Abstract
Cardiovascular reactivity is a potential mechanism underlying associations of close relationship quality with cardiovascular disease. Two models describe oxytocin as another mechanism. The “calm and connect” model posits an association between positive relationship experiences and oxytocin levels and responses, whereas the “tend and befriend” model emphasizes the effects of negative relationship experiences in evoking oxytocin release. In this study of 180 younger couples, relationship quality had a small, marginally significant inverse association with plasma oxytocin levels, and neither positive nor negative couple interactions evoked change in plasma oxytocin. Negative couple interactions evoked significant cardiovascular reactivity, especially among women. Hence, in the largest study of these issues to date, there was little support for key tenets of the “calm and connect” model, and only very modest support for the ”tend and befriend” model. However, findings were consistent with the view that CVR contributes to the effects of relationship difficulties on health.
Keywords: relationship quality, couple interaction, oxytocin, cardiovascular reactivity
1. Introduction
The availability and quality of personal relationships are robust predictors of physical health (Holt-Lunstad, Smith, & Layton, 2010). As a key instance, marriage and similar close relationships are related to subsequent cardiovascular disease (CVD). Married individuals are at reduced risk for CVD (Kaplan & Kronick, 2006), though this benefit is sometimes smaller for women (Eaker et al., 2007). Further, strain and disruption in marriage and similar relationships confer increased risk of hypertension (Tobe et al., 2007), atherosclerosis (Smith et al., 2011), coronary heart disease (De Vogli, Chandola, & Marmot, 2007; Matthews & Gump, 2002), and poor prognosis among heart patients (King & Reiss, 2012; Orth-Gomer et al, 2000).
Physiological stress responses could contribute to these associations (Kiecolt-Glaser & Newton, 2001). Conflict in close relationships evokes increases in heart rate, blood pressure, catecholamines, cortisol, and inflammatory factors (Robles & Kiecolt-Glaser, 2003; Kiecolt-Glaser et al., 2005; Kiecolt-Glaser, Gouin, & Hantsoo, 2009), whereas supportive relationships attenuate these responses (Uchino, 2006). Women often display greater reactivity to stressful marital interactions than men, perhaps contributing to a smaller marital health benefit (Kiecolt-Glaser & Newton, 2001). In the present study, we examined two mechanisms potentially linking the quality of close relationships and CVD - cardiovascular reactivity (CVR) and oxytocin, a neuropeptide related to affiliation. Specifically, we examined effects of positive and negative couple interactions on these mechanisms, and associations of oxytocin with relationship quality.
1.1 Close Relationships and Cardiovascular Reactivity
Metabolically excessive CVR in response to psychological stressors (Carroll, Phillips, & Balanos, 2009) is hypothesized to promote sustained increases in blood pressure, atherosclerosis, and the precipitation of cardiovascular events (Schwartz et al., 2003). Consistent with this view, CVR predicts hypertension (Matthews et al., 2004), atherosclerosis (Jennings et al., 2004; Matthews et al., 2006), and stroke (Everson et al., 2001), and a meta-analysis supported this association with risk of CVD (Chida & Steptoe, 2010). Negative interactions in marriage and similar close relationships evoke increases in heart rate and blood pressure (e.g., Nealey-Moore et al., 2007; Smith et al., 2009b), supporting the hypothesis that CVR may link the quality of close relationships with risk of CVD (Robles & Kiecolt-Glaser, 2003; Slatcher, 2010).
1.2 Oxytocin, Affiliation, and Health Risks
Neuroendocrine bases and effects of affiliation suggest another pathway (Knox & Uvnas-Moberg, 1998; Uvnas-Moberg, 1998). Oxytocin is a hypothalamic neuropeptide, active both in the brain and the periphery. Human studies measuring oxytocin typically assess levels in plasma, whereas human experimental studies manipulate central oxytocin levels via placebo controlled intranasal sprays. Importantly, the association between central and peripheral levels is tenuous at best and cannot be assumed (Campbell, 2010). In research with non-human mammals central oxytocin underpins a variety of affiliative behaviors, and has robust stress-dampening effects (Carter, 1998; Carter et al., 2008). Further, administration of oxytocin in animal models inhibits effects of chronic social stressors on atherosclerosis and other health outcomes (e.g., Nation et al., 2010). Two general conceptual models of oxytocin as a mechanism linking close relationships and health in humans have been proposed.
In the first, oxytocin is reciprocally associated with positive relationships (Carter, 1998; Knox & Unvas-Moberg, 1998; Unvas-Moberg, 1998). Specifically, “a warm, supportive, and friendly social environment may stimulate calm and social interaction. Such stimuli are likely to involve activation of oxytocin-related mechanisms” (Uvnas-Moberg, Arn, & Magnusson, 2005, p. 62). The release of oxytocin in turn fosters further affiliative behavior and bonding. Hence, higher levels of oxytocin are both a cause and consequence of affiliation, resulting in a positive association of oxytocin levels with the presence and quality of close relationships. Further, oxytocin attenuates negative emotions that interfere with affiliation, and physiological responses that promote CVD (Campbell, 2010; Uvnas-Moberg et al., 2005). Thus, in this perspective oxytocin underpins a biobehavioral “calm and connect” system, (Uvnas-Moberg et al., 2005), such that oxytocin “may serve to inhibit defensive behaviors associated with stress, anxiety or fear, and allow positive social interactions and the development of bonds” (Carter, 1998; p. 782).
In contrast to the “calm and connect” model, the second model emphasizes oxytocin release in response to stressors. In this view threats to or disruption of positive social relationships activate affiliative needs. This aroused social motivation, in turn, evokes a release of oxytocin, which prompts affiliative behavior (Taylor, 2006; Taylor, et al., 2000). Specifically, “oxytocin is released in response to (at least some) stressors, especially those that may trigger affiliative needs” (Taylor, 2006, p. 273). Hence, “oxytocin appears to signal relationship distress” or “gaps in positive social relationships” (Taylor, 2006, p.274), especially in women, fostering efforts toward reestablishing “an adequate level of protective and rewarding social relationships” (p.273). If successful, these affiliative efforts reduce stress responses. This “tend and befriend” pattern (Taylor et al., 2000; Taylor, 2006), characterizes stress responses of women as distinct from the “fight or flight” response more characteristic of men.
These models are not directly competing in all respects. For example, Taylor (2006) notes that the “tend and befriend” model describes “social behavior under stress and does not address larger issues concerning how oxytocin may be implicated in social activity in nonstressful times” (p.274), presumably including the positive social experiences emphasized in the “calm and connect” perspective. Similarly, Uvnas-Moberg et al (2005) note that oxytocin “can be released in response to stress” (p.63). Hence, both models acknowledge the possibility that positive and negative events could evoke oxytocin release. A recent model of the effects of oxytocin on social processes similarly includes positive and negative social processes (Kemp & Guastella, 2011). Specifically, in this view oxytocin administration or release facilitates social approach and inhibits social withdrawal. Further, social approach includes the traditionally emphasized positive processes (e.g., affiliation, warmth) but also negative processes that can reflect social approach motivation (e.g., anger, jealousy). Importantly, this effect of oxytocin on social approach (Kemp & Guastella, 2011) is consistent with descriptions of the effects of oxytocin release in both the “tend and befriend” and “calm and connect” models.
However, the perspectives differ in two respects. First, the “calm and connect” model predicts a positive association between oxytocin levels and relationship quality (Carter, 1998; Unvas-Moberg, 1998). In contrast, the “tend and befriend” model predicts an inverse association, and proponents interpret such associations as supporting the model (Taylor, 2006; Taylor et al., 2006; Taylor et al., 2010). Specifically, “gaps in positive social relationships” and “relationship distress” are associated with higher oxytocin levels, and higher plasma oxytocin is seen as a “biomarker of distress in the pair-bond relationship in women” (Taylor et al., 2010, p. 3).
The second difference is a matter of emphasis. The “calm and connect” model emphasizes the prediction that warm or supportive relationship experiences evoke an oxytocin release (Unvas-Moberg, 1998; Uvnas-Moberg et al., 2005). In contrast, the ”tend and befriend” perspective emphasizes the prediction that stressors that threaten relationship bonds and negative experiences that signal distress or disruption in close relationships evoke this response (Taylor, 2006). Specifically, oxytocin levels may rise when “the pair bond is threatened” (Taylor et al., 2010, p. 6). Again, the “calm and connect” model does not preclude oxytocin rising in response to relationship stressors, and the “tend and befriend” perspective does not preclude a release of oxytocin in response to positive relationship experiences, but the emphasis clearly differs.
Empirical support for these models is mixed. Consistent with the “calm and connect” model, higher plasma oxytocin has been associated with greater support from romantic partners (Grewen, Girdler, Amico, & Light, 2005), more affiliative behavior in relationships (Tops et al., 2007), more affection in marriage (Light, Grewen, & Amico, 2005), and more positive behavior during marital interactions (Gouin et al., 2010). Such positive associations between oxytocin levels and relationship quality are difficult to reconcile with predictions based on the “tend and befriend” model. However, supporting that perspective, oxytocin levels have been found to be associated positively with interpersonal difficulties (Cyranowski et al., 2008; Turner et al., 1999), social isolation and relationship distress, and unrelated to positive social experiences (Taylor et al., 2006; Taylor et al., 2010), findings that challenge the “calm and connect” model.
Studies examining effects of couple interactions and related experiences on short-term changes in oxytocin have also produced mixed results. Inconsistent with the “clam and connect” perspective, neither verbal social support from their male partners or standardized physical partner contact (i.e., neck and shoulder massage) altered women’s plasma oxytocin (Ditzen et al., 2007), nor did a supportive couple interaction (Gouin et al., 2010). However, a “warm touch” intervention with married couples produced an increase in salivary oxytocin, but not plasma oxytocin (Holt-Lunstad, Birmingham, & Light, 2008). Also consistent with the “calm and connect” view, plasma oxytocin levels were found to increase from before to after a period of warm partner contact, although the lack of a no-contact control complicates the interpretation of this finding (Grewen et al., 2005). Finally, relevant to the “tend and befriend” or affiliative stress view, women discussing a recent relationship transgression did not display an increase in plasma oxytocin, although the extent to which the transgression remained unforgiven was associated with increases in oxytocin (Tabak et al., 2011). Consistent with both perspectives, intranasal administration of oxytocin has been found to increase positive couple behavior, and to reduce cortisol responses during couple conflicts (Ditzen et al., 2009).
1.3 The Present Study
The inconsistent evidence regarding key tenets of the “calm and connect” and “tend and befriend” models may be due to small sample sizes in the available studies. For example, studies of associations of oxytocin levels with relationship quality have ranged from 18 to 85 participants (median n = 38). To date, we are not aware of any study testing the effect of experimentally manipulated relationship stress on momentary changes in plasma oxytocin, or any studies that permit comparison of effects of negative couple interactions on momentary oxytocin responses and CVR. Finally, experimentally administered oxytocin was found to reduce cortisol responses to couple conflicts (Ditzen et al., 2009), but the association between naturally occurring oxytocin levels and other physiological stress responses is not well-established (c.f., Grewen & Light, 2011).
To address these issues, we measured relationship quality in married and cohabiting couples, who were also randomly assigned to specific couple interaction experiences. An initial laboratory session examined the effects of warm couple contact versus no contact on plasma oxytocin. A second session tested effects of positive, neutral, or negative couple interactions on blood pressure, heart rate, plasma catecholamines, and plasma oxytocin. These responses were also measured during a subsequent anger stressor. The “calm and connect” perspective clearly predicts that positive couple interactions should evoke increases in oxytocin (Carter, 1998; Unvas-Moberg et al. 2005). The “tend and befriend” model does not make specific predictions about the effect on oxytocin release of relatively brief couple interactions that vary in valence or tone. However, to the extent that a negative couple interaction represents a stressor that “triggers affiliative needs” (Taylor, 2006, p. 273) or indicates that “the pair-bond relationship is threatened” (Taylor et al., 2010, p. 6), the “tend and befriend’ perspective would predict that such interactions will evoke an increase in oxytocin (Taylor et al., 2000; Taylor, 2006). Both perspectives predict that higher resting levels of oxytocin should be associated with reduced CVR and catecholamine response to the subsequent anger stressor (Campbell, 2010; Grewen & Light, 2011; MacDonald & MacDonald, 2010). Correlations of oxytocin levels with relationship quality tested the predictions of the positive affiliation and affiliative stress perspectives. Finally, prior research suggests that the negative couple interaction should evoke greater CVR and catecholamine release, perhaps more so for women than men (Kiecolt-Glaser & Newton, 2001).
2. Method
2.1 Participants
One hundred and eighty couples were recruited from the greater Salt Lake City, Utah area. Couples met several criteria: (a) cohabitating for at least nine-months, (b) no current cardiovascular medication (e.g., alpha and beta-blockers, calcium channel blockers, nitrates, etc.), (c) no history of chronic disease with a clear cardiovascular component (e.g., diabetes, hypertension, etc.), (d) body mass index < 35, and (e) women were not pregnant or breastfeeding. Couples had been cohabitating for an average of 41.9 months (SD = 31.2, range = 10 months to 12 years); 73% were married. The modal education level was some college (men = 33%; women = 37%) or a college degree (men = 34%; women, 26.5%). The men averaged 29.3 years of age (SD = 6.6), and the women 27.9 (SD = 6.6). For both men and women, 91% of the sample was Caucasian. Each participant was provided $100 compensation.
2.2 Overview of Design and Procedure
Participants came to the laboratory on two separate days, approximately one week apart (see Figure 1). Sessions were typically scheduled for the early evening to accommodate couples’ daily schedules, but some sessions were held in the late afternoon. On Day 1, couples were randomly assigned to either a positive interaction with their partner or to remain separate for the session. Couples were assigned to the positive interaction versus no contact conditions on a 2 to 1 basis, so as to maximize power to detect correlates of change in oxytocin during positive contact, while still permitting an appropriately sized control group. On Day 2, couples were randomly assigned in equal numbers to one of three conditions: a discussion of an event that strengthened their relationship (positive interaction); a discussion of the last “so - so” movie that they saw together (neutral interaction); or a discussion of an area of ongoing disagreement (negative interaction). All participants then individually underwent an anger stressor. Participants were placed in separate rooms for baseline and recovery periods.
Figure 1.
Schematic of experimental protocol for Day 1 and Day 2.
2.3 Measures
2.3.1 Physiological assessments
Blood was drawn seven times, using a flexible, indwelling catheter. For both days, initial baselines began 30 minutes after catheter insertion, to permit adaptation. Plasma was separated via centrifuge. Oxytocin was assessed by radioimmunoassay in the laboratory of Dr. Janet Amico (University of Pittsburgh). The intra-assay coefficient was within 10 to 12 %, with a sensitivity of 0.5 pg/ml. Catecholamine assays were performed at the University of North Carolina, Chapel Hill General Clinical Research Center, via high-performance liquid chromatography. A Minnesota Impedance Cardiograph (Model 340b) measured heart rate (HR), and a Critikon Dinamap 8100 measured systolic and diastolic blood pressure (SBP, DBP).
2.3.2 Relationship Quality
Participants independently completed the Dyadic Adjustment Scale (DAS; Spanier, 1976) and the Marital Adjustment Test (MAT; Locke & Wallace, 1954), widely-used measures with well-established reliability and validity (Snyder et al., 2005). As not all participants were married, the item wording of the MAT was modified to replace references to marriage and spouses with references to relationships and partners. DAS and MAT scores were significantly associated for women, r(180) = .54, p<.001, and men, r(180) = .42, p<.001.
2.3.3 Affective Responses to Tasks and Perceptions of Partner Behavior
Following pre-task baselines and task periods, participants completed a state affect scale (c.f., Nealey-Moore et al., 2007). The 6-item anxiety subscale includes both positively-scored items (e.g., tense, nervous) and negatively scored items (e.g., calm, relaxed). Similarly, the 6-item anger scale includes positively-scored (irritated, angry) and negatively-scored items (kind and warm-hearted, friendly). Hence, both subscales can also assess decreases from a neutral resting state through endorsement of the negatively scored items. These scales have displayed high levels of internal consistency and validity in prior research (Nealey et al., 2007; Smith et al., 2009a). The item, “I feel emotionally close to my partner” was added to assess the effectiveness of positive couple tasks. Following the couple interaction task on Day 2, participants completed the Impact Message Inventory (Kiesler, Schmidt, & Wagner, 1997). This 32-item inventory assessed participants’ perceptions of their partners’ behavior during the task along two dimensions – affiliation (i.e., warm, friendly vs. cold, hostile) and control (i.e., dominant, directive vs. submissive, accommodating). Previous marital research demonstrates the reliability and validity of this measure (e.g., Nealey-Moore et al., 2007; Smith et al., 2009a).
2.4 Task Instructions and Procedures
2.4.1 Day 1: Warm Partner Contact versus No Contact
The procedure for Day 1 is depicted in the top panel of Figure 1. Participants were seated together and provided with consent forms. One partner was randomly selected and moved to a separate room for the first 10-min baseline. They were instructed to sit quietly and relax during the baseline. Participants randomly assigned to the “no contact” condition remained separated from their partner, watching a documentary (i.e., history of the Utah territory). This was followed by a second 10-min baseline. In the positive interaction or “warm contact” condition, both participants were moved together to a single room. They were instructed to spend three minutes “thinking about their feelings of closeness” such as the first time they met, their wedding, or their anniversary. After this silent activity, they then held hands while watching a five-minute emotionally romantic (but not explicitly sexual) popular film clip (i.e., segments from “Sleepless in Seattle” and “You’ve Got Mail”). They then talked for three minutes (90-seconds each) about the feelings of closeness they experienced while watching the movie. They then hugged for 20 seconds. They were then placed in separate rooms for a second 10-min baseline. Participants completed state affect questionnaires immediately after the first baseline, and a second tine after the task period, in which they indicated their feelings during the task period. As depicted in the top panel of Figure 1, a total of three blood draws were completed - immediately after the first baseline, the no contact or warm contact task, and the second baseline.
2.4.2 Day 2: Positive, Neutral, or Negative Marital Interaction Task
The procedures for Day 2 are depicted in the bottom panel of Figure 1. Participants were in separate rooms for a 10-min baseline; SBP, DBP, and HR were recorded while they performed a minimally involving task (Smith et al., 2009b). Baseline values for SBP, DBP, and HR were averages of three values during the final three minutes of baseline. Participants completed a state affect questionnaire immediately after this baseline. They were then brought together and asked to think of events or experiences that fit the description of the positive, neutral, or negative task. Partners alternated talking for 90-second periods to control effects of speech on physiological functioning. For the first period, a randomly selected partner (partner 1) provided a description of the event or situation, followed by a 90-second response by their partner (partner 2) who was instructed to add to the provided description. During the next period, partner 2 described how the event or situation made them feel emotionally, followed by partner 1’s 90-second response to their emotions. Partner 1 then shared how the event affected their relationship. Partner 2 then responded for 90 seconds. SBP, DBP, and HR values were obtained for each 90-sec period and averaged for listening and speaking periods separately. In the positive task couples discussed an event that strengthened their relationship (e.g., birth of a child, purchasing a home). In the neutral interaction task they discussed the last “so-so” movie that they watched that was neither very positive nor very negative. In the negative task, couples discussed an on-going problem in the relationship (e.g., distribution of household chores and duties).
Following the interaction task, participants completed as second affect questionnaire, asking about their feelings during the couple task, and the rating of their partner’s behavior during the task. They were then separated for another 10-min baseline, with SBP, DBP, and HR quantified as described previously, and completed another affect questionnaire. Participants then engaged in a task in which they responded to a videotaped interaction partner. Each participant was instructed to prepare and present a speech in response to the videotaped actor. The situation placed the participant and videotaped partner in a minor auto accident, as passengers in two separate vehicles. The participant and the videotaped partner discussed the fault of the two drivers. Participants were informed that their responses would be videotaped for later evaluation.
In a one-minute preparation period, participants read a description of the scenario and prepared their responses. They then watched a 3-min videotape of the interaction partner presenting their position, in a pointedly provocative manner. Participants were then asked to present their own position and to respond to the videotaped partner’s comments, speaking for a total of three minutes. The participant then viewed another 3-minute video clip of the partner, and then responded with a second 3-min speech of their own. The video-taped interaction partner was highly abrasive in both segments. Two measurements of SBP and DBP were taken during each period of watching versus responding, HR was recorded continuously. Means for watching and responding were calculated separately for all three measures. They completed a final affect questionnaire, about their feelings during this task. As depicted in the bottom Panel of Figure 1, a total of four blood draws were taken on the second day: following the baseline period; the couple interaction task; the second baseline period; and after the anger task.
2.5 Overview of Analyses
Pearson correlations tested associations of marital quality with baseline oxytocin and changes in oxytocin during the positive couple contact condition during Day 1. Effects of warm couple contact during Day 1 on oxytocin levels were tested via 2 (Condition: Positive Contact vs. No Contact) x 2 (Partner: Women, Men) x 3 (Periods: Baseline 1, Task, Baseline 2) mixed ANOVA, treating partners as two levels of a repeated factor to accommodate dependencies within dyads. Similar mixed ANOVAs were conducted on measures of affect, ratings of spouse behavior, oxytocin, catecholamines, and CVR during Day 2. Task responses were analyzed as task – baseline change scores (Llabre et al., 1992). ANOVA results for repeated measures with three or more levels were adjusted (Greenhouse & Geisser, 1959), and significant ANOVA effects were followed with mean comparisons using the appropriate error term (Bernhardsen, 1975). Variations in degrees of freedom reflect missing data, usually due to difficulty with blood draws or cardiovascular measures.
3. Results
3.1 Plasma Oxytocin and Relationship Quality
Mean plasma oxytocin levels are presented in Table 1, as are correlations among the values for Day 1 and Day 2 experimental periods. Because the two initial resting baseline values were significantly correlated, they were averaged to provide a more stable estimate for the correlational analyses of associations with relationship quality, although results do not differ if individual baselines are used. The DAS and MAT scores of men and women were subjected to a principal components analysis, which revealed a single factor (eigenvalue = 2.13), accounting for 53.3% of the variance. A couple composite relationship quality score was calculated by unit weighting of these four measures.
Table 1.
Means (pg/ml), Standard Deviations, and Intercorrelations for Plasma Oxytocin Values
| Mean | SD | Day One Values | Day Two Values | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Mean | 1.92 | 1.87 | 1.91 | 1.92 | 1.97 | 1.79 | 1.69 | ||
| SD | (1.67) | (1.57) | (1.79) | (1.70) | (1.77) | (1.50) | (1.34) | ||
| 1 | 2.02 | (1.56) | .56 | .37 | .48 | .21 | .44 | .28 | |
| 2 | 2.13 | (1.65) | .47 | .42 | .51 | .41 | .65 | .36 | |
| 3 | 1.90 | (1.56) | .45 | .53 | .45 | .53 | .46 | .45 | |
| 4 | 1.93 | (1.48) | .49 | .44 | .39 | .48 | .65 | .41 | |
| 5 | 1.85 | (1.49) | .53 | .42 | .44 | .36 | .44 | .41 | |
| 6 | 1.99 | (1.66) | .29 | .38 | .36 | .25 | .48 | .42 | |
| 7 | 1.84 | (1.75) | .46 | .40 | .42 | .25 | .44 | .38 | |
Values for women above diagonal, men below. Day one values: 1 = resting baseline 1; 2 = post-couple contact (or no contact); 3 = resting baseline 2. Day two values: 4 = resting baseline; 5 = Post-couple interaction task; 6 = resting baseline 2; 7 = post-anger stressor. All r vales are significant at p<.05 (two-tailed). N = 177
Correlations of baseline oxytocin levels with the relationship quality composite were r(169) = −.13, p = .085 (two-tailed) for women, and r(170) = −.075, p = .326 for men. Correlations of the relationship quality composite with change in plasma oxytocin during the Day 1 warm contact condition were r(119) = −.091, p = .36, for women, and r(119) = .026, p= .79, for men. Correlations with the individual relationship quality measures were weaker than those for the composite in all cases. Hence, correlations with relationship quality provided no support for the – “calm and connect” model (i.e., no evidence of a positive association), and weak support for the “tend and befriend” model (i.e., a small, marginally significant association).
3.2 Day 1: Effects of Positive Partner Contact
3.2.1 Manipulation Checks
Means for change in self-reported affect are presented in Table 2. The mixed ANOVA of changes in the “close to my partner” item indicated a significant effect of the manipulation, F(1,174) = 26.54, p <.001, η2 = .132, such that participants in the positive contact condition reported an increase in feelings of closeness (mean = .35, std error = .051) whereas those in the no contact condition reported a non-significant decrease (mean = −.11; std error = .073),. Neither the Sex main effect or Conditions x Sex interaction approached significance, both F(1,174) < 1.0. The manipulation check was also significant for state anger, F(1, 175) = 41.47, p<.001, η2 = .192; participants in the positive contact condition showed a decrease in state anger (mean = −.65; std error = .084), whereas those in the no contact condition reported an increase (mean = .30; std error = .121). Women reported a larger decrease in state anger (mean = −.34; std error = .102) than men (mean = −.01; std error = .105), F(1, 175) = 5.06, p = .026, η2 = .028. Conditions x Sex interaction was not significant, F(1,175) = 1.45, p = .23, η = .008. Finally, the manipulation check was also significant for anxiety, F(1,175) = 4.28, p = .04, η2 = .024; participants in the positive contact condition reported a decrease (mean = −.64; std error = .259), whereas those in the no contact condition reported an nonsignificant increase (mean = .11; std. error = .299). Neither the Sex main effect or conditions x Sex interaction approached significance, both F(1,175) < 1.4, p >.24, both η < .01.
Table 2.
Means (and standard errors) for task – baseline affect change scores for day 1.
| Day One Experimental Condition
|
||||
|---|---|---|---|---|
| Positive Contact (n = 119) | No Contact (n = 58) | |||
|
|
||||
| Self-Reported Affect | Women | Men | Women | Men |
| Close and Connected | .38 (.07) | .33 (.07) | −.12 (.10) | −.09 (.10) |
| State Anger | −.72 (.12) | −.57 (.12) | .05 (.17) | .55 (.17) |
| State Anxiety | −.81 (.29) | −.48 (.30) | −.16 (.42) | .39 (.43) |
3.2.2 Oxytocin Responses
Means for oxytocin during Day 1 are presented in Table 3. Conditions x Sex x Time mixed ANOVAs revealed no effects approaching significance. Hence, although the positive contact task had expected effects on self-reported emotions, plasma oxytocin responses provided no support for the “calm and connect” perspective.
Table 3.
Means (and standard errors) for plasma oxytocin (pg/ml) in women and men during Day One Spouse contact task.
| Women | Baseline 1 | Post-Task | Baseline 2 |
|---|---|---|---|
| Positive Spouse Contact | 1.88 (.159) | 1.84 (.172) | 1.96 (.193) |
| No Contact | 1.95 (.240) | 1.91 (.259) | 1.92 (.291) |
| Men | |||
| Positive Spouse Contact | 1.96 (.156) | 2.15 (.167) | 1.93 (.155) |
| No Contact | 2.30 (.224) | 2.22 (.241) | 1.95 (.224) |
3.3 Day 2: Effects of Positive, Neutral, and Negative Couple Interactions
3.3.1 Manipulation Checks
Mean changes in state affect and ratings of spouse behavior during the task are presented in Table 4. For feelings of closeness, in a main effect for Conditions, F(2,171) = 7.93, p<.001, η2 = .085, participants in the positive condition reported an increase in closeness (mean = .44; std error= .073), whereas those in the neutral (mean = .14; std error = .074) and negative (mean = .05; std error = .071) conditions reported significantly smaller increases. Neither the Sex main effect or the Sex x Condition interaction approached significance, both F < 1.62, both p>.20, both η2 <.02.
Table 4.
Manipulation Checks for Day 2 Couple Interaction task
| Day Two Interaction Valence
|
||||||
|---|---|---|---|---|---|---|
| Positive (n = 57) | Neutral (n = 56) | Negative (n = 59) | ||||
|
|
|
|
||||
| Women | Men | Women | Men | Women | Men | |
| Close and Connected | .31 (.09) | .57 (.11) | .14 (.10) | .14 (.11) | .08 (.09) | .02 (.10) |
| State Anger | −.55 (.22) | −.38 (.22) | −.20 (.22) | −.18 (.22) | .98 (.21) | .98 (.21) |
| State Anxiety | −.12 (.34) | .23 (.39) | .66 (.34) | .66 (.39) | 2.07 (.33) | 2.20 (.38) |
| Spouse Affiliation | 5.14 (.25) | 4.55 (.22) | 4.08 (.25) | 3.59 (.23) | 2.93 (.24) | 2.99 (.22) |
| Spouse Control | 1.33 (.13) | 1.01 (.15) | 1.56 (.14) | 1.67 (.16) | 1.59 (.13) | 1.88 (.15) |
For anger, in a main effect for Conditions, F(2,171) = 18.8, p<.001, η2 = .184, participants reported a significant increase in the negative condition (mean = .98; std error = .176), a non-significant decrease in the neutral condition (mean = −.19; std error = .182), and a significant decrease in the positive condition (mean = −.46; std error = .180). The Sex main effect and Sex x Conditions interaction did not approach significance, both F <0.22, both p>.85, both η2 <.01. For anxiety, in a Conditions main effect, F(2, 171) = 15.67, p <.001, η2 = .156, participants reported a significant increase in the negative condition (mean = 2.14; std error = .269), that was larger than the neutral condition (mean = .66; std error = .276), which was greater than the positive condition (mean = .05; std error = .273). The Sex main effect and Sex x Conditions interaction did not approach significance, both F < 0.34, both p > .55, both η2 < .01.
For ratings of partner affiliation, in a main effect of Conditions, F(2,171) = 22.51, p <.001, η2 = .219, participants rated their partners as displaying more warmth during the positive task (mean = 4.84; std error = .204) than during the neutral task (mean = 3.84; std error = .212), and even less warmth during the negative task (mean = 2.91; std error = .204). Women rated their partners as warmer (mean = 4.05; std error = .143) than did men (mean = 3.67; std error = .129), F(1,171) = 7.91, p <.01, η2 = .047. The Sex x Conditions interaction was not significant, F(2,171) = 1.64, p = .20, η2 = .02.
For ratings of partner control, in a main effect for Conditions, F(2,171) = 8.33, p <.001, η2 = .092, participants rated their partners as displaying lower levels of controlling behavior during the positive task (mean = 1.17; std error = 1.03) than during the neutral task (mean = 1.62; std error = .107); those in the negative condition rated their partners as displaying the highest level of controlling behavior (mean = 1.73; std error = .103), but not significantly more so than in the neutral condition. The Sex main effect was not significant, F(1,171) = 0.07, p = .79, η2 < .01. The Sex x Conditions interaction approached significance, F(2,171) = 2.69, p = .071, η2 = .032. When examined separately, women’s ratings of their partners did not differ, F(2, 173) = 1.48, p = .23, but the Conditions main effect was significant for men’s ratings, F(2,173) = 8.80, p <.001. The Day 2 anger induction task evoked a substantial increase in reported anger over baseline levels, F(1, 165) = 265.8, p < .001, η2 = .617.
3.3.2 Oxytocin
Means for plasma oxytocin during the Day 2 experimental periods are presented in Table 5. The mixed ANOVA revealed no effects approaching significance. Hence, despite the expected effects of the positive and negative interaction manipulation on couples’ emotional experience and perceptions of partners’ behavior, the plasma oxytocin responses provided no support for either the “calm and connect” or “tend and befriend” perspectives. Further, despite the fact that the anger task evoked a large increase in self-reported anger, this stressor did not alter oxytocin levels.
Table 5.
Plasma Oxytocin (pg/ml) Mean Levels (and Standard Errors) during Day Two
| Women | Baseline 1 | Couple Task | Baseline 2 | Anger Task |
|---|---|---|---|---|
| Positive Task | 1.92 (.268) | 2.09 (.263) | 1.77 (.234) | 1.70 (.195) |
| Neutral Task | 1.84 (.278) | 2.03 (.272) | 1.94 (.242) | 1.62 (.202) |
| Negative Task | 2.11 (.251) | 1.74 (.246) | 1.71 (.219) | 1.62 (.183) |
| Men | ||||
| Positive Task | 2.08 (.194) | 1.90 (.214) | 1.92 (.236) | 2.02 (.253) |
| Neutral Task | 1.85 (.198) | 1.80 (.219) | 1.89 (.241) | 1.67 (.258) |
| Negative Task | 1.71 (.183) | 1.82 (.202) | 2.04 (.223) | 1.79 (.238) |
3.3.3 Catecholamines
Mean levels of epinephrine and norepinephrine during the day 2 tasks are presented in Table 6. Mixed ANOVAs revealed only significant effects for time for both epinephrine, F(3,384) = 21.28 p<.001, η2 = .143, and norepinephrine, F(3,384) = 45.64, p<.001, η2 = .254. In both cases, catecholamine levels declined over the first three periods, and then displayed a significant rise from the second resting baseline to the anger task. Thus, although the manipulation of the valence of couple interaction did not evoke a significant catecholamine response, the anger task did.
Table 6.
Means (and standard errors) for plasma Norepinephrine and Epinephrine (pg/ml).
| Epinephrine | Baseline 1 | Couple Task | Baseline 2 | Anger Task |
|---|---|---|---|---|
| Women | 21.5 (1.58) | 17.4 (1.24) | 18.0 (1.39) | 22.3 (1.61) |
| Men | 29.8 (2.02) | 27.9 (1.62) | 24.2 (1.98) | 35.5 (2.40) |
| Norepinephrine | ||||
| Women | 256.7 (11.2) | 259.5 (10.9) | 225.5 (9.9) | 279.1 (12.8) |
| Men | 244.1 (8.6) | 242.5 (8.3) | 208.7 (7.4) | 260.6 (11.3) |
3.3.4 Cardiovascular Reactivity
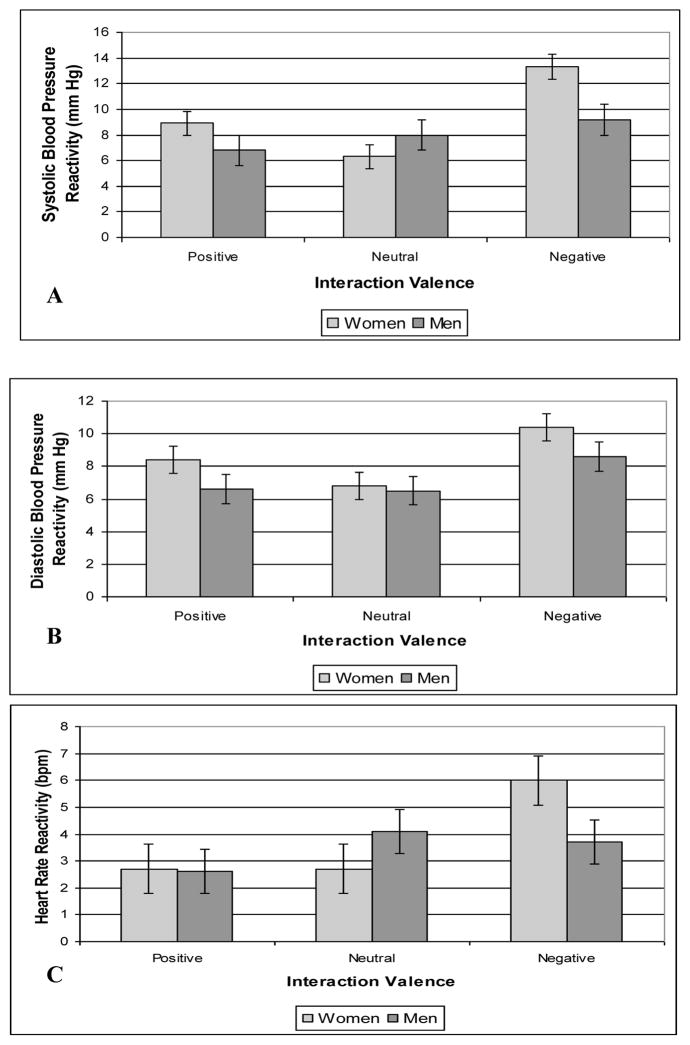
The 3 (Conditions) x 2 (Sex) x 2 (Activities: Listening vs. Speaking) mixed ANOVA of task minus baseline SBP values indicated a main effect for Activity, F(1,166) = 107.35, p <.001, η2 = .393; participants had larger increases in SBP while speaking (mean = 10.43 mm Hg; std error = .521) than listening (mean = 7.05 mmHg; std error = .445). In a significant Conditions main effect, F(2,166) = 7.95, p = .001, η2 = .087, participants in the negative condition displayed greater SBP reactivity (mean = 11.24 mm Hg; std error = .765) than did those in the neutral (mean = 7.13 mm Hg; std error = .799) and positive conditions (mean = 7.86 mm Hg; std error = .806). This difference between the negative versus neutral tasks was significant both when participants spoke (13.10 mm Hg vs. 8.83 mm Hg; std errors = .873 and .912, respectively) and while they listened (9.37 mm Hg vs. 5.43 mm Hg; std error = .746 and .779, respectively). Differences between the negative and positive conditions were similarly significant both while participants spoke (positive condition mean = 9.37 mm Hg; std error = .921) and listened (mean = 6.36 mm Hg; std error = .786).
The Sex main effect in this analysis approached significance, F(1,166) = 2.93, p = .089, η2 = .017; women tended to display greater SBP reactivity (mean = 9.51 mm Hg; std error = .548) than men (mean = 7.98 mm Hg; std error = .717). Means for a significant Sex x Conditions interaction, F(2, 166) = 3.68, p = .027, η2 = .042, are displayed in Figure 2, panel A. Follow-up comparisons indicated that women in negative condition displayed greater SBP reactivity than those in the neutral condition, t(166) = 2.65, p<.02, but men did not. Further, in the negative condition, women displayed greater SBP reactivity than men, t(166) = 2.52, p <.03, but men and women did not differ in the other conditions. Importantly, the difference between women’s SBP reactivity in the negative versus neutral conditions was significant both while they spoke (15.50 mm Hg vs. 8.00 mm Hg; std errors = 1.111 and 1.160, respectively) and listened (11.15 mm Hg vs. 4.59 mm Hg; std errors = .887 and .927, respectively). Further, women displayed greater SBP reactivity in the negative task condition than did men, both while they spoke (15. 50 mm Hg vs. 10.70 mm Hg; std errors = 1.111, 1.281, respectively) and listened (11.15 mm Hg vs. 7.60 mm Hg; std error = .887, 1.224, respectively).
Figure 2.
Means (and SE) for systolic blood pressure (Panel A), diastolic blood pressure (Panel B), and heart rate (Panel C) responses of men and women during the positive, neutral, and negative couple interaction tasks on Day 2.
The mixed ANOVA of task minus baseline changes in DBP revealed the expected effect of Activity, F(1, 166) = 111.50, p <.001, η2 = .402; participants displayed larger increases in DBP when speaking (mean = 9.17 mm Hg; std error = .361) than listening (mean = 6.62 mm Hg; std error = .317). In a significant main effect for Conditions, F(2,166) = 7.24, p = .001, η2 = .080, participants in the negative interaction condition displayed significantly greater DBP reactivity (mean = 9.51 mm Hg; std error = .532) than those in the neutral (mean = 6.66 mm Hg; std error = .556) and positive conditions (mean = 7.52 mm Hg; std error = .561). Importantly, the difference between the negative and neutral conditions was significant while participants spoke (11.20 mm Hg vs. 7.69 mm Hg; std error = .605 and .631, respectively) and listened (7.81 mm Hg vs. 5.63 mm Hg; std errors = .532 and .556, respectively). Means are presented in Figure 2, panel B. The Sex main effect in this analysis approached significance, F(1,166) = 3.03, p = .084, η2 = .018, such that women tended to display greater DBP reactivity (mean = 8.54 mm Hg; std error = .474) than men (mean = 7.25 mm Hg; std error = .504). The Sex x Condition interaction was not significant for DBP, F(2,166) = .47, p = .63, η2 <.01.
The mixed ANOVA of task minus baseline changes in HR indicated the expected effect of Activity, F(1,166) = 222.61, p<.001, η2 = .573; participants displayed larger increases in HR while speaking (mean = 6.33 bpm; std error = .465) than listening (mean = 0.96 bpm; std error = .329). In a Conditions main effect, F(2,166) = 3.35, p = .038, η2 = .038, participants in the negative condition displayed the greatest HR reactivity (mean = 4.87 bpm; std error = .604), those in the neutral condition displayed an intermediate degree (mean = 3.42 bpm; std error = .631), and those in the positive condition displayed the least (mean = 2.64 bpm; std error = .637). The Sex main effect was not significant, F(1,166) = 0.27, p = .61, η2 = .002, but the Sex x Condition interaction approached significance, F(2,166) = 2.54, p = .082, η2 = .030. Means are presented in Figure 2, panel C. In the negative condition, women displayed greater HR reactivity (mean = 6.04 bpm; std error = .888) than did men (mean = 3.69 bpm; std error = .787). Importantly, women displayed greater HR reactivity than men in the negative condition both during speaking (9.09 bpm vs. 6.32 bpm; std errors = 1.135 and .940, respectively) and listening (3.00 bpm vs. 1.06 bpm; std errors = .828 and .781, respectively). Further, women in the negative condition displayed greater HR reactivity than those in the neutral condition (mean = 2.71 bpm; std error = .928), whereas men in the negative condition did not display larger increases than in the neutral condition (mean = 4.13 bpm; std error = .822).
3.4 Responses during the Anger Task and Associations with Oxytocin
As noted above, relative to the preceding baseline, participants displayed a significant increase in state anger and catecholamines during the discussion with the abrasive videotaped interaction partner. Relative to this baseline, they also displayed significant increases over baseline for SBP (10.1 mm Hg), F(1,163) = 344.2, p <.001, η2 = .68; DBP (9.2 mm Hg), F(1,163) = 543.4, p <.001, η2 = .77; and HR (7.7 bpm), F(1,163) = 274.7, p<.001, η2 = .63. Hence, this task is an appropriate context to test the predicted association between higher oxytocin levels and smaller physiological stress responses. Among women, resting oxytocin levels were unrelated to cardiovascular and catecholamine responses to the anger stressor, all r(165) < .10. Among men, higher resting levels of plasma oxytocin were associated with smaller increases in SBP, r(165) = −.19, p = .016; DBP, r(165) = −.16, p = .043; and HR, r(165) = −.14, p = .064, but were unrelated to catecholamine responses, both r(165) <.10. Thus, resting levels of oxytocin were inversely associated with CVR for men, providing some evidence of the predicted stress-dampening effects of oxytocin, but the absence of associations for catecholamines and for CVR among women is inconsistent with the expected pattern.
3.5 Ancillary Analyses
Additional tests of associations of self-reported affect and ratings of partner affiliation and control during the Day 2 interaction tasks with oxytocin changes provided no evidence that variability in the subjective impact of these tasks was related to plasma oxytocin response. Further, the couple relationship quality index was similarly unrelated to oxytocin response to these tasks, across or within the three task conditions.
3.6 Power Analyses
Given the largely null results for the associations of relationship quality with oxytocin levels and with oxytocin responses during the Day 1 positive interaction, and for the effects of the Day 1 and Day 2 experimental manipulations on oxytocin responses, it is important to note the power to detect these effects. For associations of relationship quality with resting oxytocin, the current study was powered at .80 to detect an r of .19 or larger. In contrast, for the median sample sizes in prior studies of resting oxytocin and relationship quality described previously, they were powered at .80 to detect an r of .32 or larger. For the association of relationship quality with change in oxytocin during the Day 1 positive couple interaction task, the current study was powered at .80 to detect an association of .23 or larger. The current study was powered at .80 to detect conditions effects of f = .16 or greater and .15 or greater for the Day 1 and Day 2 tasks, respectively, and conditions x gender interactions of f = .13 and .15 or greater, respectively. In contrast, the studies of the effects of couple interactions described above were on average powered at .80 to detect effects of f= .27 or greater. Hence, the current study was well powered to detect even small effects, and considerably more so than prior studies.
4. Discussion
The present study tested predictions based on the “calm and connect” (Uvnas-Moberg et al., 2005) and “tend and befriend” (Taylor, 2006) models of oxytocin and experiences in close relationships, as well as effects of couple interactions on CVR. Before discussing the findings, it is important to note the effectiveness of our manipulations of the valence of couple interactions. During Day 1, relative to a no-contact control condition, participants in the positive partner contact condition reported increased feelings of closeness with their partner, a reduction in state anger reflecting an increase in the positive antithesis of anger (i.e., feeling “friendly” and “kind and warm-hearted”), and reduced anxiety (i.e., increased feelings of calm and relaxation). During Day 2, relative to a neutral interaction, participants in the positive condition reported increased closeness with their partner, and rated their partner as displaying much more warmth and less control during the interaction. Participants in the negative interaction condition reported substantial increases in anger, and rated their partners as displaying much less warmth (i.e., greater hostility). Also, our measures of relationship quality are well-validated in prior research and demonstrated convergent validity in the present sample. We utilized an established laboratory for oxytocin assays, and oxytocin levels showed significant associations within participants across time. Finally, our sample provided adequate power to detect even small effects, and considerably more so than prior studies.
Despite these methodological strengths, few of the predictions derived from the “calm and connect” and “tend and befriend” models were supported. First, regarding the “calm and connect” view, there was no evidence of a positive association between resting levels of oxytocin and relationship quality, no evidence of an association between relationship quality and release of oxytocin during the positive couple contact task on Day 1, and no evidence that the positive couple contact tasks on Day 1 and Day 2 evoked a momentary increase in oxytocin. Support for the “tend and befriend” model was limited to a small inverse association between relationship quality and baseline plasma oxytocin that approached but did not reach conventional levels of statistical significance. Also inconsistent with the “tend and befriend” model the negative couple interaction task on Day 2 did not evoke a momentary increase in plasma oxytocin. There was, however, some evidence that plasma oxytocin levels were associated with reduced physiological stress responses, at least in terms of CVR among men.
In contrast to these generally null findings regarding plasma oxytocin, effects of the negative couple interaction on Day 2 supported the view that heightened CVR could be a mechanism linking conflict and strain in close relationships with increased cardiovascular risk, as well as the related hypothesis that this mechanism could contribute to sex differences in the health effects of close relationships (Kiecolt-Glaser & Newton, 2001). Relative to the neutral and positive couple interactions on Day 2, the negative interaction evoked larger increases in SBP, DBP, and HR, primarily among women. For both SBP and HR, women displayed greater CVR during the negative interaction than did their male partners. The overall effects of the negative interaction on CVR and the sex differences in these responses occurred while participants spoke and while they listened to their partners. Hence, it is unlikely that the effects of negative interaction on CVR or the sex difference reflect artifacts related to speech. It is important to note that although the anger task on Day 2 evoked a substantial catecholamine response, the negative couple interaction task did not. Hence, there was no evidence that those neuroendocrine responses were altered by the tone of couple interactions, even though prior research has demonstrated associations between the degree of negativity in such conflict interactions and the magnitude of catecholamine response (Robles & Kiecolt-Glaser, 2003).
4.1 Limitations and Qualifications
Given the intrusiveness of the procedures, the laboratory interactions while couples were instrumented for blood draws and CVR assessments clearly differed from positive and negative interactions in close relationships in more naturalistic settings. Generalizations to those contexts must be made cautiously. Also, we found expected evidence of sex differences in CVR during negative couple interactions (Kiecolt-Glaser & Newton, 2001), but in other studies men and women show similarly increased CVR during such interactions (e.g., Nealey-Moore et al., 2007; Smith et al., 2009b) and in some couple contexts men display greater CVR than women (Brown & Smith, 1992; Smith et al., 1998; Smith et al., 2009b). Hence, factors that moderate such sex differences (e.g., length of relationship, age, focus of interaction task) are an important topic for future research.
The negative couple interaction task on Day 2 did not evoke a catecholamine response, even though state affect measures and ratings of partner behavior indicated that this manipulation was effective. It is possible that negative couple interactions that were sufficiently stressful to evoke catecholamine responses might have also produced different effects on cardiovascular and plasma oxytocin responses. In this regard, it is important to note again that the “tend and befriend” model (Taylor, 2006; Taylor et al., 2000, 2010) does not specifically predict that brief episodes of couple conflict as modeled here will evoke a release of oxytocin. Presumably, such conflict interactions can “trigger affiliative needs” (Taylor, 2006, p. 273) at least to some extent and pose at least mild relationship threats (Taylor et al., 2010). However, stronger and/or more prolonged relationship threats and disruptions might be required to evoke the predicted oxytocin response. Yet, if highly pointed or prolonged relationship threats and disruptions are required for adequate tests of the “tend and befriend” model in this regard, it is not clear that this central theoretical tenet is amenable to direct experimental tests within the typical guidelines for the ethical treatment of human research participants. It should also be noted that the Day 1 positive interaction task evoked the expected increase in positive affect and feeling for the partner, but the no contact control evoked an unexpected increase in negative affect. It is possible that the latter effect could have somehow masked the effect of positive couple interaction on plasma oxytocin predicted by the “calm and connect” model.
Also, more frequent measurement of plasma oxytocin might have detected additional effects given its pulsatile release and presumably short half-life (Amico, Ulbrecht, & Robinson, 1987). However, this would have made the procedure still more intrusive for couples. Also, couples participated in the present study mostly during the early evening hours, though some participated in the late afternoon. Evidence regarding diurnal patterns in plasma oxytocin release is limited and inconsistent (Amico, Tenicela, Johnson, & Robinson, 1983; Forsling, Montgomery, Halpin, Windle, & Treacher, 1998). Hence, it is possible that results might have differed at other times of the day. Although the procedures used here were consistent with current best practices, limitations of plasma oxytocin assays that result in limitations in reliability and validity of oxytocin measurement in plasma could also contribute to our null findings and inconsistencies in the literature as a whole (Sezto et al., 2011). Also, our findings are limited to plasma oxytocin. The correspondence between central oxytocin and peripheral blood levels is unclear, and many of the processes described in the “calm and connect” and “tend and befriend” models would reasonably be expected to be more evident in central levels of this neuropeptide (Campbell, 2010). Recent presentations of the “tend and befriend” model suggest that associations with relationship distress should be evident for oxytocin among women, but for vasopressin – rather than oxytocin - among men (Taylor et al., 2010). This refinement of the model is not addressed in the present study, and should be examined in future research.
Although the “tend and befriend” emphasizes threatened or disrupted pair bonds as a specific stimulus evoking a release of oxytocin, other stressors may also evoke this response. Hence, it is possible that other stressors may have produced different results, although they would be a much less precise analogue of the elements of the model we sought to test. Also, in the negative condition during the Day 2 session, participants’ partners might been seen as both the source of relationship stress and a potential target of the type of affiliative behavior activated by such stressors that is described in the “tend and befriend” model. It is possible that use of a different source of relationship stress or the availability of a different target for affiliation might have produced different effects on oxytocin. Although the model clearly predicts that threatened relational bonds in close relationships evoke a release of oxytocin, it does not directly state whether or not the motivated affiliation might be directed to that partner, as in a relationship repair focus of affiliation, or instead is only directed at a non-threatened affiliative relationship. The model also does not state if a combination of relationship threat and immediate availability of a non-threatening target for affiliation is required as a precondition of oxytocin release. It is important to note the procedure did permit a direct experimental test of the central “tend and befriend” hypothesis that stress in a close relationship evokes release of oxytocin. However, results certainly could differ if different opportunities for affiliation were available.
The intrusive nature of our procedures relative to the couple experiences we attempted to model, the possible failure to create positive or negative experiences of a sufficient intensity, infrequent or mistimed measurement of oxytocin and limitations in the related assays, the likely limited correspondence between peripheral oxytocin levels measured here and central levels each, and other specific features of our experimental design and procedure could have contributed to the null results regarding oxytocin. However, the methodological strengths noted previously should also be considered when judging implications of the results for these models. Within these two conceptual perspectives, greater specificity as to the relationship qualities associated with oxytocin levels and the relationship events, processes, and experiences that evoke oxytocin release is an important topic for theoretical refinements and future research. These results also underscore prior concerns that measurement from plasma limits the interpretation of research on close relationships, social behavior and oxytocin (Campbell, 2010).
Experimentally manipulated intranasal administration of oxytocin has important advantages in future research on close relationships (MacDonald & MacDonald, 2010), especially given that it has been found to attenuate cortisol responses during stressful couple interactions and to increase positive couple behavior (Ditzen et al., 2009). However, this approach does not address the association of naturally occurring oxytocin levels with relationship quality, or the effect of positive and negative couple interactions and experiences on oxytocin responses - central tenets of the “calm and connect” and “tend and befriend” models. Research on these issues requires measurement of oxytocin as an observed variable.
4.2 Summary, Conclusions and Future Directions
The present results provided little support for conceptual perspectives on the role of oxytocin in close relationships. Inconsistent with the “calm and connect” model, plasma oxytocin was not positively associated with relationship quality and was not affected by positive couple interactions. Consistent with the “tend and befriend” perspective, plasma oxytocin was inversely related with relationship quality, but this predicted association was quite weak and did not reach conventional significance levels. Further, plasma oxytocin was not altered by a stressful couple interaction that would presumably “trigger affiliative needs” (Taylor, 2006, p. 273) or pose a relationship threat (Taylor et al., 2010), at least to some extent. Among men, higher plasma oxytocin levels were associated with less CVR in response to a subsequent stressor. This is important evidence regarding the stress-dampening effects of oxytocin in humans, given that the very few prior studies of this issue have included smaller and less representative samples (Grewen & Light, 2011).
These results should be interpreted with appropriate caution, given the multiple limitations of the present study as described above, and future research on the “tend and befriend” and “clam and connect” perspectives should address these limitations. Presently, however, in terms of research on human couples, support for key tenets of these models regarding associations of plasma oxytocin levels with relationship quality and effects of couple interactions on changes in plasma oxytocin is perhaps best characterized as inconsistent and often contradictory in a series of small studies and absent in the single largest study of couples to date. In contrast to these null results regarding oxytocin, the findings regarding CVR were generally as predicted. Specifically, negative couple interactions evoked heightened CVR, especially for women. Hence, our results are consistent with previous suggestions that CVR is a potential link between strain in close relationships and cardiovascular risk.
Highlights.
Marital strain increases risk for cardiovascular disease.
Cardiovascular reactivity and oxytocin have been suggested as underlying mechanisms.
Plasma oxytocin was unrelated to relationship quality in 180 young married couples.
Experimental manipulation of marital interaction tone did not later plasma oxytocin.
Negative marital interactions evoked heightened cardiovascular reactivity.
Acknowledgments
This research was supported by NIH grant #RO1 HL68862
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Timothy W. Smith, University of Utah
Bert N. Uchino, University of Utah
Justin MacKenzie, University of Utah.
Angela Hicks, University of Utah.
Rebecca A. Campo, University of Utah
Maija Reblin, University of Utah.
Karen Grewen, University of North Carolina.
Janet A. Amico, University of Pittsburgh
Kathleen C. Light, University of Utah
References
- Amico J, Tenicela R, Johnston J, Robinson A. A time-dependent peak of oxytocin exists in cerebrospinal fluid but not in plasma of humans. Endocrinology and Metabolism. 1983;57:947–951. doi: 10.1210/jcem-57-5-947. [DOI] [PubMed] [Google Scholar]
- Amico J, Ulbrecht J, Robinson A. Clearance studies of oxytocin in humans using radioimmunoassay measurements of the hormone in blood and urine. Journal of Clinical Endocrinology and Metabolism. 1987;64:340–345. doi: 10.1210/jcem-64-2-340. [DOI] [PubMed] [Google Scholar]
- Bernhardsen CS. Type I error rates when multiple comparisons follow a significant F test in AVOVA. Biometrics. 1975;31:229–232. [Google Scholar]
- Brown PC, Smith TW. Social influence, marriage, and the heart: Cardiovascular consequences of interpersonal control in husbands and wives. Health Psychology. 1992;11:88–96. doi: 10.1037//0278-6133.11.2.88. [DOI] [PubMed] [Google Scholar]
- Campbell A. Oxytocin and human social behavior. Personality and Social Psychology Review. 2010;14:281–295. doi: 10.1177/1088868310363594. [DOI] [PubMed] [Google Scholar]
- Carroll D, Phillips AC, Balanos GM. Metabolically exaggerated cardiac reactions to acute psychological stress revisited. Psychophysiology. 2009;46:270–275. doi: 10.1111/j.1469-8986.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Progress in Brain Research. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular reactivity responses to laboratory stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens TL, Frank E, Seltman H, Hou-Ming C, Amico JA. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosomatic Medicine. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vogli R, Chandola T, Marmot MG. Negative aspects of close relationships and heart disease. Archives of Internal Medicine. 2007;167:1951–1957. doi: 10.1001/archinte.167.18.1951. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Neuman ID, Bodenmann von Dawans B, Turner RA, Ehlert U, Heinrichs M. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendorcinology. 2007;32:565–574. doi: 10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrchs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Eaker ED, Sullivan LM, Kelly-Hayes M, D’Agostino R, Benjamin E. Marital status, marital strain, and risk of coronary heart disease or total mortality: The Framingham Offspring Study. Psychosomatic Medicine. 2007;69:509–513. doi: 10.1097/PSY.0b013e3180f62357. [DOI] [PubMed] [Google Scholar]
- Everson SA, Lynch JW, Kaplan GA, Lakka TA, Sivenius J, Salonen JT. Stress-induced blood pressure reactivity and incident stroke in middle-aged men. Stroke. 2001;32:1263–1270. doi: 10.1161/01.str.32.6.1263. [DOI] [PubMed] [Google Scholar]
- Forsling M, Montgomery H, Halpin D, Windle R, Treacher D. Daily patterns of secretion of neurohyophysial hormones in man: effect of age. Experimental Physiology. 1998;83:409–418. doi: 10.1113/expphysiol.1998.sp004124. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving T, Stowell J, Kiecolt-Glaser J. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010;35:1082–1090. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;32:95–112. [Google Scholar]
- Grewen KM, Girdler SS, Amico JA, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine and blood pressure before and after warm partner contact. Psychosomatic Medicine. 2005;76:531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Light KC. Plasma oxytocin is related to lower cardiovascular and sympathetic reactivity to stress. Biological Psychology. 2011;87:340–347. doi: 10.1016/j.biopsycho.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on anbulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosomatic Medicine. 2008;70:976–985. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Medicine. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110:2198–2203. doi: 10.1161/01.CIR.0000143840.77061.E9. [DOI] [PubMed] [Google Scholar]
- Kaplan RM, Kronick RG. Marital status and longevity in the United States population. Journal of Epidemiology and Community Health. 2006;60:760–765. doi: 10.1136/jech.2005.037606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Guastella AJ. The role of oxytocin in human affect: A novel hypothesis. Current Directions in Psychological Science. 2011;20:222–231. [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Nueroscience and Biobehavioral Reviews. 2009 doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser Jk, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kiesler DJ, Schmidt JA, Wagner CC. A circumplex inventory of impact messages: An operational bridge between emotion and interpersonal behavior. In: Plutchik R, Conte HR, editors. Circumplex models of personality and emotions. Washington, DC: American Psychological Association; 1997. pp. 221–224. [Google Scholar]
- King KB, Reis HT. Marriage and long-term survival after coronary artery bypass grafting. Health Psychology. 2012;31:55–62. doi: 10.1037/a0025061. [DOI] [PubMed] [Google Scholar]
- Knox SS, Uvnas-Moberg K. Social isolation and cardiovascular disease: An atherosclerotic pathway. Psychoneuroendocrinology. 1998;23:877–890. doi: 10.1016/s0306-4530(98)00061-4. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA. More frequent hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biological Psychology. 2005;69:5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Llabre M, Spitzer S, Saab P, Ironson G, Schneiderman N. The reliability and specificity of delta versus residualized change as measures of cardiovascular reactivity to behavioral challenges. Psychophysiology. 1991;28:701–711. doi: 10.1111/j.1469-8986.1991.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Locke HJ, Wallace KM. Short marital-adjustment and prediction tests: Their reliability and validity. Marriage and Family Living. 1954;21:251–255. [Google Scholar]
- MacDonald K, MacDonald TM. The peptide that binds: A systematic review of oxytocin and its psychosocial effects in humans. Harvard Review of Psychiatry. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gump BB. Chronic work stress and marital dissolution increase risk of post-trial mortality in men from the Multiple Risk Factor Intervention Trial. Archives of Internal Medicine. 2002;162:309–315. doi: 10.1001/archinte.162.3.309. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Zhu S, Tucker DC, Whooley MA. Blood pressure reactivity to psychological stress and coronary calcification in the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2006;47:391–395. doi: 10.1161/01.HYP.0000200713.44895.38. [DOI] [PubMed] [Google Scholar]
- Nation DA, Szeto A, Mendez AJ, Brooks LG, Zais J, Herderick EE, Gonzales J, Noller CM, Schneiderman N, McCabe PM. Oxytocin attenuates atherosclerosis and adipose tissue inflammation in socially isolated Apoe−/− mice. Psychosomatic Medicine. 2010;72:376–382. doi: 10.1097/PSY.0b013e3181d74c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealey-Moore JB, Smith TW, Uchino BN, Hawkins MW, Olson-Cerny C. Cardiovascular reactivity during positive and negative marital interactions. Journal of Behavioral Medicine. 2007;30:505–519. doi: 10.1007/s10865-007-9124-5. [DOI] [PubMed] [Google Scholar]
- Orth-Gomer K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA. Marital stress worsens prognosis in women with coronary heart disease: The Stockholm female coronary risk study. Journal of the American Medical Association. 2000;284:3008–3014. doi: 10.1001/jama.284.23.3008. [DOI] [PubMed] [Google Scholar]
- Robles TF, Kiecolt-Glaser JK. The physiology of marriage: pathways to health. Physiology and Behavior. 2003;79:409–416. doi: 10.1016/s0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in Neuroendocrinology. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AR, Gerin W, Davidson K, Pickering T, Brosschot J, Thayer J, Christenfeld N, Linden W. Toward a causal model of cardiovascular responses to stress and the development of cardiovascular disease. Psychosomatic Medicine. 2003;65:22–35. doi: 10.1097/01.psy.0000046075.79922.61. [DOI] [PubMed] [Google Scholar]
- Sezto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCollough ME, Schneiderman N, Mendes AJ. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosomatic Medicine. 2011;73:393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatcher RB. Marital functioning and physical health: Implications for social and personality psychology. Social and Personality Psychology Compass. 2010;3:1–15. [Google Scholar]
- Smith TW, Berg CA, Florsheim P, Uchino BN, Pearce G, Hawkins M, Henry NJM, Beveridge RM, Skinner MA, Olsen-Cerny C. Conflict and collaboration in middle-aged and older couples: I: Age differences in agency and communion during marital interaction. Psychology and Aging. 2009a;24:259–273. doi: 10.1037/a0015609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TW, Gallo LC, Goble L, Ngu LQ, Stark KA. Agency, communion, and cardiovascular reactivity during marital interaction. Health Psychology. 1998;17:537–545. doi: 10.1037//0278-6133.17.6.537. [DOI] [PubMed] [Google Scholar]
- Smith TW, Uchino BN, Berg CA, Florsheim P, Pearce G, Hawkins M, Henry NJM, Beveridge RM, Skinner MA, Ko KJ, Olson-Cerny C. Conflict and collaboration in middle-aged and older married couples: II: Age, sex, and task context moderate cardiovascular reactivity during marital interaction. Psychology and Aging. 2009b;24:274–286. doi: 10.1037/a0016067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TW, Uchino BN, Florsheim P, Berg C, Hawkins M, Henry N, Beveridge R, Pearce G, Hopkins P, Yoon H. Affiliation and control during marital disagreement, history of divorce, and asymptomatic coronary artery clarification. Psychosomatic Medicine. 2011;73:350–357. doi: 10.1097/PSY.0b013e31821188ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder DK, Hetman RE, Haynes SN. Evidence-based approaches to assessing couple distress. Psychological Assessment. 2005;17:288–307. doi: 10.1037/1040-3590.17.3.288. [DOI] [PubMed] [Google Scholar]
- Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. Journal of Marriage and Family. 1976;38:15–28. [Google Scholar]
- Tabak BA, McCoullough ME, Szeto A, Mendez AJ, McCabe PM. Oxytocin indexes relational distress following interpersonal harms in women. Psychoneuroendocrinology. 2011;36:115–122. doi: 10.1016/j.psyneuen.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. Tend and befriend: Biobehavioral bases of affiliation under stress. Current Directions in Psychological Science. 2006;15:273–277. [Google Scholar]
- Taylor SE, Cousino Klein L, Lewis BP, Grunewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychobiological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein Cousino, Hu P, Greedale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosomatic Medicine. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science. 2010;21:3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Tobe SW, Kiss A, Sainsbury S, Jesin M, Geerts R, Baker B. The impact of job strain and marital cohesion on ambulatory blood pressure during 1 year: the double exposure study. American Journal of Hypertension. 2007;20:148–153. doi: 10.1016/j.amjhyper.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Tops M, Van Peer JM, Korf J, Wijers AA, Tucker DM. Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology. 2007;44:444–449. doi: 10.1111/j.1469-8986.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Turner RA, Altemus M, Enos T, Cooper B, McGuinness T. Preliminary research on plasma oxytocin in normal cycling women: Investigating emotion and interpersonal distress. Psychiatry. 1999;62:97–113. doi: 10.1080/00332747.1999.11024859. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Arn I, Magnusson D. The psychobiology of emotion: The role of the oxytocinergic system. International Journal of Behavioral Medicine. 2005;12:59–65. doi: 10.1207/s15327558ijbm1202_3. [DOI] [PubMed] [Google Scholar]