Abstract
Purpose
Chloroma (granulocytic sarcoma) is a rare, extramedullary tumor of immature myeloid cells related to acute non-lymphocytic leukemia or myelodysplastic syndrome (MDS). Radiation therapy (RT) is often used in the treatment of chloromas; however, modern studies of RT are lacking. We reviewed our experience to analyze treatment response, disease control, and toxicity associated with RT in order to develop treatment algorithm recommendations for patients with chloroma.
Materials/Methods
38 patients who underwent treatment for chloromas at our institution from 2/1990-6/2010 were identified and their medical records were reviewed and analyzed.
Results
The majority of patients that presented with chloroma at the time of initial leukemia diagnosis (78%) have not received RT as it regressed after initial chemotherapy. Yet most patients that relapsed or remained with chloroma after chemotherapy are in the RT cohort (90%). 33 courses of RT were administered to 22 patients. Radiation sub-site breakdown was: 39% head and neck, 24% extremity, 9% spine, 9% brain, 6% genitourinary, 6% breast, 3% pelvis, and 3% genitourinary. Median dose was 20 (6-36) Gy. Kaplan-Meier estimates of progression free survival (PFS) and overall survival (OS) in the RT cohort were 39% and 43%, respectively at 5 years. At a median follow-up of 11 months since RT, only one patient developed progressive disease at the irradiated site and 4 patients developed chloromas at other sites. RT was well tolerated without significant acute or late effects, and provided symptom relief in 95% of cases.
Conclusions
The majority of patients with chloromas were referred for RT when there was extramedullary progression, marrow relapse, or rapid symptom relief required. RT resulted in excellent local disease control and palliation of symptoms without significant toxicity. We recommend irradiating chloromas to at least 20 Gy, and propose 24 Gy in 12 fractions as an appropriate regimen.
Keywords: Chloroma, granulocytic sarcoma, myeloid sarcoma, extramedullary leukemia, radiation therapy
Introduction
Chloroma (also known as granulocytic sarcoma or myeloid sarcoma) is a rare, extramedullary tumor of immature myeloid cells. It was first described in 1811(1), and later coined ‘chloroma’ by King(2) in 1853 because of its green color caused by the presence of myeloperoxidase(3). The term granulocytic sarcoma was introduced later by Rappaport to describe only tumors of granulocytic origin(4); however, the term is now applied to any tumor related to acute non-lymphocytic leukemia or myelodysplastic syndrome (MDS) despite its original more limited definition.
Chloromas most frequently develop in the setting of acute myeloid leukemia (AML) but can occur in association with chronic myeloid leukemia (CML) during the accelerated phase, MDS, and rarely, in the absence of marrow involvement.(5-7) Their development can occur concomitantly, following, or rarely antedating the onset of these diseases(8). The most common locations include the skin, soft tissue, bone, periosteum and lymph nodes; however, numerous sites have been described.(6) Overall, chloromas represent a rare hematologic phenomenon with an incidence of 2.5%-9.1% in AML. (5, 9, 10) This low frequency together with their often misdiagnosis(7) and variable location has resulted in limited clinical experience, and hence in a lack of consensus treatment guidelines.
The presence of chloroma is often associated with a poor prognosis, and therefore optimization of treatment is essential.(6) While systemic therapy is often effective, RT has been used in the management of many cases. Modern information on the management of chloroma with radiation is scanty and the last comprehensive report published almost three decades ago.(11) Since that time, only few case series of chloromas have been published but information on radiotherapy and response was insufficient for developing clear treatment recommendations.(12, 13) We therefore analyzed the experience at our cancer center to examine if data may provide guidelines for this uncommon leukemia manifestation for leukemia and radiation oncology teams.
Patients & Methods
Patients
38 patients with chloroma treated at our institution between 2/1990 and 6/2010 were identified through institutional and departmental database query. The Memorial Sloan-Kettering Cancer Center IRB/Privacy board approved this retrospective review.
Review of patient information included age, diagnosis, surgery, chemotherapy, radiation, disease progression, follow-up and death. Patient gender, sites of relapse, radiation technique, dose, volume, chemotherapy agents, allogeneic transplants, major toxicities, and disease status were recorded.
Analysis
The Kaplan-Meier method was used to calculate actuarial rates of progression free survival (PFS) and overall survival (OS) from treatment completion and time of chloroma diagnosis, respectively.(14) Progression of disease was defined by progression on bone marrow evaluation, tissue biopsy, imaging and/or death in the absence of pathology or imaging. We classified patients presenting with chloroma as all cases of chloroma development at or prior to marrow involvement.
Results
Study Cohort
Clinical characteristics of the entire cohort are listed in Table 1. Clinical outcomes according to age, presentation, and chloroma location are listed in Table 2. Youngest patients between 1-20 years had the longest median survival of 35 months (range 3-108 months). Patients presenting with chloroma had a median survival of 61 months (range 3-179). In contrast, patients who developed chloromas after marrow involvement had a median survival of 14 months (range 1-57 months). Analysis by site of chloroma resulted in a wide range of median survivals with genitourinary site the longest (109 months) and the spinal axis the shortest (7 months). Five patients presented with isolated chloroma with only one receiving radiation as part of their treatment. All five patients were NED at last follow-up.
Table 1. Patient characteristics for the study cohort and by treatment group.
| Entire Cohort | Radiation | Non-Radiation | |
|---|---|---|---|
|
| |||
| Chloroma Cases | 38 | 22 | 16 |
|
| |||
| Gender | Male 25 (66%) | Male 14 (63%) | Male 11 (69%) |
| Female 13 (34%) | Female 8 (36%) | Female 5 (31%) | |
|
| |||
| Ethnicity | White 28 (74%) | White 15 (68%) | White 13 (81%) |
| Black 2 (5%) | Black 2 (9%) | Black 0 (0%) | |
| Hispanic 5 (13%) | Hispanic 4 (18%) | Hispanic 1 (6%) | |
| Asian 3 (8%) | Asian 1 (5%) | Asian 2 (13%) | |
|
| |||
| Cancer Diagnosis | AML* 29 (76%) CML† 1 (3%) MDS†† 3 (8%) Isolated Chloroma 5 (13%) |
AML 19 (86%) MDS 2 (9%) Isolated Chloroma 1 (5%) |
AML 10 (63%) CML 1 (6%) MDS 1 (6%) Isolated Chloroma 4 (25%) |
|
| |||
| Median Age at Leukemia Diagnosis (years) | 44 (1-71) | 33 (1-70) | 49 (10-71) |
|
| |||
| Median Age at Chloroma Diagnosis (years) | 44 (1-71) | 34 (1-71) | 49 (10-71) |
|
| |||
| Patients with Chloroma at Presentation | 18 (47%) | 4 (18%) | 14 (89%) |
|
| |||
| Chloroma Sites | Spine 5 Extremity 7 Pelvis 2 GU§ 4 Abdomen 5 H/N‖ 14 Chest wall/Mediastinum 3 |
Spine 4 Extremity 3 Pelvis 1 GU 1 Abdomen 1 H/N 14 |
Spine 1 Extremity 4 Pelvis 1 GU 3 Abdomen 4 Chest wall/Mediastinum 3 |
|
| |||
| Complete Response to Treatment | 35 (92%) | 22 (97%) | 13 (81%) |
|
| |||
| Patients Developing a Second Chloroma | 8 (21%) | 4 (18%) | 4 (25%) |
|
| |||
| Median Survival Since Chloroma Diagnosis (months) | 23 (1-179) | 15 (1-108) | 61 (7-179) |
Abbreviations:
= acute myeloid leukemia;
= chronic myeloid leukemia;
= myleodysplastic syndrome;
= genitourinary;
= head and neck.
Table 2. Clinical outcomes for the entire cohort.
| Groups | Number | Median Survival (months) | 5 yr PFS* | 5 yr OS† | |
|---|---|---|---|---|---|
| Age (years) | 1-20 | 10 | 35 | 67% | 70% |
| 21-50 | 13 | 26 | 44% | 51% | |
| >50 | 15 | 17 | 33% | 55% | |
| Chloroma Diagnosis | At initial presentation | 18 | 61 | 67% | 73% |
| After systemic disease | 20 | 14 | 27% | 31% | |
| Site | Head & Neck | 14 | 17 | ||
| Extremity | 7 | 17 | |||
| Abdomen | 5 | 62 | |||
| Spine | 5 | 7 | |||
| Pelvis | 2 | 40 | |||
| Chest wall/Mediastinum | 3 | 21 | |||
| Genitourinary | 4 | 109 |
Abbreviations:
= progression free survival;
= overall survival.
Clinical characteristics of the RT- and non-RT cohorts are listed in Table 1. Median age at chloroma diagnosis was 34 years (range 1-71 years) and 49 years (range 10-71 years) in the RT and non-RT cohorts, respectively. Hematopathology in the RT cohort consisted of AML in 86%, MDS in 9%, and isolated chloroma without marrow involvement in 5%. In the non-RT cohort, 63% had AML, 6% had CML, 6% had MDS and 25% had isolated chloroma.
RT-Cohort
Chloroma was biopsy proven in 45% of irradiated patients with the remainder diagnosed based on imaging and clinical features. Median interval between marrow involvement and chloroma diagnosis was 9 months (range 0-108 months). All patients in the RT cohort received chemotherapy at the initial time of leukemia or chloroma diagnosis with 18% undergoing allogeneic transplant prior to radiation. 75% of transplant patients underwent total body irradiation (TBI) as part of their conditioning regimen. Prior to the diagnosis of chloroma, 32% of patients were in remission from prior treatment for a median of 7 months (range 3-76 months). Of those patients in remission, chloroma presented concomitantly with marrow relapse in 43%. Prior to RT, 86% of patients were symptomatic from their chloroma. Soft tissue swelling or mass effect including gingival hypertrophy was observed in 50% of patients. Symptoms referable to involvement of the central nervous system (CNS), peripheral nervous system (PNS), or cranial nerves were present in 36% of patients.
Radiation Treatment
Thirty-three courses of radiation were administered in our department. Radiation technique reflected treatment era as well as location of disease. Conventional radiation therapy was used in 91% of cases. A margin of 10-20 mm was added to the gross tumor depending on anatomic boundaries. Positron emission tomography (PET)–computerized tomography (CT) simulation was used when available and appropriate to assist in treatment planning (Fig 1.). 82% of patients were treated with photons and 18% with electrons.
Fig. 1.
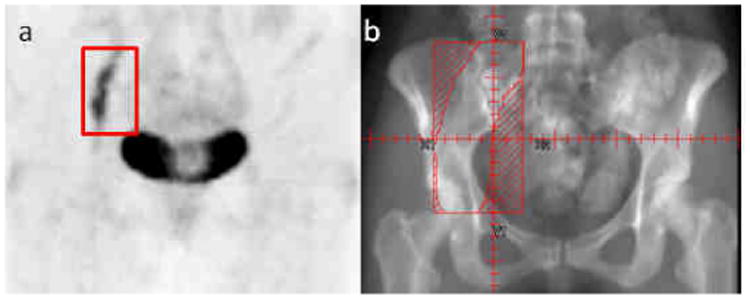
Chloroma of the right femoral nerve outlined in red on (a) pre-treatment Positron Emission Tomography (PET) treated to the field outlined in red (b) using conventional radiation therapy.
Median dose received was 20 Gy (range 6-36 Gy) with a median fraction size of 2 Gy (range 1.5-4 Gy). 82% of patients received at least 1600 cGy. Radiation sub-site breakdown was as follows: 39% head and neck, 24% extremity, 9% spine, 9% brain, 6% genitourinary, 6% breast, 3% pelvis, and 3% genitourinary. Only one patient did not complete their intended course of radiation because of general deterioration.
Local control was achieved in 97% of treatments with local failure in only one patient who received only 6 Gy to the gingiva for swelling. The patient was evaluated at an outside institution one month later for further radiation, and an additional dose of at least 20 Gy was planned to the gingiva using lateral fields according to records; no follow-up information was available. Kaplan-Meier estimates of PFS and OS were 39% and 43%, respectively at 5 years (Fig 3.). Median survival since time of RT was 12 months (range 0-105 months). Symptom relief was obtained in 95% of patients with at least 37% obtaining relief during treatment. A typical PET response to RT is demonstrated in Figure 2.
Fig. 3.
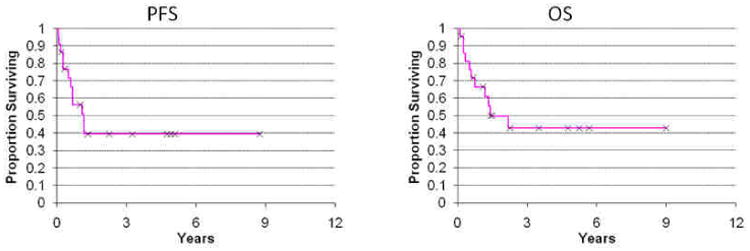
Progression free survival (PFS) since time of completion of radiation and overall survival (OS) since time of chloroma diagnosis in the radiation cohort.
Fig. 2.
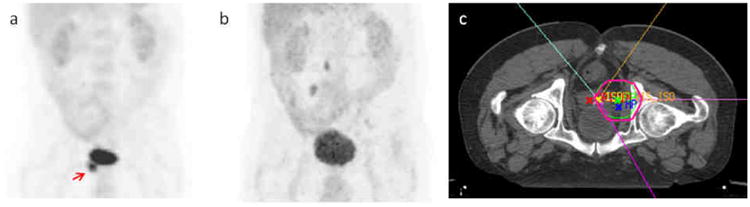
Chloroma of the right seminal vesicle on (a) pre-treatment Positron Emission Tomography (PET) indicated by red arrow demonstrating resolution (b) of hypermetabolic activity after treatment (prone position) (c) using intensity modulated radiation therapy (IMRT).
At a median follow-up of 11 months (range 0-105 months) since completion of treatment, RT was well tolerated without significant acute effects. One patient required a 2-week treatment break because of a neck abscess that developed during radiation unrelated to treatment. Long-term toxicity not necessarily as a direct consequence of RT included clinically significant hearing loss (N=1) and neurocognitive dysfunction (N=1), each in patients who underwent whole brain radiation.
After completion of RT, 4 patients developed additional chloromas at other sites, with 10 further courses of radiation administered. Notably, one patient developed multiply recurrent neurotropic chloromas all treated with radiation alone, and is currently without evidence of disease (NED).(15) Nine patients underwent allogeneic transplant after radiation with 78% undergoing TBI as part of their conditioning regimen. In total, 10 patients underwent TBI either before or after RT for chloromas with a median dose of 1375 cGy (range 450-1500 cGy).
Non-Radiation Cohort
Chloroma was biopsy proven in 81% of un-irradiated patients with the remainder diagnosed based on imaging and clinical features. Characteristics of the 16 patients with chloromas in the non-RT cohort are listed in Table 1. 89% of patients presented with chloroma at time of initial diagnosis. There were no cases of chloromas in the head and neck region, which constituted the majority in the RT cohort. Treatment in this group consisted of surgery alone in 12%, chemotherapy alone in 31%, a combination of surgery and chemotherapy in 38%, and allogeneic transplantation in 19%, with one patient receiving TBI. 81% of patients achieved a complete response (CR) to their respective treatment, 13% had stable disease, 6% had a partial response (PR), and no patients developed relapse at the initial site. Kaplan-Meier estimates of PFS and OS were 56% and 75%, respectively at 5 years (Fig 4.). Median survival since treatment completion was 50 months (range 3-179 months). Three patients relapsed with systemic disease after their initial treatment and four patients developed a chloroma at a second site. Treatment of second chloromas in the non-RT cohort included chemotherapy alone, surgery alone, allogeneic transplantation, and observation, respectively.
Fig. 4.
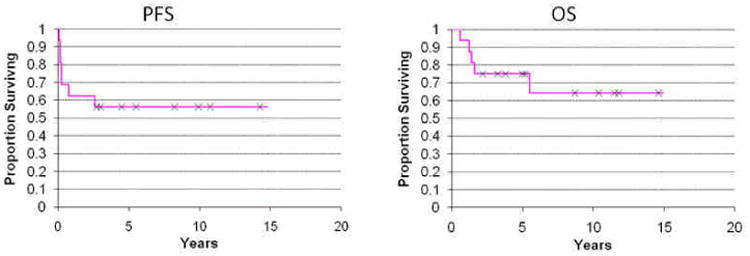
Progression free survival (PFS) since time of completion of therapy and overall survival (OS) since time of chloroma diagnosis in the non-radiation cohort.
Discussion
Treatment strategies for chloromas are largely dependent upon their response to systemic chemotherapy and whether they develop at initial diagnosis or at relapse. At our institution, patients with chloromas at initial presentation predominately received chemotherapy with or without transplantation with radiation incorporated mainly upon progression or relapse. This treatment dichotomy explains the large difference in survival patterns between the two cohorts because chloromas that develop at relapse invariably have a worse prognosis. We analyzed our data as well as the available literature to develop more guidance for consideration of RT in patients with chloroma. A suggested treatment algorithm is located in Figure 5.
Fig. 5.
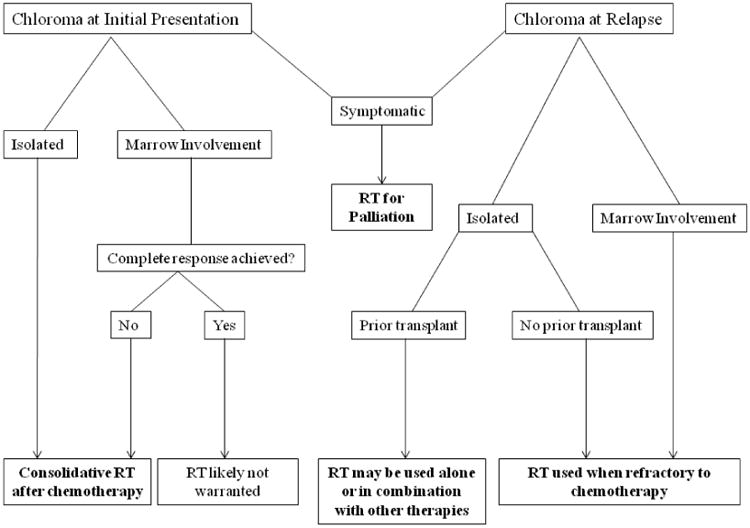
A suggested treatment algorithm for radiation (RT) in the management of chloroma.
Chloroma can rarely arise in the absence of marrow involvement.(8) The ideal timing and treatment of such cases represents a therapeutic dilemma. It has been strongly suggested that inadequately treated isolated chloroma will almost always progress to AML.(16) A recent series of nonleukemic chloroma reported that AML-type intensive chemotherapy with or without radiation was “moderately effective” in this setting.(13) Importantly, the authors note that the addition of radiotherapy was associated with a prolonged failure free survival.(13) We suggest that radiation be considered as a consolidation treatment for isolated chloroma particularly since the effective RT dose is low. Radiation site and associated toxicities are important factors for consideration.
Chloroma presenting concurrently with marrow involvement at initial diagnosis always warrants systemic treatment directed at the underlying disease. Incomplete chloroma response after chemotherapy is believed to represent a significant risk for early medullary relapse after therapy.(7) Therefore, radiation therapy should be considered in these instances in which less than a CR is achieved with chemotherapy. In our series, all 13 patients who presented with concurrent chloroma and marrow disease received chemotherapy, 3 of whom also received RT. The patients referred for RT either had an inadequate response to chemotherapy (N = 2) or were symptomatic from their chloroma (N = 1). Importantly, the two patients with less than a CR to chemotherapy who underwent consolidative RT were NED at last follow-up.
Isolated chloromas at relapse are rare, and often herald systemic relapse. Median time to marrow relapse in this setting has been reported as 7 months.(17) Treatment strategies are dependent on whether the patient relapsed after chemotherapy alone or after transplant. In our series, two patients developed isolated chloroma relapse after chemotherapy alone and were rendered NED with a combination of chemotherapy, radiation, and transplant (N=1). Chloromas developing after allogeneic transplantation are more uncommon with an incidence reported between 0.2-1.3% of patients undergoing transplantation and represent an even more difficult management problem.(18, 19) Rare isolated chloromas after allogeneic transplantation have been described.(20) Management in this setting has included donor lymphocyte infusion with clinical promise in few cases.(21) In our series, two patients developed isolated chloromas after allogeneic transplant both treated with a combination of T-cell infusion and radiation therapy with additional chemotherapy in one. Importantly, radiation rendered one patient NED and provided disease control in the second. We suggest that radiation be considered in all cases of isolated chloroma at relapse, particularly those developing after allogeneic transplant.
More frequently, chloromas develop concurrently with marrow relapse. Re-induction chemotherapy directed at the underlying disease is always warranted. Depending on the response to chemotherapy, radiation might be required to treat partially responding chloromas. In our series, 3 patients in remission developed concurrent chloroma and marrow relapse. Two of the patients underwent radiation in combination with chemotherapy and transplant and are NED. The third patient underwent chemotherapy alone and developed progressive disease resulting in death. We suggest that in this setting, radiation should be strongly considered as consolidation after chemotherapy.
Radiation is highly recommended when symptom relief is required because it provides excellent palliation. In Chak's series the majority of patients treated were symptomatic from their chloromas, with pain being the most frequent symptom.(11) In our series, 86% of patients referred for radiation were symptomatic including neurological symptoms referable to CNS, PNS, and cranial nerves in 36%. The disproportionate number of patients with chloromas in the head and neck region that received radiation probably resulted from rapid development of symptoms from space-occupying lesions in this area that required rapid intervention including orbital masses and cranial neuropathy. Importantly, symptom relief was obtained during treatment in at least 37% of patients indicating that radiation provides rapid palliation.
While there are a variety of clinical scenarios in which radiation can be utilized, a standard treatment regimen can be applied because of the excellent response of chloroma disease to low doses of radiation. In our series, there was only one local failure in 33 treatments to a site that only received 6 Gy suggesting that chloromas are radiosensitive. In contrast to older studies that support the use of at least 30 Gy, we recommend irradiating chloromas to a dose of at least 20 Gy, and propose 24 Gy in 12 fractions as an appropriate regimen given its efficacy and safety. Conventional photon treatment with 10-20 mm margins incorporating post-chemotherapy volume is warranted in most cases with electrons utilized for superficial disease. Intensity-modulated radiation therapy (IMRT) can be reserved for cases that involve re-treatment or in which toxicity is a concern because of surrounding normal structures or concurrent chemotherapy. Short and long-term toxicity were minimal with RT even in patients (N=3) that received multiple courses for additional chloromas. Notably, 10 patients underwent TBI either before or after RT for chloromas without excess morbidity indicating that radiation within the dose ranges discussed does not preclude use of TBI as a conditioning regimen or vice versa.
The presence of chloromas is usually associated with a worse outcome and a shorter survival time. (5, 7, 22) Five-year survival in both cohorts compare favorably to other series in which five-year survival rates range between 20-30%.(12, 13) While benefits in OS with radiation have not been supported in the literature (12) or by our study, we believe that radiation might contribute to improved PFS in cases of isolated chloroma (13) and in those which there was an inadequate response to chemotherapy. Patients in the pediatric age group had the longest median survival, which might reflect better leukemia outcome in general in children compared to older age groups. Analysis of chloroma site revealed a wide range median survival with meaningful conclusions difficult to establish given the small numbers in some sites.
In conclusion, patients are most often referred for radiation for chloromas at recurrence or progression; however, radiation might have a role in cases of: isolated chloroma, inadequate response to chemotherapy, recurrence after allogeneic transplant, and in circumstances that require rapid symptom relief. A low-dose regimen of 24 Gy in 12 fractions using conventional treatment can be applied to the majority of cases with excellent disease control and minimal morbidity. Given the limited experience in treatment chloromas, further study of combined modality therapy is warranted.
Acknowledgments
Lymphoma Foundation & Sports Foundation Against Cancer.
Footnotes
Conflicts of Interest Notification: The authors have no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burns A. Observations of surgical Anatomy in Head and Neck. Edinburg: Thomas Royce; 1811. [Google Scholar]
- 2.King A. A case of Chloroma. Monthly J Med. 1853;17:97. [Google Scholar]
- 3.Reardon G, Moloney WC. Chloroma and related myeloblastic neoplasms. Arch Intern Med. 1961;108:864–871. doi: 10.1001/archinte.1961.03620120048008. [DOI] [PubMed] [Google Scholar]
- 4.Rappaport H. Tumors of the hematopoietic system, in Atlas of Tumor Pathology, Section III, Fascicle 8. Armed Forces Institute of Pathology; Washington D.C.: 1967. [Google Scholar]
- 5.Neiman RS, Barcos M, Berard C, et al. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer. 1981;48:1426–1437. doi: 10.1002/1097-0142(19810915)48:6<1426::aid-cncr2820480626>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Paydas S, Zorludemir S, Ergin M. Granulocytic sarcoma: 32 cases and review of the literature. Leuk Lymphoma. 2006;47:2527–2541. doi: 10.1080/10428190600967196. [DOI] [PubMed] [Google Scholar]
- 7.Byrd JC, Edenfield WJ, Shields DJ, et al. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical review. J Clin Oncol. 1995;13:1800–1816. doi: 10.1200/JCO.1995.13.7.1800. [DOI] [PubMed] [Google Scholar]
- 8.Krause JR. Granulocytic sarcoma preceding acute leukemia: a report of six cases. Cancer. 1979;44:1017–1021. doi: 10.1002/1097-0142(197909)44:3<1017::aid-cncr2820440333>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Liu PI, Ishimaru T, McGregor DH, et al. Autopsy study of granulocytic sarcoma (chloroma) in patients with myelogenous leukemia, Hiroshima-Nagasaki 1949-1969. Cancer. 1973;31:948–955. doi: 10.1002/1097-0142(197304)31:4<948::aid-cncr2820310428>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Wiernik PH, Serpick AA. Granulocytic sarcoma (chloroma) Blood. 1970;35:361–369. [PubMed] [Google Scholar]
- 11.Chak LY, Sapozink MD, Cox RS. Extramedullary lesions in non-lymphocytic leukemia: results of radiation therapy. Int J Radiat Oncol Biol Phys. 1983;9:1173–1176. doi: 10.1016/0360-3016(83)90176-1. [DOI] [PubMed] [Google Scholar]
- 12.Lan TY, Lin DT, Tien HF, et al. Prognostic factors of treatment outcomes in patients with granulocytic sarcoma. Acta Haematol. 2009;122:238–246. doi: 10.1159/000253592. [DOI] [PubMed] [Google Scholar]
- 13.Tsimberidou AM, Kantarjian HM, Estey E, et al. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia. 2003;17:1100–1103. doi: 10.1038/sj.leu.2402958. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Bakst R, Jakubowski A, Yahalom J. Recurrent Neurotropic Chloroma: Report of a Case and Review of the Literature. Adv Hematol. 2011 doi: 10.1155/2011/854240. Article ID 854240 (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meis JM, Butler JJ, Osborne BM, et al. Granulocytic Sarcoma in Nonleukemic Patients. Cancer. 1986;58:2697–2709. doi: 10.1002/1097-0142(19861215)58:12<2697::aid-cncr2820581225>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Byrd JC, Weiss RB. Recurrent granulocytic sarcoma. An unusual variation of acute myelogenous leukemia associated with 8;21 chromosomal translocation and blast expression of the neural cell adhesion molecule. Cancer. 1994;73:2107–2112. doi: 10.1002/1097-0142(19940415)73:8<2107::aid-cncr2820730815>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Szomor A, Passweg JR, Tichelli A, et al. Myeloid leukemia and myelodysplastic syndrome relapsing as granulocytic sarcoma (chloroma) after allogeneic bone marrow transplantation. Ann Hematol. 1997;75:239–241. doi: 10.1007/s002770050350. [DOI] [PubMed] [Google Scholar]
- 19.Bekassy AN, Hermans J, Gorin NC, et al. Granulocytic sarcoma after allogeneic bone marrow transplantation: a retrospective European multicenter survey. Acute and Chronic Leukemia Working Parties of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1996;17:801–808. [PubMed] [Google Scholar]
- 20.Au WY, Chan AC, Lie AK, et al. Recurrent isolated extramedullary relapses as granulocytic sarcomas following allogeneic bone marrow transplantation for acute myeloid leukemia. Bone Marrow Transplant. 1998;21:205–208. doi: 10.1038/sj.bmt.1701043. [DOI] [PubMed] [Google Scholar]
- 21.Kottaridis PD, Ketley N, Peggs K, et al. An unusual case of intrapulmonary granulocytic sarcoma presenting as interstitial pneumonitis following allogeneic bone marrow transplantation for acute myeloid leukaemia and responding to donor lymphocyte infusion. Bone Marrow Transplant. 1999;24:807–809. doi: 10.1038/sj.bmt.1701974. [DOI] [PubMed] [Google Scholar]
- 22.List AF, Gonzalez-Osete G, Kummet T, et al. Granulocytic sarcoma in myelodysplastic syndromes: clinical marker of disease acceleration. Am J Med. 1991;90:274–276. doi: 10.1016/0002-9343(91)90559-g. [DOI] [PubMed] [Google Scholar]


