Abstract
CD19-specific chimeric antigen receptor (CAR)-modified T cells have antitumor activity in B cell malignancies, but factors that impact toxicity and efficacy have been difficult to define because of differences in lymphodepletion regimens and heterogeneity of CAR-T cells administered to individual patients. We conducted a clinical trial in which CD19 CAR-T cells were manufactured from defined T cell subsets and administered in a 1:1 CD4+:CD8+ ratio of CAR-T cells to 32 adults with relapsed and/or refractory B cell non-Hodgkin lymphoma after cyclophosphamide (Cy)-based lymphodepletion chemotherapy with or without fludarabine (Flu). Patients who received Cy/Flu lymphodepletion had markedly increased CAR-T cell expansion and persistence, and higher response rates (50% CR, 72% ORR, n=20) than patients who received Cy-based lymphodepletion without Flu (8% CR, 50% ORR, n=12). The complete response (CR) rate in patients treated with Cy/Flu at the maximally tolerated dose was 64% (82% ORR, n=11). Cy/Flu minimized the effects of an immune response to the murine scFv component of the CAR, which limited CAR-T cell expansion, persistence, and clinical efficacy in patients who received Cy-based lymphodepletion without Flu. Severe cytokine release syndrome (sCRS) and grade ≥ 3 neurotoxicity were observed in 13% and 28% of all patients, respectively. Serum biomarkers one day after CAR-T cell infusion correlated with subsequent development of sCRS and neurotoxicity. Immunotherapy with CD19 CAR-T cells in a defined CD4+:CD8+ ratio allowed identification of correlative factors for CAR-T cell expansion, persistence, and toxicity, and facilitated optimization of a lymphodepletion regimen that improved disease response and overall and progression-free survival.
INTRODUCTION
Lymphodepletion chemotherapy followed by adoptive transfer of unselected autologous T cells that are genetically modified to express a chimeric antigen receptor (CAR) specific for CD19 (CD19 CAR-T cells) has produced a high rate of complete responses (CR) in refractory B cell acute lymphoblastic leukemia (B-ALL) (1–5); however, results of therapy in refractory non-Hodgkin lymphoma (NHL) have been less impressive (6–8). Human CD4+ and CD8+ T cells are comprised of distinct subsets that differ in their capacities to proliferate, persist in vivo, and mediate antitumor effects after in vitro expansion and adoptive transfer (9–13). In preclinical studies, we demonstrated that human CD19 CAR-T cells that were manufactured from purified CD4+ or CD8+ central memory (TCM) or naïve (TN) T cells were more potent in elimination of CD19+ tumors from immunodeficient mice compared to CD19 CAR-T cells that were manufactured from effector memory (TEM) cells (13). We also observed synergistic enhancement in antitumor activity by administering a defined ratio of CD19 CAR-T cells derived from CD8+ and CD4+ T cell subsets compared to infusion of CAR-T cells derived from either subset alone, or from unselected T cells without regard for subset composition (13). Differences in the T cell subset composition of CAR-T cells prepared from unselected T cells and administered to patients with NHL in previous studies could in part have contributed to differences in efficacy in these studies (6–8,14). Moreover, heterogeneity in the subset composition of infused CAR-T cells has made it challenging in these earlier trials to discern factors that correlate with expansion and persistence of CD19 CAR-T cells, quality and durability of antitumor responses, and the toxicities of CAR-T cell therapy. We hypothesized that selecting defined subsets of T cells for genetic modification and their formulation in a defined CD4+:CD8+ ratio would provide a more uniform CAR-T cell product for clinical applications, result in reproducible in vivo activity, and facilitate identification of factors that correlate with efficacy or toxicity.
Lymphopenia and the impaired proliferative capacity of T cells from patients with B cell malignancies present challenges to CAR-T cell manufacturing. In some clinical trials, the proliferation of autologous T cells in response to a test in vitro stimulation with anti CD3/CD28 beads has been used to predict the success of manufacturing of CD19 CAR-T cells and determine patient eligibility for enrollment (15–17). Using this strategy, 24% of B-ALL patients were excluded from participation in a pediatric clinical trial, and the fraction of NHL patients that would be excluded was even higher, ranging between 75% and 100% depending on the status of the patient’s disease at the time the T cells were collected (15). We developed a strategy in which CD4+ and CD8+ T cell subsets are separately selected from apheresis products, transduced with the CD19 CAR, and then stimulated in vitro with an irradiated CD19+ EBV lymphoblastoid cell line (LCL) to selectively expand transduced T cells capable of proliferating. The CAR-T cell product was then formulated in a defined CD4+:CD8+ CAR-T cell ratio and administered in a dose escalation/de-escalation format after lymphodepletion chemotherapy. We demonstrate that this approach for manufacturing a CAR-T cell product of defined composition was feasible in heavily pre-treated patients with relapsed and refractory NHL, without excluding any patient on the basis of lymphopenia or the results of in vitro proliferation assays. The defined composition approach also allowed identification of correlative factors for CAR-T cell expansion and persistence in vivo, toxicity, and clinical response, and enabled optimization of a lymphodepletion regimen that improved disease response and overall and progression-free survival.
RESULTS
Patient characteristics
Thirty-seven patients with relapsed or refractory CD19+ NHL with a median age of 57 years (range 22 – 70) were enrolled on the study and underwent leukapheresis for CD19 CAR-T cell manufacturing. Three of the 37 patients were in CR at enrollment and were ineligible to receive lymphodepletion chemotherapy and CAR-T cells, and 2 patients had rapidly progressive disease and chose to withdraw from the study to receive hospice care before starting treatment. The thirty-two patients who proceeded to lymphodepletion chemotherapy and CD19 CAR-T cell infusion (Table 1) had previously received a median of 5 treatment regimens (range 2 – 11) for de novo large B cell lymphoma (LBCL, N = 11), large B cell lymphoma that had transformed from indolent disease (TFLBCL, N = 11), mantle cell lymphoma (MCL, n = 4), or follicular lymphoma (FL, n = 6). Sixteen patients had relapsed after autologous (n = 14) or allogeneic (n = 4) hematopoietic stem cell transplantation (HCT). Before lymphodepletion and CAR-T cell therapy, all patients had measurable disease (≥ 2 cm) in lymph nodes or other extramedullary sites. The median tumor bulk estimated by the sum of the cross-sectional areas of 6 index lesions was 2933 mm2 (range 124 – 17907 mm2, n = 30; lesion size could not be calculated for the other two patients). Nine patients had bone marrow disease, and 1 had lymphoma detected by flow cytometry in cerebrospinal fluid (CSF) that was refractory to intrathecal chemotherapy.
Table 1.
Patient characteristics, toxicity, and response
| Lymphodepletion | ||||
|---|---|---|---|---|
| Category | Cy or Cy/E | Cy/Flu | Total | |
| Patients | Evaluable | N=12 | N=20 | N=32 |
| Age (years) | Median (range) | 60 (52–68) | 53 (36–70) | 58 (36–70) |
| Sex | Male | 11 (92%) | 16 (80%) | 27/32 (84%) |
| Female | 1 (8%) | 4 (20%) | 5/32 (16%) | |
| Prior regimens | ≤2 | 4 (36%) | 3 (15%) | 7/32 (22%) |
| 3–4 | 2 (18%) | 5 (25%) | 7/32 (22%) | |
| ≥5 | 6 (50%) | 12 (60%) | 18/32 (56%) | |
| Prior autologous HCT | Yes | 6 (50%) | 8 (40%) | 14/32 (44%) |
| No | 6 (50%) | 12 (60%) | 18/32 (56%) | |
| Prior allogeneic HCT | Yes | 1 (8%) | 3 (15%) | 4/32 (13%) |
| No | 11 (92%) | 17 (85%) | 28/32 (88%) | |
| Histology | De novo aggressive B cell lymphoma | 4 (33%) | 7 (35%) | 11/32 (34%) |
| Transformed large B cell lymphoma | 4 (33%) | 7 (35%) | 11/32 (34%) | |
| Follicular lymphoma | 2 (17%) | 4 (20%) | 6/32 (19%) | |
| Mantle cell lymphoma | 2 (17%) | 2 (10%) | 4/32 (13%) | |
| Chemorefractory | Yes | 11 (92%) | 18 (90%) | 29/32 (91%) |
| No/indeterminate | 1 (8%) | 2 (10%) | 3/32 (9%) | |
| Toxicity | Evaluable | N=12 | N=20 | N=32 |
| sCRS | 2×105 EGFRt+ cells/kg | 0/2 (0%) | 0/3 (0%) | 0/5 (0%) |
| 2×106 EGFRt+ cells/kg | 0/7 (0%) | 1/11 (9%) | 1/18 (6%) | |
| 2×107 EGFRt+ cells/kg | 0/3 (0%) | 3/6 (50%) | 3/9 (33%) | |
| TOTAL | 0 (0%) | 4/20 (20%) | 4/32 (13%) | |
| Grade ≥ 3 neurotoxicity | 2×105 EGFRt+ cells/kg | 0/2 (0%) | 1/3 (33%) | 1/5 (20%) |
| 2×106 EGFRt+ cells/kg | 2/7 (29%) | 2/11 (18%) | 4/18 (22%) | |
| 2×107 EGFRt+ cells/kg | 0/3 (0%) | 4/6 (67%) | 4/9 (44%) | |
| TOTAL | 2/12 (17%) | 7/20 (35%) | 9/32 (28%) | |
| Response | Evaluable | N=12 | N=18 | N=30 |
| CR | De novo aggressive B cell lymphoma | 0/4 (0%) | 2/7 (29%) | 2/11 (18%) |
| Transformed large B cell lymphoma | 1/4 (25%) | 5/6 (83%) | 6/10 (60%) | |
| Follicular lymphoma | 0/2 (0%) | 2/3 (67%) | 2/5 (40%) | |
| Mantle cell lymphoma | 0/2 (0%) | 0/2 (0%) | 0/4 (0%) | |
| | | | | |
| 2×105 EGFRt+ cells/kg | 0/2 (0%) | 1/3 (33%) | 1/5 (20%) | |
| 2×106 EGFRt+ cells/kg | 1/7 (14%) | 7/11 (64%) | 8/18 (44%) | |
| 2×107 EGFRt+ cells/kg | 0/3 (0%) | 1/4 (25%) | 1/7 (14%) | |
| TOTAL | 1/12 (8%) | 9/18 (50%) | 10/30 (33%) | |
| ORR | De novo aggressive B cell lymphoma | 1/4 (25%) | 6/7 (86%) | 7/11 (64%) |
| Transformed large B cell lymphoma | 2/4 (50%) | 5/6 (83%) | 7/10 (70%) | |
| Follicular lymphoma | 2/2 (100%) | 2/3 (67%) | 4/5 (80%) | |
| Mantle cell lymphoma | 1/2 (50%) | 0/2 (0%) | 1/4 (25%) | |
| | | | | |
| 2×105 EGFRt+ cells/kg | 2/2 (100%) | 1/3 (33%) | 3/5 (60%) | |
| 2×106 EGFRt+ cells/kg | 3/7 (43%) | 9/11 (82%) | 12/18 (67%) | |
| 2×107 EGFRt+ cells/kg | 1/3 (33%) | 3/4 (75%) | 4/7 (57%) | |
| TOTAL | 6/12 (50%) | 13/18 (72%) | 19/30 (63%) | |
Heterogeneity of T cell subsets in NHL patients
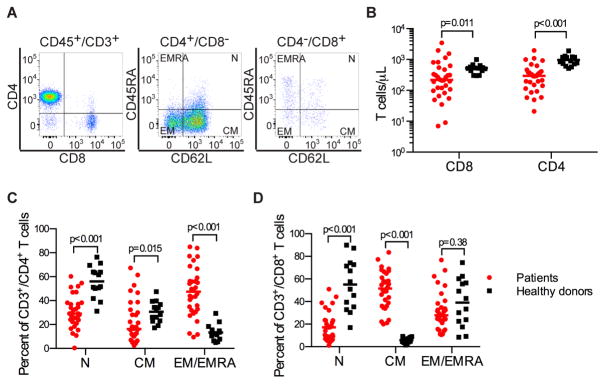
An objective of our study was to determine the feasibility of manufacturing a consistent CD19 CAR-T cell product from NHL patients by selecting defined CD8+ and CD4+ T cell subsets from leukapheresis products, transducing each subset separately, and formulating a CAR-T cell product comprised of a 1:1 ratio of CD8+ and CD4+ T cells (Figure S1). Before leukapheresis, we evaluated the proportions of CD4+ and CD8+ naïve and memory T cell subsets in the blood of each patient (Figure 1A–D). NHL patients had lower absolute numbers of CD4+ and CD8+ T cells compared to a cohort of healthy individuals (Figure 1B) and a highly variable ratio of CD4+:CD8+ T cells (median 1.47; range 0·1 – 13·7; Table S1). At screening before leukapheresis, the absolute CD4+ and CD8+ T cell counts in the blood were < 250/μL in 13 of 32 patients (41%) and 17 of 32 patients (53%), respectively. The fractions of naïve and memory subsets in the CD4+ and CD8+ compartments differed between NHL patients and healthy donors (Figure 1C, D), and there was wide variation in the percentages of each subset in individual patients. These data suggest that, particularly in NHL patients who have higher fractions of terminally differentiated TEM/EMRA and/or a very high or low ratio of CD4+:CD8+ T cells in blood, the ability to manufacture a CD19 CAR-T cell product of consistent potency may be enhanced by selection of defined T cell subsets from the leukapheresis product for CAR-modification and formulation in a defined CD4+:CD8+ CAR-T cell ratio.
Fig. 1.
Heterogeneity in distribution of TN, TCM, and TEM/EMRA cells within CD4+ and CD8+ T cell subsets in normal donors and patients with NHL. A) Representative FACS plots showing the proportion of TN (CD45RA+/CD62L+), TCM (CD45RA−/CD62L+), and TEM/EMRA (CD62L−) subsets in the CD3+/CD4+ and CD3+/CD8+ T cell populations in blood of an NHL patient. B) Absolute CD4+ and CD8+ T cell counts in blood from healthy individuals (n = 10) and NHL patients (n = 30). C) The percentages of TN, TCM, and TEM/EMRA cells in the CD3+/CD4+ T cell population. D) The percentages of TN, TCM and TEM/EMRA cells in the CD3+/CD8+ T cell population. Comparisons of continuous variables between two categories were made using the Wilcoxon rank-sum test.
CD19 CAR-T cell manufacturing
Each patient underwent a leukapheresis procedure without any serious adverse events. CD4+ and CD8+ T cells were enriched by CliniMACS selection from separate aliquots of each patient’s leukapheresis product for subsequent CAR-T cell manufacturing. Using immunomagnetic cell selection, highly enriched CD4+ T cells were isolated from leukapheresis products for CAR manufacturing from all patients. We used a two-step immunomagnetic selection procedure to enrich CD8+ TCM cells for CAR-T cell manufacturing from patients with a CD8+ TCM cell count ≥ 20/μL in blood on a screening assay (n=20; CD8+ TCM cells were isolated from 1 additional patient with 13 CD8+ TCM cells/μL). From the remaining patients with CD8+ TCM counts < 20/μL, severe lymphopenia, or high circulating lymphoma burden, we positively selected bulk CD8+ T cells (n = 10) or omitted the CD62L selection after initial depletion of CD4+, CD14+, and CD45RA+ cells (n = 1) to obtain an enriched CD8+ T cell population for transduction with the CAR.
The selected CD4+ and CD8+ T cells were transduced with a lentiviral vector that encoded the CD19 CAR and a truncated cell surface human epidermal growth factor receptor (EGFRt), which enabled identification of transduced cells by flow cytometry using the anti-EGFR monoclonal antibody, cetuximab. Transduced EGFRt+ CD4+ and CD8+ T cells were stimulated once with an irradiated allogeneic CD19+ LCL line during culture (n = 31), resulting in enrichment of the percentage of EGFRt+ cells within CD3+/CD4+ T cells from 34.3 +/− 2.6% (mean +/− standard error) to 78.8 +/− 2.1% and within CD3+/CD8+ T cells from 36.3 +/− 3.1% to 81.3 +/− 2.2% at the time of product release. LCL stimulation also dramatically increased the number of CAR-T cells at the time of harvest for product formulation and release. The median fold expansions of CD3+/CD4+ and CD3+/CD8+ T cells between anti-CD3/anti-CD28 bead stimulation and LCL stimulation were 10.4 (range 2.5 – 35.9, n = 27) and 7.5 (range 1.1 – 59.5, n = 28). Between LCL stimulation and CAR-T cell product release, the median fold expansions of CD3+/CD4+/EGFRt+ and CD3+/CD8+/EGFRt+ CAR-T cells were 230.5 (range 107.7 – 698.6, n = 28) and 198.9 (range 29.3 – 458.6, n = 29). This cell manufacturing strategy allowed a CAR-T cell product to be prepared for all patients; however, 2 of the 32 patients did not receive their target CAR-T cell dose. These 2 patients were assigned to the top dose level of 2×107 CAR-T cells/kg and received CAR-T cell doses of 8.8 × 106/kg and 7.0 × 106/kg. With the exception of 1 additional patient who received 38.8% and 100% of the assigned CD4+ and CD8+ CAR-T cell target doses, respectively, all other patients received a cell product with a CD4:CD8 CAR-T cell ratio of 1:1 in the dose specified for the cohort to which they were assigned. The infused CAR-T cells were predominantly CD45RA−/CD62L+ and CD45RA−/CD62L− (Figure S2).
Decreased anti-transgene product immune responses and enhanced CAR-T cell expansion in patients who received Cy/Flu lymphodepletion
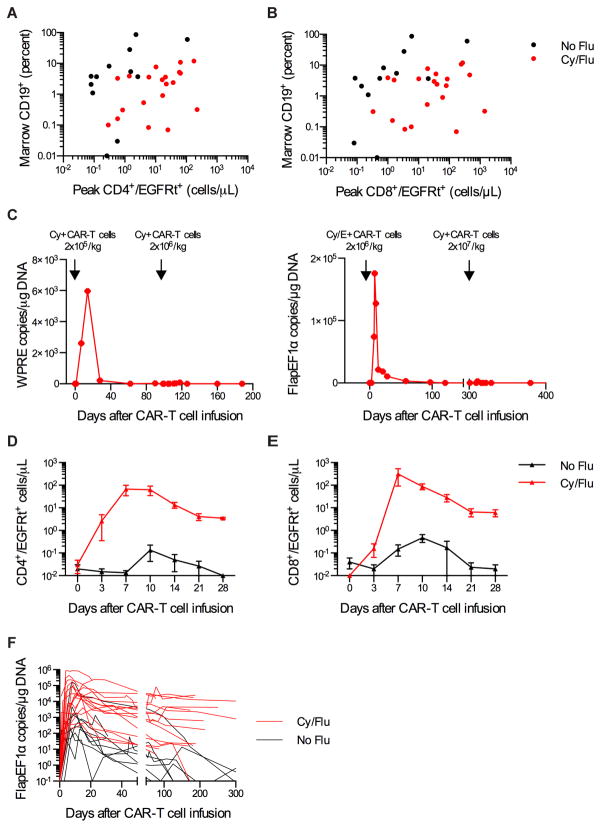
The study evaluated 3 CAR-T cell dose levels administered 36–96 hours after lymphodepleting chemotherapy. Twelve patients received chemotherapy with cyclophosphamide (Cy) or cyclophosphamide and etoposide (Cy/E) followed by infusion of CAR-T cells with an overall response rate (ORR) of 50% (one CR, five partial response, PR; Table 1). In these patients, the peak numbers of CD4+ and CD8+ EGFRt+ CAR-T cells in the blood after adoptive transfer correlated with the fraction of bone marrow CD19+ cells before lymphodepleting chemotherapy (CD4+/EGFRt+, Spearman correlation (ρ) =0·52, p=0·082; CD8+/EGFRt+, ρ=0·69, p=0·014) (Figure 2A, B). B cell aplasia, defined as <0.01% CD19+ B cells of total white cells in blood, developed after CAR-T cell infusion in 11 of these 12 patients; however, we observed a loss of CAR-T cells and recovery of B cells in blood within 100 days in 9 of 10 patients who consented to long-term monitoring. Eight of these patients developed progressive disease, suggesting that longer CAR-T cell persistence may be critical for durable antitumor efficacy. Five of the patients in this group with persistent or progressive disease received a second CAR-T cell infusion at the same dose (n=1) or a 10-fold higher CAR-T cell dose (n=4). In all 5 patients there was no observable CAR-T cell expansion or persistence immediately after infusion (Figure 2C), or measurable antitumor activity. In 5 of 5 patients, cytotoxic T cell responses that were specific for autologous T cells expressing the CAR transgene were detected in the blood after CAR-T cell therapy, and in 4 patients the peptide epitopes that were recognized were mapped to sequences in the murine scFv (Figure S3, Table S2).
Fig. 2.
Increased CD19 CAR-T cell expansion and persistence after Cy/Flu lymphodepletion. AB) Peak CD4+/EGFRt+ (A) and CD8+/EGFRt+ (B) CAR-T cell numbers after the first CAR-T cell infusion in relation to the percentage of CD19+ cells (normal and malignant CD19+ B cells) in the bone marrow before lymphodepletion chemotherapy for each patient. C) CAR-T cell persistence in blood as integrated transgene copies/μg DNA in 2 patients who received a cycle of Cy or Cy/E lymphodepletion chemotherapy and CAR-T cell infusion followed by a second cycle of Cy lymphodepletion chemotherapy and CAR-T cell infusion. Integrated transgene copies were determined by QPCR for distinct sequences located in the WPRE or Flap/EF1α regions of the lentivirus. D–E) Mean+/−SEM CD4+/EGFRt+ (D) and CD8+/EGFRt+ (E) CAR-T cell counts in blood on the indicated days after CAR-T cell infusion for patients treated with 2×107 EGFRt+ cells/kg after either Cy or Cy/E lymphodepletion (No Flu) or Cy/Flu lymphodepletion (Cy/Flu). F) CAR-T cell persistence in blood of patients who received Cy or Cy/E lymphodepletion (No Flu, black, n=9) compared to Cy/Flu lymphodepletion (red, n=18) is shown as FlapEF1α integrated transgene copies/μg DNA. CAR-T cell persistence data are truncated at the time of HCT for patients who underwent autologous or allogeneic HCT after CAR-T cell infusion.
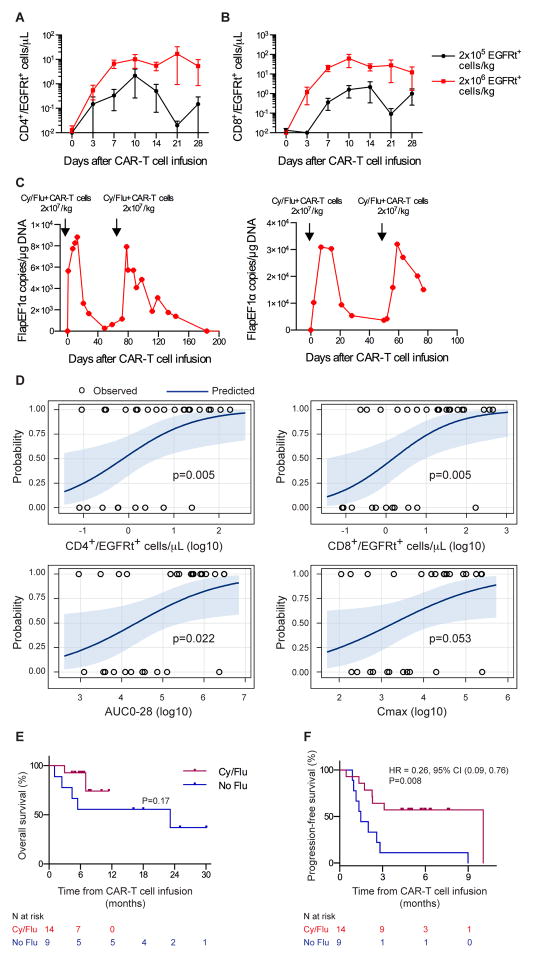
We then administered Cy and fludarabine (Flu) for lymphodepletion before CAR-T cell infusion in 20 subsequent patients to determine if intensified immunosuppression would enhance CAR-T cell expansion and persistence, and prevent or delay immune responses to the CAR. Compared to the patients without Flu in the lymphodepletion regimen, patients who received Cy/Flu had higher serum concentrations of IL-7 (p=0·014) and IL-15 (p<0·001) on the day of CAR-T cell infusion, markedly greater expansion of CD4+ and CD8+ CAR-T cells in the blood in the first 10 days after infusion, and higher numbers of CAR-T cells in blood 1 and 3 months later (Figure 2A, B, D–F). With the exception of patients who underwent autologous or allogeneic HCT after CAR-T cell infusion, in whom CAR-T cells were not detectable after HCT, 16 of the 18 patients who received Cy/Flu lymphodepletion and survived more than 28 days after CAR-T cell infusion had detectable CAR-T cells in blood by Q-PCR (>10 copies/μg DNA) at the last follow-up (range 34 – 349 days) (Figure 2F). We identified a relationship between CAR-T cell dose and in vivo expansion in Cy/Flu-lymphodepleted patients (Figure 3A, B) that was not apparent in those who received Cy or Cy/E. Furthermore, unlike patients who initially received Cy or Cy/E lymphodepletion, 3 of 4 patients who received Cy/Flu before their first CART cell infusion and had a second infusion to treat persistent disease had evidence of further tumor regression after the second infusion (Figure S4); 3 patients exhibited CAR-T cell expansion after the second infusion without developing a detectable T cell immune response to the transgene product (Figure 3C). Together, these data demonstrate that Cy/Flu lymphodepletion enhances early proliferation and persistence of both CD4+ and CD8+ CAR-T cells with kinetics that are affected by the dose of CAR-T cells infused, and enables repetitive dosing of CAR-T cells.
Fig. 3.
Improved clinical responses to CD19 CAR-T cell immunotherapy after Cy/Flu lymphodepletion. A–B) Mean +/− SEM CD4+/EGFRt+ (A) and CD8+/EGFRt+ (B) CAR-T cell counts in blood on the indicated days after CAR-T cell infusion for patients treated with Cy/Flu lymphodepletion and either 2×105 or 2×106 EGFRt+ cells/kg. C) CAR-T cell persistence in blood as integrated transgene copies/μg DNA in 2 patients who received a cycle of Cy/Flu lymphodepletion chemotherapy and CAR-T cell infusion followed by a second cycle of Cy/Flu lymphodepletion chemotherapy and CAR-T cell infusion. D) Probability curves showing the likelihood of response (CR/PR) according to the peak CD4+/EGFRt+ and CD8+/EGFRt+ cell counts in blood, the AUC0-28, and Cmax. P-values were reported from the Wilcoxon two-sample test. E–F) OS and PFS for patients who received Cy/Flu compared to Cy or Cy/E (No Flu) lymphodepletion followed by infusion of CD19 CAR-T cells at ≤ 2×106 EGFRt+ cells/kg. The median OS follow-up times for No Flu and Cy/Flu are 25 months and 6.3 months, respectively. The median PFS follow-up for Cy/Flu is 5.8 months. The median PFS for No Flu is 1.5 months. The numbers of patients at risk at each time point are indicated. Log-rank tests were used to compare between-group differences in survival curves. HR, hazard ratio. CI, confidence interval.
Improved therapeutic efficacy of CAR-T cells in patients who received Cy/Flu lymphodepletion
In the entire cohort of 32 patients, higher peak expansion and longer duration of CAR-T cell persistence in blood were associated with a greater probability of tumor regression (Figure 3D). Consistent with the increase in CAR-T cell expansion and persistence observed with Cy/Flu lymphodepletion, we found that addition of Flu in the lymphodepletion regimen was associated with improvement in the depth of response (Table 1; ordinal logistic regression, CR vs PR vs NR, p=0·02). The importance of CAR-T cell persistence for ongoing tumor eradication was highlighted by the observation that in 6 patients, the reduction in tumor burden at the initial restaging performed 1 month after CAR-T cell infusion was not the maximal response, and further tumor regression was observed on subsequent restaging studies (Figure S5). Cy/Flu lymphodepletion followed by infusion of 2×106 CAR-T cells/kg was selected as the preferred regimen for NHL patients because of the lower toxicity observed after the 2×106/kg cell dose compared to the 2×107/kg cell dose, and antitumor activity in multiple histologies (Table 1, Table S3). Although follow-up is short, patients who received CAR-T cells at ≤2×106/kg after Cy/Flu lymphodepletion had better overall survival (OS, p=0·17) and progression-free survival (PFS, p=0·008) compared to those who received Cy or Cy/E lymphodepletion (Figure 3E, F). Only one of the 9 patients who achieved CR after Cy/Flu lymphodepletion and infusion of CART cells has relapsed (follow-up 2.3 – 11.2 months).
Toxicity of CD19 CAR-T cells
Patients were evaluated for immediate and delayed toxicity of lymphodepleting chemotherapy and CAR-T cell infusion. Serious acute toxicity in the first 2 hours after CAR-T cell infusion was not observed at any CAR-T cell dose. A majority of patients developed the toxicities expected with cytotoxic chemotherapy, including bone marrow suppression, alopecia, mild mucositis, and neutropenic fever. Depletion of normal CD19+ B cells, consistent with the in vivo presence of functional CD19 CAR-T cells, was noted in 29 of 30 patients who survived until day 28.
Cytokine release syndrome (CRS) and neurotoxicity are serious toxicities have been observed in a subset of patients with B cell malignancies who are treated with CD19 CAR-T cells, and the factors that predict the patients at greatest risk for these toxicities have been difficult to define (2,4–8). We evaluated whether administering a defined composition CAR-T cell product might facilitate identifying patients who were more likely to develop toxicity. Overall, 20 of 32 patients developed increased concentrations of serum cytokines, fever, and/or hypotension consistent with CRS. Severe CRS (sCRS) requiring management in the intensive care unit (ICU) and treatment with tocilizumab (n=3) and/or corticosteroids (n=4) developed in only 4 (12.5%) patients, all of whom had received Cy/Flu conditioning (Table 1, Table S3). Severe neurotoxicity (NCI CTCAE v4.03 grade ≥ 3) was observed in 9 of 32 patients (28%), and was also more frequently observed in patients who received Cy/Flu lymphodepletion. Neurotoxicity presented as reversible encephalopathy alone (n=5), or with tremor (n=1) or speech disturbance (n=1). Choreoathetosis and fatal intracranial hemorrhage were observed in 1 patient each. T cell dose was related to the development of severe CRS and neurotoxicity, with 3 of 6 patients (50%) treated at 2×107 CAR-T cells/kg after Cy/Flu lymphodepletion developing sCRS and 4 of 6 patients (67%) developing grade ≥ 3 neurotoxicity (Table 1). Two patients who were treated with 2×107 CAR-T cells/kg died: 1 patient developed a pontine hemorrhage and 1 patient had a fatal gastrointestinal (GI) hemorrhage associated with a known gastrointestinal invasive lymphomatous mass. Thus, the CAR-T cell dose of 2×107/kg after Cy/Flu lymphodepletion was considered excessively toxic for NHL patients, and subsequent patients were treated at a lower dose level.
Biomarkers and the risk of cytokine release syndrome and neurotoxicity
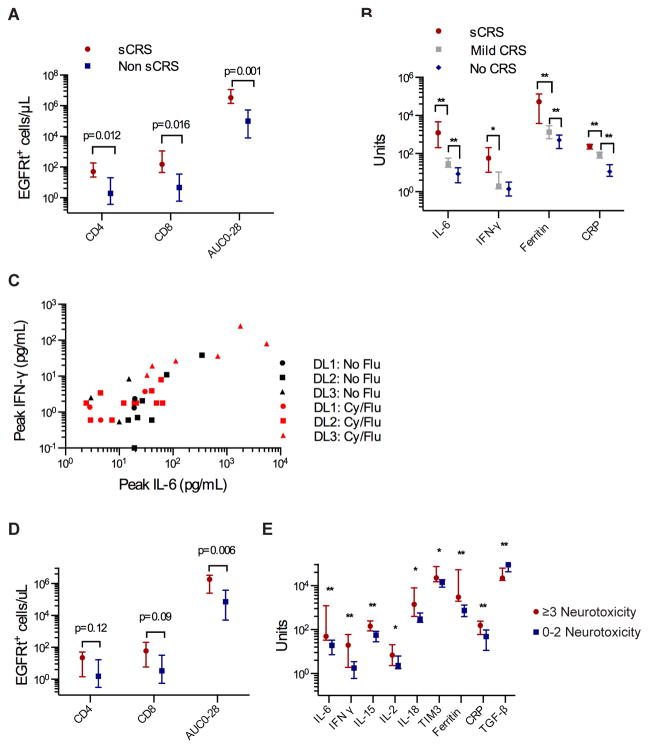
CRS is initiated by activation and proliferation of CAR-T cells after recognition of CD19+ target cells (4). The peak number of CD4+/EGFRt+ and CD8+/EGFRt+ CAR-T cells in blood, and the area under the curve of CAR-T cell numbers (transgene copies/μg DNA) between day 0 and 28 (AUC0-28) were associated with the occurrence of sCRS (Figure 4A). Peak serum concentrations of IL-6, IFN-γ, ferritin, and C-reactive protein (CRP) after CAR-T cell infusion correlated with the occurrence and severity of sCRS (Figure 4B). The highest IL-6 and IFN-γ concentrations were observed in patients who received Cy/Flu lymphodepletion followed by CAR-T cells infused at 2×107/kg (Figure 4C), consistent with the higher incidence of toxicity observed in these patients (Table 1). Patients who developed grade ≥ 3 neurotoxicity had higher AUC0-28, more peak CD4+/EGFRt+ and peak CD8+/EGFRt+ cells in blood, higher peak serum IL-6, IFN-γ, IL-15, IL-2, IL-18, TIM3, ferritin, and CRP concentrations, and lower serum TGF-β compared to those without neurotoxicity (Figure 4D–E). In multivariate analyses, peak numbers of CD4+/EGFRt+ and CD8+/EGFRt+ cells, serum ferritin, and IL-6 had the strongest associations with severe neurotoxicity (Table S4).
Fig. 4.
Factors correlating with toxicity after CD19 CAR-T cell therapy. A) Peak counts (median and interquartile range) of CD4+/EGFRt+ and CD8+/EGFRt+ cells in blood after CAR-T cell infusion and the AUC0-28 in patients with sCRS (requiring ICU) and without sCRS. B) Peak concentrations (median and interquartile range) of serum IL-6, IFN-γ, ferritin, and CRP after CAR-T cell infusion in patients with sCRS, with mild CRS (signs and symptoms of CRS, but not requiring ICU admission), and without CRS. Units shown on the y-axis are as follows: IL-6, pg/mL; IFN-γ, pg/mL; ferritin, ng/mL; CRP, mg/L. **p≤0·01, *p<0·05, Wilcoxon two sample test. P values are shown in Table S6. C) Peak concentrations of serum IL-6 and IFN-γ after CAR-T cell infusion in patients treated at dose level (DL) 1, DL2, or DL3. D) Peak counts (median and interquartile range) of CD4+/EGFRt+ and CD8+/EGFRt+ cells in blood after CAR-T cell infusion and the AUC0-28 in patients with grade ≥ 3 neurotoxicity (NT) or with grade 0–2 NT. E) Peak concentrations (median and interquartile range) of serum IL-6, IFN-γ, IL-15, IL-2, IL-18, TIM-3, ferritin, CRP, and TGF-β after CAR-T cell infusion in patients with grade ≥ 3 neurotoxicity and with grade 0–2 neurotoxicity. Units shown on the y-axis are as follows: cytokines (IL-6, IFN-γ, IL-15, IL-2, IL-18, TIM3, TGF-β), pg/mL; ferritin, ng/mL; CRP, mg/L. **p≤0·01, *p<0·05, Wilcoxon two sample test. P values are shown in Table S6.
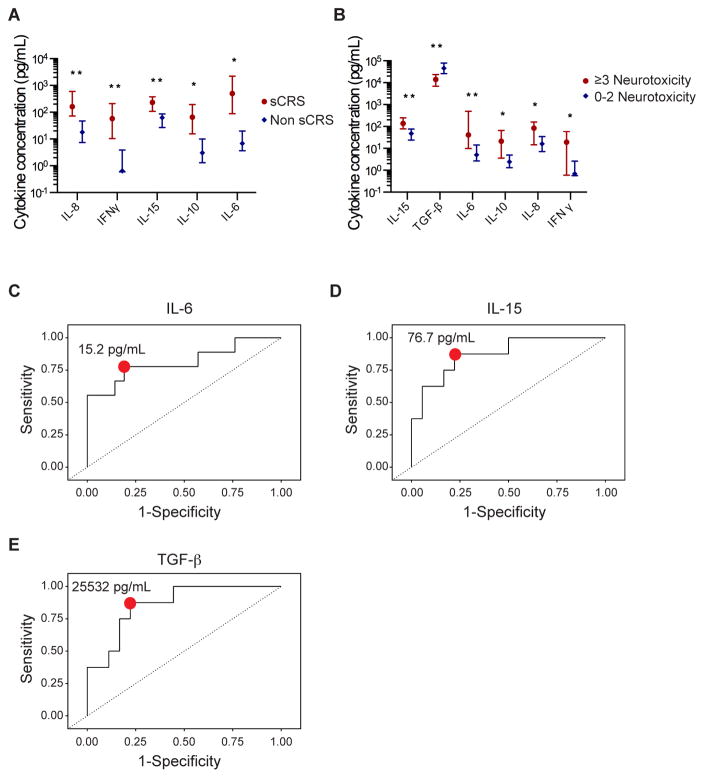
We evaluated serum biomarkers on the first day after CAR-T cell infusion to determine if patients at highest risk for subsequent severe toxicity might be identifiable for early intervention. We found higher IL-6, IFN-γ, IL-15, IL-8, and IL-10 concentrations on day 1 after CAR-T cell infusion in patients who subsequently developed sCRS (Figure 5A). Higher IL-6, IFN-γ, IL-15, IL-8, and IL-10 and lower TGF-β concentrations were found in patients who subsequently developed severe neurotoxicity compared to those who did not (Figure 5B), with the strongest associations in multivariate analyses identified in patients with higher serum IL-6 and IL-15 concentrations (Table S4). Furthermore, ROC analyses identified serum concentrations of IL-6 >15·2 pg/mL, IL-15 >76·7 pg/mL, and TGF-β <25532 pg/mL on day 1 as optimal discriminators of the risk of severe neurotoxicity (Figure 5C–E). Simultaneous or sequential use of more than 1 of these cytokine assays resulted in a net sensitivity of 97–100% and a net specificity of 95–99%, respectively (Table S5) (18). These data provide an opportunity to study the use of serum cytokine concentrations on the first day after CAR-T cell infusion to identify patients at the highest risk of subsequent toxicity who might benefit from early intervention.
Fig. 5.
Prediction of subsequent toxicity using serum biomarkers collected within 24 hours of CAR-T cell infusion. A) Concentrations (median and interquartile range) of serum IL-8, IFN-γ, IL-15, IL-10, and IL-6 on day 1 after CAR-T cell infusion in patients who did or did not subsequently develop sCRS. **p≤0·01, *p<0·05, Wilcoxon two sample test. P values are shown in Table S6. B) Concentrations (median and interquartile range) of serum IL-15, TGF-β, IL-6, IL-10, IL-8, and IFN-γ on day 1 after CAR-T cell infusion in patients who developed grade ≥ 3 neurotoxicity or grade 0–2 neurotoxicity. **p≤0·01, *p<0·05, Wilcoxon two sample test. P values are shown in Table S6. C–E) ROC curves demonstrating the interaction between serum IL-6, IL-15, and TGF-β concentrations on day 1 after CAR-T cell infusion and the occurrence of grade ≥ 3 neurotoxicity. The red point indicates the cut-points for each assay at which the following sensitivities and specificities were obtained (IL-6, 15.2 pg/mL, sensitivity 78%, specificity 81%; IL-15, 76.7 pg/mL, sensitivity 88%, specificity 78%; TGF-β, 25532 pg/mL, sensitivity 88%, specificity 78%). Data are summarized in Table S5.
DISCUSSION
CD19 CAR-T cell therapy represents an important therapeutic advance for patients with relapsed/refractory B-cell NHL; however, the optimal T cell dose and lymphodepletion regimens to achieve durable efficacy and the prediction and management of toxicities remain to be elucidated. Systematically studying these issues has been challenging because of small numbers of patients and the differences in the phenotypic composition of CAR-T cell products administered to individual patients in previous trials (2,4–7). Our data show that formulating a CAR-T cell product with a uniform CD4+:CD8+ CAR-T cell ratio for NHL patients is feasible by selecting and transducing individual T cell subsets, and if administered at the maximal tolerated dose after Cy/Flu conditioning, this approach results in marked tumor regression in a majority of patients with a low incidence of serious toxicity. The strategy of selecting CD4+ and CD8+ T cell subsets before transduction was applied to all patients on an intent to treat basis, and no patient was excluded based on the absolute lymphocyte count, tumor burden, or a pre-enrollment test expansion. This strategy was successful in obtaining a CAR-T cell product for 100% of enrolled patients, and 91% of the patients received the prescribed cell dose and formulation of CAR-T cells. This high success rate in preparing CAR-T cells in this heavily pretreated group of patients may reflect removal of inhibitory cell subsets from the leukapheresis product by selecting CD4+ and CD8+ T cells and/or the selective expansion of CAR-transduced T cells capable of proliferating by the brief in vitro expansion with CD19+ EBV-LCL before cell infusion. Additional improvements in the cell manufacturing process might be achieved using cytokines such as IL-7, IL-21, and/or IL-15 that have been reported to improve the growth of human T cells in vitro and enhance the therapeutic efficacy of adoptively transferred murine T cells in preclinical models (15,19,20). We did not observe differences in CAR-T cell expansion and persistence or clinical outcomes between patients who received CAR-T cells manufactured from CD4+ T cells formulated with either CD8+ TCM-derived CAR-T cells or bulk CD8+ T cell-derived CAR-T cells. Additional studies will be required to define the optimal T cell subset(s) and formulation for CD19 CAR-T cell therapy.
An important predictor of the antitumor efficacy of CD19 CAR-T cells in NHL is their ability to expand and persist in vivo after adoptive transfer. Our data show that CAR-T cell expansion was lower in the subset of patients who received Cy or Cy/E lymphodepletion, and persistence was shorter in these patients due to development of an anti-CAR T cell immune response. Because all CD19 CARs used in published clinical trials incorporate a murine scFv, our data provides one potential mechanism for the loss of CAR-T cells observed in other trials (2–7,21–23). Intensification of lymphodepletion by addition of Flu to Cy in our study increased the peak of expansion, the AUC0-28, and long-term persistence of infused CAR-T cells, and improved the CR rate, OS, and PFS. The specific mechanisms by which Flu increased CAR-T cell accumulation and persistence are likely multifactorial, and our data indicate that they could include increased homeostatic cytokine availability or reduction in the anti-CAR immune response. An alternative possibility is that the addition of Flu to Cy lymphodepletion might improve CAR-T cell proliferation by reducing expression of indoleamine deoxygenase in the tumor microenvironment (24). In a subset of patients, we observed an improvement in clinical response between 1 and 3 months after initial restaging. Although the timing of the peak of CAR-T cell expansion in blood did not differ between patients whose best tumor response was at the first restaging or later, it is conceivable that Cy/Flu lymphodepletion may enable prolonged persistence of functional CAR-T cells in the tumor and ongoing anti-tumor activity. It is unknown whether intensification of lymphodepletion using other chemotherapy regimens or radiation would be similarly effective in augmenting CAR-T cell proliferation and persistence.
We observed that treatment with the same lymphodepletion and CD19 CAR-T cell immunotherapy regimen resulted in lower CR rates in NHL and CLL patients compared to BALL patients (1,25). The reasons for the differences are yet to be determined, but may include differences in CAR-T cell access to tumor antigen and/or immune suppression in the tumor microenvironment in each disease. A recent report suggested that T cells isolated from patients with B-ALL, NHL and CLL differ in their capacity to proliferate in vitro to anti-CD3/anti-CD28 stimulation; however, it is not known if the reduced proliferative capacity of T cells isolated from NHL and CLL patients translates to a difference in the in vivo efficacy of infused CAR-T cell products from NHL and CLL patients compared to B-ALL patients (15). The manufacturing strategy that was used in our trial was highly successful in generating a cell product from NHL patients, even those with reduced CD4+ and CD8+ T cell numbers.
CD19 CAR-T cell therapy has been associated with toxicity in published studies, and understanding factors that predict which patients are at highest risk for toxicity is an area of intense interest (2,4–6). In our study, the use of CAR-T cells of defined composition and abrogation of the anti-CAR immune response by Cy/Flu lymphodepletion revealed a correlation between cell dose and in vivo CAR-T cell expansion, which should facilitate more predictable cell dosing and better definition of the therapeutic index. Severe toxicity due to CAR-T cells in patients treated with Cy/Flu lymphodepletion was predominantly seen at the 2×107/kg CAR-T cell dose. A reduction in the cell dose to 2×106/kg reduced toxicity and achieved CR and ORR rates of 64% and 82%, respectively, which compares favorably to the 45% CR rate observed in a recent report (6). Additional studies will be required to determine whether the higher toxicity at a given CAR-T cell dose seen in patients who received Cy/Flu lymphodepletion is due to greater lymphodepletion and in vivo CAR-T cell expansion, or whether Flu directly increases toxicity by mechanisms that are not dependent on CAR-T cell expansion. The peak CAR-T cell count in blood between day 0 – 28 after CAR-T cell infusion (Cmax) and AUC0-28 that were associated with CR were also associated with neurotoxicity and sCRS, suggesting that further reducing the CAR-T cell dose to minimize toxicity might also result in loss of efficacy. Despite delivery of a consistent CAR-T cell product at a defined optimal dose(s), severe toxicity may still occur in a minority of patients due to patient-specific biologic variation, such as may result from polymorphisms in cytokine and cytokine receptor genes. In the serum of NHL patients collected 1 day after CAR-T cell infusion, high IL-6, IL-8, IL-10, IL-15, and IFN-γ concentrations were associated with the subsequent occurrence of sCRS and neurotoxicity, and low TGF-β concentration was associated with neurotoxicity. Serum IL-6 and IFN-γ concentrations were also associated with subsequent toxicity in a cohort of B-ALL patients who received the same lymphodepletion regimens and CAR-T cell product in this study (1). Because IL-8, IL-10, IL-15, and TGF-β concentrations were not assessed in our B-ALL cohort, it remains unknown if these cytokines predict toxicity in B-ALL as well as NHL patients. Our identification of biomarkers on day 1 after CAR-T cell infusion that were associated with the subsequent occurrence of severe toxicity provides an opportunity to test in a future clinical trial whether early intervention strategies to suppress cytokine release or block cytokine receptors in high-risk patients may mitigate severe toxicity.
This study reports a large series of NHL patients treated at a single institution with CD19 CAR-T cells and demonstrates the feasibility of selecting T cell subsets for CAR engineering and formulation of defined therapeutic products from NHL patients for adoptive therapy. The results show that delivery of a consistent CAR-T cell product is feasible in an overwhelming majority of heavily pretreated patients, provides a high OR and CR rate, and allows identification of factors that are important for optimizing CAR-T cell proliferation and persistence and for reducing toxicity.
MATERIALS AND METHODS
Study design
We performed a phase 1 open label trial to evaluate the feasibility and safety of infusing a defined 1:1 ratio of CD4+:CD8+ CD19-specific CAR-T cells in patients with relapsed or refractory CD19+ B cell malignancies. This manuscript reports the data from NHL patients in the study. Because of differences in disease characteristics and the response to CAR-T cells, CLL and B-ALL patients are treated in distinct cohorts and reported separately (1,25). The study is available at https://clinicaltrials.gov/ct2/show/NCT01865617, and was conducted with approval of the Fred Hutchinson Cancer Research Center (FHCRC) institutional review board. Informed consent was obtained from all patients after a discussion of the possible risks and adverse effects of the therapy. In contrast to other studies, we did not exclude any patient based on the absolute lymphocyte count or a prior assessment of T cell function (15).
T cell subset selection and CD19 CAR-T cell manufacturing
PBMCs were collected from each patient by leukapheresis, and the product was divided into 2 aliquots for enrichment of CD4+ and CD8+ T cell subsets on a CliniMACS device for CART cell manufacturing (Figure S1) (1,26). Our preclinical studies suggested that infusion of CD8+ central memory (TCM) CAR-T cells combined with CD4+ CAR-T cells might provide optimal anti-tumor efficacy (13); therefore, CD8+ TCM cells were isolated from patients with an absolute CD8+ TCM cell count ≥ 20/μL using a two-step sequential depletion of CD4+/CD14+/CD45RA+ cells followed by selection of CD62L+ cells. For patients with an absolute CD8+ TCM cell count of <20/μL, either the CD62L+ selection was omitted or bulk CD8+ T cells were selected. Enriched CD4+ and CD8+ T cell subsets were separately stimulated with anti-CD3/anti-CD28 CTS paramagnetic beads (Dynabeads) and transduced with a lentivirus encoding a CAR comprising an FMC63-derived CD19-specific scFv fused to a modified IgG4-hinge spacer, a CD28 transmembrane domain, a 4-1BB costimulatory domain, and a CD3ζ signaling domain (13). A cell surface human EGFRt was also encoded in the lentiviral vector separated from the CAR cassette by a ribosomal skip sequence to allow precise enumeration of transduced CD4+ and CD8+ CAR-T cells by flow cytometry (27,28). After removal of CD3/CD28 beads, the CD4+ and CD8+ T cells were separately stimulated with a clinically qualified irradiated allogeneic CD19+ LCL line and cultured in medium (RPMI 1640, 10% pooled human serum, L-glutamine 3.5 mM, β-mercaptoethanol 44 μM) supplemented with IL-2 50 U/mL. CAR-T cell manufacturing was completed within approximately 15 days after initial bead stimulation. Quality assessments were performed separately on the cultured CD4+ and CD8+ CAR-T cells, and the two fractions were formulated in a 1:1 ratio of CD3+/CD4+/EGFRt+:CD3+/CD8+/EGFRt+ cells for infusion.
Lymphodepletion chemotherapy and CAR-T cell infusion
To deplete endogenous lymphocytes before adoptive transfer of CAR-T cells, patients received 1 of 4 chemotherapy regimens: cyclophosphamide (Cy) 2 – 4 g/m2 IV on day 1; Cy 2 – 4 g/m2 IV on day 1 and etoposide 100 – 200 mg/m2/day IV on days 1 – 3 (Cy/E); and Cy 60 mg/kg IV on day 1 and fludarabine 25 mg/m2/day IV on either days 2 – 4 or days 2 – 6 (Cy/Flu). Between 36 and 96 hours after completion of chemotherapy, CAR-T cells were infused (i.v.) at or as close as possible to 1 of 3 cell dose levels (2×105 EGFRt+ cells/kg; 2×106 EGFRt+ cells/kg; 2×107 EGFRt+ cells/kg) (Table 1).
Clinical response assessment
Patients underwent whole body imaging with diagnostic quality CT and concurrent PET, before and approximately 4 weeks after CAR-T cell therapy. Best responses to lymphodepletion chemotherapy and a single CAR-T cell infusion are reported according to the Lugano criteria (29). Marrow biopsies were obtained before lymphodepletion and approximately 4 weeks after CAR-T cell infusion from patients with marrow disease on initial staging. Toxicity was graded using the NCI Common Toxicity Criteria for Adverse Events v4.03, with the exception of CRS, which was defined as sCRS when ICU management was required.
CAR-T cell enumeration
Blood samples were obtained from patients before and at intervals after CAR-T cell infusion, and flow cytometry was performed to identify CD4+ and CD8+ CAR-T cells as viable CD45+/CD3+/CD4+/EGFRt+ or CD45+/CD3+/CD8+/EGFRt+ events, respectively. The absolute CAR-T cell count was determined by multiplying the percentage of CAR-T cells identified by flow cytometry in a lymphocyte FS-SS gate by the absolute lymphocyte count established by a complete blood count performed on the same day. The area under the curve of CAR-T cell numbers (transgene copies/μg DNA) between day 0 and 28 (AUC0-28) was calculated using a trapezoidal rule computational algorithm (30). Loss of CAR-T cell persistence was defined as <10 copies/μg DNA evaluated by QPCR to detect integrated transgene sequence.
Cytokine assay
Serum concentrations of IFN-γ, IL-6, IL-8, IL-10, IL-15, and TGF-β were evaluated by Luminex assay, according to the manufacturer’s instructions.
Anti-CAR immune response
We evaluated CD8+ T cell immune responses to the CAR transgene using a modification of an assay we previously described (31). Cryopreserved PBMCs collected from patients before lymphodepletion chemotherapy and approximately 4 weeks after CAR-T cell infusion were stimulated twice at 7-day intervals with autologous irradiated CAR-T cells and IL-2. The pre-infusion and post-infusion PBMC cultures were assayed for lysis of autologous CAR-T cells and autologous non-transduced T cells in a chromium release assay. An immune response against the CAR transgene was defined as the presence of specific lysis of autologous CAR-T cells by post-infusion PBMC cultures, but not of autologous non-transduced T cells or autologous CAR-T cells by pre-infusion PBMC cultures. A T cell line that exhibited specific lysis of autologous CART cells was stimulated with pools of overlapping peptides from the CAR construct, and peptide pools that induced IFN-γ secretion higher than that induced by T cells alone in an EliSPOT assay were identified. Predictions of MHC class I binding by peptides shared between the candidate pools were made using the Immune Epitope Database (IEDB) and analysis resource Consensus tool (32), which combines predictions from NetMHC (3.4) (33,34), SMM (35), and Comblib (36).
Statistical analysis
Comparisons of continuous variables between two categories were made using the Wilcoxon rank-sum test. Relationships between continuous variables were analyzed using a Spearman correlation (ρ). Univariate (with Firth correction) and stepwise multivariate logistic regression were performed to assess predictors for the occurrence of severe neurotoxicity, where log10 values were used to transform data as appropriate (for cytokine concentrations, CD4+/EGFRt+, and CD8+/EGFRt+ cell counts), with 0·01 substituting for values of 0. All p-values reported were two-sided, and no adjustments were made for multiple comparisons. Receiver operating characteristic (ROC) curves were constructed using the empirical method, and the optimal cut point was established based on the Youden index (37). For time-to-event analyses, the Kaplan-Meier method was used to estimate survival distributions, and the reverse Kaplan-Meier method was used to estimate median follow-up time (38); log-rank tests were used to compare between-group differences in survival curves.
Supplementary Material
Fig. S1: Plan of CD19 CAR-T cell manufacturing
Fig. S2: CAR-T cell product characterization
Fig. S3: Anti-CAR transgene product immune response
Fig. S4: Tumor regression after a second CAR-T cell infusion in patients who received Cy/Flu lymphodepletion
Fig. S5: Delayed normalization of imaging after CD19 CAR-T cell infusion
Table S1: Variable CD4+:CD8+ T cell ratio in blood of NHL patients
Table S2: Summary of anti-CAR transgene product immune responses
Table S3: Cytokine release syndrome and neurotoxicity
Table S4: Multivariate analyses
Table S5: Simultaneous and sequential testing of cytokines to predict toxicity
Table S6: P values for Figures 4B, 4E, 5A, 5B
Acknowledgments
The authors acknowledge the FHCRC Cell Processing Facility and Seattle Cancer Care Alliance (SCCA) Cell Therapy Laboratory, and the staff of the Program in Immunology and SCCA Immunotherapy Clinic.
FUNDING
Funding for this study was provided by: NCI R01 CA136551; NIDDK P30 DK56465; NCI P30 CA15704; Life Science Discovery Fund; Bezos Family Foundation; Juno Therapeutics. CT is a Damon Runyon Clinical Investigator.
Footnotes
AUTHOR CONTRIBUTIONS
CT and DM designed the trial and experiments, analyzed data and wrote the paper. SR designed the trial and experiments, and wrote the paper. LH, CB, MH, BP, ER, and RH performed experiments and analyzed data. LH, SC, LS, XC, BW, and SH designed experiments and analyzed data. CC collected research data. DL analyzed statistical data.
COMPETING INTERESTS
CT and CB receive research funding from Juno Therapeutics and hold patents. DM receives research funding from Juno Therapeutics. SR receives research funding from and is a co-founder of Juno Therapeutics, and holds patents. MH holds a patent. DL is an employee of and has equity in Juno Therapeutics. FHCRC receives research funding from Juno Therapeutics.
REFERENCES AND NOTES
- 1.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, Soma L, Chen X, Yeung C, Wood B, Li D, Cao J, Heimfeld S, Jensen MC, Riddell SR, Maloney DG. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DC, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, Wasielewska T, He Q, Bernal Y, Rijo IV, Hedvat C, Kobos R, Curran K, Steinherz P, Jurcic J, Rosenblat T, Maslak P, Frattini M, Sadelain M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, Raffeld M, Feldman S, Lu L, Li YF, Ngo LT, Goy A, Feldman T, Spaner DE, Wang ML, Chen CC, Kranick SM, Nath A, Nathan DA, Morton KE, Toomey MA, Rosenberg SA. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, Hakim FT, Halverson DC, Fowler DH, Hardy NM, Mato AR, Hickstein DD, Gea-Banacloche JC, Pavletic SZ, Sportes C, Maric I, Feldman SA, Hansen BG, Wilder JS, Blacklock-Schuver B, Jena B, Bishop MR, Gress RE, Rosenberg SA. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, Gea-Banacloche JC, Pavletic SZ, Hickstein DD, Lu TL, Feldman SA, Iwamoto AT, Kurlander R, Maric I, Goy A, Hansen BG, Wilder JS, Blacklock-Schuver B, Hakim FT, Rosenberg SA, Gress RE, Kochenderfer JN. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J Clin Oncol. 2016;34:1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stemberger C, Neuenhahn M, Gebhardt FE, Schiemann M, Buchholz VR, Busch DH. Stem cell-like plasticity of naïve and distinct memory CD8+ T cell subsets. Seminars in Immunology. 2009;21:62–68. doi: 10.1016/j.smim.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;117:1888–1898. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, Riddell SR. Chimeric antigen receptor-modified T cells derived from defined CD8(+) and CD4(+) subsets confer superior antitumor reactivity in vivo. Leukemia. 2015;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, Liu H, Grilley B, Rooney CM, Heslop HE, Brenner MK, Dotti G. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh N, Perazzelli J, Grupp SA, Barrett DM. Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci Transl Med. 2016;8:320ra3. doi: 10.1126/scitranslmed.aad5222. [DOI] [PubMed] [Google Scholar]
- 16.Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, Lacey SF, Melenhorst JJ, McGettigan SE, Cook DR, Zhang C, Xu J, Do P, Hulitt J, Kudchodkar SB, Cogdill AP, Gill S, Porter DL, Woyach JA, Long M, Johnson AJ, Maddocks K, Muthusamy N, Levine BL, June CH, Byrd JC, Maus MV. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127:1117–1127. doi: 10.1182/blood-2015-11-679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, Ambrose D, Grupp SA, Chew A, Zheng Z, Milone MC, Levine BL, Melenhorst JJ, June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordis L. Epidemiology. 4. Philadelphia: Saunders; 2008. [Google Scholar]
- 19.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced Leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan DA, Morgan RA, Laurencot C, Rosenberg SA. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ninomiya S, Narala N, Huye L, Yagyu S, Savoldo B, Dotti G, Heslop HE, Brenner MK, Rooney CM, Ramos CA. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood. 2015;125:3905–3916. doi: 10.1182/blood-2015-01-621474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turtle CJ, Hanafi L-A, Berger C, Gooley T, Chaney C, Cherian S, Soma L, Chen X, Yeung C, Loeb K, Wood B, Hudecek M, Sommermeyer D, Li D, Hay K, Heimfeld S, Riddell SR, Maloney DM. Rate of durable complete response in ALL, NHL, and CLL after immunotherapy with optimized lymphodepletion and defined composition CD19 CAR-T cells. J Clin Oncol. 2016;34 Abstract 102. [Google Scholar]
- 26.Terakura S, Yamamoto TN, Gardner RA, Turtle CJ, Jensen MC, Riddell SR. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119:72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, Jensen MC, Riddell SR. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3:125–135. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Chang WC, Wong CW, Colcher D, Sherman M, Ostberg JR, Forman SJ, Riddell SR, Jensen MC. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA A. L. A. L. G. Alliance, C. O. G. Eastern, M. C. L. C. European, L. F. Italian, O. F. R. European, O. C. H.-O. G. Treatment, E. D. M. Ó. Grupo, H.-G. L. S. G. German, H. S. G. German, L. S. G. Japanese, S. A. Lymphoma, C. T. G. NCIC, L. S. G. Nordic, O. G. Southwest, K. N. C. R. I. United. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh ST. Using trapezoidal rule for the area under a curve calculation. Proceedings of the Twenty-Seventh Annual SAS User Group International (SUGI) Conference. Paper 229–27; 2002. [Google Scholar]
- 31.Berger C, Flowers ME, Warren EH, Riddell SR. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107:2294–2302. doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim Y, Ponomarenko J, Zhu Z, Tamang D, Wang P, Greenbaum J, Lundegaard C, Sette A, Lund O, Bourne PE, Nielsen M, Peters B. Immune epitope database analysis resource. Nucleic Acids Res. 2012;40:W525–30. doi: 10.1093/nar/gks438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundegaard C, Lamberth K, Harndahl M, Buus S, Lund O, Nielsen M. NetMHC-3.0: accurate web accessible predictions of human, mouse and monkey MHC class I affinities for peptides of length 8–11. Nucleic Acids Res. 2008;36:W509–12. doi: 10.1093/nar/gkn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen M, Lundegaard C, Worning P, Lauemøller SL, Lamberth K, Buus S, Brunak S, Lund O. Reliable prediction of T-cell epitopes using neural networks with novel sequence representations. Protein Sci. 2003;12:1007–1017. doi: 10.1110/ps.0239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters B, Sette A. Generating quantitative models describing the sequence specificity of biological processes with the stabilized matrix method. BMC Bioinformatics. 2005;6:132. doi: 10.1186/1471-2105-6-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sidney J, Assarsson E, Moore C, Ngo S, Pinilla C, Sette A, Peters B. Quantitative peptide binding motifs for 19 human and mouse MHC class I molecules derived using positional scanning combinatorial peptide libraries. Immunome Res. 2008;4:2. doi: 10.1186/1745-7580-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.YOUDEN WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 38.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 39.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1: Plan of CD19 CAR-T cell manufacturing
Fig. S2: CAR-T cell product characterization
Fig. S3: Anti-CAR transgene product immune response
Fig. S4: Tumor regression after a second CAR-T cell infusion in patients who received Cy/Flu lymphodepletion
Fig. S5: Delayed normalization of imaging after CD19 CAR-T cell infusion
Table S1: Variable CD4+:CD8+ T cell ratio in blood of NHL patients
Table S2: Summary of anti-CAR transgene product immune responses
Table S3: Cytokine release syndrome and neurotoxicity
Table S4: Multivariate analyses
Table S5: Simultaneous and sequential testing of cytokines to predict toxicity
Table S6: P values for Figures 4B, 4E, 5A, 5B