Abstract
Purpose:
The endothelial nitric oxide synthase (eNOS) G894T polymorphism has been reported to cause endothelial dysfunction and may have a role in the development of coronary artery disease (CAD). The aim of the present study was to investigate the association of eNOS G894T genetic polymorphism and plasma levels of nitric oxide (NO) with CAD risk in an Iranian population.
Materials and Methods:
We studied 200 patients with angiographically documented CAD and 100 matched controls. Analysis of G894T genetic polymorphism of eNOS was performed by polymerase chain reaction-restriction fragment length polymorphism method. Plasma levels of NO were determined using Griess method. Biochemical analysis was conducted by routine colorimetric methods.
Results:
Plasma levels of NO were significantly lower in CAD patients than control subjects (41.60±12.70 vs. 55.48±16.57, P=0.001). Also, the mean plasma levels of NO were significantly lower in T allele carriers of eNOS G894T polymorphism than G allele carriers (P<0.001). The genotype distribution and minor T allele frequency of eNOS G894T polymorphism significantly differed between CAD patients and control subjects (P<0.05). However, no significant association was found between the eNOS G894T polymorphism and the severity of CAD (number of diseased vessel) or the lipid profile of CAD patients (P>0.05).
Conclusion:
Reduced plasma level of NO is associated with increased risk of CAD in our population. Moreover, eNOS G894T polymorphism is a significant risk factor for CAD development via reducing the plasma levels of NO. However, eNOS G894T polymorphism is not a contributing factor for the severity of CAD.
Keywords: Nitric oxide synthase type III, Coronary artery disease, Polymerase chain reaction-restriction fragment length polymorphism, Single nucleotide polymorphism G894T
INTRODUCTION
Coronary artery disease (CAD) as a common multifactorial disease is the leading cause of morbidity and mortality in developed as well as developing countries [1]. Despite the high prevalence of CAD worldwide, its underlying mechanism has not been fully understood. The main cause of CAD is atherosclerosis that is characterized by defective endothelial function and plaque formation in the inner wall of the artery [1].
Endothelial dysfunction is a common finding in CAD that occurs in response to numerous cardiovascular risk factors and precedes the development of atherosclerosis. One of the main regulators of vascular endothelial cell function is nitric oxide (NO), which acts as an important relaxing factor and atheroprotective agent [2]. NO also inhibits several key steps in atherosclerosis including platelet activation, platelet and leukocyte adhesion, adhesion molecule and chemokine expression, inflammatory cell infiltration, and smooth muscle cell migration and proliferation [3,4].
NO is produced in endothelial cells by oxidation of L-arginine to L-citrulline, a reaction catalyzed by endothelial nitric oxide synthase (eNOS). The eNOS is encoded by a 26 exongene located on chromosome 7q35 to 36 [5]. Several alterations have been reported in the eNOS gene that changes gene expression levels and functional status of eNOS. A point mutation of guanine (G) to thymine (T) at nucleotide 894 in exon 7 of the eNOS gene changes the coding sequence of the eNOS from glutamic acid to aspartic acid at codon 298 (Glu298Asp, also known as G894T, rs1799983) [6]. This variant results in reduced eNOS enzymatic activity and decreased production of NO by endothelial cells [6]. The Asp allele of G894T polymorphism of eNOS gene has been reported as a risk factor for CAD development with conflicting results [7–13]. These controversial results are probably due to differences in the ethnic background of the studied population [14]. Moreover, the association between eNOS G894T polymorphism and the plasma levels of NO is not consistent. Some studies have shown reduced NO production in genotypes carrying the Asp allele [9] while other studies did not report such an association [10]. Currently, there is limited data regarding the role of eNOS G894T polymorphism as a risk factor for CAD development from the northwest of Iran. So, the aim of the present study was to investigate the association of eNOS G894T genetic polymorphism and CAD development. Also, intergenotypic variations of plasma NO levels were determined in the study population.
MATERIALS AND METHODS
1. Study population
The present case control study included 200 patients with angiographically documented CAD (more than 50% detectable stenosis in at least one major coronary artery) and 100 healthy matched controls. All of the control subjects were selected after careful inspection by a cardiovascular specialist. For all subjects, a complete medical history including questions about smoking habits, history of hypertension and diabetes was obtained by questionnaire. The mean age of CAD patients and controls was 58.3±27.6 and 56.7±29.5 years, respectively. After 12 hours fasting, 5 mL of venous blood were collected in ethylenediamine tetraacetic acid containing tubes and immediately centrifuged. The plasma fraction was separated and stored at −40°C until biochemical analysis and the cellular fraction was used for DNA and RNA extractions. The study was approved by the Ethic Committee of Zanjan University of Medical Sciences (IRB no. ZUMS.REC.1394.324), Zanjan, Iran.
2. eNOS G894T polymorphism analysis
Genomic DNA was isolated using a commercially available kit (Geno Plus Genomic DNA Mini, GG2001; Viogene, Grunwaldzka, Poland) and subsequently was stored at −40°C until analysis.
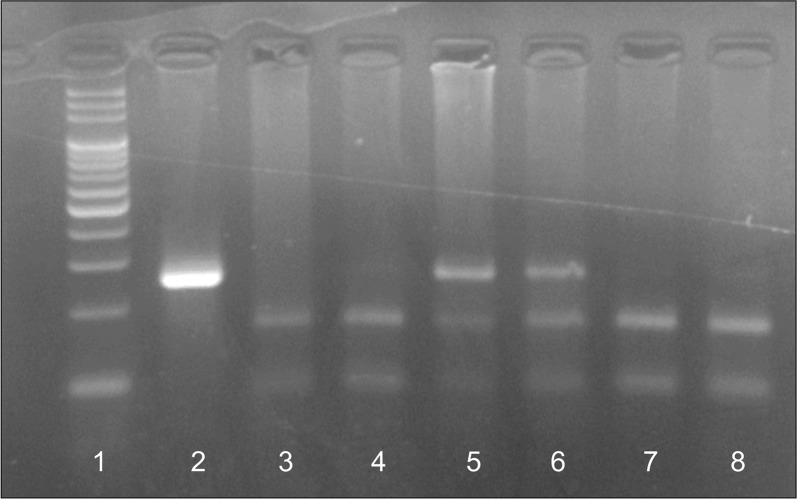
Analysis of eNOS G894T polymorphism was conducted by polymerase chain reaction (PCR)-restriction fragment length polymorphism method as previously described [15]. The sequences of primers used for PCR reaction were as follows, forward: 5′-AAGGCAGGAGACAGTGGATGG-3′ and reverse: 5′-CCCAGTCAATCCCTTTGGTGCT CA-3′. Restriction digestion was performed in a total volume of 20 μL including 8 μL PCR amplicons, 2 μL digestion buffer and 2 units of BanII (EURx Ltd., Gdansk, Poland) restriction enzyme and appropriate volumes of DNase-free water. Then, samples were incubated for 5 hours at 37°C. The digested PCR products were subjected to electrophoresis on a 2.5% agarose gel and subsequently stained with Sybr green dye. Digestion of the PCR product in the presence of T allele resulted in a single non-cleaved 248 bp fragment, while in the presence of G allele, the 248 bp amplicon was cleaved into 163 bp and 85 bp fragments (Fig. 1).
Fig. 1.
Analysis of endothelial nitric oxide synthase G894T polymorphism. The polymerase chain reaction products were digested by restriction enzyme BanII (EURx Ltd., Gdansk, Poland). Lane 1: ladder 100 bp, Lane 2: mutant homozygote genotype (894TT), Lanes 5, 6: heterozygote genotype (894GT), Lanes 3, 4, 7, 8: wild type genotype (894GG).
3. Estimation of plasma NO levels
Determination of NO in plasma samples was performed indirectly using the Griess reaction as previously described with slight modification [16]. Since NO is rapidly converted to nitrite (NO2−) and nitrate (NO3−) in biological fluids, the total concentration of nitrite and nitrate can be determined as a quantitative measure of NO production.
Briefly, plasma samples were deproteinized with 30% zinc sulfate solution. Then, 100 µL of deproteinized plasma samples were incubated with 100 µL Griess reagent at 37°C for 30 min, during which the magenta colored azo dye was developed from the stable decomposition product, nitrite and the Griess reagent. Then, the absorbance of reactions was measured on a microplate reader at 543 nm. An appropriate series of standard solutions were run and the acquired absorbance of standards was used for constructing of a standard curve. The concentration of NO in the plasma sample was determined using the standard curve.
4. Estimation of plasma lipids levels
Plasma total cholesterol (TC), triglycerides (TG), high density lipoprotein-cholesterol (HDL-C) and fasting glucose levels were measured using commercially available enzyme assay kits (Pars Azmoon kit; Pars Azmoon Co., Tehran, Iran) and Mindray auto-analyzer (BS-200; Shenzhen Mindray Bio-Medical Electronics Co., Ltd, Shenzhen, China). The plasma low density lipoprotein-cholesterol (LDL-C) was calculated using the Friedewald and Fredrickson’s formula [17].
5. Statistical analysis
Numerical data were presented as mean±standard deviation and were compared using independent Student’s t-test. Association between different categorical variables was done using chi-square (X2) test. All statistical analysis was performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) with a statistical significance level of P<0.05.
RESULTS
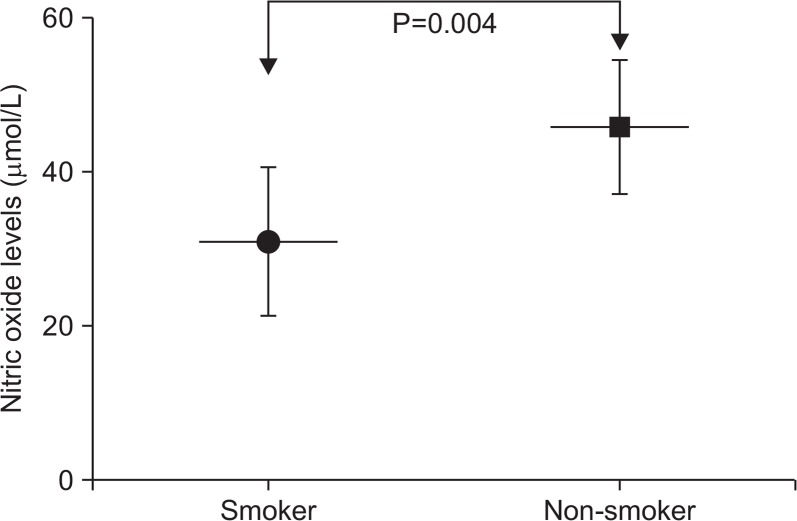
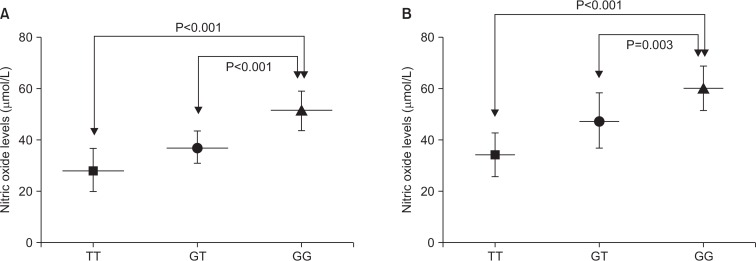
The clinical and demographic characteristics of the CAD patients and control subjects indicated no significant differences in the mean ages, sex distribution and triglyceride levels between the two groups (P>0.05) (Table 1). However, compared with control subjects, CAD patients had significantly higher plasma levels of TC and LDL-C and lower plasma levels of HDL-C (P<0.05). Also, there were significantly higher percentages of patients with diabetes (as defined by fasting blood glucose >125 mg/dL) (P=0.022), hypertension (systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg) (P=0.016) and smoking habit (P=0.009) in the CAD patients in comparison to control subjects. The mean plasma levels of NO was significantly lower in CAD patients than control subjects (41.60±12.70 vs. 55.48±16.57, P=0.001) (Table 1). Since smoking is one of the most important causes of cardiovascular disease, we performed a subgroup analysis to assess the effect of smoking on mean plasma levels of NO. Results revealed that mean plasma levels of NO was significantly lower in smoker compared with non-smoker CAD patients (P=0.004) (Fig. 2). However, no significant correlation was seen between plasma NO levels and plasma TC levels, TG levels, HDL-C and LDL-C levels (P>0.05). Additionally, the intergenotypic differences of plasma NO levels were evaluated in patients with and without eNOS G894T polymorphism. Results indicated that plasma levels of NO was significantly lower in GT heterozygote and mutant TT homozygote genotype of eNOS G894T polymorphism when compared to wild type GG genotype (P<0.001) (Fig. 3A). Also, significant intergenotypic differences of plasma NO levels were observed in control subjects (Fig. 3B).
Table 1.
Clinical characteristics of the coronary artery disease (CAD) patients and the control subjects included in our study
| Variable | CAD group (n=200) | Control group (n=100) | P-value |
|---|---|---|---|
| Age (y) | 58.7±24.3 | 56.7±29.5 | 0.475 |
| Sex (male/female) | 110/90 | 51/49 | 0.478 |
| Triglyceride (mg/dL) | 181.2±99.7 | 169.4±75.7 | 0.395 |
| Total cholesterol (mg/dL) | 195.8±76.3 | 167.3±49.5 | 0.002 |
| High density lipoprotein (mg/dL) | 36.8±11.1 | 43.3±14.8 | 0.010 |
| Low density lipoprotein (mg/dL) | 104.3±54.9 | 85.5±43.2 | 0.041 |
| Hypertension | 44 (22) | 9 (9) | 0.016 |
| Diabetes | 48 (24) | 11 (11) | 0.022 |
| Smoking | 58 (29) | 12 (12) | 0.009 |
| Nitric oxide (μmol/L) | 41.60±12.70 | 55.48±16.57 | 0.001 |
Values are presented as mean±standard deviation, number only, or number (%).
Fig. 2.
Plasma levels of nitric oxide in smoker and non-smoker coronary artery disease patients.
Fig. 3.
The association between different genotypes of endothelial nitric oxide synthase G894T polymorphism and mean plasma levels of nitric oxide in coronary artery disease patients (A) and control subjects (B). TT, homozygote; GT, heterozygote; GG, wild type.
The genotypic distribution of eNOS G894T polymorphism in CAD patients and control subjects were evaluated. The distribution of heterozygote genotype and mutant homozygote genotype between CAD patients and control subjects were significantly different (P=0.016; P=0.009, respectively) (Table 2). Also, the frequency of the T allele of the G894T polymorphism was significantly higher in CAD patients than control subjects (odds ratio [OR], 2.1; 95% confidence interval [CI], 1.3–3.1; P=0.001) (Table 2).
Table 2.
Genotype and allele distribution of eNOS G894T polymorphism in CAD patients and control subjects
| eNOS G894T polymorphism | CAD group (n=200) | Control group (n=100) | OR (95% CI) | P-value |
|---|---|---|---|---|
| GG genotype | 97 (48.5) | 67 (67.0) | 1 | Ref. |
| GT genotype | 80 (40.0) | 29 (29.0) | 1.9 (1.1–3.2) | 0.016 |
| TT genotype | 23 (11.5) | 4 (4.0) | 4 (1.3–12) | 0.009 |
| G allele | 274 (68.5) | 163 (81.5) | 1 | Ref. |
| T allele | 126 (31.5) | 37 (18.5) | 2.1 (1.3–3.1) | 0.001 |
Values are presented as number (%) or OR (95% CI).
eNOS, endothelial nitric oxide synthase; CAD, coronary artery disease; OR, odds ratio; CI, confidence interval; GG, wild type; GT, heterozygote; TT, homozygote; G, guanine; T, thymine.
Additionally, the association between eNOS G894T gene polymorphism and CAD risk was investigated under the recessive genetic model and dominant genetic model. Results indicated that G894T gene polymorphism increased CAD risk in both the recessive genetic model (OR, 3.1; 95% CI, 1.05–9.3; P=0.032) and dominant genetic model (OR, 2.2; 95% CI, 1.3–3.6; P=0.020) (Table 3). Moreover, the association between eNOS G894T genotype distribution and the severity of CAD (number of diseased vessel) were analyzed. Results showed no significant association between genotype distribution of eNOS G894T polymorphism and CAD severity (P>0.05) (Table 4).
Table 3.
Analysis of eNOS G894T gene polymorphism in the CAD group and control group using dominant and recessive genetic models
| Genetic model | Genotype | CAD group (n=200) | Control group (n=100) | OR (95% CI) | P-value |
|---|---|---|---|---|---|
| Dominant | GG | 97 (48.5) | 67 (67.0) | 1 | Ref. |
| GT+TT | 103 (51.5) | 33 (33.0) | 2.2 (1.3–3.6) | 0.020 | |
| Recessive | GG+GT | 177 (88.5) | 96 (96.0) | 1 | Ref. |
| TT | 23 (11.5) | 4 (4.0) | 3.1 (1.05–9.3) | 0.032 |
Values are presented as number (%) or OR (95% CI).
eNOS, endothelial nitric oxide synthase; CAD, coronary artery disease; OR, odds ratio; CI, confidence interval; GG, wild type; GT, heterozygote; TT, homozygote.
Table 4.
Number of CAD patients with 1, 2, and 3 diseased vessels according to various genotypes of eNOS G894T polymorphism
| eNOS G894T polymorphism | 1 DV (n=67) | 2 DV (n=85) | 3 DV (n=48) | P-value (2DV vs. 1DV) | P-value (3DV vs.1DV) |
|---|---|---|---|---|---|
| GG | 31 (46.3) | 40 (47.1) | 26 (54.2) | Ref. | Ref. |
| GT | 30 (44.8) | 36 (42.4) | 14 (29.2) | 0.833 | 0.160 |
| TT | 6 (8.9) | 9 (10.5) | 8 (16.6) | 0.795 | 0.439 |
| (GT+TT) | 36 (53.7) | 45 (52.9) | 22 (45.8) | 0.923 | 0.403 |
Values are presented as number (%).
CAD, coronary artery disease; eNOS, endothelial nitric oxide synthase; DV, diseased vessel; GG, wild type; GT, heterozygote; TT, homozygote.
The genotype distribution of eNOS G894T polymorphism was evaluated between smoker and non-smoker CAD patients. Results indicated that out of 58 CAD patients who were smokers, 13 patients (22.4%) had 894TT genotype while among 142 non-smoker CAD patients, 10 patients (7.1%) had 894TT genotype. There was a significant difference in the distribution of 894TT genotype between smoker and non-smoker CAD patients (OR, 3.18; 95% CI, 1.3–7.6; P=0.01).
DISCUSSION
The study was planned to determine the role of eNOS G894T polymorphism and plasma NO levels in CAD development and also to determine the effect of this polymorphism on plasma NO levels in CAD patients. Our main findings were as follows: (i) reduced plasma levels of NO is a significant risk factor for development of CAD in the Iranian population; (ii) the eNOS G894T polymorphism is a contributing factor for decreased plasma levels of NO; (iii) the eNOS G894T polymorphism is a significant risk factor for development but not for severity of CAD in the Iranian population.
The association of eNOS G894T gene polymorphism with CAD risk has been investigated in different populations; however, variable results of association [7–11] and lack of association [12,13,18] have been reported. The results of the present study strongly suggest that eNOS G894T polymorphism is a risk factor for CAD. Analysis of eNOS G894T polymorphism revealed a positive association between heterozygous and homozygous carriers of the T allele and the occurrence of CAD in the studied population. The increased CAD risk observed in the carriers of heterozygous and mutant homozygous genotypes of eNOS G894T polymorphism suggests that the risk of CAD posed by the 894T allele is expressed both dominantly and recessively, as indicated in Table 3. Therefore, our study was in accordance with studies by Kerkeni et al. [19] and Saini et al. [7] that showed significant association between this polymorphism and CAD risk.
There are some evidence in carriers of the 894T allele of eNOS G894T polymorphism that can predispose them to CAD occurrence including (i) an enhanced systemic pressor response to phenylephrine [20], (ii) a reduced blood pressure fall after exercise training [21], (iii) lower basal blood flow and reduced vasodilation to adenosine in coronary arteries [22], and (iv) a reduced flow-mediated dilatation of the brachial artery [23] that collectively makes carriers of this polymorphism more prone to suffer from coronary events. The synergistic effects between these effects coupled with decreased bioavailability of NO in T allele carriers and also the presence of some gene-gene and gene-environmental interactions modulating the NO levels may accelerate the process of endothelial damage and subsequent emergence of overt CAD among T allele carriers (Asp allele) of the eNOS G894T gene polymorphism [7,9]. Also, greater red blood cell aggregability potential has been reported in CAD patients carrying the T allele of eNOS G894T polymorphism [24]. However, our results were inconsistent with the findings from some other studies that have reported a negative association between this polymorphism and CAD [12,13,18]. The reasons for these contradictory results may be related to variation in study design, different selection criteria for cases and controls, heterogeneity in sample size, and numerous known and unknown gene-gene and gene-environmental interactions [25]. Also, ethnic differences may influence the impact of this polymorphism on CAD risk [14].
Several evidences suggest that alterations in the NO pathway might be involved in endothelial dysfunction and atherosclerosis [7,19]. The results of the present study also indicated a reduced level of NO in CAD patients. This may have resulted as a consequence of increased occurrence of the T allele in CAD patients compared with control subjects (31.5% vs. 18.5%). Decreased NO levels in atherosclerotic vessels may be caused by oxidative stress in CAD [26]. Oxidative stress leads to generation of reactive oxygen species which scavenge NO, thereby reducing NO bioavailability [27]. Therefore, impaired endothelial function caused by reduced NO levels in CAD patients may play a role in the pathogenesis of cardiovascular events.
Moreover, our study indicated that eNOS G894T polymorphism is associated with decreased plasma concentration of NO in the CAD patients, which is consistent with some previously published studies that reported reduced bioavailability of NO in carriers of eNOS G894T polymorphism [7,9]. The possible mechanism by which the 894T allele (Asp298) results in reduced NO levels in the body may be the selective proteolytic cleavage of the Asp298 in the endothelial cells and vascular tissues. The resultant cleaved fragments especially among the carriers of homozygous mutants of this polymorphism lead to diminished activity of the enzyme and decreased NO biosynthesis [28].
Moreover, in our study the 894TT genotype was more common among CAD patients who were smokers compared with CAD patients who were non-smokers (P=0.01). These data, possibly suggest that cigarette smoking as an established underlying causes of endothelial dysfunction may act synergistically with this common polymorphism to attenuate endothelial function and predispose patients to increased CAD risk. Therefore, our study provided an evidence for a gene-environment interaction in modulating predisposition to CAD risk. Similar results were reported by Dafni et al in a study of 204 patients with a history of myocardial infarction in the Greek population [8].
Also, similar to the study by Kerkeni et al. [19], our study indicated no significant differences in the genotype distribution of eNOS G894T polymorphism between CAD patients with one, two and three diseased vessels. These results confirmed the ineffectiveness of this common polymorphism as a determining factor for the severity of disease in CAD patients.
CONCLUSION
Reduced plasma level of NO is associated with increased risk of CAD in our population. Moreover, eNOS G894T polymorphism is a significant risk factor for CAD development but not for the severity of CAD, which acts by reducing the plasma levels of NO. Also, cigarette smoking, by synergistic combination with eNOS G894T polymorphism, may predispose to the development of CAD.
Acknowledgments
The present study was supported by a grant from Zanjan University of Medical Sciences (ZUMS), Deputy of Research and Technology (grant number A-12-119-7) Zanjan, Iran.
REFERENCES
- 1.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 2.Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10:4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- 3.Badimon L, Padró T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care. 2012;1:60–74. doi: 10.1177/2048872612441582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitiello L, Spoletini I, Gorini S, Pontecorvo L, Ferrari D, Ferraro E, et al. Microvascular inflammation in atherosclerosis. IJC Metabolic & Endocrine. 2014;3:1–7. doi: 10.1016/j.ijcme.2014.03.002. [DOI] [Google Scholar]
- 5.Marsden PA, Heng HH, Scherer SW, Stewart RJ, Hall AV, Shi XM, et al. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem. 1993;268:17478–17488. [PubMed] [Google Scholar]
- 6.Hirata RD, Salaza LA, Cavalli SA, Yoshioka KK, Matsumoto LO, Santos ST, et al. A method to detect the G894T polymorphism of the NOS3 gene. Clinical validation in familial hypercholesterolemia. Clin Chem Lab Med. 2002;40:436–440. doi: 10.1515/CCLM.2002.074. [DOI] [PubMed] [Google Scholar]
- 7.Saini V, Bhatnagar MK, Bhattacharjee J. Association of endothelial dysfunction with endothelin, nitric oxide and eNOS Glu298Asp gene polymorphism in coronary artery disease. Dis Markers. 2011;31:215–222. doi: 10.1155/2011/419708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dafni C, Drakoulis N, Landt O, Panidis D, Reczko M, Cokkinos DV. Association of the eNOS E298D polymorphism and the risk of myocardial infarction in the Greek population. BMC Med Genet. 2010;11:133. doi: 10.1186/1471-2350-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saini V, Bhatnagar MK, Bhattacharjee J. Endothelial nitric oxide synthase Glu298Asp (G894T) gene polymorphism in coronary artery disease patients with type 2 diabetes mellitus. Diabetes Metab Syndr. 2012;6:106–109. doi: 10.1016/j.dsx.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Angeline T, Isabel W, Tsongalis GJ. Endothelial nitric oxide gene polymorphisms, nitric oxide production and coronary artery disease risk in a South Indian population. Exp Mol Pathol. 2010;89:205–208. doi: 10.1016/j.yexmp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Rai H, Parveen F, Kumar S, Kapoor A, Sinha N. Association of endothelial nitric oxide synthase gene polymorphisms with coronary artery disease: an updated meta-analysis and systematic review. PLoS One. 2014;9:e113363. doi: 10.1371/journal.pone.0113363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelhedi R, Kharrat N, Bouayed NA, Abid L, Abdelmouleh W, Sahnoun IT, et al. Lack of association of NOS3 and ACE gene polymorphisms with coronary artery disease in Southern Tunisia. Biochem Genet. 2013;51:92–100. doi: 10.1007/s10528-012-9545-x. [DOI] [PubMed] [Google Scholar]
- 13.Younan H, Razek GA, Elkhashab K, Abdelrasol H, Saad M. Relationship of endothelial nitric oxide synthase gene polymorphism with atherosclerotic coronary and carotid arterial disease in Egyptian population. Egypt Heart J. 2015;67:225–232. doi: 10.1016/j.ehj.2014.01.003. [DOI] [Google Scholar]
- 14.Luo JQ, Wen JG, Zhou HH, Chen XP, Zhang W. Endothelial nitric oxide synthase gene G894T polymorphism and myocardial infarction: a meta-analysis of 34 studies involving 21,068 subjects. PLoS One. 2014;9:e87196. doi: 10.1371/journal.pone.0087196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Liao S, Lu R, Dang H, Zhao J, Ding X. Endothelial nitric oxide synthase gene polymorphism is associated with Legg-Calvé-Perthes disease. Exp Ther Med. 2016;11:1913–1917. doi: 10.3892/etm.2016.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grisham MB, Johnson GG, Lancaster JR., Jr Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996;268:237–246. doi: 10.1016/S0076-6879(96)68026-4. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Andrikopoulos GK, Grammatopoulos DK, Tzeis SE, Zervou SI, Richter DJ, Zairis MN, et al. Association of the 894G>T polymorphism in the endothelial nitric oxide synthase gene with risk of acute myocardial infarction. BMC Med Genet. 2008;9:43. doi: 10.1186/1471-2350-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerkeni M, Addad F, Chauffert M, Myara A, Ben Farhat M, Miled A, et al. Hyperhomocysteinemia, endothelial nitric oxide synthase polymorphism, and risk of coronary artery disease. Clin Chem. 2006;52:53–58. doi: 10.1373/clinchem.2005.057950. [DOI] [PubMed] [Google Scholar]
- 20.Philip I, Plantefeve G, Vuillaumier-Barrot S, Vicaut E, LeMarie C, Henrion D, et al. G894T polymorphism in the endothelial nitric oxide synthase gene is associated with an enhanced vascular responsiveness to phenylephrine. Circulation. 1999;99:3096–3098. doi: 10.1161/01.CIR.99.24.3096. [DOI] [PubMed] [Google Scholar]
- 21.Rankinen T, Rice T, Pérusse L, Chagnon YC, Gagnon J, Leon AS, et al. NOS3 Glu298Asp genotype and blood pressure response to endurance training: the HERITAGE family study. Hypertension. 2000;36:885–889. doi: 10.1161/01.HYP.36.5.885. [DOI] [PubMed] [Google Scholar]
- 22.Naber CK, Baumgart D, Altmann C, Siffert W, Erbel R, Heusch G. eNOS 894T allele and coronary blood flow at rest and during adenosine-induced hyperemia. Am J Physiol Heart Circ Physiol. 2001;281:H1908–H1912. doi: 10.1152/ajpheart.2001.281.5.H1908. [DOI] [PubMed] [Google Scholar]
- 23.Savvidou MD, Vallance PJ, Nicolaides KH, Hingorani AD. Endothelial nitric oxide synthase gene polymorphism and maternal vascular adaptation to pregnancy. Hypertension. 2001;38:1289–1293. doi: 10.1161/hy1201.097305. [DOI] [PubMed] [Google Scholar]
- 24.Bor-Kucukatay M, Demir S, Akbay R, Dursunoglu D, Akdag B, Semiz E. Relationship between hemorheology and Glu(298)Asp polymorphism of endothelial nitric oxide synthase gene in patients with coronary artery disease. Mol Biol Rep. 2010;37:171–178. doi: 10.1007/s11033-009-9572-9. [DOI] [PubMed] [Google Scholar]
- 25.Gorroochurn P, Hodge SE, Heiman GA, Durner M, Greenberg DA. Non-replication of association studies: “pseudo-failures” to replicate? Genet Med. 2007;9:325–331. doi: 10.1097/GIM.0b013e3180676d79. [DOI] [PubMed] [Google Scholar]
- 26.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tesauro M, Thompson WC, Rogliani P, Qi L, Chaudhary PP, Moss J. Intracellular processing of endothelial nitric oxide synthase isoforms associated with differences in severity of cardiopulmonary diseases: cleavage of proteins with aspartate vs. glutamate at position 298. Proc Natl Acad Sci U S A. 2000;97:2832–2835. doi: 10.1073/pnas.97.6.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]