Abstract
Chronic stages of paracoccidioidomycosis (PCM) are characterized by granulomatous lesions which promote the development of pulmonary fibrosis leading to the loss of respiratory function in 50% of patients; in addition, it has been observed that neutrophils predominate during these chronic stages of P. brasiliensis infection. The goal of this study was to evaluate the role of the neutrophil during the chronic stages of experimental pulmonary PCM and during the fibrosis development and tissue repair using a monoclonal specific to this phagocytic cell. Male BALB/c mice were inoculated intranasally with 1.5x106 P. brasiliensis yeast cells. A monoclonal antibody specific to neutrophils was administered at 4 weeks post-inoculation followed by doses every 48h during two weeks. Mice were sacrificed at 8 and 12 weeks post-inoculation to assess cellularity, fungal load, cytokine/chemokine levels, histopathological analysis, collagen and expression of genes related to fibrosis development. Depletion of neutrophils was associated with a significant decrease in the number of eosinophils, dendritic cells, B cells, CD4-T cells, MDSCs and Treg cells, fungal load and levels of most of the pro-inflammatory cytokines/chemokines evaluated, including IL-17, TNF-α and TGF-β1. Recovery of lung architecture was also associated with reduced levels of collagen, high expression of TGF-β3, matrix metalloproteinase (MMP)-12 and -14, and decreased expression of tissue inhibitor metalloproteinase (TIMP)-2, and MMP-8. Depletion of neutrophils might attenuate lung fibrosis and inflammation through down-regulating TGF-β1, TNF-α, IL-17, MMP-8 and TIMP-2. These results suggest that neutrophil could be considered as a therapeutic target in pulmonary fibrosis induced by P. brasiliensis.
Introduction
Pulmonary fibrosis is the final outcome of a progressive, uncontrolled and irreversible process of tissue regeneration triggered by exposure to multiple agents such as environmental toxins, radio/chemotherapy and some pathogens, including dimorphic fungi such as Paracoccidiodes spp., the causal agent of paracoccidioidomycosis (PCM) [1,2].
PCM is a systemic fungal infection of great importance in Latin America, mainly in Brazil, Colombia, and Venezuela. It is estimated that 10 million people are infected with this pathogen, of which only about 1–2% will develop the mycosis [3,4], mostly with chronic and progressive form of the disease (90%) [5]. This chronic stage of PCM is characterized by a granulomatous inflammatory response and progressive damage in the lung tissue as a response to the fungus, which remains even after treatment and promotes the pulmonary fibrosis with loss of respiratory function in 50% of the patients [6].
Multiple studies have demonstrated the relevance of neutrophils in the pathogenesis of PCM, especially during the early stages of infection [7,8]. Recently, it has been suggested that neutrophils may modulate the innate and adaptive immune response against P. brasiliensis infection through the production of cytokines and lipid mediators that lead the immune system toward a protective response mediated by a Th1-like pattern [9,10]. On the other hand, Pina et al. [11] using an antibody-mediated neutrophil depletion strategy in an animal model of pulmonary PCM demonstrated that functionality of neutrophils was dependent on the host genetic pattern; thus, when compared resistant (A/J) and susceptible (B10.A) mice to P. brasiliensis infection and treated with an anti-neutrophil monoclonal antibody (mAb) anti-Gr1 (clone RB6-8C5), susceptible animals showed a decrease in survival time, increase on fungal burden in the lung, liver, and spleen as well as higher levels of interleukin (IL)-4, IL-12, hepatic cytokines and synthesis of antibodies associated with Th1 and Th2 profiles; in contrast, resistant mice treated with the mAb showed higher levels of IL-12, GM-CSF and Th1-associated antibodies with diminished levels of hepatic cytokines, thus evidencing the importance of neutrophils according to the host genetic background [11]. More recently, and using an intermediate susceptible mouse model to P. brasiliensis infection, we demonstrated that neutrophils are essential for protection and also important to regulate immunopathology in PCM during the early stages of infection [12].
Actually, it has not been demonstrated how the neutrophil exerts its role during the development of the granulomatous response in P. brasiliensis infection and fibrotic outcome in PCM. Therefore, we aimed to determine the role of neutrophils during the chronic stage of PCM in BALB/c strain mice which have intermediate susceptibility to P. brasiliensis infection [13] treated with the anti-neutrophils mAb anti-Ly6G (clone 1A8). Our results showed that neutrophil depletion during these chronic stages of P. brasiliensis infection was associated with a decrease in the granulomatous inflammatory response and fungal load as well as with recovery of lung architecture with attenuation of pulmonary fibrosis, thus indicating a detrimental role of these phagocytic cells in the chronic stages of PCM and during development of fibrosis process.
Materials and Methods
Mice
BALB/c male mice of eight week-old were obtained from the breeding colony maintained at Corporación para Investigaciones Biológicas, CIB (Medellín, Colombia). Two experimental groups of mice were gathered which consisted of infected or non-infected control mice. Mice from both groups were split into sub-groups in order to undergo the following treatment regimens for each evaluation time: untreated, isotype-treated and neutrophil-depleted animals.
Ethics statement
This study was carried out following the Colombian (Law 84/1989, Resolution No. 8430/1993), European Union, and Canadian Council on Animal Care regulations. The protocol was approved by the Institutional Ethics Committee of the Corporación para Investigaciones Biológicas (# PRE00501023085, Acta No.92).
Paracoccidioides brasiliensis yeasts
In this study, a highly virulent P. brasiliensis isolate (Pb18) was used. Yeast cells were subcultured every week on slant agar tubes with supplemented medium; Sabouraud Dextrose Agar (Difco Laboratories, Detroit, MI, USA), 0.14% L-asparagine (Sigma-Aldrich, Saint Louis, MO, USA), 0.01% thiamine hydrochloride (Sigma-Aldrich, Saint Louis, MO, USA) and 100 U/ml Penicillin– 100 μg/ml Streptomycin (GIBCO Invitrogen Corporation, Carlsbad, CA, USA), and incubated at 36°C in 5% CO2. Yeast cells were transferred into 100 ml supplemented medium as previously described and incubated during 4 days at 36°C at 150 rpm. Then, yeast suspension was pelleted by centrifugation (1400g, 10°C for 10 min), washed twice with 1X PBS, pH = 7.4 (GIBCO Invitrogen Corporation, Carlsbad, CA, USA) and passed 20 times through a 21G x 1.5 inch needle using a 10 ml syringe in order to obtain between 70 to 80% individual yeast cells. The yeast suspension was allowed to settle during 10 min and supernatant was transferred to a new tube. Cell suspension was concentrated by centrifugation and counted in a hemocytometer to determine the percentage of individual yeast cells and their viability using Janus Green vital dye (Acros Organics, Geel, Belgium). The number of yeast was adjusted to 1.5x106 cells contained in 60μl to infect the mice.
Paracocccidioides brasiliensis infection
Intranasal inoculation of cell suspension previously described (1.5x106 yeast cells in 60μl PBS) was performed in anesthetized animals by intramuscular injection of 50μl Ketamine (80mg/kg) (Laboratorios Biosano, Santiago, Chile)–Xylazine (8mg/kg) (Bayer S.A., Bogotá, Colombia) solution. The total inoculum was split into two equal doses instilled intranasally within a 5–10 min period. Non-infected (control) mice were inoculated with 60μl of PBS.
Depletion of neutrophils
Mice were injected intraperitoneally with 200μl solution containing 200μg of the mAb anti-Ly6G, clone 1A8 (Bio X Cell, West Lebanon, NH, USA). Control mice were injected with an equivalent amount of an isotype control IgG2a, clone 2A3 (Bio X Cell, West Lebanon, NH, USA). During the chronic phase of the disease, mAb treatment was started at 4 or 8 weeks post-inoculation (with PBS or P. brasiliensis), followed by additional doses every 48h over a period of two weeks.
Colony forming units determination (CFU)
Mice groups were sacrificed at 12 weeks post-infection after undergoing the corresponding treatment. Lungs, livers, and spleens were removed, weighed and homogenized in 2ml sterile 1X PBS-100 U/ml Penicillin-100 μg/ml Streptomycin solution using a gentleMACS Dissociator (Miltenyi Biotec, Teterow, Germany). Homogeneous suspensions of those tissues were diluted (1:10, 1:100 and 1:1000) and 0.5ml of each dilution was plated on Petri dishes added with Brain Heart Infusion (BHI) agar medium (BD BBL, Franklin Lakes, NJ, USA) supplemented with 0,5% D-(+)-Glucose (Sigma-Aldrich, Saint Louis, MO, USA), 4% horse serum (GIBCO Invitrogen Corporation, Carlsbad, CA, USA) previously heating inactivated at 56°C for 30 min, and 300μM EDTA (Sigma-Aldrich, Saint Louis, MO, USA), followed by incubation at 36°C, 5% CO2. CFU counts were performed during the first 11 days after plating homogeneous suspensions. In order to determine the normalized CFU count, it was applied the following formula: CFU/g of tissue = total number of colonies multiplied by the total dilution factor which was obtained by multiplying dilution factors for each of the following parameters: homogenized tissue (total volume of homogenized tissue/g of tissue), volume plated on Petri dishes (0.5ml) and diluted homogeneous suspension plated on Petri dishes. Data was transformed into Log10 CFU/g of tissue.
Determination of cytokines and chemokines
Mice were sacrificed at 12 weeks post-infection after undergoing the corresponding treatment. Lungs were removed and homogenized as described previously, homogeneous suspension was added with protease inhibitor cocktail (Roche Applied Science, Mannheim, Germany), mixed and centrifuged (3,000 rpm, 4°C for 5 min). Aliquots of supernatants were stored at 70°C until being used. The following cytokines and chemokines were determined from those supernatants: CC chemokine ligand (CCL)-2 (monocyte chemoattractant protein 1, MCP-1), CCL-3 (macrophage-inflammatory protein 1α, MIP-1α), CCL-4 (MIP-1β), CCL-5 (RANTES), CCL-11 (Eotaxin), CXC chemokine ligand (CXCL)-1 (keratinocyte chemoattractant, KC), CXCL-2 (MIP-2), CXCL-5 (lipopolysaccharide induced CXC chemokine, LIX), CXCL-9 (MG), CXCL-10 (interferon-inducible protein-10, IP-10), granulocyte colony-stimulating factor (G-CSF), monocyte colony-stimulating factor (M-CSF), GM-CSF, IFNγ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17, TNFα, leukemia inhibitory factor (LIF), and vascular endothelial growth factor (VEGF). Determination of these molecules was performed by a multiplex assay using a commercial kit and the Luminex 200 system (EMD Millipore, Billerica, MA, USA).
Flow cytometry
Lungs from mice were removed and homogenized using 40 and 70μm sterile cell strainers (Thermo Fisher Scientific Inc, Waltham, MA, USA) in RPMI plus 1% FBS (Sigma-Aldrich, Saint Louis, MO, USA) previously heating inactivated at 56°C for 30 min. Cell suspension was pelleted by centrifugation (1500 rpm, 10°C for 10 min), red blood cells were lysed by ACK Lysing Buffer for 3–5 min (GIBCO Invitrogen Corporation, Carlsbad, CA, USA), and RPMI plus 1% FBS was added to stop the reaction and wash the cells. Then, cell suspension was pelleted again, resuspended in RPMI plus 10% FBS and counted in a hemocytometer. The cell suspension was divided among several wells on a 96-well plate in order to determine the following cell populations: neutrophils, eosinophils, DCs, macrophages, B cells, CD8 T, CD4 T and NK cells. Fc receptors were blocked using a purified rat anti-mouse CD16/CD32 (BD Pharmingen, San Diego, CA, USA) and cells were immunostained in FACS buffer (1X PBS/0.5% FBS) using fluorescent mAbs against murine surface molecules and isotype controls as follows: Fluorescein isothiocyanate (FITC)-Rat IgG2bκ (A95-1), Phycoerythrin (PE)-Rat IgG2aκ (R35-95), Allophycocyanin (APC)-Rat IgG1κ (R3-34), V450-Rat IgG2bκ (A95-1), APC-Cy7-Rat IgG1κ (R3-34), FITC-anti-CD45 (30-F11), APC-anti-Ly-6G (1A8), APC-Cy7-anti-Ly-6G and Ly-6C (RB6-8C5), PE-anti-CD11b (M1/70), APC-anti-CD11c (HL3), PE-anti-Siglec-F (E50-2440), PE-anti-Mac-3 (M3/84), PE-anti-CD23 (B3B4), APC-anti-IgM (II-41), PE-anti-CD3 (17A2), PerCP-anti-CD8a (53–6.7), PerCP-Cy5.5-anti-CD4 (RM4-5), PE-anti-CD25 (PC61), APC-anti-NK-1.1 (PK136), PE-Cy7-anti-IFNγ (XMG1.2), PE-Cy7-anti-IL-4 (11B11), APC-Cy7-anti-IL-17a (TC11-18H10), V450-anti-IL-10 (JES5-16E3), and Alexa Fluor 647-anti-FoxP3 (MF23) (BD Pharmingen, San Diego, CA, USA), PE-anti-IL-22 (Poly5164) (Biolegend). For Treg cells and intracellular cytokines analysis, cells were treated with Cytofix/Cytoperm™ solution—Perm/Wash™ solution (BD Pharmingen, San Diego, CA, USA) and intracellular staining. After washing cell suspensions in FACS buffer, they were fixed with FACS buffer/1% PFA (Carlo Erba, Barcelona, Spain). Analysis was determined using a FACS Canto II system (BD Biosciences, San Jose, CA, USA) and FlowJo V10 software (FlowJo, LLC, Data Analysis software, Ashland, OR, USA). Cell populations were analyzed as follows: (a) cell events in region 1 (R1) were gated by forward scatter versus side scatter areas; (b) CD45+ events were gated from R1 by side scatter area versus CD45 staining to establish the R2 region, from which (c) cell events were gated according to the specific surface marker to determine the specific cell subpopulation and specific intracellular markers. The total number of leukocytes was obtained multiplying the counted of cell suspension by the percentage of CD45+ cells. The number of each leukocyte subpopulation was determined by multiplying the percentage of each gated subpopulation by the total number of leukocytes (CD45+ population). Leukocytes subpopulations were defined as follows: neutrophils: CD45+/CD11b+/Gr1+/Ly6G+, eosinophils: CD45+/CD11c-/SiglecF+, myeloid-derived suppressor cells: CD45+/CD11b+/Gr1+/Ly6G-, alveolar macrophages: CD45+/CD11c+/CD11blow/med, DCs: CD45+/CD11c+/CD11bhigh, tissue macrophages: CD45+/CD11c+/Mac3+, B cells: CD45+/CD23+/IgM+, CD8 T cells: CD45+/CD3+/CD8+, CD4 T cells: CD45+/CD3+/CD4+, NK cells: CD45+/CD3-/NK1.1+, CD4 T helper-1 cells: CD45+/CD4+/IFNγ+, CD4 T helper-2 cells: CD45+/CD4+/IL-4+, CD4 T helper-17a cells: CD45+/CD4+/IL-17a+, CD4 T helper-22 cells: CD45+/CD4+/IL-22+ and Treg cells: CD45+/CD4+/CD25+/FoxP3+.
Real time PCR analysis
Groups of mice were sacrificed at 12 weeks post-infection. Lungs were removed and homogenized as described above and total RNA was obtained using TRIzol® (Invitrogen, Carlsbad, CA, USA) and treating samples with DNase I (Thermo Fisher Scientific Inc, Waltham, MA, USA). cDNA was synthesized using 500ng of total RNA using the Maxima First Strand cDNA synthesis kit for RT-qPCR according to the manufacturer’s instructions (Thermo Fisher Scientific Inc, Waltham, MA, USA). Real-time PCR was done using Maxima SYBR Green/Fluorescein qPCR Master Mix (2X) according to the manufacturer’s instructions (Thermo Fisher Scientific Inc, Waltham, MA USA). The CFX96 Real-Time PCR Detection System (Bio-Rad, Headquarters Hercules, California, USA) was employed to measure gene expression levels. Melting curve analysis was performed after the amplification phase of real time PCR assays to eliminate the possibility of non-specific amplification or primer-dimer formation. Validation of housekeeping genes for normalization mRNA expression was performed before gene expression analysis. Fold changes in target gene mRNA expression were quantified relative to Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; housekeeping gene previously defined). Each experiment was conducted in duplicate and the expression level measured in triplicate. Primers sequences are listed in Table 1.
Table 1. Primers sequences.
| Gene | Primer forward (5´—3´) | Primer reverse (5´—3´) |
|---|---|---|
| GAPDH | CATGGCCTTCCGTGTTCCTA | GCGGCACGTCAGATCCA |
| 18S RNA | GGTCGGCGTCCCCCAACTTCT | CGTGCAGCCCCGGACATCTAA |
| β2-microglobulin | AAGTATACTCACGCCACCCACCG | TTTTTTCCCGTTCTTCAGCATTTG |
| Collagen-1α2 | CGCTCCCAGCCTTCACTCAGAC | CACGTGCGAGCAGGGTTCTTTC |
| Collagen-3α1 | GGATGCAGCCACCTTGGTCAGT | GGCAGTCTAGTGGCTCCTCATCAC |
| TGF-β1 | TACTGCCGCTTCTGCTCCCACTCC | TCGATGCGCTTCCGTTTCACCAG |
| TGF-β3 | GGGGCGTCTCAAGAAGCAAAAG | GGGCCCTCTTCTTCCTCTGACTG |
| TGF-βRc2 | CCGGGGCATCGCTCATCTC | CACACAGGCAACAGGTCAAGTCGT |
| Tissue inhibitor metalloproteinase-1 (TIMP-1) | GCTAAAAGGATTCAAGGCTGTGGG | CTCTTCACTGCGGTTCTGGGACTT |
| TIMP-2 | CCTCTGGATGGACTGGGTCAC | CGGGTCCTCGATGTCAAGAAACTC |
| Matrix metalloproteinase-1a (MMP-1a) | TGCCAAAAGCAGTTGTGGAAGATG | GTGGAAGGAGAGCACAATATCGCC |
| MMP-8 | GCCACGATGGTTGCAGAGAAGC | CACTCCACATCGAGGCATTTCCA |
| MMP-12 | GGGCTAGAAGCAACTGGGCAACTG | CCATCTTGACCTCTGGGGCACTG |
| MMP-13 | CCACAGTTGACAGGCTCCGAGAA | GTATTCACCCACATCAGGCACTCC |
| MMP-14 | CGATGAGGGGACTGAGGAGGAGAC | CAGCAGTAGTACCGGCAGGACCAC |
| MMP-15 | AACGCGGGTGACAAGGATGAGG | CCAGGCCCAGAATACAGAGCAACA |
| CXCR-4 | CGATAGCCTGTGGATGGTGGTGTT | TCTGGTGGCCCTTGGAGTGTGAC |
| CXCL-12 | TCCCCATTCTCCTCATCCTCATCT | CTCCCTGCAAAGCCACCGTCTAT |
| T-bet | CCGGGCCTGGCGAGTTCTT | GCGGGCAATGCGTAGTCCTC |
| GATA-3 | GGTCGGCCAGGCAAGATGAGAAA | TAATGGGGTGGTGGGCTGAGGAT |
| RORc | CGCCGCAAGCCAGCAGTGTAAT | TCGGGACATGCCCAGAGCCA |
| Ahr | GGCTGCCAGGCAGGGTTGTGA | GCTGGCCGCGGAGGAGAAAC |
| FoxP3 | CCAACGCCCCAACAAGTGCTC | TCATCGCCCGGTTTCCATAGGTA |
| Inducible Nitric oxide Synthase-2 (iNOS2) | GCCGCATGAGCTTGGTGTTTG | GCAGCCGGGAGTAGCCTGTGT |
| Arginase-1 | CCTTGGCTTGCTTCGGAACTCA | CTTGGGAGGAGAAGGCGTTTGC |
Histopathological analysis
Before detaching the tissue from the mouse, lungs were perfused using 10ml of 1X PBS in order to wash out red blood cells followed by 10ml of formalin (4% Formaldehyde solution, EM Science, Gibbstown, NJ), 0.15M sodium dihydrogen phosphate (Merck, Darmstadt, Germany), and 0,11M sodium hydroxide (Sigma-Aldrich, Saint Louis, MO, USA) to fix the tissue. Lungs were removed and submerged in 4% formalin until ready to be processed. Fixed tissues were embedded in paraffin, cut and stained with Hematoxylin and eosin (H&E) in order to determine the lung inflammatory response, methenamine silver to identify P. brasiliensis yeast cells and Masson’s trichrome to differentiate collagen fibers. Sliced and stained tissues were analyzed using a Nikon Eclipse Ci-L microscope (Nikon Instruments Inc., Melville, USA) and acquired using a Nikon DS-Fi2 digital camera and NIS Elements 4.30.02 Laboratory Image Software (Nikon Instruments Inc., Melville, USA). Image processing and quantitative analysis of inflammatory response and granulomatous cellular infiltrate by total pulmonary area was performed using ImageJ Software (National Institutes of Health, NIH, Maryland, USA). The percentage of injured area was calculated by dividing the total injured area, which includes cellular infiltrates and inflammatory lesions, by the total area of lung. The average size of the granuloma was obtained from each section of lung tissue per experimental group.
Total collagen
Groups of mice were sacrificed at 12 weeks post-infection and lungs were removed and homogenized as describe above. Isolation and concentration of total collagen was performed using the homogenized suspension with acid neutralizing reagent (0.5M acetic acid, 0.1 mg/ml pepsin) (Sigma-Aldrich, Saint Louis, MO, USA) and collagen isolation & concentration reagent (Biocolor, Northern Ireland, U.K.) and centrifuged at 12,000 rpm, 4°C, 10 min. Then, the pellets were resuspended with Sircol Dye reagent (Biocolor, Northern Ireland, U.K.) and proceed with the Sircol Soluble Collagen Assay according to the manufacturer instructions.
Statistical analysis
Data analysis was performed using Graph Pad Prism software version 5 (GraphPad Software, Inc., La Jolla, CA, USA). Medians and IQR were used to analyze fungal load, flow cytometry, cytokines, and chemokines levels. The Mann-Whitney test was used for comparisons between groups in all developed methodologies. Values of P<0.05 were considered to be significant.
Results
Neutrophils were efficiently depleted using the specific mAb anti-Ly6G during the chronic stages of PCM
Previous studies in our lab showed that the mAb anti-Ly6G (clone 1A8) depleted specifically neutrophils in BALB/c mice inoculated with PBS or 1,5x106 P. brasiliensis yeasts cells at different early stages (48 and 96h, see Results in [12]).
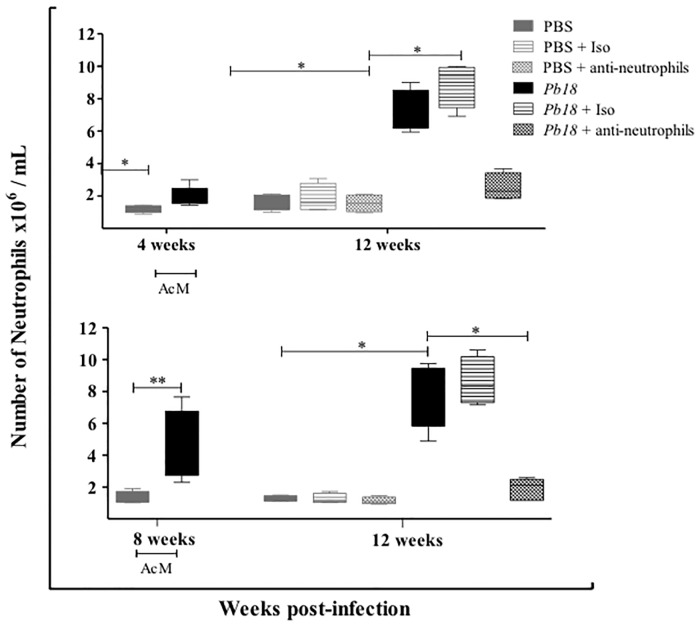
In order to investigate the efficiency of the treatment to deplete neutrophils during the chronic stages of experimental pulmonary PCM, a flow cytometry assay was carried out for lung homogenates at 12 weeks post-infection. We observed that the number of neutrophils remained significantly fewer in infected mice treated with the specific mAb anti-Ly6G, starting such treatment at week 4 or 8 post-infection and analyzed later (12 weeks post-infection), when compared to control mice (Fig 1).
Fig 1. The anti-Ly6G mAb efficiently depleted neutrophils during chronic stage of PCM.
BALB/c mice were intranasally inoculated with PBS or 1.5x106 P. brasiliensis (Pb18) yeast cells, treated with an isotype control Ab or the anti-Ly6G specific mAb against neutrophils during the chronic phase of P. brasiliensis infection (4 or 8 week post-challenge). Neutrophils were assessed by flow cytometry as described in the Materials and Methods section. Data shown represent median and IQR (n = 4–5 mice/group; representative of two independent experiments). *, P<0.05 and **, P<0.01 comparing infected-untreated mice versus control mice or comparing infected-anti-Ly6G mAb-treated mice versus infected-untreated mice. PBS, control mice; PBS + Iso, control mice treated with isotype control Ab (clone: 2A3); PBS + anti-neutrophils, control mice treated with anti-Ly6G mAb (clone: 1A8); Pb18, infected-untreated mice; Pb18 + Iso, infected mice treated with isotype control Ab; Pb18 + anti-neutrophils, infected mice treated with anti-Ly6G mAb.
Depletion of neutrophils was associated with a decreased number of CD4 T cells, B cells, eosinophils, DCs and MDSc in lungs of mice at the chronic stage of the PCM
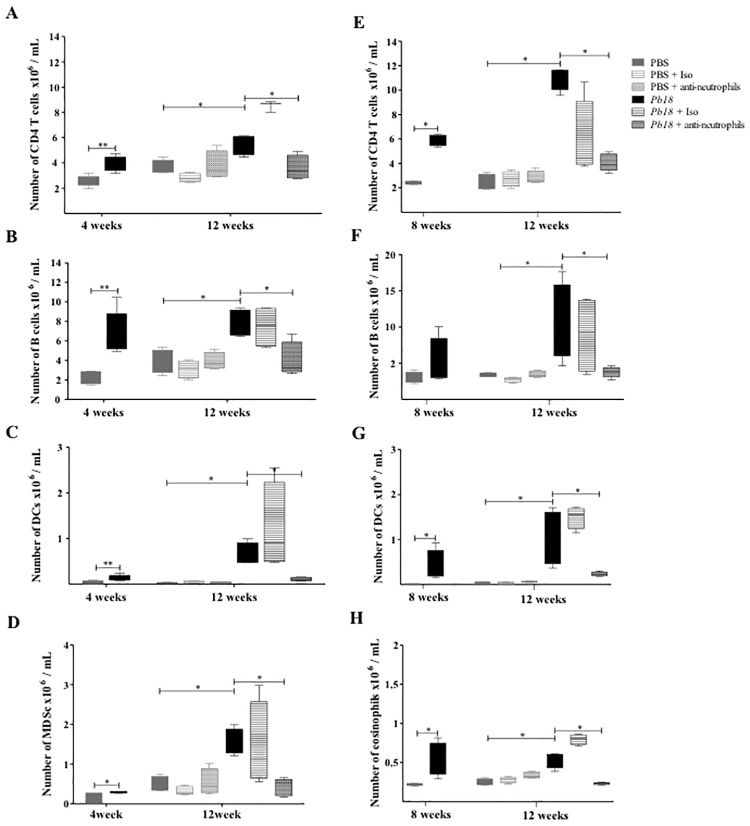
During the chronic stage of the disease, populations of cells were assessed by flow cytometry as described in Materials and Methods. Interestingly, the number of CD4 T cells, B cells, dendritic cells (DCs) and myeloid-derived suppressor cells (MDSc) were significantly lower in the neutrophil-depleted mice treated at week 4 post-infection and analyzed at 12 weeks post-infection compared to untreated or isotype-treated mice (Fig 2A–2D). When the mAb treatment started at 8 weeks post-infection, numbers of CD4 T cells, B cells, DCs and eosinophils also remained significantly lower in those animals compared to control mice at 12 weeks post-infection (Fig 2E–2H).
Fig 2. Depletion of neutrophils is associated with a decreased number of CD4 T cells, B cells, eosinophils, DCs and MDSc during the chronic stages of P. brasiliensis infection.
BALB/c mice were inoculated with PBS or 1.5x106 P. brasiliensis (Pb18) yeast cells and treated with an isotype control Ab or anti-Ly6G mAb at 4 weeks (A, B, C, D) or 8 weeks (E, F, G, H) post-challenge and analyzed at 12 weeks post-infection. Cell populations from lungs of mice were assessed by flow cytometry. CD4 T cells (A, E), B cells (B, F) DCs (C, G), myeloid suppressor cells (D) and eosinophils (H) were identified as described in the Materials and Methods section. Data shown represent median and IQR (n = 4–5 mice/group; representative of two independent experiments). *, P<0.05; comparing infected untreated mice versus control mice, or comparing infected-anti-Ly6G mAb treated mice versus infected-untreated mice. PBS, control mice; PBS + Iso, control mice treated with isotype control Ab (clone: 2A3); PBS + anti-neutrophils, control mice treated with anti-Ly6G mAb (clone: 1A8); Pb18, infected-untreated mice; Pb18 + Iso, infected mice treated with isotype control Ab; Pb18 + anti-neutrophils, infected mice treated with anti-Ly6G mAb.
Neutrophil depletion is associated with low levels of pro-inflammatory cytokines and chemokines in lungs of mice during the chronic stages of PCM
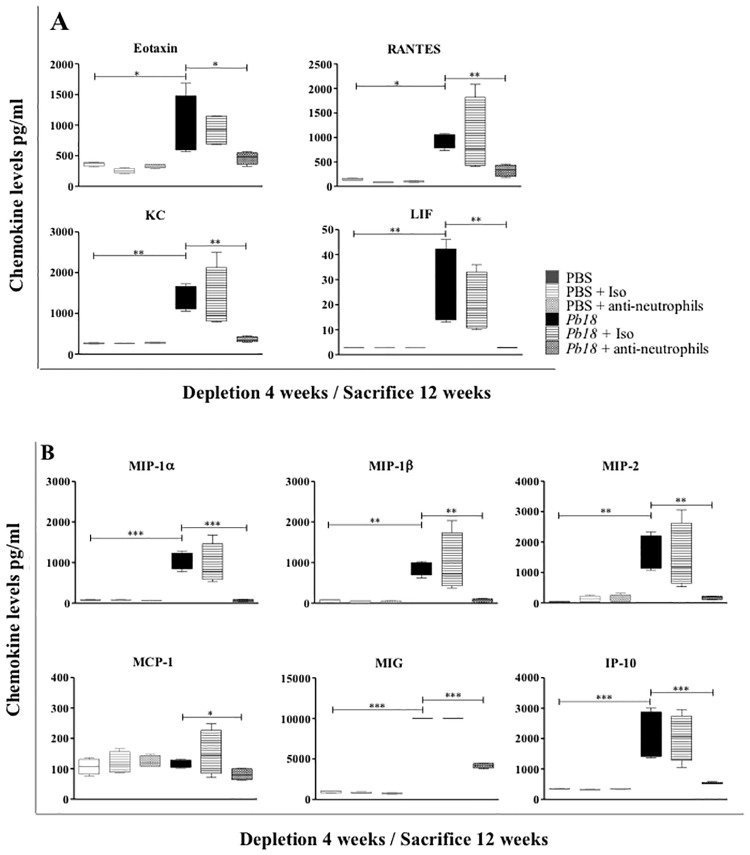
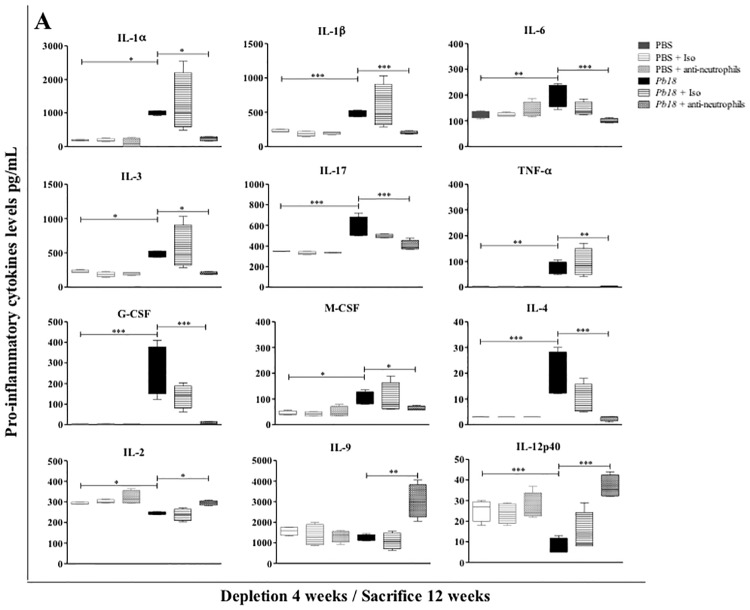
Regarding cytokine and chemokine profiles, mice in which treatment with the mAb against neutrophils started at 4 weeks post-infection and evaluated at 12 weeks post-infection showed a significant decrease in levels of chemokines such as CCL11 (eotaxin), CCL5 (RANTES), CXCL1 (KC), LIF, CCL3 (MIP-1α), CCL4 (MIP-1β), CXCL2 (MIP-2), CCL2 (MCP-1), CXCL9 (MIG) and CXCL10 (IP-10) (Fig 3A and 3B), as well as most of the pro-inflammatory cytokines evaluated: IL-1α, IL-1β, IL-3, IL-4, IL-6, IL-17, G-CSF, M-CSF and TNF-α (Fig 4A), when compared to control mice. In addition, a significant increase was observed in levels of IL-2, IL-9 and IL-12p40 in the same group of mice (Fig 4B). When mice were depleted of neutrophils at 8 weeks post-infection and evaluated at 12 weeks post-infection, they showed significant decrease in levels of CCL3 (MIP-1α), CCL4 (MIP-1β), CXCL1 (KC), LIF, G-CSF and M-CSF (Data not shown).
Fig 3. Effect of neutrophil depletion on chemokine levels in lungs during the chronic stage of P. brasiliensis infection.
BALB/c mice were infected with 1.5x106 P. brasiliensis (Pb18) yeast cells and treated with an isotype control Ab or anti-Ly6G mAb at 4 weeks post-challenge and analyzed at 12 weeks post-infection. Chemokines levels from lungs of mice were assessed by Luminex 200 system as described in the Materials and Methods section. A) Chemokines associated with the recruitment of granulocyte cells (neutrophils, eosinophils and basophils); B) chemokines associated with the recruitment of mononuclear cells (lymphocytes, monocyte/macrophage and dendritic cells). Data shown represent median and IQR (n = 4–5 mice/group; representative of two independent experiments). *, P<0.05, **, P<0.01 and ***, P<0.001 comparing infected untreated mice versus control mice, or comparing infected-anti-Ly6G mAb treated mice versus infected-untreated mice. PBS, control mice; PBS + Iso, control mice treated with isotype control Ab (clone: 2A3); PBS + anti-neutrophils, control mice treated with anti-Ly6G mAb (clone: 1A8); Pb18, infected-untreated mice; Pb18 + Iso, infected mice treated with isotype control Ab; Pb18 + anti-neutrophils, infected mice treated with anti-Ly6G mAb.
Fig 4. Effect of neutrophils depletion on cytokine levels in lungs during the chronic stage of P. brasiliensis infection.
BALB/c mice were infected with 1.5x106 P. brasiliensis (Pb18) yeast cells and treated with an isotype control Ab or anti-Ly6G mAb at 4 weeks post-challenge and analyzed at 12 weeks post-infection. Cytokines levels associated with inflammatory response from lungs of mice were measured by Luminex 200 system as described in the Materials and Methods section. Data shown represent median and IQR (n = 4–5 mice/group; representative of two independent experiments). *, P<0.05, **, P<0.01 and ***, P<0.001 comparing infected untreated mice versus control mice, or comparing infected-anti-Ly6G mAb treated mice versus infected-untreated mice. PBS, control mice; PBS + Iso, control mice treated with isotype control Ab (clone: 2A3); PBS + anti-neutrophils, control mice treated with anti-Ly6G mAb (clone: 1A8); Pb18, infected-untreated mice; Pb18 + Iso, infected mice treated with isotype control Ab; Pb18 + anti-neutrophils, infected mice treated with anti-Ly6G mAb.
Depletion of neutrophils was correlated with a reduction in parameters related to Th immunity profiles
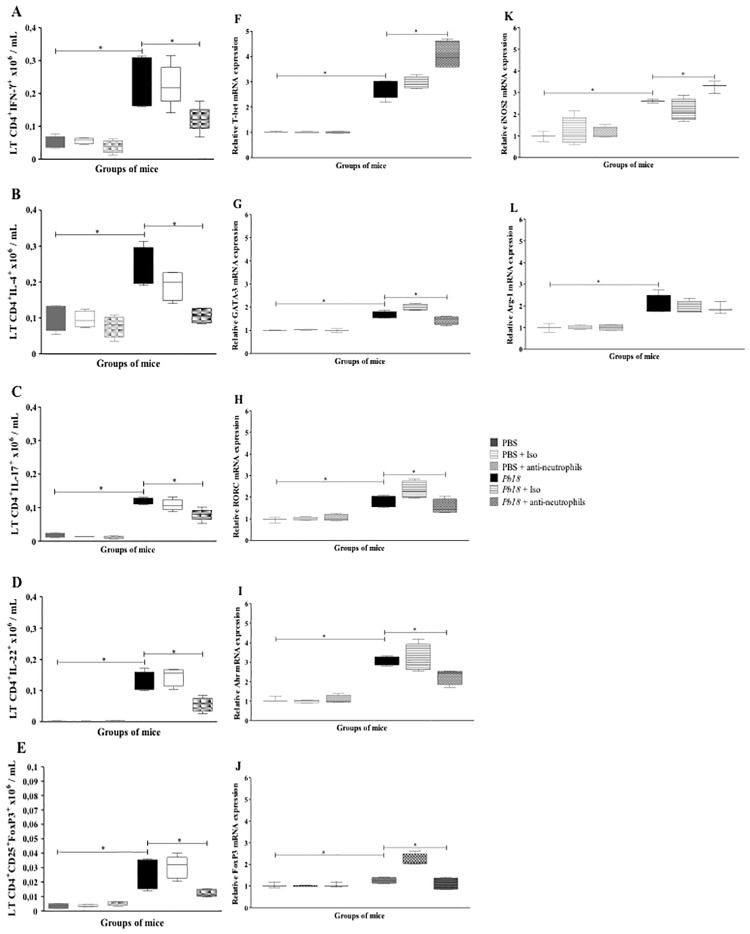
We evaluated the effect of neutrophils depletion on the Th-related parameters, in terms of cytokines production and transcription factors expression. Overall, P. brasiliensis-infected mice treated with mAb anti-neutrophils at 4 weeks post-infection showed a decrease in the number of Th1, Th2, Th17, Th22 and Treg subsets population (Fig 5A–5E). Likewise, this neutrophil-depleted group of mice demonstrated a lower mRNA expression of specific transcription factors GATA-3 (Th2), RORc (Th17), Ahr (Th22) and FoxP3 (Treg) (Fig 5G–5J) when compared with infected-isotype control or untreated mice, except for T-bet transcription factor mRNA (Th1) which was found overexpressed in neutrophils-depleted mice (Fig 5F).
Fig 5. Neutrophil depletion is associated with decreased intracellular cytokine levels and changes in transcription factor mRNA expression related to T helper subsets during the chronic stages of P. brasiliensis infection.
BALB/c mice infected with 1.5x106 P. brasiliensis yeast cells and treated with an isotype control Ab or anti-Ly6G mAb at 4 weeks post-infected and analyzed at 12 weeks post-infection. Flow cytometry was performed to identify the following lymphocytes subsets: A) Th1 (LT CD4+IFNγ+), B) Th2 (LT CD4+IL-4+), C) Th17 (LT CD4+IL-17+), D) Th22 (LT CD4+IL-22+), and E) Treg cells (LT CD4+CD25+ FoxP3+). Data shown represent median and IQR (n = 4–5 mice/group; representative of two independent experiments). Relative quantification of the mRNA expression for T-bet (F), GATA-3 (G), RORc (H), Ahr (I), FoxP3 (J), iNOS2 (K) and Arginase-1 (L) were measured in lungs of mice. Results are expressed as mean ± SEM (n = 4–5 mice/group; representative of two independent experiments). *, P<0.05 comparing infected untreated mice versus control mice, or comparing infected-anti-Ly6G mAb treated mice versus infected-untreated mice. PBS, control mice; PBS + Iso, control mice treated with isotype control Ab (clone: 2A3); PBS + anti-neutrophils, control mice treated with anti-Ly6G mAb (clone: 1A8); Pb18, infected-untreated mice; Pb18 + Iso, infected mice treated with isotype control Ab; Pb18 + anti-neutrophils, infected mice treated with anti-Ly6G mAb.
We also analyzed the mRNA expression of two other Th1- and Th2-related genes: the inducible nitric oxide synthase-2 (iNOS2) and Arginase-1 (Arg-1), respectively. Our results demonstrated that the iNOS2 mRNA expression was increased in infected and mAb-treated mice when compared to control mice (Fig 5K), meanwhile the Arg-1 mRNA was expressed in similar levels among the different groups of infected mice analyzed (Fig 5L).
Neutrophil-depleted mice showed a better control of P. brasiliensis infection during the chronic phase of PCM
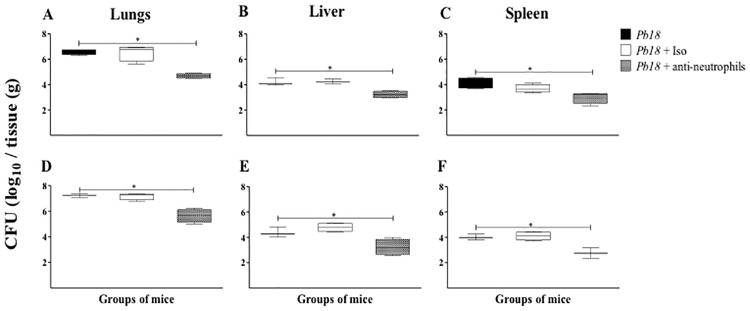
Our next step was to determine the effect of neutrophils depletion on the infection control. The fungal burden was evaluated at 12-week post-infection in lungs, liver and spleen of infected and untreated, isotype control or mAb anti-neutrophils treated mice at 4 or 8-week post-infection. All neutrophil-depleted group of mice showed a significant reduction of fungal burden in all organs assessed compared to infected-untreated or infected-isotype control treated mice (Fig 6A–6F).
Fig 6. Depletion of neutrophils is associated wit a reduction on the fungal burden in lungs, liver, and spleen from mice infected with P. brasiliensis at the chronic stages of infection.
Fungal load measurement was performed in lung, liver and spleen of mice at 12 weeks post-challenge with 1.5x106 P. brasiliensis (Pb18) yeast cells and treated with an isotype control Ab or anti-Ly6G mAb at 4 weeks (A, B, C) or 8 weeks (D, E, F) post-challenge. Data shown represent median and IQR (n = 4–5 mice/group; representative of two independent experiments). A statistically significant reduction in fungal burden was observed in the lungs of mice infected and treated with the anti-Ly6G mAb (*, P<0.05) compared to the infected untreated mice and the infected-isotype control Ab treated mice. Pb18, infected, untreated mice; Pb18 + Iso, infected mice treated with isotype control Ab (clone: 2A3); Pb18 + anti-neutrophils, infected mice treated with anti-Ly6G mAb (clone: 1A8).
Depletion of neutrophils was associated with reduction of the granulomatous inflammatory response and recovery of pulmonary architecture in mice infected with P. brasiliensis
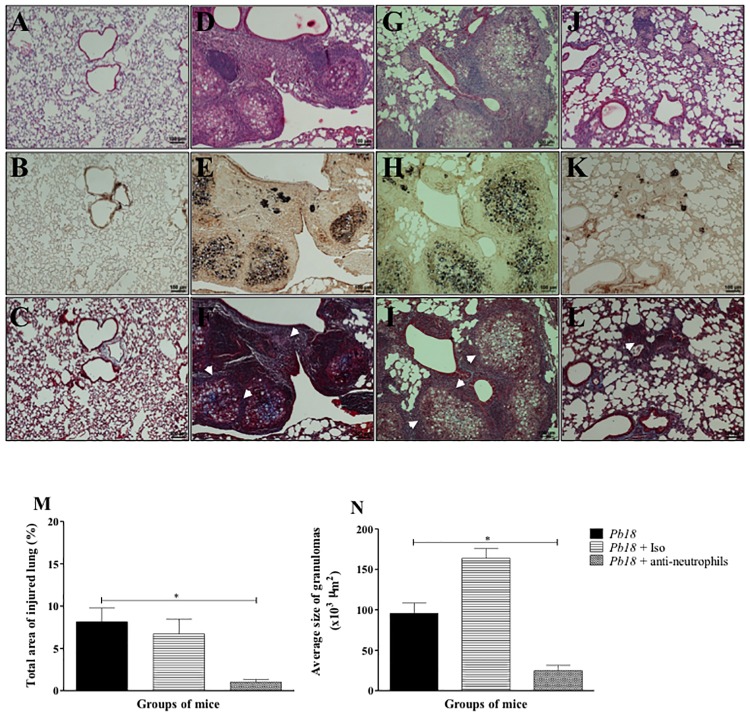
In order to evaluate the effect of neutrophil-depletion strategy in the lung parenchyma (in situ), lungs were processed for histopathological analysis as described in the Materials and Methods section. It was found a granulomatous cellular infiltrate (Fig 7D and 7G) with abundant parasitic yeast form surrounded by collagen fibers (Fig 7E, 7H, 7F and 7I) in the untreated- or isotype treated- and infected mice groups. Meanwhile, infected and treated mice with the anti-neutrophils mAb at 4 weeks post-infection evidenced an apparent decrease in the total area of injured lung (Fig 7M), as well as reduction in the size of granulomas (Fig 7N) and composition of cellular infiltrates (Fig 7J), accompanied by a significant decrease in the amount of P. brasiliensis yeasts and collagen fibers (Fig 7K and 7L).
Fig 7. Depletion of neutrophils is associated with a reduction in both granulomatous inflammatory response and fibrosis in lungs from mice challenged with P. brasiliensis.
Microphotographs are representative of lungs from uninfected (A, B, C), infected and untreated mice (D, E, F) or infected and treated with an isotype control Ab (G, H, I) or an anti-Ly6G mAb specific to neutrophils (J, K, L) at 12 weeks post-challenge and obtained from groups of 4–5 mice. Lungs were fixed, embedded in paraffin, cut and stained using H&E staining to determine lung inflammatory response (A, D, G, J), methenamine silver staining (B, E, H, K) to identify P. brasiliensis’ yeast cells and Masson’s trichrome (C, F, I, L) to identify and differentiate collagen fibers as described in the Materials and Methods section. Arrowheads indicate collagen fibers (in white). Quantitative analysis of total injured lung area (M) and size of granuloma (N) were assessed by ImageJ software as described in the Materials and Methods section. These results are representative of two independent experiments. Magnification 10X.
Recovery of the pulmonary architecture was associated to low level of total soluble collagen, an overexpression of collagen-1 mRNA and a decrease expression of collagen-3 gene
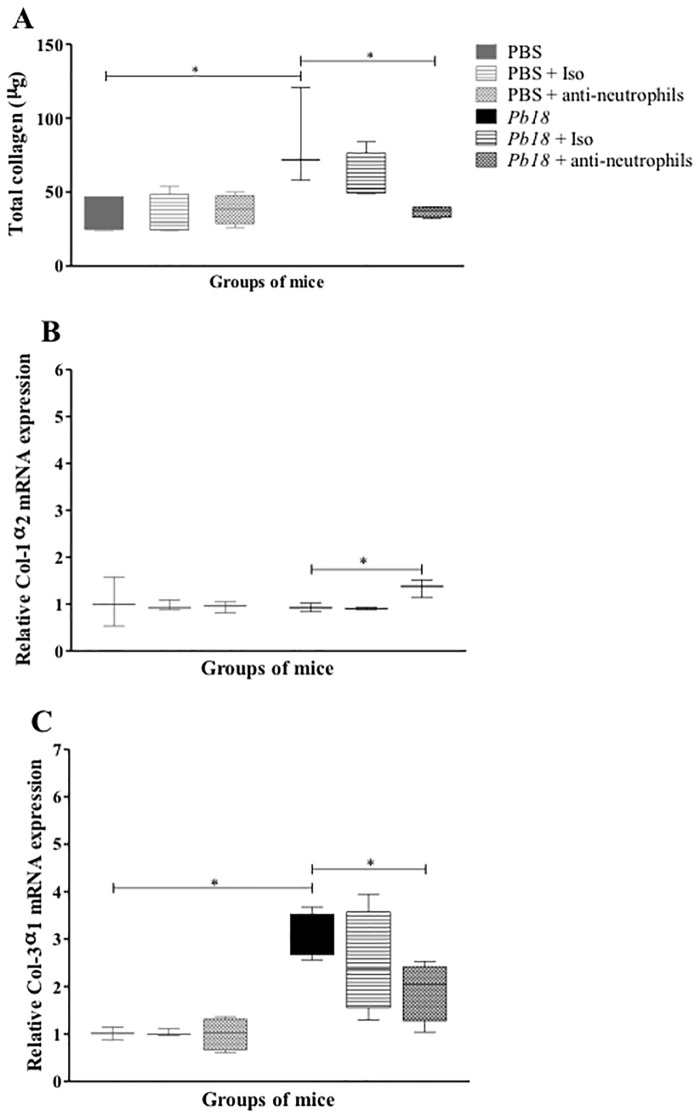
In order to determine if the collagen, considered as the most important extracellular matrix protein (ECMp) involved in the fibrosis development, was associated with the controlled granulomatous inflammatory process and the recovery of pulmonary architecture in P. brasiliensis-infected mice, we determined the production and expression of this ECMp in situ. It was observed that the total amount of soluble collagen was diminished in neutrophils-depleted mice (Fig 8A) compared to control animals. Interestingly, neutrophil-depleted mice showed an increase expression of collagen-I and a decrease expression of collagen-III mRNA (Fig 8B–8C).
Fig 8. Neutrophils depletion is associated with a decrease of collagen levels and changes on mRNA level expression of Colagen-1 and Colagen-3 in lungs of mice infected with P. brasiliensis yeast cells.
BALB/c mice were infected with 1.5x106 P. brasiliensis (Pb18) yeast cells and treated with an isotype control Ab or anti-Ly6G mAb at 4 weeks post-challenge and analyzed at 12 weeks post-infection. Total collagen (A) and relative quantification of the mRNA expression for Col-1α2 (B) and Col-3α1 (C) were performed in lungs of mice (n = 4–5 mice/group; representative of two independent experiments). Results are expressed as median ± IQR. *, P<0.05 comparing infected untreated mice versus control mice, or comparing infected-anti-Ly6G mAb treated mice versus infected untreated mice. PBS, control mice; PBS + Iso, control mice treated with isotype control Ab (clone: 2A3); PBS + anti-neutrophils, control mice treated with anti-Ly6G mAb (clone: 1A8); Pb18, infected, untreated mice; Pb18 + Iso, infected mice treated with isotype control Ab; Pb18 + anti-neutrophils, infected mice treated with anti-Ly6G mAb.
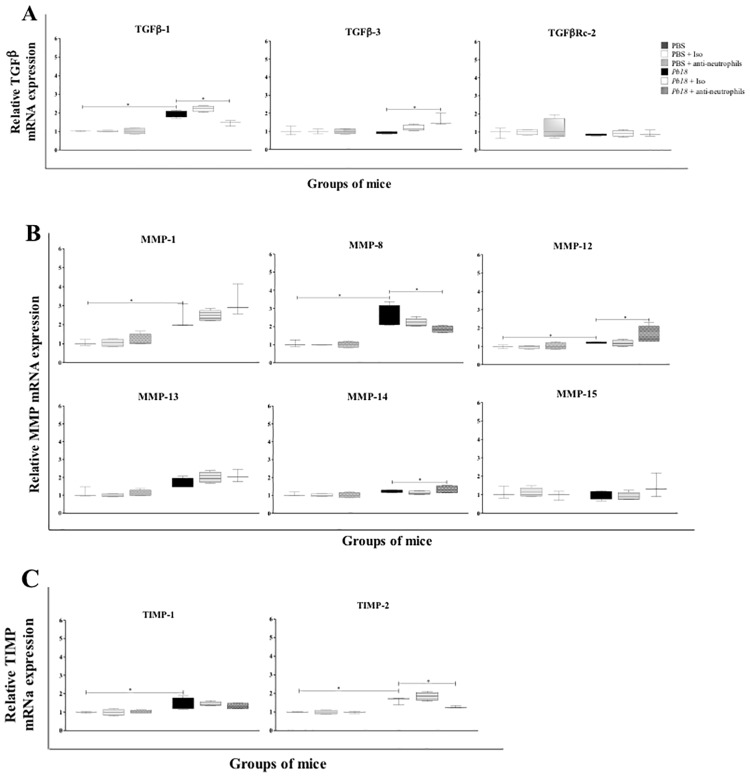
Reduction of granulomatous inflammatory response in lungs of neutrophil-depleted and infected mice was associated with an altered expression pulmonary fibrosis-related genes: TGF-β, MMP and TIMP
Finally, we evaluated the effect of neutrophils-depletion treatment on the mRNA expression of genes associated with the development of pulmonary fibrosis. Our results demonstrated that the mRNA expression of TGF-β1, Matrix metalloproteinase (MMP)-8 and Tissue inhibitor metalloproteinase (TIMP)-2 genes were significantly diminished in infected and mAb treated-animals compared to control ones (Fig 9). In contrast, the mRNA of TGF-β3, MMP-12 and MMP-14 genes were found overexpressed in this group of mice (Fig 9).
Fig 9. Reduction of the granulomatous inflammatory response in lungs of neutrophil-depleted mice challenged with P. brasiliensis during the chronic course of infection is associated with a decrease of expression of TGF-β1 and MMP-8 and overexpression of TGF-β3, MMP-12 and MMP-14.
BALB/c mice were infected with 1.5x106 P. brasiliensis (Pb18) yeast cells and treated with an isotype control Ab or anti-Ly6G mAb at 4 weeks post-challenge and analyzed at 12 weeks post-infection. Relative quantification of the mRNA expression was performed in lungs of mice for the following genes: A) TGF-β1, TGF-β3 and TGF-βRc2; B) MMP-1, MMP-8, MMP-12, MMP-13, MMP-14 and MMP-15; and C) TIMP-1 and TIMP-2 (n = 4–5 mice/group; representative of two independent experiments). Results are expressed as median ± IQR. *, P<0.05 comparing infected untreated mice versus control mice, or comparing infected-anti-Ly6G mAb treated mice versus infected untreated mice. PBS, control mice; PBS + Iso, control mice treated with isotype control Ab (clone: 2A3); PBS + anti-neutrophils, control mice treated with anti-Ly6G mAb (clone: 1A8); Pb18, infected, untreated mice; Pb18 + Iso, infected mice treated with isotype control Ab; Pb18 + anti-neutrophils, infected mice treated with anti-Ly6G mAb.
Discussion
Several studies have been highlighting the importance of neutrophils in the pathogenesis of PCM, most of them dedicated to evaluate the role of these cells during early stages of infection and their influence on the adaptive immune response [8,11,12]. However, to our knowledge, there are no studies focused on evaluating neutrophil involvement in the chronic stages or in the development of fibrosis observed in this mycosis. In the present study, we describe for the first time that the depletion of neutrophils during chronic stages of PCM is associated with a better control of P. brasiliensis infection, reduction in the inflammatory and granulomatous response, and attenuation of the fibrosis process as observed by recovery of the pulmonary architecture with decreased collagen fibers and lower expression of genes associated with development of fibrosis.
Neutrophils are considered pivotal as the first line of innate immunity and as promoter of adaptive immune response that contribute to the development of inflammatory reaction against a wide range of infections [14–16]. Many strategies have been used to understand neutrophil functions during recognition and elimination of a pathogenic microorganism [17–19]. Those strategies include genetic manipulation (Gfi-1-/- knockout) [20] or the implementation of monoclonal antibodies such as anti-Gr1 (RB6-8C5) or anti-Ly6G (1A8) in animal models inducing depletion of neutrophils [21], the latter being most widely used to evaluate the in vivo functions of these cells during the development of immune response against infections caused by Toxoplasma gondii [22], Leishmania amazonensis [23], and fungal infections caused by Aspergillus fumigatus [24], Cryptococcus neoformans [25,26] and Candida albicans [27]. In the case of P. brasiliensis infection, a study involving resistant (A/J) or susceptible (B10.A) mice to fungus and treated with the anti-Gr1 mAb during the early stages of infection showed that neutrophils exert a protective role on the innate phase of immunity only in the susceptible host infected with the fungus [11]. Similar results were obtained in our laboratory using a new experimental PCM model in BALB/c mice infected i.n. with P. brasiliensis yeast and treated with the specific anti-neutrophils mAb (anty-Ly6G; clone 1A8) during the acute phase of infection, demonstrating a decrease in survival time complemented with an increased fungal burden and exacerbation of the inflammatory response [12].
The goal of the present study was to evaluate the effect of neutrophils depletion during the chronic stage of PCM and specially during the development of fibrosis process; herein, we demonstrated that depletion of these phagocytic cells was associated with a better control of infection, as shown by lower fungal burden in their lungs, livers, and spleens, suggesting that the presence of neutrophils may be contributing to the persistence of P. brasiliensis. Several in vitro studies have described the interaction between P. brasiliensis yeasts and neutrophils hypothesizing about how this phagocytic cell promotes the replication of fungus allowing the infection to be spread from lungs to other organs and systems. Kurita et al [28–30] suggested that P. brasiliensis yeast could evade the immune response and survive inside the neutrophil. In addition, Acorci et al [31] reported that the ability of yeast cells to inhibit apoptosis and prolong the lifetime of neutrophils via IL-8. Moreover, it has been demonstrated that neutrophils from patients with PCM have a significant deficiency to digest in vitro viable P. brasiliensis yeasts without prior activation by IFN-γ, GM-CSF and/or IL-1β [32,33].
Likewise, our findings may indicate that the absence of neutrophils promotes the development of an effective immune response leading to reduced tissue damage. Similar results have been reported in an animal model of C. neoformans infection, in which it was found that neutrophils depletion increased levels of pro- and anti-inflammatory cytokines, favoring the infection control [34]. Additionally, Park et al [35] using an animal model of neutropenic invasive aspergillosis postulated that the absence of neutrophils in infected tissue enhances the inflammatory cytokine milieu and cellular response to Aspergillus in the host. Similarly, Dunay et al [22] observed that T. gondii-infected mice treated with the anti-neutrophils mAb were able to control the parasite replication, increase their survival to infection accompanied by a reduction in number of CD4 and CD8 T cells. Interestingly, in the present study we observed that neutrophil depletion in P. brasiliensis-infected mice was associated with diminished number of CD4 T, B cells, DCs, and Treg cells as well as pro-inflammatory cytokines and chemokines. Thus, these results confirm that neutrophils play an important immunoregulatory role in the pathogenesis of PCM.
On this line, several authors have demonstrated the ability of neutrophils to regulate leukocyte trafficking through multiple chemokine production [36,37]. In our case, this phenomenon could be linked to the significant reduction in the levels of several chemokines in infected mAb-treated mice, which are involved in the recruitment of mononuclear and polimorphonuclear cells (Fig 3) [38,39]. It is also clear that neutrophils produce a large number of cytokines during infection [40,41], many of which, in our case, were found significantly decreased in neutrophil-depleted mice. Chen et al [42] observed from a cerebral malaria animal model, that in the absence of neutrophils the expression of pro-inflammatory cytokines and the development of cerebral microhaemorrhages were reduced, supporting the hypothesis that this phagocytic cell plays a critical role in coordinating the inflammatory response and is determinant in the pathogenesis of malaria infection.
Regarding cell populations, our results showed a lower number in eosinophils and MDSc on neutrophil-depleted and infected mice compared to control animals. Some authors have associated these populations of cells with a Th2-profile [43,44] and suppression of adaptive immunity [45] that could promote the development of severe forms of PCM [46,47]. Of note, eosinophilia has been associated with worsening and a poor prognosis of fungal infections including PCM and coccidioidomycosis, a process linked to a Th2 immunity pattern, which is less efficient to control infection; additionally, the toxic radical molecules stored in their granules could be also associated to the tissue damage observed [43,48–51]. On the other hand, MDSc are characterized by their capacity to suppress T cell response and subsequently modulate the outcome of fungal infections [52]. Thus, in the present study, depletion of neutrophils was associated with a diminished number in eosinophils and MDSc accompanied by a reduction on fungal burden and an improvement of control infection as well as of recovery of damaged tissue.
Furthermore, some reports have evidenced that resistance to P. brasiliensis infection depends mainly upon Th1 and Th17 cells [46,53,54]. However, in some cases, this T cell response can lead to massive destruction of lung tissue affecting its functionality, as seen in patients with PCM and fibrosis [1,6]. Likewise, it has been suggested the importance of neutrophils in adaptive immunity and pulmonary host defenses. Loures et al [55] showed that Toll-like receptor (TLR)-2-deficient mice infected with P. brasiliensis yeasts develop a prevalent Th17 immunity with a diminished presence of Tregs, allowing control fungal growth but also inducing tissue damage mediated by neutrophils. Later on, the same group reported that TLR4-deficient mice were unable to control the infection and developed an inefficient immune response by decreased expansion of Th17 cells and elevated differentiation of Treg, which could negatively control the expansion and migration of P. brasiliensis-specific T cells to the lungs [56]. In contrast, our findings showed that neutrophil-depletion treatment was associated with a reduction of CD4 T cell subsets, including Th17 and Tregs accompanied by higher levels of IL-2, IL-9 and IL-12 cytokines and the overexpression of T-bet and iNOS genes, thus supporting our hypothesis of the development of an effective immune response that controls not only the fungal growth but also the intensity of the tissue damage.
Finally, regarding pulmonary fibrosis induced by P. brasiliensis infection, previous reports obtained from animal models have described the formation of granulomatous infiltrates starting at 4 weeks post-infection [57,58] and increased amounts of collagen, TGF-β and TNF- α levels [59,60] as observed in the present work. Interestingly, after treatment with the anti-neutrophils mAb, we observed a decrease in the amount of collagen, the inflammatory area, the size of granulomas, the number of Tregs, and the expression of TGF-β1. This might be explained by an indirect mechanism in which the neutrophil produces IL-17 a pivotal cytokine that subsequently induces the recruitment and activation of Treg cells, as well as the induction of the TGF-β production by type II macrophages which in turn activate fibroblast to produce collagen. Thus, we previously demonstrated that neutrophils are an important source of IL-17 [12]. Moreover, in a previous report it was demonstrated that the usage of a combined itraconazole-pentoxifylline treatment in P. brasiliensis-infected mice, initiated at 4 and 8 weeks post-infection, reduced both the inflammatory response (including neutrophils) and fibrosis [61]; herein, we confirmed that reduction of the fibrosis process was accompanied by a lower number of neutrophils as well as IL-17, Th-17, and Treg cells.
An additional hypothesis suggests that neutrophil induces the production and over-activation of proteolytic enzymes, such as MMPs, responsible for extracellular matrix (mainly collagen) remodeling and degradation [62,63]. In pathological conditions, the MMPs are associated with tissue destruction, weakening of extracellular matrix (ECM) and fibrosis [64,65]; once this happens, TIMPs may act in the tissue environment to neutralize the MMP, thereby preventing excessive degradation [62,66]. In the present study, we observed a decrease in mRNA expression of MMP-8 (collagenase-III), known as “neutrophil collagenase” [67,68]. Additionally, those infected and treated mice showed an increased level of collagen-I, TIMP-2, MMP-12 and MMP-14 genes, associated with a significant reduction of the fibrotic sequelae and a tissue remodeling process [69]. Although for many years fibrosis was thought to be a progressive and irreversible process, recent studies have challenged this theory. It is possible that a controlled inflammatory response could facilitate ECM degradation and remodeling [70].
In summary, we demonstrated that neutrophils play a dual role during P. brasiliensis infection, being protecting during early infection and harmful during the chronic phase of disease ([12] and the present study). Thus, we could hypothesize that during early infection neutrophils interact with macrophages and DCs through production of chemokines and cytokines, which in turn induce their recruitment, activation, differentiation and maturation. In the case of macrophages, the IFN-γ produced by neutrophils [12] and/or other innate cells, activate and enable them to kill the fungus through potent antifungal mechanisms, whereas interactions with DCs could promote their maturation with the subsequent development and expansion of T cell-mediated immunity, as described for A. fumigatus infection [71]. In contrast, during the chronic phase of fungal infection, the recruitment of neutrophils induces an uncontrolled immune reaction, leading to a severe tissue inflammatory pathology due to the excessive release of IL-17, TNF-α and enzymes contained in their granules [14,72]. Additionally, neutrophils may harbor the fungus, acting as “Trojan horses”, allowing it to survive inside these phagocytic cells and disseminate from the site of infection to other organs and systems, as described for other pathogens [14,73,74]. Moreover, different neutrophil subsets have been described and characterized by the cytokine and chemokine production, and by different surface antigens expression, as well [75]. However, the identification of neutrophil subpopulations has not been described in fungal infections so far. In consequence, it could not be ruled out that different neutrophil subsets act at different phases of fungal infection, leading to an opposite immune response.
Conclusions
The above results indicate that neutrophil depletion was associated with a better control of infection and decrease of the granulomatous inflammatory response and fibrosis during the chronic stages of experimental PCM, suggesting that this phagocytic cell exert a harmful role in the immune response to P. brasiliensis infection and could influence the development of fibrosis in affected individuals. Thus, neutrophil could be considered as a possible therapeutic target in PCM.
Acknowledgments
We thank Dr. Lina Salazar for her support in providing the histopathological analysis.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by Departamento Administrativo de Ciencia, Tecnología e Innovación (COLCIENCIAS), Bogotá, Colombia, Project 183-2010 (Code number 2213-519-28621), and the Research Committee (CODI) of Universidad de Antioquia through the Sustainability Strategy Program 2014–2015. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hardie WD, Glasser SW, Hagood JS (2009) Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol 175: 3–16 10.2353/ajpath.2009.081170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Restrepo A, Gonzalez A, Agudelo CA (2011) Paracoccidioidomycosis In Essentials of Clinical Mycology. Kauffman A.C., Pappas G.P., Sobel D.J., and Dismukes E.W., editors. Springer New York, New York, NY: pp. 367–85 10.1007/978-1-4419-6640-7_21 [DOI] [Google Scholar]
- 3.Brummer E, Castaneda E, Restrepo A (1993) Paracoccidioidomycosis: an update. Clin Microbiol Rev 6: 89–117 10.1128/CMR.6.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marques SA (2013) Paracoccidioidomycosis: epidemiological, clinical, diagnostic and treatment up-dating. An Bras Dermatol 88: 700–711 10.1590/abd1806-4841.20132463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo AL, Tobon A, Restrepo A, Queiroz-Telles F, Nucci M (2011) Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 49: 785–798 10.3109/13693786.2011.577821 [DOI] [PubMed] [Google Scholar]
- 6.Tobon AM, Agudelo CA, Osorio ML, Alvarez DL, Arango M, Cano LE, et al. (2003) Residual pulmonary abnormalities in adult patients with chronic paracoccidioidomycosis: prolonged follow-up after itraconazole therapy. Clin Infect Dis 37: 898–904 10.1086/377538 [DOI] [PubMed] [Google Scholar]
- 7.Bedoya V, McEwen JG, Tabares AM, Jaramillo FU, Restrepo A (1986) Pathogenesis of paracoccidioidomycosis: a histopathological study of the experimental murine infection. Mycopathologia 94: 133–144 10.1007/BF00454591 [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez A, Cano LE (2001) Participación del polimorfonuclear neutrófilo en la respuesta inmune contra Paracoccidioides brasiliensis. Biomedica 21: 264–274 10.7705/biomedica.v21i3.1117 [DOI] [Google Scholar]
- 9.Balderramas HA, Penitenti M, Rodrigues DR, Bachiega TF, Fernandes RK, Ikoma MR, et al. (2014) Human neutrophils produce IL-12, IL-10, PGE2 and LTB4 in response to Paracoccidioides brasiliensis. Involvement of TLR2, mannose receptor and dectin-1. Cytokine 67: 36–43 10.1016/j.cyto.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues DR, Fernandes RK, Balderramas HdeA, Penitenti M, Bachiega TF, Calvo SA, et al. (2014) Interferon-gamma production by human neutrophils upon stimulation by IL-12, IL-15 and IL-18 and challenge with Paracoccidioides brasiliensis. Cytokine 69: 102–109 10.1016/j.cyto.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 11.Pina A, Saldiva PH, Restrepo LE, Calich VL (2006) Neutrophil role in pulmonary paracoccidioidomycosis depends on the resistance pattern of hosts. J Leuk Biol 79: 1202–1213 10.1189/jlb.0106052 [DOI] [PubMed] [Google Scholar]
- 12.Pino-Tamayo PA, Puerta-Arias JD, Lopera D., Urán-Jiménez ME, Gonzalez A (2016) Depletion of neutrophils exacerbate the early inflammatory immune response in ling of mice infected with Paracoccidioides brasiliensis. Mediators Inflamm 2016:3183285, 10.1155/2016/3183285 10.1155/2016/3183285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer-Vermes LM, Sakamoto TN, Vaz CA, Calich VL (1995) Influence of the genetic pattern and sex of mice in experimental paracoccidioidomycosis. Clin Exp Immunol 101: 114–120 10.1111/j.1365-2249.1995.tb02286.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mocsai A (2013) Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med 210: 1283–1299 10.1084/jem.20122220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan C (2006) Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6: 173–182 10.1038/nri1785 [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Arase H (2014) Regulation of immune responses by neutrophils. Ann N Y Acad Sci 1319: 66–81 10.1111/nyas.12445 [DOI] [PubMed] [Google Scholar]
- 17.Janardhan KS, Sandhu SK, Singh B (2006) Neutrophil depletion inhibits early and late monocyte/macrophage increase in lung inflammation. Front Biosci 11: 1569–1576 10.2741/1904 [DOI] [PubMed] [Google Scholar]
- 18.Lewis SM, Khan N, Beale R, Treacher DF, Brown KA (2013) Depletion of blood neutrophils from patients with sepsis: treatment for the future? Int Immunopharmacol 17: 1226–1232 10.1016/j.intimp.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 19.Zuluaga AF, Salazar BE, Rodriguez CA, Zapata AX, Agudelo M, Vesga O (2006) Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect Dis 6: 55 10.1186/1471-2334-6-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hock H, Hamblen MJ, Rooke HM, Traver D, Bronson RT, Cameron S, et al. (2003) Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity 18: 109–20 10.1016/S1074-7613(02)00501-0 [DOI] [PubMed] [Google Scholar]
- 21.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE (2008) Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leuk Biol 83: 64–70 10.1189/jlb.0407247 [DOI] [PubMed] [Google Scholar]
- 22.Dunay IR, Fuchs A, Sibley LD (2010) Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect Immun 78: 1564–1570 10.1128/IAI.00472-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sousa LM, Carneiro MB, Resende ME, Martins LS, Dos Santos LM, Vaz LG, et al. (2014) Neutrophils have a protective role during early stages of Leishmania amazonensis infection in BALB/c mice. Parasite Immunol 36: 13–31 10.1111/pim.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mircescu MM, Lipuma L, van Rooijen N, Pamer EG, Hohl TM (2009) Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J Infect Dis 200:647–656 10.1086/600380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wozniak KL, Kolls JK, Wormley FL Jr (2012) Depletion of neutrophils in a protective model of pulmonary cryptococcosis results in increased IL-17A production by gammadelta T cells. BMC Immunol 13: 65 10.1186/1471-2172-13-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M, Sun D, Liu G, Wu H, Zhou H, Shi M (2016) Real-time in vivo imaging reveals the ability of neutrophils to remove Cryptococcus neoformans directly from the brain vasculature. J Leukoc Biol 99:467–473 10.1189/jlb.4AB0715-281R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huppler AR, Conti HR, Hernandez-Santos N, Darville T, Biswas PS, Gaffen SL (2014) Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J Immunol 192: 1745–1752 10.4049/jimmunol.1302265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurita N, Biswas SK, Oarada M, Sano A, Nishimura K, Miyaji M (1999) Fungistatic and fungicidal activities of murine polymorphonuclear leucocytes against yeast cells of Paracoccidioides brasiliensis. Med Mycol 37: 19–24. 10.1111/j.1365-280X.1999.00194.x [DOI] [PubMed] [Google Scholar]
- 29.Kurita N, Oarada M, Ito E, Miyaji M (1999) Antifungal activity of human polymorphonuclear leucocytes against yeast cells of Paracoccidioides brasiliensis. Med Mycol 37: 261–267 10.1111/j.1365-280X.1999.00229.x [DOI] [PubMed] [Google Scholar]
- 30.Kurita N, Oarada M, Brummer E (2005) Fungicidal activity of human peripheral blood leukocytes against Paracoccidioides brasiliensis yeast cells. Med Mycol 43: 417–422 [DOI] [PubMed] [Google Scholar]
- 31.Acorci MJ, Dias-Melicio LA, Golim MA, Bordon-Graciani AP, Peracoli MT, Soares AM (2009) Inhibition of human neutrophil apoptosis by Paracoccidioides brasiliensis: role of interleukin-8. Scan J Immunol 69: 73–79 10.1111/j.1365-3083.2008.02199.x [DOI] [PubMed] [Google Scholar]
- 32.Goihman-Yahr M, Pereira J, Isturiz G, Viloria N, Carrasquero M, Saavedra N, et al. (1992) Relationship between digestive and killing abilities of neutrophils against Paracoccidioides brasiliensis. Mycoses 35: 269–274 10.1111/j.1439-0507.1992.tb00873.x [DOI] [PubMed] [Google Scholar]
- 33.Kurita N, Oarada M, Miyaji M, Ito E (2000) Effect of cytokines on antifungal activity of human polymorphonuclear leucocytes against yeast cells of Paracoccidioides brasiliensis. Med Mycol 38: 177–182 10.1080/714030926 [DOI] [PubMed] [Google Scholar]
- 34.Mednick AJ, Feldmesser M, Rivera J, Casadevall A (2003) Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur J Immunol 33: 1744–1753 10.1002/eji.200323626 [DOI] [PubMed] [Google Scholar]
- 35.Park SJ, Burdick MD, Brix WK, Stoler MH, Askew DS, Strieter RM, et al. (2010) Neutropenia enhances lung dendritic cell recruitment in response to Aspergillus via a cytokine-to-chemokine amplification loop. J Immunol 185: 6190–6197 10.4049/jimmunol.1002064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pliyev BK (2008) Chemotactically active proteins of neutrophils. Biochemistry 73: 970–984 10.1134/S0006297908090034 [DOI] [PubMed] [Google Scholar]
- 37.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA (2000) The neutrophil as a cellular source of chemokines. Immunol Rev 177: 195–203 10.1034/j.1600-065X.2000.17706.x [DOI] [PubMed] [Google Scholar]
- 38.Rollins BJ (1997) Chemokines. Blood 90: 909–928 [PubMed] [Google Scholar]
- 39.Zlotnik A, Yoshie O (2000) Chemokines: a new classification system and their role in immunity. Immunity 12: 121–127 10.1016/S1074-7613(00)80165-X [DOI] [PubMed] [Google Scholar]
- 40.Cassatella MA (1999) Neutrophil-derived proteins: selling cytokines by the pound. Adv Immunol. 73: 369–509 10.1016/S0065-2776(08)60791-9 [DOI] [PubMed] [Google Scholar]
- 41.Denkers EY, Del Rio L, Bennouna S (2003) Neutrophil production of IL-12 and other cytokines during microbial infection. Chem Immunol Allergy 83: 95–114 10.1159/000071557 [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Zhang Z, Sendo F (2000) Neutrophils play a critical role in the pathogenesis of experimental cerebral malaria. Clin Exp Immunol 120: 125–133 10.1046/j.1365-2249.2000.01196.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mamoni RL, Nouer SA, Oliveira SJ, Musatti CC, Rossi CL, Camargo ZP, et al. (2002) Enhanced production of specific IgG4, IgE, IgA and TGF-beta in sera from patients with the juvenile form of paracoccidioidomycosis. Med Mycol 40: 153–159 [DOI] [PubMed] [Google Scholar]
- 44.Shikanai-Yasuda MA, Higaki Y, Uip DE, Mori NS, Del Negro G, Melo NT, et al. (1992) Bone marrow involvement and eosinophilia in paracoccidioidomycosis. Rev Inst Med Trop Sao Paulo 34: 85–90 [PubMed] [Google Scholar]
- 45.Kallberg E, Stenstrom M, Liberg D, Ivars F, Leanderson T (2012) CD11b+Ly6C++Ly6G- cells show distinct function in mice with chronic inflammation or tumor burden. BMC Immunol 13: 69 10.1186/1471-2172-13-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Castro LF, Ferreira MC, da Silva RM, Blotta MH, Longhi LN, Mamoni RL (2013) Characterization of the immune response in human paracoccidioidomycosis. J Infect 67: 470–485 10.1016/j.jinf.2013.07.019 [DOI] [PubMed] [Google Scholar]
- 47.Pereira RM, Bucaretchi F, Barison Ede M, Hessel G, Tresoldi AT (2004) Paracoccidioidomycosis in children: clinical presentation, follow-up and outcome. Rev Inst Med Trop Sao Paulo 46: 127–131 10.1590/S0036-46652004000300002 [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez A, Hung CY, Cole GT (2011) Absence of phagocyte NADPH oxidase 2 leads to severe inflammatory response in lungs of mice infected with Coccidioides. Microb Pathog 51: 432–441 10.1016/j.micpath.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Echols RM, Palmer DL, Long GW (1982) Tissue eosinophilia in human coccidioidomycosis. Rev Infect Dis 4: 656–664 10.1093/clinids/4.3.656 [DOI] [PubMed] [Google Scholar]
- 50.Wagner JM, Franco M, Kephart GM, Gleich GJ (1998) Localization of eosinophil granule major basic protein in paracoccidioidomycosis lesions. Am J Trop Med Hyg 59: 66–72 [DOI] [PubMed] [Google Scholar]
- 51.Muniz VS, Baptista-Dos-Reis R, Neves JS (2013) Functional extracellular eosinophil granules: a bomb caught in a trap. Int Arch Allergy Immunol 162: 276–282 10.1159/000354934 [DOI] [PubMed] [Google Scholar]
- 52.Rieber N, Singh A, Öz H, Carevic M, Bouzani M, Amich J, et al. (2015) Pathogenic fungi regulate immunity by inducing neutrophilic myeloid-derived suppressor cells. Cell Host Microbe 17: 507–514 10.1016/j.chom.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cano LE, Kashino SS, Arruda C, Andre D, Xidieh CF, Singer-Vermes LM, et al. (1998) Protective role of gamma interferon in experimental pulmonary paracoccidioidomycosis. Infect Immun 66: 800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Souto JT, Figueiredo F, Furlanetto A, Pfeffer K, Rossi MA, Silva JS (2000) Interferon-gamma and tumor necrosis factor-alpha determine resistance to Paracoccidioides brasiliensis infection in mice. Am J Pathol 156: 1811–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loures FV, Pina A, Felonato M, Calich VL (2009) TLR2 is a negative regulator of Th17 cells and tissue pathology in a pulmonary model of fungal infection. J Immunol 183: 1279–1290 10.4049/jimmunol.0801599 [DOI] [PubMed] [Google Scholar]
- 56.Loures FV, Pina A, Felonato M, Araujo EF, Leite KR, Calich VL (2010) Toll-like receptor 4 signaling leads to severe fungal infection associated with enhanced proinflammatory immunity and impaired expansion of regulatory T cells. Infect Immun 78: 1078–1088 10.1128/IAI.01198-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cock AM, Cano LE, Velez D, Aristizabal BH, Trujillo J, Restrepo A (2000) Fibrotic sequelae in pulmonary paracoccidioidomycosis: histopathological aspects in BALB/c mice infected with viable and non-viable Paracoccidioides brasiliensis propagules. Rev Inst Med Trop Sao Paulo 42: 59–66 10.1590/S0036-46652000000200001 [DOI] [PubMed] [Google Scholar]
- 58.Kerr IB, Araripe JR, Oliveira PC, Lenzi HL (1988) Paracoccidioidomycosis: a sequential histopathologic study of lesions in experimentally-infected rats. Rev Inst Med Trop Sao Paulo 30: 336–350 10.1590/S0036-46651988000500003 [DOI] [PubMed] [Google Scholar]
- 59.Franco L, Najvar L, Gomez BL, Restrepo S, Graybill JR, Restrepo A (1998) Experimental pulmonary fibrosis induced by Paracoccidioides brasiliensis conidia: measurement of local host responses. Am J Trop Med Hyg 58: 424–430 [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez A, Restrepo A, Cano LE (2008) Pulmonary immune responses induced in BALB/c mice by Paracoccidioides brasiliensis conidia. Mycopathologia 165: 313–330 10.1007/s11046-007-9072-1 [DOI] [PubMed] [Google Scholar]
- 61.Naranjo TW, Lopera DE, Diaz-Granados LR, Duque JJ, Restrepo AM, Cano LE (2011) Combined itraconazole-pentoxifylline treatment promptly reduces lung fibrosis induced by chronic pulmonary paracoccidioidomycosis in mice. Pul Pharmacol Ther 24: 81–91 10.1016/j.pupt.2010.09.005 [DOI] [PubMed] [Google Scholar]
- 62.Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274: 21491–21494 10.1074/jbc.274.31.21491 [DOI] [PubMed] [Google Scholar]
- 63.Stamenkovic I (2003) Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathology 200: 448–464 10.1002/path.1400 [DOI] [PubMed] [Google Scholar]
- 64.Amalinei C, Caruntu ID, Giusca SE, Balan RA (2010) Matrix metalloproteinases involvement in pathologic conditions. Rom J Morphol Embryol 51: 215–228 [PubMed] [Google Scholar]
- 65.Ohbayashi H (2002) Matrix metalloproteinases in lung diseases. Curr Prot Pept Sci 3: 409–421 10.2174/1389203023380549 [DOI] [PubMed] [Google Scholar]
- 66.Bode W, Fernandez-Catalan C, Grams F, Gomis-Ruth FX, Nagase H, Tschesche H, et al. (1999) Insights into MMP-TIMP interactions. Ann N Y Acad Scien 878: 73–91 10.1111/j.1749-6632.1999.tb07675.x [DOI] [PubMed] [Google Scholar]
- 67.Horwitz AL, Hance AJ, Crystal RG (1977) Granulocyte collagenase: selective digestion of type I relative to type III collagen. Proc Natl Acad Sci 74: 897–901 10.1073/pnas.74.3.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu KG, Stultz CM (2013) Insight into the degradation of type-I collagen fibrils by MMP-8. J Mol Biol 425: 1815–1825 10.1016/j.jmb.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 69.Shapiro SD (1998) Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol 10: 602–608 10.1016/S0955-0674(98)80035-5 [DOI] [PubMed] [Google Scholar]
- 70.Cox TR, Erler JT (2011) Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Models Mech 4: 165–178 10.1242/dmm.004077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park SJ, Burdick MD, Mehrad B (2012) Neutrophils mediate maturation and efflux of lung dendritic cells in response to Aspergillus fumigatus germ tubes. Infect Immun 80: 1759–1765. 10.1128/IAI.00097-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cruz A, Fraga AG, Fountain JJ, Rangel-Moreno J, Torrado E, Saraiva M, et al. (2010) Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med 207: 1609–1616. 10.1084/jem.20100265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang Q, Ghose P, Ismail N (2013) Neutrophils mediate immunopathology and negatively regulate protective immune responses during fatal bacterial infection-induced toxic shock. Infect Immun 81: 1751–1763. 10.1128/IAI.01409-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, et al. (2005) Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood 106: 1843–1850. 10.1182/blood-2005-03-1281 [DOI] [PubMed] [Google Scholar]
- 75.Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F (2004) Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 21:215–226. 10.1016/j.immuni.2004.07.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.