Abstract
Background & Aims
Cirrhosis is associated with blunted cardiovascular response to stimuli such as hemorrhage, but the mechanism remains unclear. We aimed to clarify the role of endocannabinoids in blunted hemorrhage response in cirrhotic rats.
Methods
Cirrhosis was induced by bile duct ligation (BDL). Hemodynamics were measured. Cannabinoid receptor-1 (CB1) antagonist, AM251, and macrophage inhibitor gadolinium chloride (GdCl3) were administered. Myocardial levels of anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) were measured and resident monocytes and macrophages quantified by immuno-histochemistry. Isolated cardiomyocyte contractility was measured before and after incubation with monocytes from BDL and sham controls.
Results
Hemorrhage significantly decreased arterial pressure and left ventricular dP/dT. After hemorrhage, these changes quickly reversed in controls, but were severely prolonged in BDL rats. Chronic AM251 treatment restored this impaired response. AEA and 2-AG levels were increased in BDL hearts and further increased after hemorrhage. Sham hearts showed virtually no monocytes or macrophages before or after hemorrhage, whereas BDL hearts had significantly more white blood cells which further increased after hemorrhage. GdCl3 treatment significantly reduced cardiac endocannabinoid levels both at baseline and after hemorrhage. This treatment also restored cardiovascular response to hemorrhage in BDL rats but did not affect sham controls. Monocytes isolated from BDL rats more potently inhibited cardiomyocyte contractility than sham control monocytes.
Conclusions
The cirrhotic heart showed increased monocyte recruitment and endocannabinoid levels. CB1 blockade or GdCl3 treatment restored blunted cardiovascular response to hemorrhage. Endocannabinoids released by monocytes blunt cardiac response to hemorrhage. Preventing monocyte recruitment or blocking endocannabinoid signaling may improve cardiovascular homeostasis in cirrhosis.
Keywords: Cirrhosis, Cardiomyopathy, Endocannabinoids, AM251, Hemorrhage, CB-1, Monocytes, Macrophages
Introduction
In cirrhotic patients, cardiac output is increased at baseline; however, ventricular contractile response to pharmacological, surgical or physiological stimuli is blunted. The attenuated cardiac contractile responsiveness in the face of increased baseline cardiac output is known as cirrhotic cardiomyopathy [1].
Cardiovascular dysfunction is responsible for 7–15% of mortality after liver transplantation in cirrhotic patients [2]. Moreover, hemorrhagic events like rupture of esophageal varices are a significant cause of morbidity and mortality in cirrhotic patients and impaired cardiac response could result in poorer outcomes [3].
Exogenous cannabinoids have been known for centuries with substances like hashish and marijuana. Endogenous cannabinoids are lipid-like substances and include arachidonoyl ethanolamide or anandamide, and 2-arachidonoylglycerol [4].
We previously demonstrated the increased endocannabinoid tone in blunted cardiac contractility in a cirrhotic rat model. Furthermore, we found an increase in local cardiac production of endocannabinoids in cirrhotic rats [5].
Circulating macrophages/monocytes are an important source of endocannabinoids, the production of which is increased in various forms of shock [6,7] and liver cirrhosis [8,9] through the action of elevated plasma levels of bacterial endotoxin [8,10]. Activated macrophages depress myocardial contractility through increased adherence to myocardiocytes and the release of soluble mediators [11,12]. The present study was to investigate the possible role of macrophage-derived endocannabinoids in cardiac dysfunction in cirrhotic rats in an in vivo setting and in particular, their role in altered cardiac response to hemorrhage.
Materials and methods
Animal model
The study protocols were approved by the Animal Care Committee of the University of Calgary, Faculty of Medicine, under the guidelines of the Canadian Council on Animal Care. Male Sprague–Dawley rats (Charles River, St Laurent, QC, Canada) weighing 200–250 g were used. Rats had free access to rat chow and water and were maintained in a 12-hour light/dark cycle. Common bile duct ligation (BDL) was performed to induce cirrhosis as described previously [13]. Briefly, the common bile duct was exposed by a midline abdominal incision under pentobarbital anesthesia. The duct was doubly-ligated and sectioned between the ligatures. Sham operated rats were treated in the same manner without BDL. All studies were performed 4 weeks after BDL or sham operation.
Hemodynamic indices
A left ventricular pressure-volume loop was constructed by placing a microtip pressure–volume catheter (SPR-839; Millar Instruments, Houston, TX) in the left ventricle via the right carotid artery as described previously [14]. Measurement of mean arterial pressure and drug administration were performed by placing PE-50 and PE-10 polyethylene cannulae into the left femoral artery and right jugular vein, respectively. After stabilization for 30 min, the signals were continuously recorded at a sampling rate of 1000 Hz using an ARIA pressure–volume conductance system (Millar Instruments) coupled to a Powerlab/4SP A/D converter (AD Instruments, Mountain View, CA), and then stored and displayed on a computer. All pressure–volume loop data were analyzed with a locally developed data acquisition program (CVWorks1.2; Advanced Measurements Inc., Calgary, AB, Canada). Although theoretically this setup could be calibrated for absolute volume measurement using standard saline-filled cylindrical holes, in our setting volume measurements were not reproducible between different animals. However, relative volume measured in each animal was reliable which allowed us to calculate percent changes of the volume-related indices for each rat in a single experiment, but absolute values of these indices in different animals could not be compared with each other.
End-systolic pressure-volume relationship (ESPVR) was calculated by gradually decreasing the preload by applying pressure on the inferior vena cava and calculating the slope of the line that passes through the end-systolic points of the PV loop. This intervention was performed by placing a saline-soaked cotton tip applicator on the inferior vena cava through an abdominal incision. Changes in the slope of the ESPVR are well-accepted as a relatively preload- and after-load-independent measure of contractility in a single experiment [15]. Again because of the involvement of volume measurement in this index, it was not possible to compare the results among different animals.
Controlled hemorrhage was produced by withdrawal of blood through the femoral artery (2 ml/kg/min × 3 min) by a syringe connected to a motorized withdrawal pump.
Drugs
The cannabinoid receptor-1 (CB1) antagonist, AM251 (Sigma Chemicals), was administered either acutely (3 mg/kg, iv) in the experiments involving ventricular pressure-volume studies and measurement of changes in ESPVR or chronically (3 mg/kg/day, SC × 2 days) in the hemorrhage studies. The CB-2 receptor antagonist AM630 (Sigma) was administered acutely (3 mg/kg iv) in ventricular pressure-volume experiments. Gadolinium chloride (12 mg/kg iv daily × 2 days, Sigma) was used to deplete monocytes and macrophages from cardiac tissue.
Monocyte isolation and effect on cardiomyocyte contractility
Heparinized blood (15–30 unit/ml) 5 ml was carefully layered over 5 ml Histopaque-1077 solution (sigma) and centrifuged at 400 g for 20 min. The mononuclear fraction (a white layer at the interface) was aspirated, resuspended in 2 ml of phosphate-buffered saline (PBS), and centrifuged at 800 g for 20 min. The resulting pellet was resuspended in 0.5 ml PBS and used for subsequent experiments. Unloaded cell shortening was quantified using a video sarcomere detector (Ionoptix, Milton, MA, USA). After recording the baseline contractility of cardiomyocytes, 30 μl of monocyte suspension was directly applied to the recording chamber which contained 300 μl Tyrode solution and cardiomyocytes. Ten minutes after incubation, the contractility was recorded in the same cell.
Quantification of endocannabinoids in cardiac tissue
The heart was rapidly removed from sacrificed rats. Left ventricular tissue was divided into roughly 100 mg pieces and frozen in liquid nitrogen. Following pulverization of the frozen sample under liquid nitrogen, the samples were homogenized in 0.5 ml of an ice-cold solution of methanol/Tris buffer (50 mM, pH 8.0), 1:1, containing 7 ng per 100 mg tissue weight of d4-anandamide, synthesized as described [16]. To each homogenate, 2 ml of ice-cold chloroform/methanol (1:1) and 0.5 ml of 50 mM Tris buffer, pH 8.0, was added. The homogenate was centrifuged at 4 °C (500 × g for 2 min), the chloroform phase was recovered and transferred to a borosilicate tube, and the water phase was extracted two more times with ice-cold chloroform. The combined extract was evaporated to dryness at 32 °C under a stream of nitrogen. The dried residue was reconstituted in 110 μl of chloroform, and 2 ml of ice-cold acetone was added. The precipitated proteins were removed by centrifugation (1800 × g, 10 min), and the clear supernatant was removed and evaporated to dryness. The dry residues were reconstituted in 50 μl of ice-cold methanol, of which 35 μl was used for analysis by liquid chromatography/in line mass spectrometry, by using an Agilent 1100 series LC-MSD, equipped with a thermostated autosampler and column compartment. Liquid chromatographic separation of endocannabinoids was achieved by using a guard column (Discovery HS C18, 2 cm × 4.0 mm, 3 μm, 120A) and analytical column (Discovery HS C18, 7.5 cm × 4.6 mm, 3 μm) at 32 °C with a mobile phase of methanol/water/acetic acid (85:15:0.1, vol/vol/vol) at a flow of 1 ml/min for 12 min followed by 8 min of methanol/acetic acid (100:0.1, vol/vol). The MSD (model LS) was set for atmospheric pressure chemical ionization, positive polarity, and selected ion monitoring to monitor ions m/z 348 for AEA, 352 for d4-AEA, and 379 for 2-arachidonoylglycerol (2-AG). The spray chamber settings were as follows: vaporizer, 400 °C; gas temperature, 350 °C; drying gas, 5.0 L/min; and nitrogen was used as the nebulizing gas with a pressure of 60 psig. Calibration curves were produced by using synthetic anandamide and 2-AG (Cayman Chemical, Ann Arbor, MI). The amounts of AEA and 2-AG in the samples were determined by using inverse linear regression of standard curves. Values are expressed as fmol per mg wet tissue.
Immunohistochemistry
The removed heart was retrogradely perfused with ice-cold saline followed by ice-cold 4% paraformaldehyde. After the hearts were embedded in paraffin, transverse sections were cut and stored for immunohistochemistry. 5 μm sections of the heart were deparaffinized, and treated with 0.3% hydrogen peroxide in methyl alcohol for 30 min to block endogenous peroxidases. After three washes in phosphate buffer, the sections were exposed to normal goat serum, and then incubated with a monocyte/macrophage-specific primary antibody (mouse anti-rat anti-CD68 antibody also known as ED1) diluted in PBS for 1 hour at room temperature. After washing, the sections were sequentially treated with biotinylated goat anti-mouse immunoglobulin and avidin-biotin peroxidase complex; developed with diaminobenzidine-hydrogen peroxide solution and finally counterstained with hematoxylin. Quantification of CD68-positive cells was performed by calculating the average number of cells per high power field (20×).
Statistical analysis
The results are expressed as mean ± SE. Student’s t test was used to compare the differences between two groups and multiple comparisons for three or more groups were analyzed by one-way or two-way ANOVA, followed by a Newman-Keuls post hoc test, where appropriate. A p value <0.05 was considered to be significantly different.
Results
Effects of CB1 blockade on PV loop and ESPV relationship
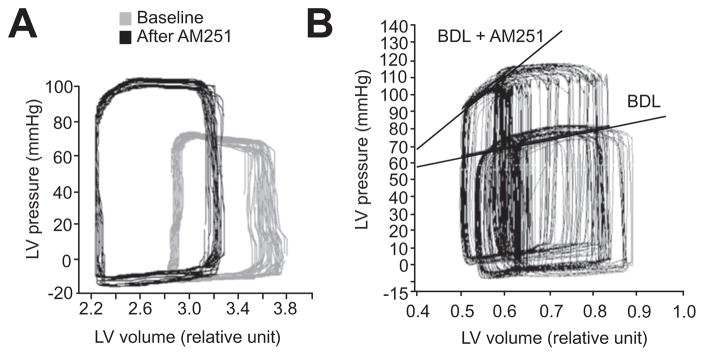
Fig. 1 shows an example of left ventricular pressure-volume loop in a BDL rat before and after administration of AM251. This treatment resulted in a significant increase in left ventricular end-systolic pressure and a significant decrease in left ventricular end-diastolic volume in the BDL group. However, this treatment did not change pressure-volume loop in sham control animals (Fig. 1A). AM251 administration also resulted in a significant increase in ESPVR in BDL rats compared to baseline but it did not affect the sham control rats (Fig. 1B). Administration of a CB2 antagonist, AM630, did not change end-systolic pressure, end-diastolic volume, or ESPVR in either sham control or BDL animals (data not shown).
Fig. 1. The effect of CB1 antagonist, AM251, on cardiac contractility.
(A) Left ventricular pressure-volume loop. AM251 (3 mg kg−1) treatment increased end-systolic ventricular pressure and reduced end-diastolic volume in BDL animals but did not have a significant effect in sham control rats (n = 3). (B) AM251 treatment significantly increased ESPVR in the BDL group while it had no significant effect in sham control animals (n = 3). ESPVR: end-systolic pressure-volume relationship.
Hemodynamic response to hemorrhage
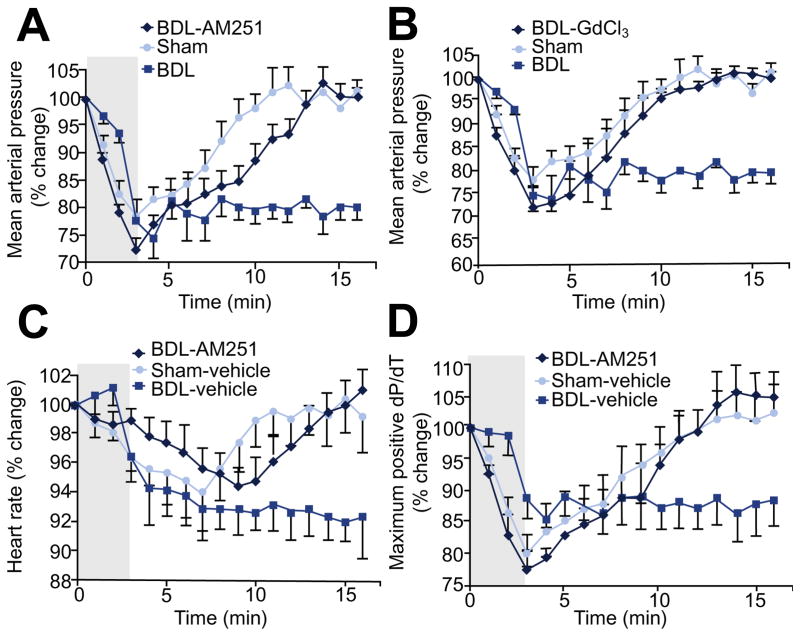
Controlled hemorrhage resulted in a significant decline in mean arterial pressure (MAP) in both sham control and BDL groups. However, the rate of decline was slower in the BDL group (Fig. 2). Once hemorrhage stopped at 3 min, blood pressure immediately started rising in the sham control group and was restored within 10 min while it remained unchanged in the BDL group and did not return back to baseline values for the duration of the observation period (Fig. 2A and B). Surprisingly, heart rate began to drop shortly after hemorrhage started in both sham control and BDL groups (Fig. 2C). This drop in heart rate started earlier in the sham control group. Regardless of the protocol used in the pilot studies, any degree of hemorrhage that induced a significant drop in blood pressure was associated with bradycardia in sham controls. Heart rate started rising in sham control animals after the hemorrhage stopped, but the rise in heart rate followed the rise in blood pressure. In the BDL group, similar to the blunted MAP response, heart rate did not increase and remained at the same level (Fig. 2C). Although left ventricular dP/dT is not a very reliable index for measurement of cardiac contractility, especially in the presence of changing preload, it was the only available option in our setting as hemorrhage moved the pressure-volume loops outside the sensitivity range of the probe for volume measurement. Hemorrhage resulted in a drop in maximum dP/dT in both sham control and BDL groups; however, it recovered in sham control animals after hemorrhage stopped but did not change in the BDL group (Fig. 2D).
Fig. 2. The role of endocannabinoids in response to hemorrhage.
(A and B) Mean arterial pressure (MAP) returned to baseline once hemorrhage (2 ml/min/kg for 3 min) stopped in sham control and BDL-AM251, BDL-GdCl3 groups but remained at the same level in BDL rats (n ≥4). (C) Heart rate (HR) returned to baseline once hemorrhage stopped in sham control and BDL-AM251 groups but remained at the same level in BDL rats (n ≥4). (D) Left ventricular contraction velocity (dP/dT) returned to baseline once hemorrhage stopped in sham control and BDL-AM251 groups but remained at the same level in BDL rats (n ≥4).
Treatment with AM251 did not alter the response to hemorrhage in sham control animals (data not shown) but significantly changed the pattern of cardiovascular responses in the BDL group. The drop in blood pressure in response to hemorrhage in AM251-treated BDL animals was similar to that of the BDL-vehicle group. However, unlike the BDL-vehicle group, once the hemorrhage stopped, blood pressure started rising and returned to baseline values (Fig. 2A). GdCl3 had the similar effect as AM251 on MAP response to hemorrhage (Fig. 2B). Heart rate also dropped in response to hemorrhage but quickly returned to baseline. Similar to the response in sham control group, restoration of heart rate chronologically followed the rise in blood pressure (Fig. 2C). Changes in dP/dT where more variable among different animals; it started rising in the AM251-treated group after hemorrhage whereas it did not change in the BDL-vehicle group (Fig. 2D).
Quantification of cardiac endocannabinoids
Cardiac concentration of anandamide was significantly increased in the BDL animals compared to that of sham control rats (7.9 ± 0.5 and 4.97 ± 0.31 fmol/mg, respectively) (Table 1). Hemorrhagic stress did not change the cardiac levels of anandamide in sham control subjects but resulted in a significant increase in BDL rats (Table 1). GdCl3 significantly decreased the cardiac levels of anandamide in hemorrhage stimulation. 2-AG levels in the cirrhotic hearts were significantly higher than those of sham control subjects (98.3 ± 11.3 and 51.3 ± 5.2 fmol/mg, respectively) but hemorrhagic stress did not change 2-AG levels in either sham control or BDL groups (data not shown). GdCl3 did not induce a significant change in 2AG levels in either sham control or BDL groups neither at baseline nor after hemorrhage stress (data not shown). GdCl3 treatment restored the blunted response of the BDL rats to hemorrhage (Fig. 2B) but did not affect sham control animals (data not shown).
Table 1.
Ventricular anandamide concentration (fmol/mg).
| Groups | Baseline | HMRG | GdCl3 | HMRG + GdCl3 |
|---|---|---|---|---|
| Sham | 5.0 ± 0.3 | 5.0 ± 0.3 | ||
| BDL | 7.5 ± 0.5* | 12.4 ± 1.6† | 6.1 ± 0.2 | 7.8 ± 0.2‡ |
Data are expressed as mean ± SE (n = 6 in each group). HMRG, hemorrhage; GdCl3, gadolinium chloride; BDL, Bile duct ligation;
p <0.05 compared with sham baseline;
p <0.05 compared with BDL baseline;
p <0.05 compared with HMRG.
Presence of monocytes and macrophages in the heart
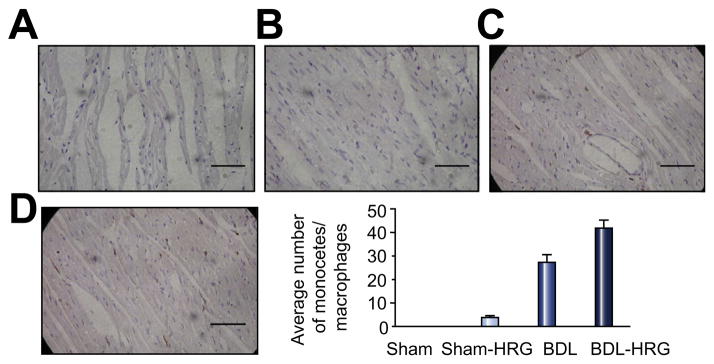
Monocytes and macrophages were virtually absent in the sham control hearts but were abundant in the cirrhotic hearts. Hemorrhagic stress slightly increased the number of monocytes and macrophages in the sham control group but significantly increased their numbers in the BDL rats (Fig. 3).
Fig. 3. Immunohistochemical staining of a selective marker for rat monocytes and macrophages – CD68 – in the hearts of sham control and BDL animals at resting state and after hemorrhagic stress.
The bar graph shows average number of CD68 positive macrophages counted in four fields at 20× magnification. Scale bar represents 20 μm. (This figure appears in colour on the web.)
Effect of monocytes on contractility in cardiomyocytes
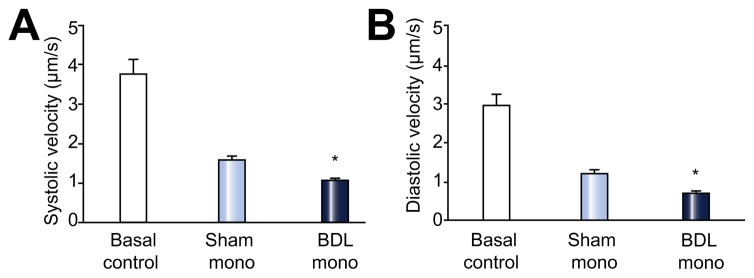
The basal contractile and relaxation velocities were the same in the two groups of cells (Fig 4). After 10 min incubation, the contractile velocity was significantly attenuated in the cells incubated with monocytes from BDL rats. Cells incubated with monocytes from sham rats also decreased the contractility, but to a lesser extent than the BDL monocytes (n = 6, p <0.05). The changes of diastolic velocity showed a similar pattern as systolic velocity (Fig. 4).
Fig. 4. The effect of monocyte incubation on cardiomyocyte contraction and relaxation.
Monocytes from BDL rats had more inhibitory effects on contraction and relaxation velocities compared with those from sham operation animals (n = 6 in each group, *p <0.05).
Discussion
Cardiac dysfunction in cirrhosis when initially reported more than four decades ago was presumed to be caused by alcoholic cardiotoxicity. It is now clear that it is the cirrhosis per se that leads to cirrhotic cardiomyopathy [3,17]. Non-alcoholic murine cirrhotic models, such as BDL [18], carbon tetrachloride [19], and thioacetamide [13] all demonstrate cardiac dysfunction. In the present study, we used the BDL rat model as it reliably produces biliary cirrhosis with cardiovascular disturbances that mirror the human condition of cirrhosis. Moreover, it avoids the use of any toxin such as CCl4 or thioacetamide that might complicate interpretation of the results.
The increasing use of invasive procedures that stress the heart in cirrhotic patients, such as TIPS and liver transplantation, has highlighted the clinical significance of cardiovascular dysfunction in cirrhosis. Cardiac dysfunction causes significant mortality and morbidity after orthotopic liver transplantation [20,21] and may contribute to the pathogenesis of hepatorenal syndrome [22,23]. Moreover, bleeding is common in cirrhotic patients and an inadequate cardiac response may contribute to poor outcomes [3].
In this study the increased blood pressure and reduced left ventricular end-diastolic volume after AM251 suggested an increase in cardiac contractility in cirrhotic rats after CB1 blockade. However, these indices are load-dependent and could be affected by changes in cardiac preload and afterload [24]. We therefore studied ESPVR, a load-independent measure of cardiac contractility. The increased ESPVR in BDL rats after AM251 suggests that endocannabinoids inhibit cardiac contractility via CB1 receptors. These in vivo data are in line with our previous in vitro findings in BDL rats [5] and in vivo results in a rat model of CCl4-induced cirrhosis [25].
Since AM251 is a highly selective CB1 receptor antagonist, its application immediately blocks the inhibitory effect of endocannabinoids on cardiac contractility via blockage of CB1 receptor, not through a decrease of anandamide synthesis or release [25].
A blunted cardiovascular response to vasoconstrictive stimuli such as hemorrhage in cirrhosis has traditionally been ascribed to an attenuated arterial response to humoral vasoconstrictors such as catecholamines [26,27]. This mechanism is likely to be involved but a purely arterial vascular effect is an oversimplification. Indeed, many studies over the past two decades demonstrate abnormalities at several levels of the cardiovascular system in cirrhosis or portal hypertension: 1) abnormal central neural cardiovascular-regulatory areas in the brainstem and hypothalamus [28]; and 2) inability to mobilize venous blood reservoirs in the liver [29], spleen [30], and gut [31]. The present study now demonstrates that the left ventricular response to hemorrhage is also blunted.
The hemorrhage data clearly demonstrated a blunted cardiovascular response in the cirrhotic animals by all parameters examined including arterial pressure, heart rate and ventricular contractility (dP/dT). Chronic treatment with AM251 restored the responsiveness to hemorrhage in BDL rats. This suggests that blunted cardiovascular responsiveness to hemorrhage in cirrhosis is mediated by endocannabinoids via CB1.
We previously provided indirect pharmacological evidence of increased local production of endocannabinoids in the cirrhotic heart [5]. In the present study, cardiac levels of both anandamide and 2-AG were significantly higher in BDL animals. This finding is in line with the results of Batkai et al. in a CCl4-induced rat model of cirrhosis [25]. However, in their study only the levels of anandamide and not 2-AG were increased in the cirrhotic heart. This difference may be due to the different rat models used in the two studies.
Our previous in vitro experiments also pointed to the possibility of increased local cardiac endocannabinoid production in response to cardiovascular stress [5]. In order to test this hypothesis, we measured cardiac endocannabinoid concentrations not only at baseline, but also after hemorrhagic stress. Hemorrhage did not significantly affect endocannabinoid levels in sham control hearts. In a non-cirrhotic shock model, when hemorrhage was more profound and longer lasting than in the present experiments, circulating macrophages produced elevated levels of anandamide and CB1 blockade reversed the hypotension [6]. The present findings indicate that a similar mechanism is activated in cirrhosis, and when both hemorrhage and cirrhosis are present, there is an additive increase in tissue anandamide levels. The observation that hemorrhage in the cirrhotic rats significantly increased levels of anandamide but not 2-AG suggests different regulatory mechanisms for cardiac metabolism of anandamide and 2-AG, and also agrees with a recent study of peripheral and hepatic levels in cirrhotic patients in whom anandamide but not 2-AG was elevated [32].
In almost all tissues studied to date including brain and heart, concentrations of 2-AG are markedly higher compared with anandamide (30-fold higher in heart [33], 200-fold higher in brain [34]). Our data showed that 2-AG was 12-fold higher than anandamide in the BDL heart. A logical question may be posed why such massively higher levels of 2-AG would not overwhelm the CB1 receptors, leaving no physiological role for the relatively puny levels of anandamide? Or, in other words, could the hemorrhage-induced doubling of the anandamide concentration in the cirrhotic heart be pathophysiologically relevant given a background log-order higher 2-AG concentration? Recent reviews by Piomelli [34], and Alger and Kim [35] that underscore the complexity of endocannabinoid physiology have suggested answers to these questions. In brief, it is believed that endocannabinoid signaling via CB1 receptors depends on several factors, some of them more important than the measurable total concentrations of 2-AG and anandamide. 2-AG plays important roles in lipid metabolism and other intracellular processes independent of its effect on CB1 receptors. Endocannabinoids are contained in several compartments or pools, and the releasable pool that activates CB1 receptors may only represent a small fraction of the total, particularly in the case of 2-AG. For example, in the brain, a significant fraction of 2-AG serves a housekeeping intracellular function and is not available for signaling via CB1 [34].
Interestingly, hemorrhage and hypotension resulted in a heart rate decline in both sham and BDL groups. In pilot experiments, this bradycardiac response occurred even with the least amount of hemorrhage that could induce measurable hypotension. This phenomenon has been reported before in both humans and animal models of hemorrhagic shock [36,37]. Although the exact mechanism for this paradoxical bradycardia is not completely understood, it might be explained by the Bezold-Jarisch reflex [38]. Normally, in response to mild to moderate bleeding, heart rate and cardiac output increase to maintain the systemic blood pressure. However, during severe hemorrhage, the reduced blood volume results in diminished cardiac filling. This is followed by a baroreceptor-mediated increase in parasympathetic activity and decrease in sympathetic tone which paradoxically result in decreased heart rate and cardiac output, and further declines in blood pressure.
The observation that heart rate and blood pressure consistently declined slower in the BDL group compared to sham animals also remains unexplained. This finding was surprising because the cardiovascular response to stress is known to be blunted in cirrhotic subjects. We speculate that in the BDL heart, increased endocannabinoid tone reduces contractility and attenuates the Bezold-Jarisch reflex. This hypothesis is supported by the observation that AM251 and gadolinium treatment both dramatically increased the hemorrhage-induced rate of decline in BDL rats.
To our knowledge, the presence of monocytes and macrophages in the cirrhotic heart has never been reported before. Our BDL-cirrhotic hearts had large numbers of monocytes and macrophages, which further increased after hemorrhage, whereas control hearts showed very few. This pattern of change parallels the alterations in anandamide levels with hemorrhage. These results suggest a significant role for transmigrated monocytes and macrophages in the increased cardiac production of endocannabinoids. Although the stimulus for macrophage activation has not been directly examined in the present study, bacterial endotoxin likely plays a role. Both cirrhosis and hemorrhage have been associated with ‘leaky gut’ and increased plasma endotoxin levels [39,40]. When we applied the monocytes from BDL rats to cardiomyocytes, we demonstrated that monocytes directly inhibited the contractility and relaxation.
While the above experiments are suggestive, they do not prove a causal relationship. We therefore used gadolinium to disrupt the mononuclear cells. This treatment is known to markedly reduce secretory function of monocytes and macrophages [41]. Gadolinium treatment significantly reduced cardiac levels of anandamide in BDL rats after hemorrhage and essentially normalized their blunted cardiovascular response, but did not affect the hemorrhage response in control animals. Therefore these results in conjunction with our other data offer strong evidence that endocannabinoids secreted by monocytes and macrophage in the heart mediate the blunted hemorrhage stress response in the BDL-cirrhotic rat.
Our study has important potential therapeutic implications. Hemorrhage from gastroesophageal varices or other causes is frequent in patients with cirrhosis, and their cardiovascular ‘fragility’ to such insults might be reduced by treatment with CB1 antagonists. Randomized trials of a CB1 antagonist rimonabant showed that it effectively suppressed appetite, but induced severe depression in some patients after prolonged use, and therefore further development was stopped [42]. We speculate that a few doses of rimonabant or a similar CB1-blocker may help stabilize hemodynamics during the acute bleeding episode; the drug would not be used long-term thus avoiding the neuro-psychiatric adverse effects. Alternatively, CB1-antagonists that selectively target only peripheral CB1 receptors may offer potential new strategies to manage cardiovascular disturbances in cirrhotic patients.
Acknowledgments
Financial support
SAG was supported by a Canadian Association for Study of Liver fellowship award. The study was funded by a Canadian Liver Foundation research grant.
Abbreviations
- BDL
bile duct ligation
- CB1
Cannabinoid receptor-1
- GdCl3
gadolinium chloride
- AEA
anandamide
- 2-AG
2-arachidonoyl glycerol
- ESPVR
end-systolic pressure-volume relationship
Footnotes
Authors’ contributions
S.S. Lee originated the idea, helped design the protocols and analyzed the data. S.A. Gaskari and H. Liu designed the protocols, performed the majority of the experiments, analyzed the data and wrote the draft of the manuscript. C. D’Mello performed the monocyte immunohistochemistry. G. Kunos measured the endocannabinoid concentrations and helped analyze the data. All authors helped revise the first draft and revision, and gave final approval to submissions.
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect.
References
- 1.Ma Z, Miyamoto A, Lee SS. Role of altered beta-adrenoceptor signal transduction in the pathogenesis of cirrhotic cardiomyopathy in rats. Gastroenterology. 1996;110:1191–1198. doi: 10.1053/gast.1996.v110.pm8613009. [DOI] [PubMed] [Google Scholar]
- 2.Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaskari SA, Honar H, Lee SS. Therapy insight: cirrhotic cardiomyopathy. Nat Clin Pract Gastroenterol Hepatol. 2006;3:329–337. doi: 10.1038/ncpgasthep0498. [DOI] [PubMed] [Google Scholar]
- 4.De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaskari SA, Liu H, Moezi L, Li Y, Baik SK, Lee SS. Role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Br J Pharmacol. 2005;146:315–323. doi: 10.1038/sj.bjp.0706331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner JA, Varga K, Ellis EF, Rzigalinski BA, Martin BR, Kunos G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- 7.Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- 8.Batkai S, Jarai Z, Wagner JA, Goparaju SK, Varga K, Liu J, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- 9.Ros J, Claria J, To-Figueras J, Planaguma A, Cejudo-Martin P, Fernandez-Varo G, et al. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology. 2002;122:85–93. doi: 10.1053/gast.2002.30305. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Batkai S, Pacher P, Harvey-White J, Wagner JA, Cravatt BF, et al. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J Biol Chem. 2003;278:45034–45039. doi: 10.1074/jbc.M306062200. [DOI] [PubMed] [Google Scholar]
- 11.Balligand JL, Kelly RA, Marsden PA, Smith TW, Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993;90:347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simms MG, Walley KR. Activated macrophages decrease rat cardiac myocyte contractility: importance of ICAM-1-dependent adhesion. Am J Physiol. 1999;277:H253–H260. doi: 10.1152/ajpheart.1999.277.1.H253. [DOI] [PubMed] [Google Scholar]
- 13.Ma Z, Zhang Y, Huet PM, Lee SS. Differential effects of jaundice and cirrhosis on beta-adrenoceptor signaling in three rat models of cirrhotic cardiomyopathy. J Hepatol. 1999;30:485–491. doi: 10.1016/s0168-8278(99)80109-3. [DOI] [PubMed] [Google Scholar]
- 14.Pacher P, Liaudet L, Bai P, Virag L, Mabley JG, Hasko G, et al. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther. 2002;300:862–867. doi: 10.1124/jpet.300.3.862. [DOI] [PubMed] [Google Scholar]
- 15.Summers RL. Noninvasive estimation of the end systolic pressure-volume relationship using impedance cardiography. J Miss State Med Assoc. 2000;41:575–578. [PubMed] [Google Scholar]
- 16.Giuffrida A, Rodriguez de Fonseca F, Piomelli D. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal Biochem. 2000;280:87–93. doi: 10.1006/abio.2000.4509. [DOI] [PubMed] [Google Scholar]
- 17.Alqahtani SA, Fouad TR, Lee SS. Cirrhotic cardiomyopathy. Semin Liver Dis. 2008;28:59–69. doi: 10.1055/s-2008-1040321. [DOI] [PubMed] [Google Scholar]
- 18.Nam SW, Liu H, Wang JZ, Feng AY, Chu G, Merchant N, et al. Cardiomyocyte apoptosis contributes to pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated mice. Clin Sci. 2014;127:516–526. doi: 10.1042/CS20130642. [DOI] [PubMed] [Google Scholar]
- 19.Yang CH, Ting WJ, Day CH, Ju DT, Yeh YL, Chung LC, et al. SHSST cyclodextrin complex prevents the fibrosis effect on CCl4-induced cirrhotic cardiomyopathy in rats through TGF-beta pathway inhibition effects. Int J Mol Sci. 2014;15:8037–8048. doi: 10.3390/ijms15058037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rayes N, Bechstein WO, Keck H, Blumhardt G, Lohmann R, Neuhaus P. Cause of death after liver transplantation: an analysis of 41 cases in 382 patients. Zentralbl Chir. 1995;120:435–438. [PubMed] [Google Scholar]
- 21.Myers RP, Lee SS. Cirrhotic cardiomyopathy and liver transplantation. Liver Transpl. 2000;6:S44–S52. doi: 10.1002/lt.500060510. [DOI] [PubMed] [Google Scholar]
- 22.Lee SS. Cardiac dysfunction in spontaneous bacterial peritonitis: a manifestation of cirrhotic cardiomyopathy? Hepatology. 2003;38:1089–1091. doi: 10.1053/jhep.2003.50489. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-del-Arbol L, Urman J, Fernandez J, Gonzalez M, Navasa M, Monescillo A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210–1218. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]
- 24.Radovits T, Korkmaz S, Loganathan S, Barnucz E, Bomicke T, Arif R, et al. Comparative investigation of the left ventricular pressure-volume relationship in rat models of type 1 and type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2009;297:H125–H133. doi: 10.1152/ajpheart.00165.2009. [DOI] [PubMed] [Google Scholar]
- 25.Batkai S, Mukhopadhyay P, Harvey-White J, Kechrid R, Pacher P, Kunos G. Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am J Physiol Heart Circ Physiol. 2007;293:H1689–H1695. doi: 10.1152/ajpheart.00538.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laleman W, Landeghem L, Wilmer A, Fevery J, Nevens F. Portal hypertension: from pathophysiology to clinical practice. Liver Int. 2005;25:1079–1090. doi: 10.1111/j.1478-3231.2005.01163.x. [DOI] [PubMed] [Google Scholar]
- 27.Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology. 2008;134:1715–1728. doi: 10.1053/j.gastro.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Song D, Liu H, Sharkey KA, Lee SS. Hyperdynamic circulation in portal-hypertensive rats is dependent on central c-fos gene expression. Hepatology. 2002;35:159–166. doi: 10.1053/jhep.2002.30417. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Liu H, Gaskari SA, McCafferty DM, Lee SS. Hepatic venous dysregulation contributes to blood volume pooling in cirrhotic rats. Gut. 2006;55:1030–1035. doi: 10.1136/gut.2005.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamza SM, Kaufman S. Role of spleen in integrated control of splanchnic vascular tone: physiology and pathophysiology. Can J Physiol Pharmacol. 2009;87:1–7. doi: 10.1139/Y08-103. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Liu H, Gaskari SA, Tyberg JV, Lee SS. Altered mesenteric venous capacitance and volume pooling in cirrhotic rats are mediated by nitric oxide. Am J Physiol Gastrointest Liver Physiol. 2008;295:G252–G259. doi: 10.1152/ajpgi.00436.2007. [DOI] [PubMed] [Google Scholar]
- 32.Caraceni P, Viola A, Piscitelli F, Giannone F, Berzigotti A, Cescon M, et al. Circulating and hepatic endocannabinoids and endocannabinoid-related molecules in patients with cirrhosis. Liver Int. 2009;30:816–825. doi: 10.1111/j.1478-3231.2009.02137.x. [DOI] [PubMed] [Google Scholar]
- 33.Iannotti FA, Piscitelli F, Martella A, Mazzarella E, Allara M, Palmieri V, et al. Analysis of the “endocannabinoidome” in peripheral tissues of obese Zucker rats. Prostaglandins Leukot Essent Fatty Acids. 2013;89:127–135. doi: 10.1016/j.plefa.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 35.Alger BE, Kim J. Supply and demand for endocannabinoids. Trends Neurosci. 2011;34:304–315. doi: 10.1016/j.tins.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rea RF, Thames MD. Neural control mechanisms and vasovagal syncope. J Cardiovasc Electrophysiol. 1993;4:587–595. doi: 10.1111/j.1540-8167.1993.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 37.Kirkman E, Watts S. Haemodynamic changes in trauma. Br J Anaesth. 2014;113:266–275. doi: 10.1093/bja/aeu232. [DOI] [PubMed] [Google Scholar]
- 38.Aviado DM, Guevara Aviado D. The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes. Ann N Y Acad Sci. 2001;940:48–58. [PubMed] [Google Scholar]
- 39.Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology. 1988;8:232–236. doi: 10.1002/hep.1840080207. [DOI] [PubMed] [Google Scholar]
- 40.Yao YM, Tian HM, Sheng ZY, Wang YP, Yu Y, Sun SR, et al. Inhibitory effects of low-dose polymyxin B on hemorrhage-induced endotoxin/bacterial translocation and cytokine formation in rats. J Trauma. 1995;38:924–930. doi: 10.1097/00005373-199506000-00018. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad N, Gardner CR, Yurkow EJ, Laskin DL. Inhibition of macrophages with gadolinium chloride alters intercellular adhesion molecule-1 expression in the liver during acute endotoxemia in rats. Hepatology. 1999;29:728–736. doi: 10.1002/hep.510290324. [DOI] [PubMed] [Google Scholar]
- 42.Ruilope LM, Despres JP, Scheen A, Pi-Sunyer X, Mancia G, Zanchetti A, et al. Effect of rimonabant on blood pressure in overweight/obese patients with/without co-morbidities: analysis of pooled RIO study results. J Hypertens. 2008;26:357–367. doi: 10.1097/HJH.0b013e3282f2d625. [DOI] [PubMed] [Google Scholar]