage 26–27 [31]) from a pond in Crawford County, Pennsylvania. Tadpoles were transported to the laboratory and immediately separated into five plastic containers (35.6 x 20.3 x 11.7 cm) at a density of approximately 6 tadpoles/L. The containers were gently aerated and were held in an environmental chamber at 28°C (± 1°C) on a 12: 12 light: dark photoperiod. The tadpoles were fed an ad libitum diet that consisted of a mixture of Sera Micron® (an algal based food) and spinach 4–5 times per week. We changed approximately 75% of the water from each container on a weekly basis.
The tadpoles completed metamorphosis between 02 June 2014 and 09 June 2014. Throughout the larval stage, we checked the containers 3 x each day and placed individuals with 4 legs into vented rectangular plastic containers (12.5 x 10 x 5 cm) that contained a tri-fold paper towel and approximately 15 mL of aged tap water. The containers were placed at a 20° angle so that the metamorphosing toad had access to water and also a damp substrate. Once the toad had fully absorbed its tail, we considered the individual a “juvenile” and moved it to an environmental chamber at 18°C (±1°C) on a 12: 12 light: dark photoperiod. Juvenile toads were housed in vented circular plastic containers (7.5 cm deep and 11 cm diameter) on tri-fold paper towels that were soaked with 12mL of aged tap water. Juvenile toads were fed fruit files (Drosophila sp.) ad libitum 2–3 times per week until they were large enough to consume crickets. At that point, and throughout the remainder of the experiment, they were fed 4–6 crickets (0.318–0.635 cm) per week. We replaced the paper towel bedding with fresh bedding on a weekly basis.
Experimental design
Our experiment utilized a 3 x 3 fully factorial design in which we randomly assigned 90 juvenile toads to one of the 9 resulting treatments (n = 10/treatment) and tested for main and interactive effects of CORT and Bd on toad survival, resistance, and tolerance. On 23 June 2014, we exposed toads to one of three CORT treatments: 0.1 μM solution, 0.01 μM solution, or an ethanol control. We followed the protocol of Searle et al. [28] to make our CORT solutions. In brief, we dissolved 0.0056 g or 0.0561 g of CORT into one of two vials that contained 9.72 mL of ethanol (representing our low and high CORT treatments). We then applied the designated CORT treatment (or ethanol) and 15 mL of water to a fresh tri-fold paper towel every other day for 10 consecutive days (i.e., 5 applications). This volume was sufficient to fully saturate the paper towel so that the toad was always in contact with solution. The application of exogenous CORT directly on the bedding of post-metamorphic anurans at similar levels and durations (0.5 μM daily for 15 days) has been shown to elevate circulating levels of whole-body CORT to physiologically relevant levels [28]. Twenty four hours after the last CORT application, we conducted a bedding change and placed each toad on a paper towel that contained 12 mL of aged tap water. The toad was held on this paper towel for an additional 24 hours to avoid measuring any remaining exogenous CORT from the bedding in our water-borne CORT assay (see below).
Quantification of water-borne CORT
We extracted and quantified water-borne CORT following the protocol of Gabor et al. [32], which has been validated on a number of species of frogs, toads, and salamanders [27, 32, 33] and also in fish (reviewed in [34]). After the toads were in their focal containers for 25 hours, we transferred toads to individual, sterilized, Petri® dishes (diameter: 140 mm; depth: 25 mm) filled with 50 mL of water for 60 m. All toads were sampled at the same day and time to avoid their potentially confounding effects. The water samples were stored at -20°C until the extraction of the water-borne CORT.
We randomly selected 30 toads (n = 10 from each of the three CORT treatments) and analyzed the total (free + conjugated) CORT released in the water samples. We first thawed each water sample and extracted CORT from the entire sample by passing water through a sterilized 60mL syringe (309653; Becton Dickinson, NJ, USA) into C18 solid phase extraction columns (SEP-PAK, Waters Inc.). Prior to use, the columns were primed with 4 mL HPLC-grade methanol and 4 mL of millipore filtered water. We eluted the columns with 4 mL methanol into borosilicate vials and the methanol was evaporated under a stream of nitrogen gas at 37°C using an Evap-O-Rac (Cole-Palmer). Samples were re-suspended in 20 μL of 100% ethanol, vortexed for 1 m, and then in 380 μL EIA buffer (Cayman Chemicals Inc., MI, USA) for a final volume of 400 μL. CORT was measured using an enzyme-immunoassay (EIA) kit (Cayman Chemicals Inc.). All samples were run in duplicate. All CORT data are presented as pg/sample (pg/mL multiplied by 0.40 mL, which accounts for the amount of EIA buffer used to reconstitute the sample).
Bd exposures
The Bd isolate that we used in our experiment (JEL 660) was isolated from a wild-collected anuran in Ohio and cryopreserved at the University of Maine since isolation. The stock culture used in our experiment has been held in 1% tryptone broth at 5C in the laboratory at Allegheny College since 2014. Throughout this time, we transferred the culture to new media every 4–5 months (approximately 3 times prior to the start of the experiment).
Immediately after removing the toads from their Petri® dishes (above), we placed them back into their respective focal container and exposed them to one of three Bd treatments by pipetting 2.0 mL of tryptone broth that contained either 8.0x104 zoospores, 2.0x104 zoospores, or a sham exposure that did not contain any zoospores onto the dorsal side of each toad. Excess innocula was allowed to drip onto the paper towel substrate, which formed a thin layer of innocula. The toads were held on this substrate for 48 hours, after which we replaced the Bd-exposed (or tryptone) bedding with fresh bedding as described above. To prevent accidental transfer of Bd zoospores between toads, the experimenter used a different nitrile glove for each animal when conducting the bedding changes. Throughout the experiment, all equipment was either autoclaved or placed in a 10% bleach solution to kill Bd [35].
Bd is implicated in global amphibian declines and infections are expected to induce symptoms of chytridiomycosis and also death. Throughout the duration of the experiment, we monitored the toads twice daily for a proper righting response or for mortality. Animals that did not exhibit a righting response were deemed pre-moribund and euthanatized to alleviate pain and discomfort. Toads that lost their righting response were placed individually in a glass beaker that contained approximately 50 mL of an overdose of MS222 (≥ 250 mg/L) to anesthetize and euthanize. Any dead toad or toad that lost its righting response was massed (to the nearest 0.01 g), immediately swabbed for Bd quantification (see below), and placed in a -20°C freezer. We terminated the experiment 28 days after the toads were exposed to Bd, at which all surviving toads were massed and then euthanatized with an overdose of MS222 (described above).
Swabbing and qPCR details
Bd infection on the toads was measured by swabbing each animal on Day 7 post-exposure or on the day a toad died. We passed a sterile swab (Medical Wire & Equipment; MW 113) across the dorsal surface of each toad a total of 10 times. We also recorded the mass of each toad (to the nearest 0.01 g) on Day 7. To prevent cross-contamination with Bd or Bd DNA, the experimenter used a different pair of nitrile gloves while swabbing and massing each toad.
Our DNA extractions and qPCR analyses followed the methods of Boyle et al. [36] and modified by Hyatt et al. [37]. Test samples were run singly instead of triplicate to control costs, as recommended by Kriger et al. [38]. We added TaqMan® Exogenous Internal Positive Control (Exo IPC) Reagents (Applied Biosystems, Foster City, CA) to every reaction well to assess inhibition of the PCR reaction [37]. The Exo IPC system uses a standardized concentration of an artificial DNA sequence that is added to each reaction well with its own set of primers and a separate fluorescent probe. The strength of this reaction is used to assess overall reaction inhibition. Extractions were diluted 1:100 and processed in an Applied Biosystems Step One Real-time PCR system. None of our samples were inhibited and thus did not require any further dilution. We considered infection intensity as the number of Bd zoospore equivalents per sample. Zoospore equivalents were calculated by multiplying the genome equivalent values generated by the qPCR assay by 80, which accounts for the fact that the DNA from the swabs was diluted 80-fold during extraction and qPCR preparation. We considered a sample Bd positive when zoospore equivalents were ≥ 1 [39, 40].
Statistical analyses
Our first statistical analyses tested for differences in CORT release in the water (pg/sample) in toads from the three CORT treatments (categorical responses: none, low, and high). We added mass (taken that day) as a co-variate in the analysis to account for size differences in the toads. We used a liner model ("lm" function in R statistical software) and assessed statistical significance (p < 0.05) using the "Anova" function in the "car" package of R statistical software. Although the toads given high CORT had higher CORT release into the water compared to the other two treatments, there was no difference between the CORT release in the water between the toads given no CORT and low CORT (see Results and Fig 1), we combined the toads from the no CORT and low CORT treatments into a single treatment in the following statistical analyses (see S1 Appendix for the statistical procedures and results using three CORT treatments instead of two).
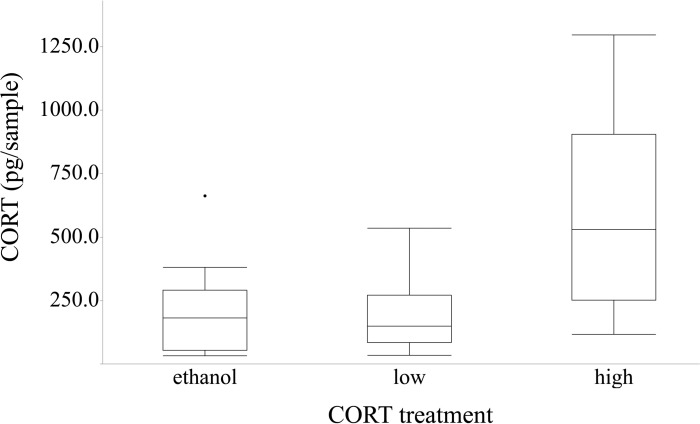
Fig 1. Corticosterone (CORT) release rates in toads.
Corticosterone (CORT) release rates of American toads (Anaxyrus [= Bufo] americanus) exposed to either an ethanol control, low CORT (0.01 μM), or high CORT (0.1 μM). Toads in the high CORT treatment had significantly higher CORT than the toads in either the ethanol or low CORT treatments.
We used the “coxph” function in the “survival” package in R, which uses a Cox proportional hazards regression model. This statistical model censors all individuals surviving on day 28 to account for our lack of information about their true times of death, a standard approach to down-weight the influence of these individuals in the survival analysis. In this analysis, we tested for main and interactive effects of the categorical response variables CORT (none/low and high) and Bd dose (none, low, and high) on toad survival. We assessed statistical significance (P < 0.05) using the “Anova” function in the “car” package of R.
Our next statistical models compared differences in host resistance using Bd abundance (i.e., the infection intensity of all Bd-exposed toads, including those with a "0" infection burden) as a response variable. Our first model tested for differences in resistance on Day 7. Here, we excluded the Bd-exposed toads that died prior to Day 7 (n = 11). We tested for the main and interactive effects of CORT exposure (none/low and high) and Bd dose (low and high) on our response variable (log transformed Bd+1). By definition, "resistance" refers to the ability of a host to prevent or eliminate an infection [2] and non-exposed animals are not considered in analyses of resistance because one would not expect them to have any pathogen burden. We included the pre-experiment mass of each toad and the number of days that the toad survived as covariates in the analysis because large toads could have more Bd simply because of their body size. We used number of days alive to avoid the potentially confounding factor of differences in the number of days that Bd could grow on the toads. In our second model, we used the maximum Bd infection, which we defined as the maximum Bd abundance we detected on a swab from either Day 7 or when the toad died (if it died). We used the same predictors as described above. We assessed statistical significance (P < 0.05) in both models using the "Anova" function in the "car" package of R statistical software.
To test for differences in tolerance, we used the percentage of mass change throughout the experiment (or when the toad died) as a proxy for host fitness because anuran metamorphic mass is generally a good predictor of fitness [41]. To account for differences in vigor (i.e., fitness in the absence of parasite exposure), we included all of the toads that were given a sham (control) Bd dose in our analysis. We used the "lm" function in R and tested for main and interactive effects of CORT exposure (none/low and high) and log-transformed maximum detected Bd abundance (i.e., the highest Bd infection value obtained from Day 7 or the date of death) on host fitness. We also included the number of days alive as a covariate in the analysis. We assessed statistical significance (p < 0.05) using the “Anova” function in the “car” package of R.
Ethical approval
All applicable institutional and national guidelines for the care and use of animals were followed. The protocol was approved by the Institutional Animal Care and Use Committee (through the Animal Research Committee) of Allegheny College. American toad tadpoles were collected through a Scientific Collectors Permit granted to MDV through the PA Fish and Boat Commission.
Results
The CORT release into the water differed among treatment groups (ANOVA, F2,26 = 7.505, P = 0.003), even after controlling for the effects of mass (F1,26 = 0.261, P = 0.614) on CORT release. Toads exposed to ethanol and a low dose of CORT had similar CORT release and toads exposed to a high dose of CORT had a higher CORT release than the other two treatments (Fig 1). The intra-assay coefficient of variation for the plate was 12.5% (determined by the dividing standard deviation for each duplicate sample by the sample mean to obtain a sample coefficient of variation and then averaging all of the individual coefficient of variation values) and the standards had a good fit to the standard curve (r2 = 0.9896).
None of the toads that were given a sham exposure were infected with Bd (0% prevalence and 0.00 zoospores). The average infection intensity of toads infected with Bd (excluding the exposed but non-infected toads) was 189.29 (SE ± 69.82) and 1089.11 (SE ± 367.05) zoospores in the low and high treatments, respectively.
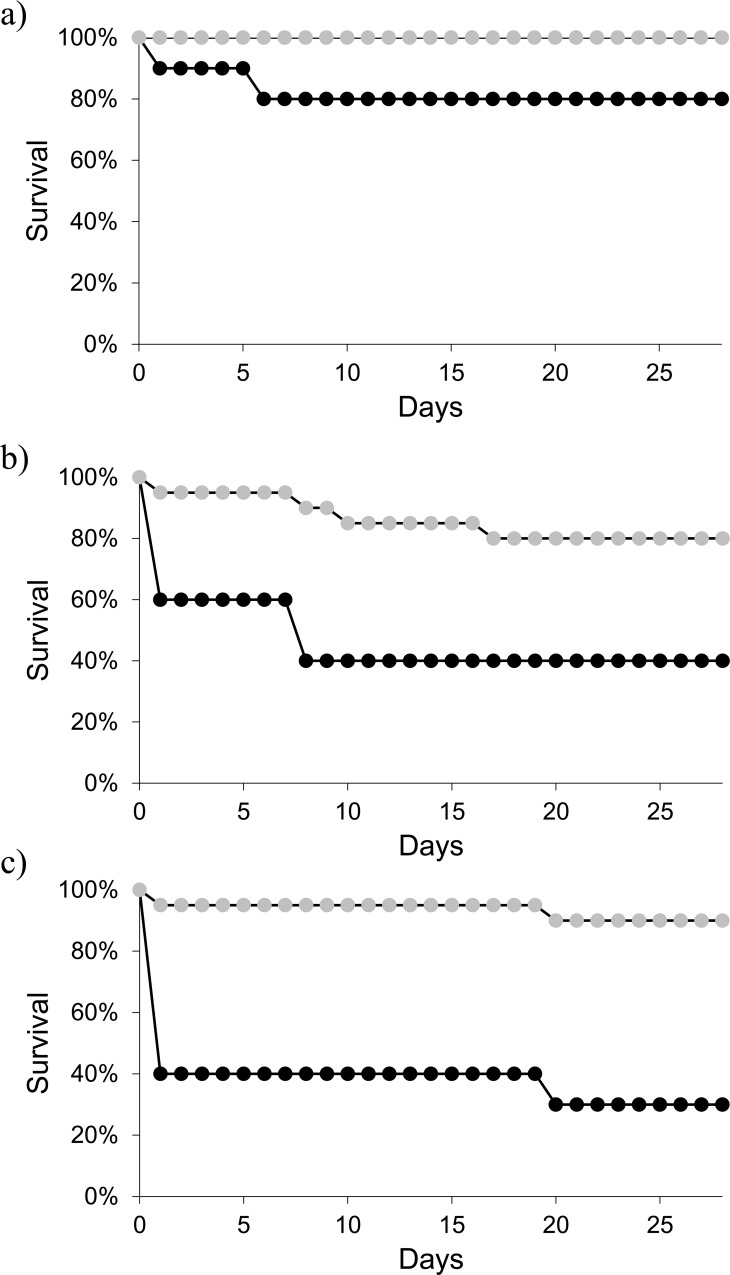
Exposure to Bd increased toad mortality (Cox, X2 = 12.204, P = 0.002) and the overwhelming majority of the toads that died during the experiment died within 24 hours of exposure to Bd (Fig 2B and 2C). Irrespective of their CORT treatment, toads that were exposed to Bd had higher mortality (~33% in the low Bd treatment and ~37% in the high Bd treatment compared to non-exposed toads (~6%). Toads that were exposed to CORT had significantly higher mortality than did toads not exposed to CORT (Cox, X2 = 17.848, P < 0.001). Although the interaction between Bd dose and CORT was nonsignificant (Cox, X2 = 1.494, P = 0.474). See S1 Appendix and S1 Fig for the results using three CORT doses.
Fig 2. Survival plots of toads.
Survival plots of American toads (Anaxyrus [= Bufo] americanus) exposed to (a) a control treatment without Batrachochytrium dendrobatidis ("Bd), (b) a low dose of Bd, and (c) a high dose of Bd. Grey circles represent toads exposed to the ethanol/low CORT dose (n = 20 per panel); black circles represent toads exposed to a high CORT dose (n = 10 per panel). Exposure to Bd and CORT independently reduced survival, but the two factors did not interact to affect survival.
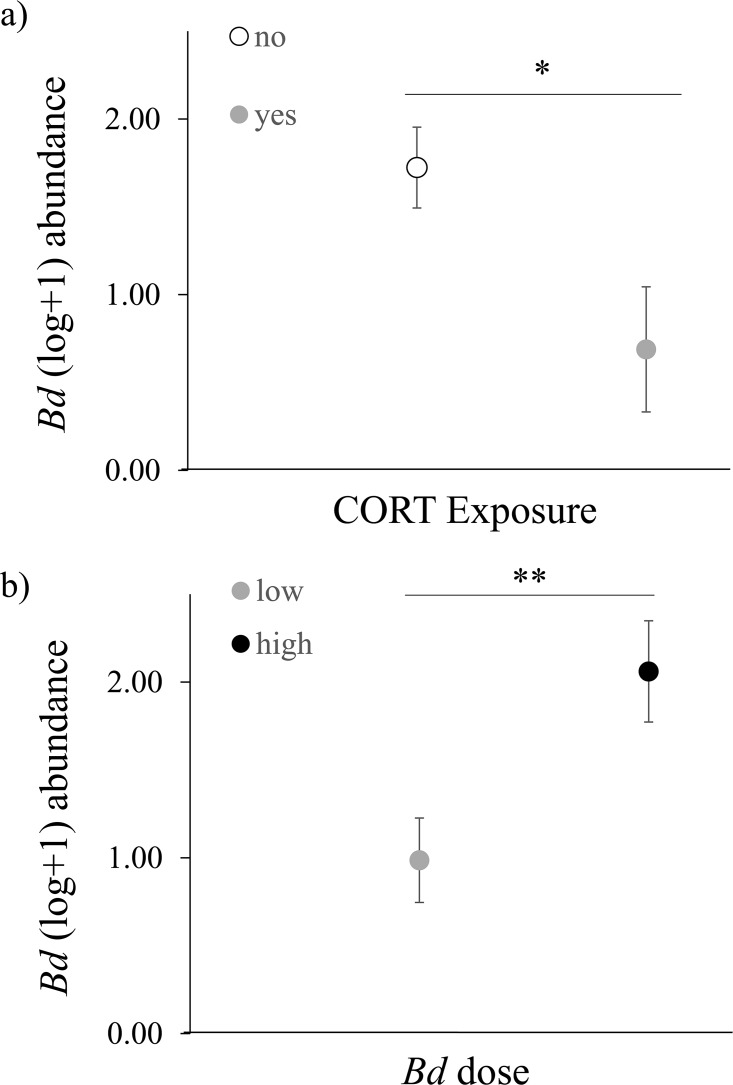
When examining toad resistance to Bd, we detected quantitatively similar main and interactive effects of Bd dose and CORT when using the Day 7 swabs and the maximum Bd detected as response variables (Table 1 and S1 Table), indicating that our findings are robust. As such, we presented the results and figures using Day 7 data. Bd dose significantly affected toad resistance to Bd (Table 1) in a dose dependent fashion, where toads exposed to a high level of Bd had significantly higher Bd abundance compared to toads exposed to a low level of Bd (Fig 3A). We also found a significant main effect of CORT exposure on resistance to Bd (Table 1). Toads exposed to CORT had a lower Bd abundance compared to toads exposed to an ethanol control/low CORT (Fig 3B). Exposure to CORT and Bd dose did not significantly interact to affect resistance (Table 1). See S2 Fig for the results using three CORT doses.
Table 1. The main and interactive effects of CORT exposure (none/low, and high) and Bd dose (low, high) on toad resistance, as measured by Day 7 Bd abundance.
| Predictor | F value | d.f. | P value |
|---|---|---|---|
| CORT exposure | 4.974 | 1 | 0.026 |
| Bd dose | 8.443 | 1 | 0.004 |
| Mass | 1.578 | 1 | 0.194 |
| Days Alive | 1.044 | 1 | 0.213 |
| CORT x Bd | 1.172 | 1 | 0.296 |
| Error | 42 |
Fig 3. Effects of Corticosterone (CORT) on toad resistance.
Significant main effects of (a) CORT exposure and (b) Bd dose on American toad (Anaxyrus [= Bufo] americanus) resistance, as measured by Batrachochytrium dendrobatidis (Bd) abundance. Values represent the average log-transformed Bd abundance for each treatment. Error bars indicate 1 S.E. Statistical differences between treatments are indicated as * = 0.026 and ** = 0.004. Sample sizes for the CORT exposure treatments were 38 and 10 (no CORT and CORT exposed, respectively). Sample sizes for the Bd dose treatments were 25 and 23 (low and high Bd, respectively).
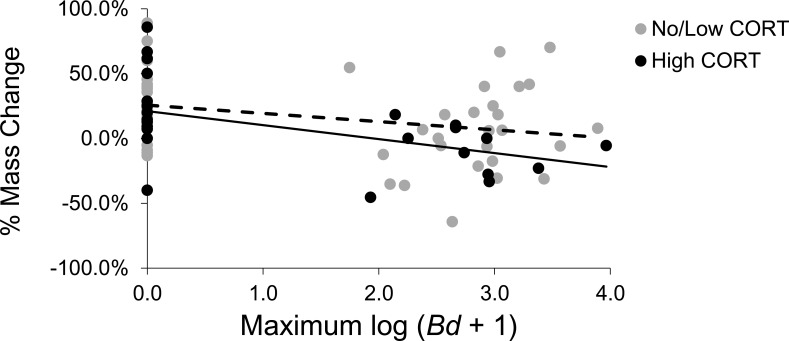
There was a significant negative relationship between maximum Bd abundance and the percentage of mass change by toads (linear model, F1,85 = 6.183, P = 0.015), indicating that toads are not tolerant to Bd. However, exposure to CORT did not affect toad tolerance to Bd (linear model, CORT x max Bd: F1,85 = 0.176, P = 0.676; Fig 4). See S3 Fig for the results using three CORT doses.
Fig 4. Effects of Corticosterone (CORT) on toad tolerance.
The effect of CORT exposure on American toads (Anaxyrus [= Bufo] americanus) tolerance to Batrachochytrium dendrobatidis (Bd), as measured by the relationship between maximum Bd infection intensity and percentage of mass change.
All data are available in S1 Dataset.
Discussion
The idea that glucocorticoids, such as CORT, are immunosuppressive when applied at high concentrations for long periods of time is one of central paradigms in disease ecology and eco-physiological. Although connections between stress, physiology, and immunology are well supported [11, 12], these principles have rarely been used to explain causal relationships between stress and disease outcomes in wild vertebrates. Our fully factorial laboratory experiment using field-collected American toads (Anaxyrus [Bufo] americanus) exposed to physiologically relevant doses of CORT and the pathogenic chytrid fungus revealed that CORT can mediate an outcome of infectious diseases.
Contrary to our predictions, and much of the literature on stress physiology disease risk, toads exposed to CORT had a lower Bd abundance compared to toads given no CORT/low CORT (Fig 3B). Although the association between glucocorticoids and their physiological consequences are frequently thought of as suppressive, glucocorticoids have many actions including permissive and preparative capacities [10]. The most plausible explanation for our finding was that our high CORT dose (0.1 μM) did not chronically elevate circulating levels of CORT in the toads, but were instead more similar to an acute stressor and were thus preparative. Preparative actions are thought to modulate an organism’s stress response by enhancing responsiveness to a subsequent stressor, such as infectious diseases [42]. Evidence from the mammal literature demonstrates that glucocorticoids can enhance the production of immunoglobulins [43], T cell function [44], and cytokine-mediated immune responses [45]. This rationale is also supported by a recent study by Tatiersky et al. [46] who found that injection of Northern leopard frogs (Lithobates pipiens) with CORT increased gene expression of cutaneous antimicrobial peptides, which are known to increase amphibian resistance to Bd [47–50]. Recent research in the amphibian-Bd model system also demonstrates that anuran resistance to Bd is also associated with leukocyte abundance and proliferation [22]. Circulating leukocytes can change in response to exogenous CORT [51] and thus changes in leukocyte abundance are another possible mechanism underlying our findings. Although we did not measure changes in any immune parameters in our experiment, the evidence outlined above provides possible CORT-mediated mechanisms for our finding. Future research that further test how glucocorticoids enhance (or suppress) specific immune responses in wild vertebrates are needed to fully understand the outcomes of disease.
Our result that Bd resistance is influenced by CORT is opposite the results presented in a similar paper in which Searle et al. [28] found that exposure to CORT did not affect Bd infections in three species of amphibians (including a toad of the same genus as the ones we used). However, there are a number of reasons for our different results. First, disease outcomes are context dependent [52] and our experiment used a different Bd isolate than did Searle et al. [28]. Each Bd isolate was regional to the location in which the respective host species were collected and likely represent ecologically and evolutionarily relevant host-pathogen interactions. Bd isolates differ in their virulence [53] and whether/how CORT mediates host resistance to Bd isolates with different virulence is unknown. Second, the majority of amphibians used by Searle et al. [28] were tadpoles whereas our study used metamorphic amphibians. Amphibian resistance to pathogens changes through development [7, 54], most likely due to the reorganization of the amphibian immune system after metamorphosis [55], which is mediated by glucocorticoids [56]. Thus, the different patterns observed across studies might be associated with different life stages. However, Searle et al. [28] did use metamorphic anurans in one experiment and also found no effect of CORT on Bd infection. This difference is most likely explained by the fact that our CORT doses differed (our dose of 0.1 μM versus 0.5 μM in 28). Lastly, our average infection intensity was at least three orders of magnitude higher than the juvenile anurans used in the former study (the average Bd infection intensity of the toads in our experiment was 1333.99 genome equivalents compared to 0.84 genome equivalents reported in [28]). One possible reason that Searle et al. [28] failed to detect a significant effect of CORT on host resistance was because of the lack of variation across the samples due to the fact that the frogs were infected with less than a single zoospore.
Tolerance is an important, yet often overlooked, aspect of an animal’s defense strategy. One way in which a host can increase their tolerance to disease is by reducing self-damage associated with inflammatory responses directed at pathogens (e.g., increasing anti-inflammatory cytokines or transforming growth factor beta which induce anti-inflammatory T-cells [15]). Although few studies have actually identified mechanisms of host tolerance in wild vertebrates, recent evidence links glucocorticoid-mediated anti-inflammatory responses with reductions in immunopathology as drivers of tolerance [57]. We found no evidence that CORT improved toad tolerance to Bd. In fact, if anything, the qualitative observation that exposure to CORT decreased toad tolerance to Bd (Fig 4) is opposite the predictions based on the literature. There is evidence that some species of amphibians respond to Bd by increasing inflammatory cells in the skin [58, 59] and genomic analyses have found that genes associated with inflammation are upregulated in Bd-infected amphibians [60, 61]. For instance, histological evidence of anurans infected with Bd show mild to moderate infiltrates of inflammatory cells within the dermis, occasionally extending into the epidermis [62]. Thus, although it is plausible that CORT-mediated anti-inflammatory responses could promote tolerance to Bd, our results do not support that hypothesis. More research is needed to understand the mechanisms of amphibian tolerance to Bd, including understanding the link between CORT, anti-inflammatory cytokines, and host tolerance.
Although infection with Bd significantly increased toad mortality compared to non-exposed toads, we did not find any differences in mortality between the low- and high-exposure treatments (Fig 2B and 2C) despite significant differences in average Bd abundance between these two treatments (Fig 3B). This result is even more surprising considering that previous researchers found dose-dependent patterns of mortality observed in recently metamorphosed toads of the same genus [63]. The Bd-infected toads that died in our experiment had an average infection intensity of 1470 zoospores (±530 SE), which is orders of magnitude lower than the 10,000 zoospore mortality threshold proposed by other researchers [64], which suggests that amphibian species or life-stage and Bd isolate, or their interaction, can cause result in amphibian mortality at much lower infection intensities.
In addition to the main effect of Bd on toad mortality, we found that toads exposed to exogenous CORT had higher mortality than toads exposed to no CORT/low CORT, irrespective of the Bd treatment. It is difficult to reconcile the notion that exposure to CORT can have beneficial (increased resistance to Bd; Fig 3) and negative (increased mortality; Fig 2) effects when applied at the same dose for the same duration of time. However, the patterns of mortality in the CORT treatments seem to be driven primarily by Bd. Of the 20 toads in the CORT treatment that were exposed to Bd, 13 died (n = 7 and 7 in the low and high Bd treatments, respectively; Fig 2B and 2C) whereas only 2 of the 10 toads that were not exposed to Bd but received a high dose of CORT died (Fig 2A). The fact that we did not detect a significant interaction between CORT and Bd on survival is likely an issue of statistical power. An alternative explanation to this pattern that is independent of Bd is that toads exposed to CORT experienced an increased metabolic rate [65, 66] and died from an energetic/caloric deficit. Our test subjects were recently metamorphosed amphibians and likely did not have a surplus of energetic reserves. Given this logic, one could predict that the toads exposed to CORT but died would experience a mass loss whereas toads that were exposed to high CORT but survived would gain mass. Although our sample size for these data is too limited to test statistically, our results follow this pattern: surviving toads that were exposed to CORT increased their mass by 21.6% compared to an 8.3% mass loss for toads that were exposed to CORT and died. Taken collectively with our data on CORT and Bd resistance, these findings could suggest that the toads healthy enough to survive exposure to CORT were also the ones that were the most resistant to Bd.
Understanding how physiological stressors affect host resistance and tolerance to pathogens is an essential component to the ecology of infectious diseases. However, few studies have tested how CORT affects host responses to pathogens using wild vertebrates (but see [16, 17–19]) and to our knowledge, our study is the first to extend this conceptual framework to tolerance to infection. Our results show that host physiological condition can change disease outcomes, but that these changes are not always straightforward. For example, exposure to high levels of CORT generally increased toad mortality, enhanced resistance to Bd, but did not affect tolerance. Our results emphasize the need for more studies that test how host physiological condition can affect resistance and tolerance to infection. In general, a larger sample size could help tease apart some of the patterns that we observed (e.g., an interaction between CORT dose and Bd on mortality, the effect of CORT on host tolerance, the effect of exogenous CORT exposure on CORT release) but lacked the proper statistical power to rigorously test. In addition, understanding how different exogenous doses of CORT relate to the various physiological levels of endogenous CORT will be important to understand whether hosts are in the reactive scope of their stress response. In addition, future studies that inhibit CORT secretion would help our understanding of the relationship between stress physiology and disease resistance and tolerance in wild vertebrates.
Supporting Information
(PDF)
(CSV)
Survival plots of American toads (Anaxyrus [= Bufo] americanus) exposed to (a) a control treatment without Batrachochytrium dendrobatidis (Bd), (b) a low dose of Bd, and (c) a high dose of Bd. Open circles represent toads exposed to the ethanol treatment (n = 10 per treatment), grey circles represent toads exposed to the low CORT treatment (n = 10 per treatment); black circles represent toads exposed to the high CORT treatment (n = 10 per treatment). Exposure to Bd and CORT independently reduced survival, but the two factors did not interact to affect survival.
(PDF)
Main effects of (a) CORT exposure and (b) Batrachochytrium dendrobatidis (Bd) dose on American toad (Anaxyrus [= Bufo] americanus) resistance, as measured by Bd abundance. Values represent the average log-transformed Bd abundance for each treatment. Error bars indicate 1 S.E.
(PDF)
The effect of CORT exposure on American toads (Anaxyrus [= Bufo] americanus) tolerance to Batrachochytrium dendrobatidis (Bd), as measured by the relationship between maximum Bd infection intensity and percentage of mass change.
(PDF)
(PDF)
Acknowledgments
We thank Stephan Schoech and Travis Wilcoxen for providing feedback on earlier drafts of this manuscript and to Jason Rohr for statistical advice. We also thank Caitlin Gabor for assistance with the water-borne CORT assay. We also thank Liz Caskey for ordering supplies used in this project.
Data Availability
All relevant data are located in the Supporting Information file 'S1 Dataset'.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Raberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Philosophical Transactions of the Royal Society B-Biological Sciences. 2009;364(1513):37–49. 10.1098/rstb.2008.0184 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318(5851):812–4. 10.1126/science.1148526 . [DOI] [PubMed] [Google Scholar]
- 3.Roy BA, Kirchner JW. Evolutionary dynamics of pathogen resistance and tolerance. Evolution. 2000;54:51–63. 68. 10.1111/j.0014-3820.2000.tb00007.x [DOI] [PubMed] [Google Scholar]
- 4.Little TJ, Shuker DM, Colegrave N, Day T, Graham AL. The Coevolution of Virulence: Tolerance in Perspective. PLOS Pathogens. 2010;6(9). 10.1371/journal.ppat.1001006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholthof KBG. The disease triangle: pathogens, the environment and society. Nature Reviews Microbiology. 2007;5(2):152–6. 10.1038/Nrmicro1596 . [DOI] [PubMed] [Google Scholar]
- 6.Klein SL. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunology. 2004;26(6–7):247–64. 10.1111/j.0141-9838.2004.00710.x . [DOI] [PubMed] [Google Scholar]
- 7.Johnson PTJ, Kellermanns E, Bowerman J. Critical windows of disease risk: amphibian pathology driven by developmental changes in host resistance and tolerance. Functional Ecology. 2011;25(3):726–34. 10.1111/j.1365-2435.2010.01830.x [DOI] [Google Scholar]
- 8.Lee KP, Cory JS, Wilson K, Raubenheimer D, Simpson SJ. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proceedings of the Royal Society B-Biological Sciences. 2006;273(1588):823–9. 10.1098/rspb.2005.3385 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamal SM, Khalifa KE. Immune modulation by helminthic infections: worms and viral infections. Parasite Immunology. 2006;28(10):483–96. 10.1111/j.1365-3024.2006.00909.x . [DOI] [PubMed] [Google Scholar]
- 10.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21(1):55–89. 140. 10.1210/edrv.21.1.0389 [DOI] [PubMed] [Google Scholar]
- 11.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nature Reviews Immunology. 2006;6(4):318–28. 10.1038/Nri1810 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology. 2005;5(3):243–51. 10.1038/Nri1571 . [DOI] [PubMed] [Google Scholar]
- 13.Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Stress-induced changes in blood leukocyte distribution—Role of adrenal steroid hormones. Journal of Immunology. 1996;157(4):1638–44. . [PubMed] [Google Scholar]
- 14.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Neuroendocrine Immune Basis of the Rheumatic Diseases Ii, Proceedings. 2002;966:290–303. 10.1111/j.1749-6632.2002.tb04229.x . [DOI] [PubMed] [Google Scholar]
- 15.Sears BF, Rohr JR, Allen JE, Martin LB. The economy of inflammation: when is less more? Trends in Parasitology. 2011. 10.1016/j.pt.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 16.Belden LK, Moore IT, Wingfield JC, Blaustein AR. Corticosterone and growth in Pacific Treefrog (Hyla regilla) tadpoles. Copeia. 2005;2005(2):424–30. 166. 10.1643/cp-04-139r [DOI] [Google Scholar]
- 17.Norris DO, Donahue S, Dores RM, Lee JK, Maldonado TA, Ruth T, et al. Impaired adrenocortical response to stress by brown trout, Salmo trutta, living in metal-contaminated waters of the Eagle River, Colorado. General and Comparative Endocrinology. 1999;113(1):1–8. 10.1006/gcen.1998.7177 . [DOI] [PubMed] [Google Scholar]
- 18.Owen JC, Nakamura A, Coon CAC, Martin LB. The effect of exogenous corticosterone on West Nile virus infection in Northern Cardinals (Cardinalis cardinalis). Veterinary Research. 2012;43 Artn 3410.1186/1297-9716-43-34. 10.1186/1297-9716-43-34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickering AD, Pottinger TG. Stress responses and disease resistance in salmonid fish: effects of chronic elevation of plasma cortisol. Fish Physiology and Biochemistry. 1989;7:253–8. 10.1007/BF00004714 [DOI] [PubMed] [Google Scholar]
- 20.Voyles J, Rosenblum EB, Berger L. Interactions between Batrachochytrium dendrobatidis and its amphibian hosts: a review of pathogenesis and immunity. Microbes and Infection. 2011;13(1):25–32. 10.1016/j.micinf.2010.09.015 . [DOI] [PubMed] [Google Scholar]
- 21.Rohr JR, Raffel TR, Romansic JM, McCallum H, Hudson PJ. Evaluating the links between climate, disease spread, and amphibian declines. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(45):17436–41. 10.1073/pnas.0806368105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMahon TA, Sears BF, Venesky MD, Bessler SM, Brown JM, Deutsch K, et al. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosupression. Nature. 2014;511:224–7. 10.1038/nature13491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rollins-Smith LA, Reinert LK, Burrowes PA. Coqui frogs persist with the deadly chytrid fungus despite a lack of defensive antimicrobial peptides. Diseases of Aquatic Organisms. 2015;113(1):81–3. 10.3354/dao02823 . [DOI] [PubMed] [Google Scholar]
- 24.Savage AE, Sredl MJ, Zamudio KR. Disease dynamics vary spatially and temporally in a North American amphibian. Biological Conservation. 2011;144(6):1910–5. 10.1016/j.biocon.2011.03.018 . [DOI] [Google Scholar]
- 25.Garner TWJ, Walker S, Bosch J, Leech S, Rowcliffe JM, Cunningham AA, et al. Life history tradeoffs influence mortality associated with the amphibian pathogen Batrachochytrium dendrobatidis. Oikos. 2009;118(5):783–91. 10.1111/j.1600-0706.2008.17202.x . [DOI] [Google Scholar]
- 26.Venesky MD, Wilcoxen TE, Rensel MA, Rollins-Smith L, Kerby JL, Parris MJ. Dietary protein restriction imparis growth, immunity, and disease resistance in southern leopard frog tadpoles. Oecologia. 2011;169:23–31. 10.1007/s00442-011-2171-1 [DOI] [PubMed] [Google Scholar]
- 27.Gabor CR, Fisher MC, Bosch J. A non-invasive stress assay shows that tadpole populations infected with Batrachochytrium dendrobatidis have elevated corticosterone levels. Plos One. 2013;8(2). ARTN e5605410.1371/journal.pone.0056054. 10.1371/journal.pone.0056054 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Searle CL, Belden LK, Du P, Blaustein AR. Stress and chytridiomycosis: exogenous exposure to corticosterone does not alter amphibian susceptibility to a fungal pathogen. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology. 2014;321:243–53. 10.1002/jez.1855 [DOI] [PubMed] [Google Scholar]
- 29.Kindermann C, Narayan EJ, Hero JM. Urinary corticosterone metabolites and chytridiomycosis disease prevalence in a free-living population of male Stony Creek frogs (Litoria wilcoxii). Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology. 2012;162(3):171–6. 10.1016/j.cbpa.2012.02.018 . [DOI] [PubMed] [Google Scholar]
- 30.Peterson JD, Steffen JE, Appel AG, Rollins-Smith LA, Mendonca MT. Chytridiomycosis is metabolically costly and induces sickness behavior and immune effects that are linked to increased corticosterone secretion. Integrative and Comparative Biology. 2011;51:E108–E. . [Google Scholar]
- 31.Gosner KL. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16:183–90. [Google Scholar]
- 32.Gabor CR, Bosch J, Fries JN, Davis DR. A non-invasive water-borne hormone assay for amphibians. Amphibia-Reptilia. 2013;34(2):151–62. 10.1163/15685381-00002877 . [DOI] [Google Scholar]
- 33.Gabor CR, Fisher MC, Bosch J. Elevated corticosterone levels and changes in amphibian behavior are associated with Batrachochytrium dendrobatidis (Bd) infection and Bd lineage. Plos One. 2015;10(4). UNSP e012268510.1371/journal.pone.0122685. 10.1371/journal.pone.0122685 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott AP, Ellis T. Measurement of fish steroids in water—a review. General and Comparative Endocrinology. 2007;153(1–3):392–400. 10.1016/j.ygcen.2006.11.006 . [DOI] [PubMed] [Google Scholar]
- 35.Johnson ML, Berger L, Philips L, Speare R. Fungicidal effects of chemical disinfectants, UV light, desiccation and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms. 2003;57(3):255–60. 10.3354/dao057255 . [DOI] [PubMed] [Google Scholar]
- 36.Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Diseases of Aquatic Organisms. 2004;60:141–8. 158. 10.3354/dao060141 [DOI] [PubMed] [Google Scholar]
- 37.Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, Obendorf D, et al. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms. 2007;73:175–92. 120. 10.3354/dao073175 [DOI] [PubMed] [Google Scholar]
- 38.Kriger KM, Hero JM, Ashton KJ. Cost efficiency in the detection of chytridiomycosis using PCR assay. Diseases of Aquatic Organisms. 2006;71(2):149–54. 10.3354/dao071149 . [DOI] [PubMed] [Google Scholar]
- 39.Venesky MD, Liu X, Sauer EL, Rohr JR. Linking manipulative experiments to field data to test the dilution effect. Journal of Animal Ecology. 2014;83(3):557–65. 10.1111/1365-2656.12159 . [DOI] [PubMed] [Google Scholar]
- 40.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(21):9689–94. 10.1073/pnas.0914111107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Earl JE, Whiteman HH. Are commonly used fitness predictors accurate? A meta-analysis of amphibian size and age at metamorphosis. Copeia. 2015;103:297–309. 10.1643/ch-14-128 [DOI] [Google Scholar]
- 42.Dhabhar FS. A hassle a day may keep the pathogens away: The fight-or-flight stress response and the augmentation of immune function. Integrative and Comparative Biology. 2009;49(3):215–36. 10.1093/Icb/Icp045 . [DOI] [PubMed] [Google Scholar]
- 43.Grayson J, Dooley NJ, Koski IR, Blaese RM. Immunoglobulin production induced in vitro by glucocorticoid hormones: T cell-dependent stimulation of immunoglobulin production without B cell proliferation in cultures of human peripheral blood lymphocytes. Journal of Clinical Investigation. 1981;68:1539–47. 10.1172/jci110408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barber AE, Coyle SM, Marano MA, Fischer E, Calvano SE, Fong YM, et al. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. Journal of Immunology. 1993;150(5):1999–2006. . [PubMed] [Google Scholar]
- 45.Jensen LE, Whitehead AS. Regulation of serum amyloid A protein expression during the acute-phase response. Biochemical Journal. 1998;334:489–503. 10.1042/bj3340489 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tatiersky L, Rollins-Smith LA, Lu R, Jardine C, Barker IK, Clark ME, et al. Effect of glucocorticoids on expression of cutaneous antimicrobial peptides in northern leopard frogs (Lithobates pipiens). Bmc Veterinary Research. 2015;11 Artn 19110.1186/S12917-015-0506-6. 10.1186/s12917-015-0506-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rollins-Smith LA, Carey C, Longcore J, Doersam JK, Boutte A, Bruzgal JE, et al. Activity of antimicrobial skin peptides from ranid frogs against Batrachochytrium dendrobatidis, the chytrid fungus associated with global amphibian declines. Developmental and Comparative Immunology. 2002;26(5):471–9. Pii S0145-305x(01)00088-X. 10.1016/s0145-305x(01)00088-x . [DOI] [PubMed] [Google Scholar]
- 48.Rollins-Smith LA, Conlon JM. Antimicrobial peptide defenses against chytridiomycosis, an emerging infectious disease of amphibian populations. Developmental and Comparative Immunology. 2005;29(7):589–98. 10.1016/j.dci.2004.11.004 . [DOI] [PubMed] [Google Scholar]
- 49.Rollins-Smith LA, Reinert LK, O'Leary CJ, Houston LE, Woodhams DC. Antimicrobial peptide defenses in amphibian skin. Integrative and Comparative Biology. 2005;45(1):137–42. 10.1093/icb/45.1.137 [DOI] [PubMed] [Google Scholar]
- 50.Woodhams DC, Ardipradja K, Alford RA, Marantelli G, Reinert LK, Rollins-Smith L. Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Animal Conservation. 2007;10:409–17. 121. 10.1111/j.1469-1795.2007.00130.x [DOI] [Google Scholar]
- 51.Davis AK, Maerz JC. Effects of exogenous corticosterone on circulating leukocytes o f a salamander (Ambystoma talpoideum) with unusually abundant eosinophils. International Journal of Zoology. 2008;2010:735937 10.1155/2010/735937 [DOI] [Google Scholar]
- 52.Daskin JH, Alford RA. Context-dependent symbioses and their potential roles in wildlife diseases. Proceedings of the Royal Society B-Biological Sciences. 2012;279(1733):1457–65. 10.1098/rspb.2011.2276 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher MC, Garner TWJ, Walker SF. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annual Review of Microbiology. 2009;63:291–310. 10.1146/annurev.micro.091208.073435 . [DOI] [PubMed] [Google Scholar]
- 54.Rohr JR, Raffel TR, Hall CA. Developmental variation in resistance and tolerance in a multi-host-parasite system. Functional Ecology. 2010;24(5):1110–21. 10.1111/j.1365-2435.2010.01709.x . [DOI] [Google Scholar]
- 55.Rollins-Smith LA. Metamorphosis and the amphibian immune system. Immunological Reviews. 1998;166:221–30. 10.1111/j.1600-065x.1998.tb01265.x . [DOI] [PubMed] [Google Scholar]
- 56.Rollins-Smith L, Barker KS, Davis T. Involvement of glucocorticoids in the reorganization of the amphibian immune system at metamorphosis. Developmental Immunology. 1997;5:145–52. 10.1155/1997/84841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adelman JS, Kirkpatrick L, Grodio JL, Hawley DM. House finch populations differ in early inflammatory signaling and pathogen tolerance at the peak of Mycoplasma gallisepticum infection. American Naturalist. 2013;181(5):674–89. 10.1086/670024 . [DOI] [PubMed] [Google Scholar]
- 58.Berger L, Speare R, Skerratt LF. Distribution of Batrachochytrium dendrobatidis and pathology in the skin of green tree frogs Litoria caerulea with severe chytridiomycosis. Diseases of Aquatic Organisms. 2005;68(1):65–70. 10.3354/dao068065 . [DOI] [PubMed] [Google Scholar]
- 59.Davidson EW, Parris MJ, Collins JP, Longcore JE, Pessier AP, Brunner JL. Pathogenicity and transmission of chytridiomycosis in Tiger Salamanders (Ambystoma tigrinum). Copeia. 2003;2003:601–7. 21. 10.1643/cp-02-120r1 [DOI] [Google Scholar]
- 60.Ellison AR, Tunstall T, DiRenzo GV, Hughey MC, Rebollar EA, Belden LK, et al. More than skin deep: functional genomic basis for resistance to amphibian chytridiomycosis. Genome Biology and Evolution. 2015;7(1):286–98. 10.1093/gbe/evu285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ribas L, Li MS, Doddington BJ, Robert J, Seidel JA, Kroll JS, et al. Expression profiling the temperature-dependent amphibian response to infection by Batrachochytrium dendrobatidis. Plos One. 2009;4(12):e8408 10.1371/Journal.Pone.0008408 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reeder NM, Pessier AP, Vredenburg VT. Reservoir species for the emerging amphibian pathogen Batrachochytrium dendrobatidis thrives in a landscape decimated by disease. Plos One. 2012;7(3):e33567 10.1371/journal.pone.0033567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carey C, Bruzgul JE, Livo JL, Walling ML, Kuehl KA, Dixon BF, et al. Experimental exposures of Boreal Toads (Bufo boreas) to a pathogenic chytrid fungus (Batrachochytrium dendrobatidis). EcoHealth. 2006;3:5–21. 12. 10.1007/s10393-005-0006-4 [DOI] [Google Scholar]
- 64.Kinney VC, Heemeyer JL, Pessier AP, Lannoo MJ. Seasonal pattern of Batrachochytrium dendrobatidis infection and mortality in Lithobates areolatus: Affirmation of Vredenburg's "10,000 Zoospore Rule". Plos One. 2011;6(3):e16708 10.1371/journal.pone.0016708 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romero LM. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. General and Comparative Endocrinology. 2002;128(1):1–24. 10.1016/s0016-6480(02)00064-3 . [DOI] [PubMed] [Google Scholar]
- 66.Wack CL, DuRant SE, Hopkins WA, Lovern MB, Feldhoff RC, Woodley SK. Elevated plasma corticosterone increases metabolic rate in a terrestrial salamander. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology. 2012;161(2):153–8. 10.1016/j.cbpa.2011.10.017 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(CSV)
Survival plots of American toads (Anaxyrus [= Bufo] americanus) exposed to (a) a control treatment without Batrachochytrium dendrobatidis (Bd), (b) a low dose of Bd, and (c) a high dose of Bd. Open circles represent toads exposed to the ethanol treatment (n = 10 per treatment), grey circles represent toads exposed to the low CORT treatment (n = 10 per treatment); black circles represent toads exposed to the high CORT treatment (n = 10 per treatment). Exposure to Bd and CORT independently reduced survival, but the two factors did not interact to affect survival.
(PDF)
Main effects of (a) CORT exposure and (b) Batrachochytrium dendrobatidis (Bd) dose on American toad (Anaxyrus [= Bufo] americanus) resistance, as measured by Bd abundance. Values represent the average log-transformed Bd abundance for each treatment. Error bars indicate 1 S.E.
(PDF)
The effect of CORT exposure on American toads (Anaxyrus [= Bufo] americanus) tolerance to Batrachochytrium dendrobatidis (Bd), as measured by the relationship between maximum Bd infection intensity and percentage of mass change.
(PDF)
(PDF)
Data Availability Statement
All relevant data are located in the Supporting Information file 'S1 Dataset'.