Abstract
Background
Psychological stress has long been recognized as a contributing factor to asthma symptom expression and disease progression. Yet, the neural mechanisms that underlie this relationship have been largely unexplored in research addressing the pathophysiology and management of asthma. Studies that have examined the mechanisms of this relationship in the periphery suggest that it is the superimposition of acute stress on top of chronic stress that is of greatest concern for airway inflammation.
Methods
We compared asthmatic individuals with high and low levels of chronic life stress in their neural and peripheral physiological responses to the Trier Social Stress Test and a matched control task. We used FDG-PET to measure neural activity during performance of the two tasks. We used both circulating and airway-specific markers of asthma-related inflammation to assess the impact of acute stress in these two groups.
Results
Asthmatics under chronic stress had a larger HPA-axis response to an acute stressor, which failed to show the suppressive effects on inflammatory markers observed in those with low chronic stress. Moreover, our PET data suggest that greater activity in the anterior insula during acute stress may reflect regulation of the effect of stress on inflammation. In contrast, greater activity in the mid-insula and perigenual anterior cingulate seems to reflect greater reactivity and was associated with greater airway inflammation, a more robust alpha amylase response, and a greater stress-induced increase in proinflammatory cytokine mRNA expression in airway cells.
Conclusions
Acute stress is associated with increases in markers of airway inflammation in asthmatics under chronic stress. This relationship may be mediated by interactions between the insula and anterior cingulate cortex, that determine the salience of environmental cues, as well as descending regulatory influence of inflammatory pathways in the periphery.
Introduction
Asthma is a disease characterized by chronic inflammation of the airways and acute episodes of airway constriction. The initiation, persistence, and overall pattern of airway inflammation in asthma are complex and vary from individual to individual. These characteristics are influenced by many factors, including allergic sensitization, infections, and psychological stress. Although considerable insight has been gained into the mechanisms by which allergic sensitization and respiratory infections influence airway inflammation, the neural mechanisms through which psychological stress contributes to airway inflammation in humans are largely unknown.
Experimental designs that consider the temporal dynamics of stress in asthma suggest that chronic stress, as opposed to short episodic stress, is of primary concern. In rodent models, several studies have shown that while an acute stressful episode causes a decrease in inflammatory markers in the lungs of sensitized animals, chronic stress leads to an increase in these markers (e.g. Forsythe et al., 2004; Okuyama et al., 2007). Further, when the effects of the stress hormone corticosterone are blocked, the decrease in inflammatory markers following acute stress is abolished, whereas the increase observed following chronic stress is unchanged (Forsythe et al., 2004). This phenomenon has been observed in humans as well. Marin et al. (2009) studied children with asthma over a two-year period and found that mononuclear cells from children who reported chronic life stress coupled with an acute stressful event produced elevated levels of asthma-promoting cytokines compared to asthmatic children without chronic stress or healthy controls. Likewise, Liu et al. (2002) demonstrated that undergraduate asthmatic subjects had greater airway inflammation and a larger decrement in lung function in response to allergen challenge during final examination week—a period of prolonged stress—compared to an identical challenge during a relatively stress-free period. Moreover, in the absence of allergen challenge, these participants had elevated levels of circulating eosinophils during the final exam period, suggesting a priming of the asthmatic response.
The fact that psychological factors can influence asthma symptoms underscores the critical role of the brain, since it is only through the brain that such influences can be transduced and exert downstream changes on peripheral biological systems important in the expression of asthma. Yet, research directed toward understanding the pathophysiology of asthma has mostly overlooked the role of the brain (Bonsignore et al., 2015; Carr and Kraft, 2015). Therefore, the goal of this experiment was to determine the role of the brain, and regions involved in emotion processing in particular, in modulating asthma-related inflammation in response to an acute stressor, in individuals with high and low levels of chronic life stress. Because our hypotheses specifically address efferent effects of the bi-directional brain-immune pathways, our design employs an acute stressor in the absence of immune provocation.
Based on our prior research, our primary hypotheses concerning the constituents of the neural circuitry involved in this interaction were focused on the insula and anterior cingulate cortex (ACC). We have shown these regions to be differentially activated by illness-specific cognitive information, in an inflammatory condition relative to a period where no inflammation was present. Moreover, the degree of activation in these regions predicted the magnitude of subsequent airway inflammation and lung function decline (Rosenkranz et al., 2012, 2005). These findings are consistent with those reported by a separate group in a sample of healthy individuals (Harrison et al., 2009a, 2009b). In this study, the insula and ACC were significantly more activated during an inflammatory state, induced by typhoid vaccination, than during placebo vaccination and activity in these regions, in the inflammatory condition only, predicted inflammation-induced fatigue and mood deterioration.
Chronic stress is a common prelude to mood and anxiety disorders (Caspi et al., 2003; McEwen, 2004) and the incidence of these conditions is elevated in individuals with asthma (Kuehn, 2008). Further, asthma severity and control are often worse in individuals with depression and anxiety (Strine et al., 2008). Thus, our hypotheses concerning peripheral inflammatory pathways involved in this interaction were focused on the expression of genes in the IL-1β/IL-17 pathway. Cytokines in this pathway have been implicated, not only in asthma pathogenesis and corticosteroid insensitivity (Esnault et al., 2012; Vazquez-Tello et al., 2013), but also in depressive- and anxiety-like behavior (Beurel et al., 2013; Ying Chen et al., 2011; Rossi et al., 2012). An additional focus, to address clinical relevance, included fraction of exhaled nitric oxide (FeNO; parts per billion). FeNO has been shown to be a useful marker of airway inflammation in asthma, in particular because its rise frequently precedes that of lung function decline and thus is valuable in preventative care (Hoffmeyer et al., 2009).
Materials and Methods
Participants
Our participants included 30 individuals with mild allergic asthma (average age 26.23 ± 6.04 years, 12 female) and this group was comprised of 15 individuals with high chronic stress and 15 with low chronic stress, as determined by the UCLA Life Stress Interview (Hammen, 1991). Participants were recruited within Madison, WI and the surrounding community using an established database of asthmatic individuals, flyers, and online advertisements. All participants had a physician’s diagnosis of asthma for at least 6 months prior to study entry and a positive skin test to at least one common aeroallergen. All participants were otherwise medically healthy, as determined by physical examination, and were free of respiratory infection for the previous 4 weeks. Participants were excluded if they used medication for anxiety or depression, or required asthma medication beyond an inhaled β-agonist on an as-needed basis. Participants were also excluded if they were a current smoker, a former smoker with a history exceeding 5 pack years, pregnant, breastfeeding, or had any history of bipolar or schizophrenic disorders, brain damage or seizures. UW-Madison’s Health Sciences Institutional Review Board approved the protocol, and all participants provided informed consent and were given monetary compensation for their participation.
Chronic Stress
Determination of high and low chronic stress was made using the UCLA Life Stress Interview (LSI) for Adults (Hammen, 1991). This semi-structured interview covers a range of domains including: intimate relationships, close friendships, social life, family of origin relationships, relationships with one’s children (if applicable), work, finances, health of self, and health of family members. Function over the previous six months, in each of these domains, is assessed on a 1 (high) to 5 (low) point scale, in half-point increments. Interviews were conducted by trained interviewers and were scored by an independent team. High chronic stress was defined as an average score above 2.5, coupled with no domain score less than 2 and at least 1 domain score of 3.5 or above. Low chronic stress was defined as an average score below 2 and all single domain scores below 3. High and low stress groups differed significantly in mean LSI score (t(28) = 12.42, p < .001).
Study Design
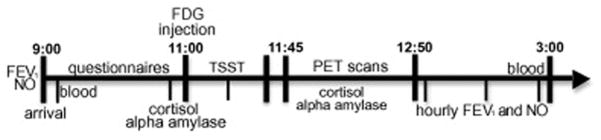
All data were collected during three consecutive days of lab visits. This three-day visit occurred twice, once for each condition (stress and control). Condition order was randomly assigned. On the first day of each set of visits, sputum and blood samples were collected for assessment of inflammatory cell differentials and a physical exam was performed to rule out respiratory infection and recent asthma exacerbation. On the second day, the experimental manipulation (stress or control) was performed (see Fig 1). To prepare for the [F-18]fluorodeoxyglucose (FDG) positron emission tomography (PET) scans and stress hormone measures, participants fasted and were asked to abstain from caffeine for 4–5h before the experiment. Forced expiratory volume (FEV1) and FeNO were measured to establish baseline lung function and inflammation values. After insertion of a catheter for blood draw and FDG injection, participants rested quietly for 1h, while completing self-report questionnaires. After the 1h rest, a saliva sample was collected, immediately before injection of the FDG, for measurement of baseline cortisol and alpha amylase. Immediately following FDG injection, participants were escorted to a nearby room where they performed the Trier Social Stress Task or control task for about 30 min, which spans the bulk of the FDG uptake time. One saliva sample was collected 15 min into the task, and an additional sample was collected immediately after task completion. Participants were then positioned in the PET scanner. PET scans measuring FDG uptake into the brain were collected for 30 min1. During PET scanning, saliva samples were collected every 10 min for 50 min post-task, for a total of 8 samples. After completion of PET imaging, hourly measurements of FeNO and FEV1 were obtained. At 4h post-task, a final blood sample was collected. On the third day, participants returned to provide samples of sputum, blood, and measurements of FEV1 and FeNO. A structural MRI scan was also collected during one of the study visits.
Figure 1. Measures collected and schedule of a typical challenge day.
Note: The FDG brain scan was only 30 min in duration. FDG lung scans were also acquired, but not reported on here.
Experimental Conditions
An extended version of the Trier Social Stress Test (TSST) was used in the stress condition (Kirschbaum et al., 1993). This is a well-validated and standardized social stressor with proven consistency in evoking a physiological stress response. The version used here extends the duration of the original task (from 15 to 30 min), in order to accommodate the bulk of the radiotracer uptake into the brain, by adding an additional verbal performance and mental arithmetic task. The second verbal task involved defining difficult words. This extended version has been validated for evocation of a robust stress response, assessed by both self-report and salivary cortisol (Kern et al., 2008). As in the original version, the TSST is performed standing in front of a microphone, before a panel of two stern judges and a video camera. Participants were given 5 minutes to prepare a speech after a topic was revealed, but were not allowed to use their notes during the speech.
The control task was designed to match the TSST in duration, cognitive function (e.g. performing mental arithmetic, accessing working memory), and physical activity (standing, speaking, etc.), with the stress-provoking aspects removed. The panel of judges and microphone were absent, and participants were told explicitly that no one could hear them speaking and that their performance would not be recorded or evaluated. A sound pressure meter was used to confirm that participants spoke during the entire task. Otherwise, the conceptual framework and environment was identical to that of the stress task. Participants prepared for 5 min. and then spoke for 5 min. about a recent book they read, movie they had seen, or trip they had taken. For the specific instructions given, see Supplemental Information. After each 5 min. segment, the experimenter entered the room and gave instructions for the next segment. The second verbal task and both mental arithmetic tasks were identical to that of the stress condition, but were far less difficult (e.g. serial subtraction in increments of 2, rather than 17).
Brain Imaging
Anatomical MRI data were acquired, for localization of the PET signal, on a 3.0-T MR750 scanner (GE Healthcare, Waukesha, WI, USA) with an 8-channel head coil. A three-dimensional magnetization-prepared rapid gradient echo (3D MPRAGE) image was acquired using the following parameters: inversion time/echo time/repetition time, 450/ 3.2/8.2 ms; flip angle, 12°; 1mm slice thickness, field of view, 256 mm; and acquisition matrix size, 256 × 256, 160 × 1 mm slices. The T1 anatomical images were skull stripped using FreeSurfer 5.1 (http://surfer.nmr.mgh.harvard.edu). Participants were familiarized with both PET and MRI scanning environments prior to data acquisition.
Regional cerebral glucose metabolism was measured using FDG PET with a Siemens ECAT EXACT HR+ PET scanner in three-dimensional mode (Brix et al., 1997). A bolus injection of 5–7 mCi of FDG was administered, via an indwelling catheter placed in the antecubital vein, prior to the TSST and control task. Thirty-five minutes after injection, and following task performance, participants were positioned in the PET scanner with the canthomeatal line parallel to the in-plane field of view (FOV). A dynamic time series sequence of six 5-min emission frames of FDG data was acquired. A 6-minute transmission scan was then acquired for attenuation correction. Data were reconstructed with an iterative reconstruction algorithm (4 iterations, 16 subsets) and a 4mm gaussian filter using ECAT version 7.2 software, with corrections for random events, dead time, attenuation, and scanner normalization. The final images had dimensions of 128 × 128 × 63, corresponding to voxel dimensions of 2.57 × 2.57 × 2.43mm3.
All pre-processing of PET data was accomplished using SPM12. Control and stress task images were independently motion corrected and summed across the 30 minute FDG acquisition. The stress image was then co-registered to the control image for each individual. Each PET image was then co-registered to the participant’s MRI, skull stripped, and scaled to a standard value, based on the injected dose of FDG. Finally, the anatomical MRI and scaled PET images were normalized to MNI template space and smoothed using a 8mm kernel.
Stress Hormones
Levels of two salivary stress hormones, cortisol and alpha amylase (AA), provided measures of the magnitude of the stress response to the TSST and control task. These hormones were chosen as markers of activity in the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system, respectively. Participants provided samples of saliva at baseline, using salivettes. Subsequent saliva samples were collected 15 min. into each task, immediately after the end of each task, as well as every 10 minutes for the next 50 minutes, for a total of 8 saliva samples. Salivary cortisol and AA levels were measured by Dr. Nicolas Rohleder at Brandeis University, using standard assay techniques. Cortisol was measured using a commercially available luminescence immunoassay (CLIA; IBL-Hamburg, Hamburg, Germany) with a detection limit of .138 nmol/L, and AA was measured using an enzyme-kinetic assay, with a detection limit of .03 U/ml, using reagents provided by Roche Diagnostics (Indianapolis, IN, USA) as previously described (Rohleder and Nater, 2009). Intra- and inter-assay coefficients of variation for cortisol were 4.51 and 6.08, respectively, and 4.39 and .67 for AA.
Lung Function and Inflammatory Measures
Lung function was measured using portable spirometry equipment, according to American Thoracic Society standards (Anonymous, 1995) and quantified as the volume of air forcibly exhaled in the first 1s of effort (FEV1). Though we did not expect that an acute stressor would result in a decline in lung function, we measured spirometry to ensure safety. FeNO was measured in breath condensate, following American Thoracic Society guidelines (Silkoff et al., 1997) at a flow rate of 50 mL/s, with a rapid-response chemiluminescent analyzer (NIOX System; Aerocrine, Solna, Sweden; Silkoff et al., 2004). Both spirometry and FeNO measurements were performed before each task, to obtain a baseline, and hourly after PET scanning, to evaluate changes in lung function and airway inflammation. Both measures were also collected approximately 24h post-stress to monitor persistence of airway change.
Both blood and sputum samples were collected to quantify the magnitude of inflammation. The measurement of immune cells in lung sputum provides critical information about local immune responses at the site of inflammation, while the blood measurements provide information about inflammatory potential. For collection of sputum, participants were pre-treated with a beta-agonist to protect against bronchospasm and then inhaled a nebulized 3% buffered saline solution mist. They were asked to attempt to produce sputum at 4 min intervals. Sputum and blood were collected approximately 24h before and after each experimental challenge. An additional blood sample was collected at 4h post-challenge. From these samples, inflammatory cell differentials were computed. The sputum was diluted 1:1 with a 1:10 concentration of dithiothreitol (DTT – SPUTOLYSIN® Reagent, Calbiochem). After shaking in a 37° water bath and centrifugation, cytospins were prepared and stained with Giemsa to determine cell distributions. The total number of nucleated cells was counted on a Neubauer hemocytometer. Count of 300 cells was used for white blood cell differential to determine the number of eosinophils in blood. Difference scores (post-pre) were computed for each challenge to reflect the change in cell number in response to challenge. Cell differential was not obtained for sputum samples containing > 80% squamous cells. Cells (up to 1×106 cells/aliquot) were stored in 1 ml of Trizol Reagent (Invitrogen, Carlsbad, CA) for RNA purification.
For measurement of gene expression of cytokines in the IL-1β/IL-17 pathway, total RNA was extracted according to the Trizol Reagent manufacturer’s recommendations. Typically, 400–800 ng of total RNA were recovered from the sputum samples. The reverse transcription reaction was performed using the Superscript III system (Invitrogen/Life Technologies, Grand Island, NY, USA). Expression of mRNA was determined by qPCR using SYBR Green Master Mix (SABiosciences, Frederick, MD, USA) and human IL-17, IL-23A, and IL-1R1 forward and reverse specific primers (see Supplemental Table I for primer sequences) were designed using Primer Express 3.0 (Applied Biosystems, Carlsbad, CA, USA) and blasted against the human genome to determine specificity using http://www.ncbi.nlm.nih.gov/tools/primer-blast. The reference genes, β-glucuronidase (GUSB) and ribosomal protein S26 were used to normalize the samples. Standard curves were performed and efficiencies were determined for each set of primers. Efficiencies ranged between 92 and 114%. PCR was performed for 40 cycles using a calculated ~30 ng of total RNA. Data are expressed as average −ΔCt for GUSB and S26 using the comparative cycle threshold (ΔCT) method as described previously (Esnault et al., 2012). Difference scores were computed using these −ΔCt values, to reflect change from baseline in stress relative to control conditions ((stress-baseline)-(control-baseline)).
Self-report measures
Self-report instruments completed at each set of visits included the State-Trait Anxiety Inventory (STAI; Spielberger, 1983), Beck Depression Inventory (BDI; Beck and Ward, 1961), Perceived Stress Scale (PSS; Cohen et al., 1983), Positive and Negative Affect Scale (PANAS; Watson et al., 1988) and Asthma Control Questionnaire (ACQ; Juniper et al., 1999).
Data analysis
Regional cerebral glucose metabolism with FDG-PET
Analysis of PET data was carried out using the Randomise tool in FSL (Winkler et al., 2014). These analyses were done in two stages. First, a priori region of interest (ROI) analyses, within Harvard-Oxford atlas-defined masks of the insular cortex and ACC (see Fig 1 in Supplementary Materials), were performed. Linear mixed effects models were used to test the group x challenge interactions in these ROIs. Linear regression was used to test the association between the difference in regional glucose metabolism in the stress and control conditions (stress-control), in each ROI, with peripheral physiological and self-report measures. Second, discovery analyses were conducted using a whole-brain voxel-wise application of the methods described for the ROI analyses. Nonparametric permutation tests using the threshold free cluster enhancement (TFCE) approach (Smith and Nichols, 2009), within the Randomise tool, were used to correct for multiple comparisons in both whole-brain analyses, as well as in those restricted to a priori ROIs.
Stress Hormones, Inflammatory Measures, and Lung Function
For all peripheral physiological measures, repeated measures mixed models were used to examine the effects of group, challenge condition, time, and their interactions. A random intercept was used to adjust for repeated measures within-subject and models were estimated using the lmer function (Bates et al., 2015) of the lme4 library of the R software package (www.r-project.org). P-values were computed according to the calculation of Satterthwaite’s approximation, as implemented in the lmer Test library in R (SAS Technical Report R-101: Tests of Hypotheses in Fixed-Effects Linear Models, 1978). A random effect for time was also considered, but was not found to be significant and thus was omitted. Likewise, to identify the most parsimonious model, we started with full models, including a group x challenge x time interaction (and higher order polynomial terms when necessary), and omitted insignificant interaction terms through a backwards model selection. Other parameter estimates remained stable during model selection. Because a quadratic trend over time was present in cortisol and FEV1,, we included quadratic terms, as well as their interactions, in these models. In the AA data, both quadratic and cubic trends over time were present and were included in this model.
As a way to capture overall HPA-axis and SNS response to challenge condition, area under the curve (AUC) with respect to ground was computed for each stress hormone, as described in Pruessner et al., 2003. Cortisol AUC values were log-transformed to normalize their distribution. AA values were normally distributed. Log cortisol and AA AUC variables were used in subsequent correlation and regression analyses to examine associations among variables.
Independent samples t-tests were used to compare change from baseline to post-challenge ((stress-baseline)-(control-baseline)) between groups for mRNA expression of each cytokine assessed in the IL-1β/IL-17 pathway.
Self-report Measures
Self-report data were analyzed using mixed-model (2 group x 2 challenge) repeated measures ANOVA with SPSS v23 software.
Relationships among variables
The relationship among variables with predicted associations (e.g. asthma control and depression) were assessed using Pearson’s correlation, with SPSS v23 software. When a difference in relationship by group was suspected, hierarchical regression analyses were used instead, where LSI score, as a continuous variable indexing chronic stress, and the IV were entered on the first step and their interaction was entered on the second step.
Correction for multiple comparisons
To correct for multiple comparisons across all peripheral measures, a Bonferroni correction was applied, such that all p-values < .002 (.05/22) meet a corrected threshold.
Results
Stress Hormones
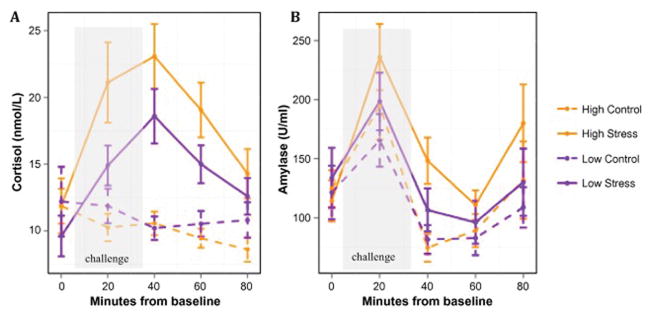
The final model for the cortisol data included all main effects and the following 2-way interactions: group x challenge, challenge x time, and challenge x time2. Results of the repeated measures mixed models analysis showed a significant main effect of challenge (B = 7.83, t(443) = 7.56, p < .001), as well as a significant group x challenge interaction (B = 4.34, t(442) = 3.63, p < .001), indicating that the high stress group had a larger cortisol response to the TSST than the low stress group, but the groups did not differ in their response to the control task (Fig 2A). In addition, a significant challenge x time2 interaction (B = −.005, t(438) = −5.65, p < .001) was present, indicating that there is an inverted U-shaped pattern in the cortisol response to the stress condition, but not in the control condition. For AA, the final model included all main effects and the following interactions: group x time, challenge x time, group x time2, challenge x time2, and group x time3. Results showed significant main effects of challenge, time, and quadratic and cubic trends over time, as well as significant group x time, challenge x time2, and group x time3 interactions (see Table 1 for statistics). Interpretation of the interactions is best understood visually. Essentially this analysis shows that the groups differ over time in expression of AA (see Fig 2B), and the addition of the quadratic and cubic terms further separates the groups within each challenge condition (see Supplementary Figs. 2A & 2B), particularly during the period encompassing the peak and initial post-peak decline of AA response.
Figure 2. Stress hormone responses to the TSST and control task.
Means displayed by group and challenge for (A) cortisol data and (B) alpha amylase data. Error bars represent standard error of the mean.
Table 1.
Linear mixed model statistics for alpha amylase
| Variable | Coefficient | 95% CI | SE | T | P |
|---|---|---|---|---|---|
| (Intercept) | 91.696 | (50.019, 133.388) | 21.376 | 4.29 | 0.0001 |
| Group | 21.661 | (−37.000, 80.324) | 30.039 | 0.721 | 0.476 |
| Challenge | 46.828 | (30.551, 63.109) | 8.372 | 5.593 | <0.0001 |
| Time | −3.029 | (−3.826, −2.232) | 0.41 | −7.388 | <0.0001 |
| Time2 | 0.039 | (0.022, 0.056) | 0.009 | 4.439 | <0.0001 |
| Time3 | 0.002 | (0.001, 0.002) | 0.0003 | 6.641 | <0.0001 |
| Group x Time | −1.253 | (−2.337, −0.169) | 0.558 | −2.247 | 0.025 |
| Challenge x Time | −0.342 | (−0.797, 0.113) | 0.234 | −1.461 | 0.145 |
| Group x Time2 | −0.00005 | (−0.02, 0.02) | 0.01 | −0.005 | 0.996 |
| Challenge x Time2 | −0.024 | (−0.041, −0.008) | 0.008 | −2.862 | 0.004 |
| Group x Time3 | 0.0009 | (0.0004, 0.002) | 0.0004 | 2.502 | 0.013 |
Lung function and Inflammatory Measures
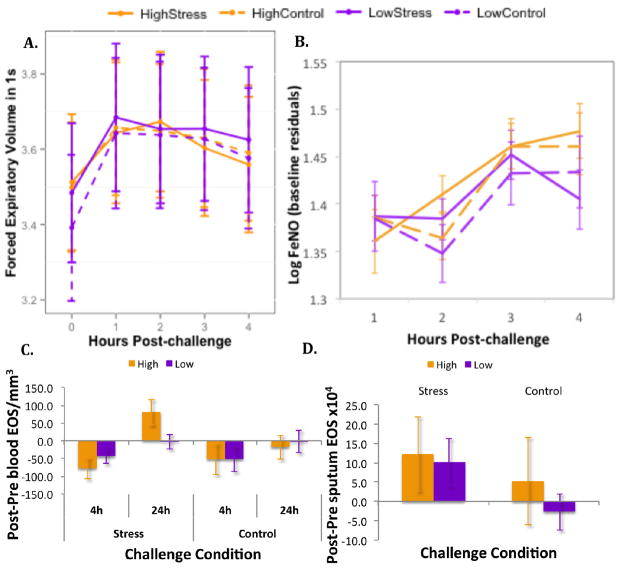
At baseline, the groups did not differ in lung function (p > .5), though they did differ significantly in log FeNO (t(28) = 2.13, p < .05), such that those with low levels of chronic stress had greater levels of FeNO before the stress challenge than those with high chronic stress. The final model for FEV1 included the main effects and a quadratic trend over time. Analysis of this model revealed significant main effects of time (B =.15, t(271) = 9.23, p < .001), and the quadratic trend over time (B = −.03, t(271) = −8.16, p < .001). These effects indicate that FEV1 changed following both challenges, such that lung function increased in both groups post-challenge, but began returning to baseline levels, particularly for those with high stress following the TSST. Importantly, FEV1 was not affected by challenge condition (Fig 3A).
Figure 3. Change in lung function and asthma-related inflammatory markers in response to stress and control tasks.
Means displayed by group and challenge for (A) FEV1, (B) FeNO, (C) blood EOS, and (D) sputum EOS. Error bars represent standard error of the mean.
For log FeNO, baseline values were included in the model as covariates to account for pre-challenge differences. The final model for FeNO data included main effects of time and group, and the interaction between group and time, plus baseline as a covariate. The outcome of this analysis showed a significant group x time interaction (B = .03, t(199) = −2.31, p < .05), indicating that FeNO values differed over time between groups, such that at the 4h measurement, FeNO values in the low stress group tended to drop whereas those of the high stress group tended to rise (Fig 3B).
The final models for both blood and sputum EOS contained only main effects. The outcome of these linear mixed-effects analyses showed a significant effect of time (B = 71.03, t(88) = 3.39, p = .001) for blood EOS and a significant effect of challenge (B = 11.52, t(24) = 2.21, p = .037) for sputum EOS. These effects indicate that, the change in number of EOS in blood in response to challenge was more positive at 24h than at 4h and this effect was driven primarily by the increase in blood eosinophils following the TSST in the high stress group (Fig 3C). In sputum, the effect of challenge indicates that eosinophil number increased more in response to the TSST than the control task, in both groups (Fig 3D).
Unfortunately, only 21 of 30 (10 high stress) individuals had sputum samples of sufficient quality to perform mRNA analyses. No significant group differences were observed in change from baseline to post-stress, relative to control, for any of the 3 cytokines examined.
Self-report
The outcome of repeated measures ANOVA analyses of self-report data revealed main effects of group for asthma control (ACQ; F(1, 28) = 6.02, p = .021), depression (BDI; F(1, 28) = 19.08, p < .001), and anxiety (STAI; F(1, 28) = 8.34, p < .01), such that those in the high stress group reported more symptoms of anxiety (High: M = 40.8, SE = 3.0; Low: M = 31.2, SE = 2.6) and depression (High: M = 6.7, SE = .8; Low: M = 1.8, SE = .8) and poorer asthma control (High: M = 1.2, SE = .3; Low: M = .3, SE = .3) than those in the low stress group. There were no main effects of challenge or group by challenge interactions for any of these measures. On the other hand, PANAS positive and negative affect, which was assessed immediately following each challenge, showed a challenge by valence interaction where positive affect did not differ following stress and control conditions, but negative affect was significantly higher following the stress, relative to control task (PANAS; F(1, 28) = 14.73, p = .001). No main effect of group or interactions involving group were present, indicating that the groups did not differ with respect to their reporting of positive affect, negative affect, or affect in general, irrespective of challenge condition. Neither a main effect of challenge or group, nor any interactions were observed for perceived stress (PSS) scores.
FDG-PET
ROI Analyses
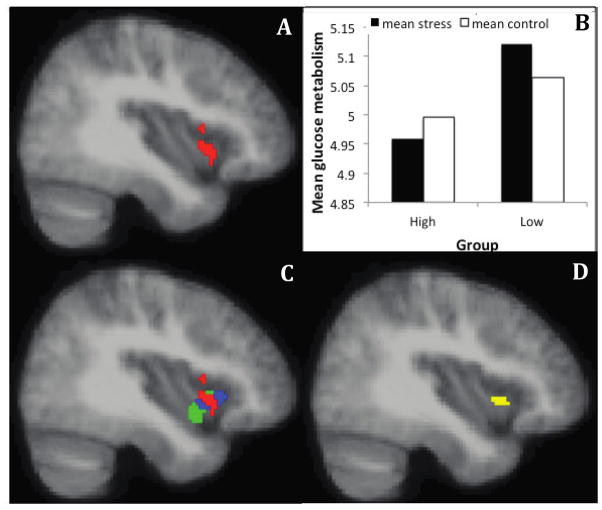
The results of the linear mixed effects analyses revealed no clusters within the anatomical insula or ACC ROIs showing a group x challenge interaction at a corrected p < .05. However, at a threshold of p < .05 uncorrected, a cluster showing a group x challenge interaction (see Fig 4A) is present in the anterior insula, that largely overlaps with clusters we have reported in previous research (Fig 4B & 4D); Rosenkranz et al., 2012). This interaction is significant at p < .05 using an a priori empirical mask taken from the overlap of clusters reported in Rosenkranz et al. (2012), and is primarily driven by an increase in glucose metabolism during the stress, relative to control condition, in the low stress group (see Fig 4C). In addition, a cluster showing a group x challenge interaction in the mid-cingulate cortex (MCC) is also present at a threshold of p < .05 uncorrected (Fig 5A), which is primarily driven by a reduction in glucose metabolism during the stress condition, relative to the control condition, in the low stress group (Fig 5B).
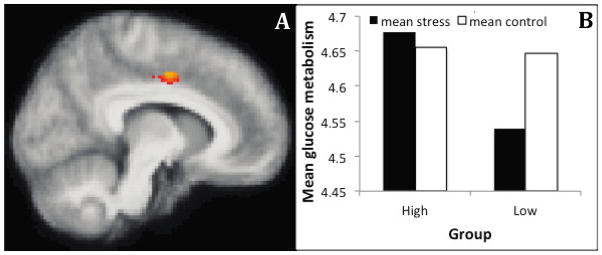
Figure 4. Individuals with asthma who have low levels of chronic life stress show increased glucose metabolism in the anterior insula during a social stressor, relative to a control task, compared to asthmatic individuals with high levels of chronic life stress.
(A) Cluster showing a group x challenge interaction in the insula ROI analysis. MNI coordinates of the peak voxel in mm (34, 16, −16), 286 voxels. (B) Mean glucose metabolism extracted from the cluster shown in (A) for each condition and group. This plot is provided only to show the data over the significant voxels, and inferences are not provided since the voxel-wise analysis and ROI-averaged analyses utilize the same contrast, which is a case of circular analysis (Kriegeskorte et al., 2009). (C) Overlap of the cluster showing a group x challenge interaction in the right insula in the current data (red) with the cluster showing a group x challenge x valence interaction (blue), and correlation with sputum EOS (green) published in Rosenkranz et al., 2012..(D) Intersection of the three clusters. Image A is thresholded at p < .05, uncorrected.
Figure 5. Individuals with asthma who have low levels of chronic life stress show decreased glucose metabolism in the mid-cingulate cortex (MCC) during a social stressor, relative to a control task, compared to asthmatic individuals with high levels of chronic life stress.
(A) Cluster showing a group x challenge interaction in the ACC ROI analysis, thresholded at p < .05, uncorrected. MNI coordinates of the peak voxel in mm (10, −4, 44), 102 voxels. (B) Mean glucose metabolism extracted from the cluster shown in (A) for each condition and group. This plot is provided only to show the data over the significant voxels, and inferences are not provided since the voxel-wise analysis and ROI-averaged analyses utilize the same contrast, which is a case of circular analysis (Kriegeskorte et al., 2009).
The results of linear regression analyses within the insular cortex, that examined relationships between change in glucose metabolism and peripheral measures, showed a positive correlation between FDG-PET signal in the mid-insula and mean FeNO (p < .05 corrected). This indicates that individuals with greater FeNO had greater glucose metabolism in this region during the stress, relative to the control condition (Fig 6). Regression analyses within the ACC ROI revealed a positive association between glucose metabolism (stress - control) in MCC and the increase from baseline levels of IL23A mRNA expression following stress, relative to control (p < .05, corrected). This association indicates that greater MCC metabolism during the TSST, relative to control, is associated with a larger increase in IL23A mRNA expression following the TSST, relative to control task (Fig 7A). An analogous association, at trend level, was present between glucose metabolism in the perigenual ACC and the increase from baseline levels of IL1R1 mRNA expression following stress, relative to control (p < .06, corrected; Fig 7B). Cluster sizes and coordinates of peak voxels can be found in Tables 2 & 3. In order to provide a complete picture of the data, the results of all ROI regression analyses, thresholded at p < .05 uncorrected, are also included in Tables 2 & 3.
Figure 6. Activity in the mid-insula is positively associated with mean FeNO.
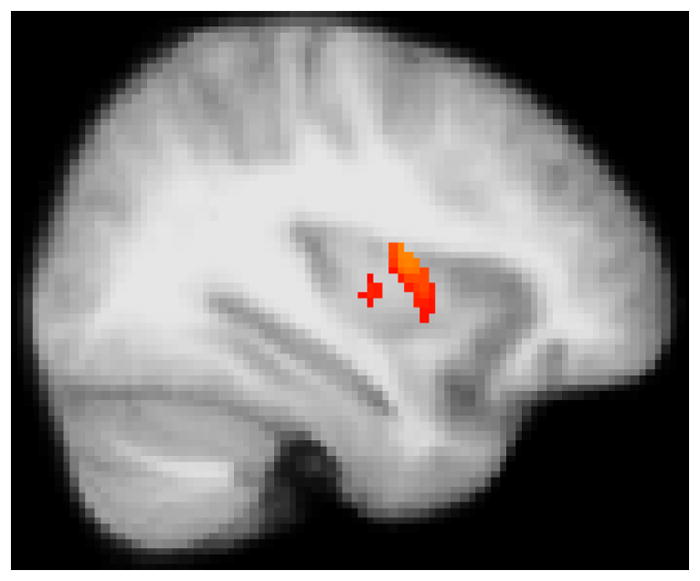
Glucose metabolism in the mid-insula during performance of the TSST, relative to the control challenge (stress – control), and FeNO averaged across measurements. MNI coordinates of the peak voxel in mm (34, 0, 14), 128 voxels. Image thresholded at p < .05, corrected.
Figure 7. Activity in the cingulate cortex is positively associated stress-related change in mRNA expression of genes in the IL1β/IL-17 pathway.
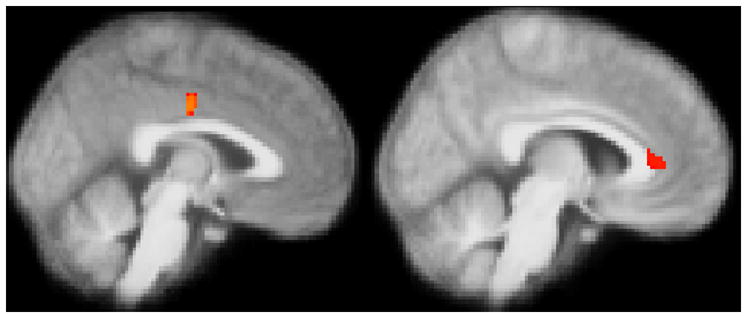
(A) Glucose metabolism in the MCC during performance of the TSST, relative to the control task (stress – control), and the increase in IL23A from baseline to post-stress, relative to post-control. MNI coordinates of peak voxel in mm (−2, −14, 36), 39 voxels (B) Glucose metabolism in the perigenual ACC during performance of the TSST, relative to the control task (stress – control), and the increase in IL1R1 from baseline to post-stress, relative to post-control. MNI coordinates of peak voxel in mm (8, 36, 6), 65 voxels. Images thresholded at (A) p < .05 and (B) p < .06, corrected.
Table 2.
Insula ROI regression cluster descriptives
| Region | Regressor | Direction of association | Coordinates of peak voxel (mm) | Cluster size |
|---|---|---|---|---|
| R mid | Mean FeNO | + | 34, 0, 14 | 128* |
| Bi-lateral mid | IL1R1 change (stress-control) | + | 30, 8, −16 | 593 |
| R posterior | Blood EOS 24h Post(stress-control)- Pre(stress-control | + | 32, −28, 8 | 230 |
| R ventral posterior | Cortisol AUC (stress-control) | + | 38, −20, −2 | 26 |
| R anterior | Sputum EOS 24h Post(stress-control)- Pre(stress-control | − | 42, 24, −2 | 186 |
| Bi-lateral ventral anterior to mid | ACQ | − | 40, −6, −12 | 420 |
| L anterior | BDI | − | −26, 16, 16 | 423 |
| L anterior | STAI | − | −32, 14, −8 | 391 |
| L anterior | Cortisol AUC (stress-control) | − | −24, 24, 4 | 216 |
| L anterior/ frontal operculum | IL1R1 change (stress-control) | − | −34, 16, 18 | 86 |
indicates p < .05 corrected threshold. All other clusters reported at p < .05, uncorrected. All p-values reported are uncorrected for multiple comparisons across regression analyses, but the corresponding Bonferroni threshold would be 0.05/11 = 0.005.
Table 3.
ACC ROI regression cluster descriptives
| Region | Regressor | Direction of association | Coordinates of peak voxel (mm) | Cluster size |
|---|---|---|---|---|
| MCC | IL23A change (stress-control) | + | −2, −14, 36 | 39* |
| perigenual | IL1R1 change (stress-control) | + | 8, 36, 6 | 65* |
| dorsal | Blood EOS 24h Post(stress-control)-Pre(stress-control | + | −4, 22, 34 | 533 |
| supragenual and perigenual | Amylase AUC (stress-control) | + | −18, 26, 24 6, 34, −6 |
199 97 |
| MCC | BDI | + | −14, −14, 38 | 54 |
| MCC | Mean FeNO | + | 4, −6, 30 | 266 |
| MCC | Amylase AUC (stress-control) | − | 16, −6, 36 | 38 |
| subgenual | STAI | − | −10, 4, −16 | 260 |
| subgenual | FeNO 4h post-task (stress-control) | − | 12, 28, −18 | 74 |
| subgenual | Sputum EOS 24h Post(stress-control)-Pre(stress-control | − | 10, 18, −16 | 31 |
indicates p < .05 corrected threshold. All other clusters reported at p < .05, uncorrected. All p-values reported are uncorrected for multiple comparisons across regression analyses, but the corresponding Bonferroni threshold would be 0.05/11 = 0.005.
Whole brain analyses
The results of whole-brain voxel-wise regression analyses showed that the difference in FeNO at 4h post-challenge (stress-control) is positively associated with glucose metabolism (stress - control) in a region spanning the precentral and postcentral gyri (p < .05, corrected) and negatively associated with glucose metabolism in a region covering the middle temporal gyrus, as well as a small region of the orbital frontal cortex (p < .05, corrected). Cluster sizes and coordinates of peak voxels for whole-brain analyses can be found in Table 3. A complete list of results from whole-brain voxel-wise regressions, thresholded at p < .01 uncorrected, can also be found in Table 4.
Table 4.
Whole-brain voxel-wise regression cluster descriptives
| Region | Regressor | Direction of association | Coordinates of peak voxel (mm) | Cluster size |
|---|---|---|---|---|
| Precentral/Postcentral gyri | FeNO 4h post-task (stress-control) | + | −40, −26, 54 | 431* |
| TPJ/SMA | BDI | + | 42, −62, 24 | 1234 |
| TPJ/LOC | Mean FeNO | + | −20, −60, 28 | 955 |
| Precuneus/PCC | Amylase AUC (stress-control) | + | −28, −38, 14 | 958 |
| Precuneus/PCC | Cortisol AUC (stress-control) | + | (L) −16, 54, 10 (R) 6, −56, 4 |
132 21 |
| Cerebellum/PHG | Mean FeNO | + | −26, −56, −38 | 5098 |
| IFG/OFC | FeNO 4h post-task (stress-control) | + | −38, 26, −10 | 474 |
| Thalamus | Amylase AUC (stress-control) | + | −14, −24, 4 | 81 |
| Frontal pole | Amylase AUC (stress-control) | + | 28, 72, 2 | 275 |
| Middle Temporal gyrus | FeNO 4h post-task (stress-control) | − | 68, −20, −16 | 194* |
| OFC | FeNO 4h post-task (stress-control) | - | −24, 18,−24 | 65 |
indicates p < .05 corrected threshold. Otherwise, cluster threshold is p < .01, uncorrected. All p-values reported are uncorrected for multiple comparisons across regression analyses, but the corresponding Bonferroni threshold would be 0.05/11 = 0.005. SMA = Supplementary Motor Area; TPJ = temporal parietal junction; LOC = lateral occipital cortex; PHG = parahippocampal gyrus; IFG = inferior frontal gyrus; OFC = orbital frontal cortex
Relationships among peripheral measures
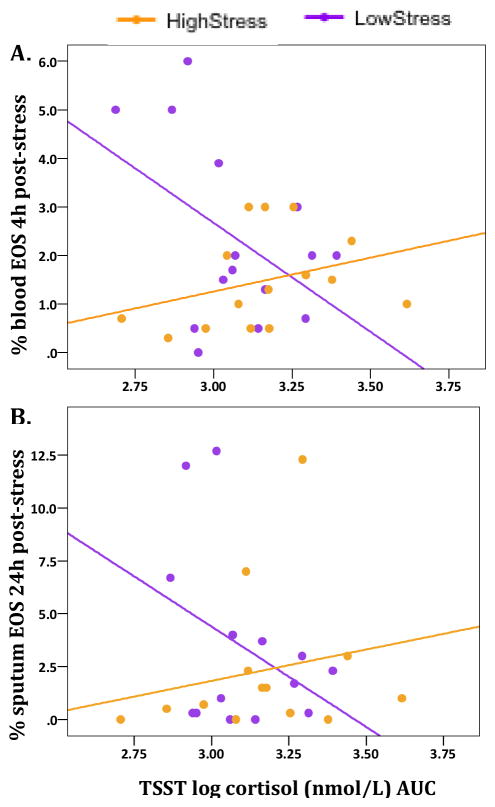
Results of regression analyses showed a significant interaction between LSI score (group) and log cortisol AUC post-stress challenge on 4h post-stress percentage of blood eosinophils (B = 4.63, t=2.21, p = .036), such that for those with high LSI scores, greater cortisol response was associated with higher blood eosinophils at 4h post-stress, whereas this relationship was opposite for those with low LSI scores (Fig. 8A). This same pattern, at trend-level significance, was observed with 24h post-stress sputum eosinophils as the outcome measure (Fig. 8B; B = −.24, t = −1.98, p = .059). In contrast, positive associations were observed, across groups, between the cortisol and AA response to the TSST, relative to control, and the relative change in mRNA expression following stress vs. control for IL1R1 (cortisol: B = 1.57, t = 2.10, p = .05; AA: B = 6.67 × 10−5, t = 2.76, p = .012) and IL17A (cortisol: B = 2.8, t = 2.10, p = .054).
Figure 8. Relationship between cortisol response to stress and percent EOS post-stress differs by chronic stress group.
Interaction between Life Stress Interview (LSI) score and log cortisol AUC following stress challenge on (A) % blood EOS at 4h post-stress (B) % sputum EOS at 24h post-stress
Analysis of relationships between asthma control and psychological symptoms showed that poorer asthma control (higher ACQ score) is associated with greater symptoms of depression (BDI; r= .77, p < .0001) and anxiety (STAI; r (29) = .82, p < .0001). In addition, a negative association (r= −.48, p < .01) between asthma control and the difference in positive affect (PANAS) following the stress condition and the control condition was observed, indicating that the bigger the decrement in positive affect following stressor, relative to the control condition, the poorer the asthma control.
Discussion
The data presented here confirm and extend previous findings that chronic stress is associated with increased expression of asthma symptoms, as well as symptoms of anxiety and depression in asthmatic individuals. In addition, our data show that poor asthma control is highly associated with greater symptoms of anxiety and depression. Those with high chronic stress were also more reactive to an acute stressor, as demonstrated by a greater TSST-induced cortisol response and greater self-reported post-stress negative affect. Further, we show that in those with high levels of chronic stress, a greater cortisol response to an acute stressor is associated with increases in blood and sputum EOS, whereas these relationships are opposite in those with low chronic stress. These data mirror those reported by Bailey et al. (2009) and others (Curry et al., 2010; Powell et al., 2013) who demonstrate that chronic social stress causes a potentiation in airway inflammation, via increased bone marrow production of inflammatory immune cells, increased recruitment of EOS to the airway, and a reduction in the ability of glucocorticoids to inhibit inflammation in rodent models of airway inflammation, and suggest mechanisms through which chronic stress may contribute to more severe asthma and poorer asthma control (Chen and Miller, 2007; Cohen et al., 2008; Wright and Steinbach, 2001).
Elevated FeNO in asthmatic individuals is associated with increased eosinophilia in sputum, poorer asthma control, and more severe asthma (Aytekin and Dweik, 2012; Dweik et al., 2010; Schleich et al., 2014; Yamamoto et al., 2012). Previous research on the effects of psychological perturbation on FeNO in asthma has shown conflicting results (Ritz and Trueba, 2014). For instance, in response to an acute laboratory stressor, similar to the one employed in this study, FeNO levels consistently rise, but this change is driven by those with low levels of depression and the rise is inversely related with cortisol response to the stressor (Ritz et al., 2014, 2011). On the other hand, during a more prolonged period of stress, such as during a period of examinations, FeNO has been shown to fall from baseline levels, with a stronger decline observed in those with a greater increase in cortisol and greater symptoms of depression (Ritz et al., 2015; Trueba et al., 2013). Following both challenge conditions, FeNO levels rose significantly across groups in our sample, but sharply declined in the low chronic stress group at 4h post-TSST. This observation corroborates a previous finding by Chen et al. (2010), who reported a rise in FeNO in response to an acute laboratory stressor, the magnitude of which increased as SES decreased. Therefore, our data fit nicely within and tie together the current literature2, and suggest that while response to acute stressors may reduce expression of markers of airway inflammation in asthma, when superimposed on a background of chronic stress, these same stress responses may potentiate their expression.
Initial efforts to identify the primary mediators of stress-enhanced inflammation in asthma have focused on Th2 cytokines, and there is evidence for their involvement under certain circumstances (Chen and Miller, 2007; Chen et al., 2006; Marin et al., 2009). However, IL-1β/IL-17 pathways are also emerging as promising candidates. The release of IL-1β by EOS causes the increased expression of IL-17A in CD4+ cells (Esnault et al., 2012). IL-17 has been linked to asthma severity and decreased responsiveness to corticosteroids (Vazquez-Tello et al., 2013). Moreover, in both human and rodent models, psychological stress evokes increases in peripheral IL-1β (Brydon et al., 2005), and an IL-1β infusion induces anxiety-like behavior (Rossi et al., 2012). Similarly, IL-17 promotes depressive-like behavior in mice (Beurel et al., 2013) and is associated with depression in humans (Ying Chen et al., 2011). Though there was not an overall effect of stress on the expression of genes in the IL-17 pathway in the current study, our data do provide support for a role for these cytokines in the mechanisms that underlie the impact of psychological stress on airway inflammation in asthma, in that individual differences in cortisol and AA responsivity to the TSST were positively associated with increased post-stress expression of IL-17A and IL-1R1 in cells obtained from the airway.
In response to the stress vs. control condition, glucose metabolism in the anterior insula showed a challenge x valence interaction. This region has substantial overlap with regions identified by our group in a previous fMRI study (Rosenkranz et al., 2012), where this region was more responsive following inhaled allergen challenge and predicted the magnitude of the airway inflammatory response. In the current PET study, the interaction is driven by an increase in TSST-evoked anterior insula activity in those with low chronic stress. Stress-induced increases in glucose metabolism in this region were also associated with a less reactive profile across a range of measures, though only at an uncorrected threshold. Hannestad et al. (2012) reported a similar pattern of observations from a study that used FDG-PET to image the change in neural activity associated with endotoxin-induced inflammation and found that the more this region was activated by endotoxin challenge, the less severe was the loss of social interest reported by participants in response to endotoxin and the smaller the increase in proinflammatory cytokines. Together, these data suggest that the anterior insula is engaged during disruptions in homeostasis, and its net activity, across the period of FDG uptake into the brain, may reflect a regulatory role.
In addition to the difference in neuroimaging modality, an important distinction between the design of the current study and that described in Rosenkranz et al., 2012, is that the current study involved an explicit and intense psychologial stressor, whereas our 2012 study did not. Instead, it compared the neural response to asthma-relevant words, in those who would go on to develop an inflammatory airway response, to those who would not. This suggests the possibility that, for those who went on to develop the inflammatory response, these words (e.g. “wheeze”, “suffocate”) were more cognitively salient or threatening, and required the invocation of regulatory resources, whereas for those who did not develop an inflammatory response, no regulatory resources were required. Therefore, the discordance with our previously published results may reflect a need to regulate vs. no need to regulate in Rosenkranz et al., 2012, in contrast to greater mobilization of regulatory resources by those with low chronic stress in the current study, in a context where all participants had a need to regulate. This is consistent with the Hannestad (2012) study, where all participants had a need to regulate consequent to the ensuing inflammatory response generated by endotoxin challenge, and those with the greatest increase in anterior insula metabolism were those with the least severe adverse symptoms.
In addition to the interaction, a significant association between glucose metabolism (stress-control) in the mid-insula and mean FeNO level was observed. This relationship is consistent with our previous work and that of others (Harrison et al., 2009a; Ochsner et al., 2008; von Leupoldt et al., 2009). In two separate studies with asthmatic participants, we showed that reactivity of the mid-insula, following allergen provocation, was associated with a subsequent larger airway inflammatory response and fall in lung function (Rosenkranz et al., 2012, 2005). Activation in this area has been heavily attributed to interception (Avery et al., 2015; Ochsner et al., 2008; Simmons et al., 2013), and its subcortical connectivity, particularly with autonomic control centers, suggests that the mid-insula may have a role in modulating efferent output. Indeed, stimulation of this region causes various autonomic-driven effects, including changes in heart rate, blood pressure, respiration and gastric motility (Augustine, 1996; Bagaev and Aleksandrov, 2006; Yasui et al., 1991). Thus activity in this region may more generally reflect, perhaps in tandem with the ACC, descending responses to threat or disrupted homeostasis (James et al., 2013).
In analyses that focused on the cingulate cortex, increased glucose metabolism during the TSST (stress-control) in the mid-cingulate cortex (MCC) was associated with increased expression of sputum cell IL23A mRNA (p < .05 corrected), as well as several other indicators of both psychological and physiological reactivity to stress, at an uncorrected threshold. The cluster showing these effects lies on the border between the anterior and posterior MCC3, as defined in Cavanagh and Shackman (2014). This region, particularly the anterior aspect, has been identified as a convergence zone for processing information related to “…pain, threat, and other more abstract forms of potential punishment” (Cavanagh and Shackman, 2014) and seems to be exquisitely sensitive to prediction errors during social interactions, when the outcomes of one’s efforts fail to meet their goals in interacting with others (Apps et al., 2013). Moreover, during the physiological perturbation associated with typhoid vaccination, activity in the MCC is positively related to symptoms of fatigue and lethargy (Harrison et al., 2009a). This region of the ACC is closely connected with the mid-insula (Deen et al., 2011; Mesulam and Mufson, 1982a, 1982b) and other regions important in guiding motivated behavior during threat or uncertainty (Shackman et al., 2011). Given this proposed role, we might expect that those with high levels of life stress would show a potentiation of activity in this region during the TSST, relative to control, and relative to those with low life stress. To the contrary, a group x challenge interaction was observed in this region, albeit at a p < .05 uncorrected threshold, which was driven primarily by a decrease in activity during the TSST, relative to control, in those with low chronic stress. Given the liberal threshold at which this effect is observed, its interpretation is tenuous, but could reflect the consequences of active reappraisal during the TSST and down-regulation of activity in this region.
In the perigenual region of the ACC, glucose metabolism during the TSST (stress-control) was associated with increased expression of IL1R1. This region is nearly identical to that which showed a positive relationship between reactivity following allergen challenge and the allergen-induced increase in sputum EOS and corticosteroid insensitivity of peripheral blood leukocytes in our previous work (Rosenkranz et al., 2005). Further, activation of this region has been shown previously in response to social evaluative stressors, particularly in individuals under chronic stress, and this activation was associated with greater perceived stress and cortisol reactivity (Akdeniz et al., 2014; Kern et al., 2008). This area of the ACC has high expression of receptors for glucocorticoids (Herman et al., 2005) and its function during social stress has been attributed to emotional and efferent autonomic responses (Akdeniz et al., 2014). Together with our previous findings, these data suggest that the perigenual ACC may be a critical region at the interface of immune modulation based on cognitive or emotional context.
The relationships between glucose metabolism and peripheral inflammatory markers observed in our data, though correlative, build upon related mechanistic models in rodents and may begin to delineate the brain-immune pathways through which chronic stress contributes to poorer asthma outcomes in humans. As discussed above, activity in both the perigenual ACC and mid-insula is associated with increased sympathetic activation (see also Critchley et al., 2003; Maihöfner et al., 2011). In our data, greater perigenual ACC activity was related to greater stress-induced release of AA (at an uncorrected threshold). Sympathetic nerves directly innervate bone marrow and their activity can influence hematopoiesis (Katayama et al., 2006). In an elegant set of studies, Powell et al., (2013) demonstrated the involvement of sympathetic activity in the increase in proinflammatory gene expression observed in individuals of low SES, by employing a parallel rodent model of chronic stress. They were able to show that the increase in proinflammatory gene expression (including IL-1β) was mediated by β-adrenergic-induced increases in myelopoiesis and a selective expansion of immature inflammatory monocytes and granulocytes. Release of IL-1β causes increased expression of IL-17A from T cells (Esnault et al., 2012). IL-17 contributes to allergic inflammation in asthma in many ways, including recruitment of eosinophils and neutrophils to the airway (Park and Lee, 2010). In addition, IL-17 promotes corticosteroid resistance through enhancement of the expression of glucocorticoid receptor-beta (GR- β; Vazquez-Tello et al., 2013)). Both IL-1β and IL-17 have been linked to mood and anxiety disorders, as well (Baune et al., 2010; Beurel et al., 2013; Bufalino et al., 2013; Yili Chen et al., 2011; Liu et al., 2012; Waisman et al., 2015). Our data parallel many of these observations. First, TSST exposure led to an increase in eosinophils in both blood and sputum, particularly in those under chronic stress. Second, a larger cortisol and AA response to stress was associated with a greater increase in mRNA expression of IL-17A and IL-1R14, and the suggestion of corticosteroid insensitivity was found for those under chronic stress. In addition to AA levels, activity in the perigenual ACC was associated with increased expression of IL1R1, as was activity in the mid-insula. Therefore, we can begin to piece together a proposed pathway whereby stress-related activation in cingulate and insular subregions leads to increased sympathetic outflow, and stimulation of production and release into circulation of a primed and biased set of inflammatory immune cells. The expression of IL-1β from these cells, coupled with the increased IL-1β-induced expression of IL-17 could lead to the poorer asthma control, as well as increased symptoms of depression and anxiety observed in our participants with high levels of chronic stress.
FDG-PET was chosen as the neuroimaging modality for this design because it allowed us to measure the neural activity that occurred during the experience of a social stressor. We contrasted glucose metabolism during performance of the TSST with that of a control task that matched the TSST in major elements (e.g. cognitive function, motor involvement, duration), but with the stressful aspects removed. Though every attempt was made to minimize the differences between these two tasks in every way except the stressfulness, differences in glucose metabolism that are unrelated to stress are impossible to avoid, and contribute noise to the measurement that makes detection of the true stress signals difficult. This is evident in the paucity of results that survive correction for multiple comparisons. For this reason, we reported effects at a both a conservative and a liberal threshold, which should be considered when situating these results in the current literature. Given the increased risk for false positives in a small study, these results should be interpreted with caution.
Nonetheless, the findings presented here extend our understanding of the relationship between stress and airway inflammation in asthma and provide further evidence for the involvement of the insula and ACC in this relationship. Further, these data may provide new insight into the roles of the subregions within the insula and ACC. Our data also lend support to the hypothesis that the IL-1β/IL-17 pathway is involved in the interrelationship between psychological factors and asthma. Together, this work highlights both central and peripheral targets for intervention directed at buffering the effects of stress on chronic inflammatory disease, particularly in individuals under chronic stress.
Supplementary Material
Highlights.
Chronic stress is associated with increased depression and anxiety and poorer asthma control.
Asthmatics with high chronic stress have an increased cortisol response to the TSST.
Glucose metabolism during stress in the insula and ACC predict greater sympathetic reactivity and enhanced expression of airway inflammatory markers
Acknowledgments
The authors wish to thank Michele Wolff, Holly Eversoll, Eveyln Falibene, and Miranda Hyde for their indispensible contribution to collecting and analyzing data, and Jeanette Mumford for guidance and expertise in statistical analysis and display of data. This research was supported by the National Center for Complementary and Integrative Health (K01-AT006202 to Rosenkranz), a core grant to the Waisman Center from the National Institute of Child Health and Human Development (NICHD) P30HD003352 to Albee Messing, Altermed Research Foundation, Evjue Foundation, and generous donations from individuals to the Center for Investigating Healthy Minds. No donors, either anonymous or identified, have participated in the design, conduct, or reporting of research results in this manuscript.
Footnotes
The FDG brain scan was only 30 min in duration. FDG lung scans were also acquired for an additional 25 min, but not reported on here.
It is noteworthy that these previous studies did not employ a control task with which to compare FeNO response, and though the control task was not associated with an increased cortisol response, the increase in FeNO in our data was comparable to that of the stress task suggesting that perhaps the control task was somewhat stressful as well.
Given the resolution and smoothing of these data, we cannot be precise in our localization of this cluster and our description is meant as an approximation. Nonetheless, the neural correlates of this cluster are more consistent with those of the anterior aspect of this region.
Note IL-1R1, the receptor for IL-1β, was measured because measurement of IL-1β mRNA cannot distinguish between the cleaved, biologically active form and the immature isoform of IL-1β protein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akdeniz C, Tost H, Streit F, Haddad L, Wüst S, Schäfer A, Schneider M, Rietschel M, Kirsch P, Meyer-Lindenberg A. Neuroimaging Evidence for a Role of Neural Social Stress Processing in Ethnic Minority–Associated Environmental Risk. JAMA Psychiatry. 2014;71:672. doi: 10.1001/jamapsychiatry.2014.35. [DOI] [PubMed] [Google Scholar]
- Anonymous. Standardization of spirometry. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm/137.2.493c. [DOI] [PubMed] [Google Scholar]
- Apps MaJ, Lockwood PL, Balsters JH. The role of the midcingulate cortex in monitoring others’ decisions. Front Neurosci. 2013;7:251. doi: 10.3389/fnins.2013.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine J. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–244. doi: 10.1016/S0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Avery Ja, Kerr KL, Ingeholm JE, Burrows K, Bodurka J, Simmons WK. A common gustatory and interoceptive representation in the human mid-insula. Hum Brain Mapp. 2015;36:2996– 3006. doi: 10.1002/hbm.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytekin M, Dweik Ra. Nitric oxide and asthma severity: towards a better understanding of asthma phenotypes. Clin Exp Allergy. 2012;42:614–6. doi: 10.1111/j.1365-2222.2012.03976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagaev V, Aleksandrov V. Visceral-related area in the rat insular cortex. Auton Neurosci. 2006;125:16–21. doi: 10.1016/j.autneu.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Kierstein S, Sharma S, Spaits M, Kinsey SG, Tliba O, Sheridan JF, Panettieri RA, Haczku A. Social Stress Enhances Allergen-Induced Airway Inflammation in Mice and Inhibits Corticosteroid Responsiveness of Cytokine Production. J Immunol. 2009;182:7888–7896. doi: 10.4049/jimmunol.0800891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Machler M, Bolker BM, Walker SC. Fitting Linear Mixed-Effects Models using lme4. J Stat Softw. 2015;67 [Google Scholar]
- Baune BT, Dannlowski U, Domschke K, Janssen DGA, Jordan MA, Ohrmann P, Bauer J, Biros E, Arolt V, Kugel H, Baxter AG, Suslow T. The interleukin 1 beta (IL1B) gene is associated with failure to achieve remission and impaired emotion processing in major depression. Biol Psychiatry. 2010;67:543–9. doi: 10.1016/j.biopsych.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH. An inventory for measuring depression. Arch Gen Psychiatry. 1961:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry. 2013;73:622–30. doi: 10.1016/j.biopsych.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignore MR, Profita M, Gagliardo R, Riccobono L, Chiappara G, Pace E, Gjomarkaj M. Advances in asthma pathophysiology: stepping forward from the Maurizio Vignola experience. Eur Respir Rev. 2015;24:30–9. doi: 10.1183/09059180.10011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix G, Zaers J, Adam LE, Bellemann ME, Ostertag H, Trojan H, Haberkorn U, Doll J, Oberdorfer F, Lorenz WJ. Performance evaluation of a whole-body PET scanner using the NEMA protocol. National Electrical Manufacturers Association J Nucl Med. 1997;38:1614–1623. [PubMed] [Google Scholar]
- Brydon L, Edwards S, Jia H, Mohamed-Ali V, Zachary I, Martin JF, Steptoe A. Psychological stress activates interleukin-1beta gene expression in human mononuclear cells. Brain Behav Immun. 2005;19:540–6. doi: 10.1016/j.bbi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Bufalino C, Hepgul N, Aguglia E, Pariante CM. The role of immune genes in the association between depression and inflammation: A review of recent clinical studies. Brain Behav Immun. 2013;31:31–47. doi: 10.1016/j.bbi.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Carr TF, Kraft M. Update in Asthma 2014. Am J Respir Crit Care Med. 2015;192:157–163. doi: 10.1164/rccm.201503-0597UP. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene. Science (80- ) 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. J Physiol Paris. 2014 doi: 10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. J Allergy Clin Immunol. 2006;117:1014–20. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain Behav Immun. 2007;21:993–9. doi: 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Strunk RC, Bacharier LB, Chan M, Miller GE. Socioeconomic status associated with exhaled nitric oxide responses to acute stress in children with asthma. Brain Behav Immun. 2010;24:444–50. doi: 10.1016/j.bbi.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Jiang T, Chen P, Ouyang J, Xu G, Zeng Z, Sun Y. Emerging tendency towards autoimmune process in major depressive patients: A novel insight from Th17 cells. Psychiatry Res. 2011;188:224–230. doi: 10.1016/j.psychres.2010.10.029. http://dx.doi.org/10.1016/j.psychres.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang P, Li F, Kijlstra A. The effects of Th17 cytokines on the inflammatory mediator production and barrier function of ARPE-19 cells. PLoS One. 2011;6:e18139. doi: 10.1371/journal.pone.0018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RT, Canino GJ, Bird HR, Celedón JC. Violence, abuse, and asthma in Puerto Rican children. Am J Respir Crit Care Med. 2008;178:453–459. doi: 10.1164/rccm.200711-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983;24:385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Curry JM, Hanke ML, Piper MG, Bailey MT, Bringardner BD, Sheridan JF, Marsh CB. Social disruption induces lung inflammation. Brain Behav Immun. 2010;24:394–402. doi: 10.1016/j.bbi.2009.10.019. http://dx.doi.org/10.1016/j.bbi.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey Ka. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21:1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweik Ra, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair Saa, Bleecker E, Busse W, Calhoun WJ, Castro M, Chung KF, Israel E, Jarjour N, Moore W, Peters S, Teague G, Gaston B, Erzurum SC. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010;181:1033–41. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnault S, Kelly EAB, Nettenstrom LM, Cook EB, Seroogy CM, Jarjour NN. Human eosinophils release IL-1β and increase expression of IL-17A in activated CD4+ T lymphocytes. Clin Exp Allergy. 2012;42:1756–64. doi: 10.1111/j.1365-2222.2012.04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe P, Ebeling C, Gordon JR, Befus aD, Vliagoftis H. Opposing effects of short- and long-term stress on airway inflammation. Am J Respir Crit Care Med. 2004;169:220–6. doi: 10.1164/rccm.200307-979OC. [DOI] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. J Abnorm Psychol. 1991;100:555–561. doi: 10.1037/0021-843X.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hannestad J, Subramanyam K, DellaGioia N, Planeta-Wilson B, Weinzimmer D, Pittman B, Carson RE. Glucose Metabolism in the Insula and Cingulate Is Affected by Systemic Inflammation in Humans. J Nucl Med. 2012;53:601–607. doi: 10.2967/jnumed.111.097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray Ma, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009a;66:415–22. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009b;66:407–14. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–13. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer F, Raulf-Heimsoth M, Brüning T. Exhaled breath condensate and airway inflammation. Curr Opin Allergy Clin Immunol. 2009;9:16–22. doi: 10.1097/ACI.0b013e32831d8144. [DOI] [PubMed] [Google Scholar]
- James C, Macefield VG, Henderson LA. Real-time imaging of cortical and subcortical control of muscle sympathetic nerve activity in awake human subjects. Neuroimage. 2013;70:59–65. doi: 10.1016/j.neuroimage.2012.12.047. [DOI] [PubMed] [Google Scholar]
- Juniper EF, Buist aS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest. 1999;115:1265–70. doi: 10.1378/chest.115.5.1265. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–29. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test” - A tool for investigating psychobiologicalstress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–40. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn BM. Asthma linked to psychiatric disorders. JAMA. 2008;299:158–60. doi: 10.1001/jama.2007.54-a. [DOI] [PubMed] [Google Scholar]
- Liu L, Coe C, Swenson C. School Examinations Enhance Airway Inflammation to Antigen Challenge. Am J Respir Crit Care Med. 2002;168:1062–7. doi: 10.1164/rccm.2109065. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho RC, Mak A. The role of interleukin ( IL ) -17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis. 2012;15:183–187. doi: 10.1111/j.1756-185X.2011.01673.x. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Seifert F, Decol R. Activation of central sympathetic networks during innocuous and noxious somatosensory stimulation. Neuroimage. 2011;55:216–24. doi: 10.1016/j.neuroimage.2010.11.061. [DOI] [PubMed] [Google Scholar]
- Marin TJ, Chen E, Munch Ja, Miller GE. Double-exposure to acute stress and chronic family stress is associated with immune changes in children with asthma. Psychosom Med. 2009;71:378–84. doi: 10.1097/PSY.0b013e318199dbc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protection and Damage from Acute and Chronic Stress: Allostasis and Allostatic Overload and Relevance to the Pathophysiology of Psychiatric Disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the Old World Monkey. II: Afferent Cortical Input and Comments on the Claustrum. J Comp Neurol. 1982a;212:23–37. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982b;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Zaki J, Hanelin J, Ludlow DH, Knierim K, Ramachandran T, Glover GH, Mackey SC. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Soc Cogn Affect Neurosci. 2008;3:144–160. doi: 10.1093/scan/nsn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama K, Ohwada K, Sakurada S, Sato N, Sora I, Tamura G, Takayanagi M, Ohno I. The distinctive effects of acute and chronic psychological stress on airway inflammation in a murine model of allergic asthma. Allergol Int. 2007;56:29–35. doi: 10.2332/allergolint.O-06-435. [DOI] [PubMed] [Google Scholar]
- Park SJ, Lee YC. Interleukin-17 regulation: an attractive therapeutic approach for asthma. Respir Res. 2010;11:78. doi: 10.1186/1465-9921-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JMG, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110:16574–9. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Ritz T, Ayala ES, Trueba AF, Vance CD, Auchus RJ. Acute stress-induced increases in exhaled nitric oxide in asthma and their association with endogenous cortisol. Am J Respir Crit Care Med. 2011;183:26–30. doi: 10.1164/rccm.201005-0691OC. [DOI] [PubMed] [Google Scholar]
- Ritz T, Trueba AF. Airway nitric oxide and psychological processes in asthma and health: a review. Ann allergy, asthma Immunol. 2014;112:302–308. doi: 10.1016/j.anai.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Ritz T, Trueba AF, Liu J, Auchus RJ, Rosenfield D. Exhaled Nitric Oxide Decreases during Academic Examination Stress in Asthma. Ann Am Thorac Soc. 2015;12:150908081522008. doi: 10.1513/AnnalsATS.201504-213OC. [DOI] [PubMed] [Google Scholar]
- Ritz T, Trueba AF, Simon E, Auchus RJ. Increases in Exhaled Nitric Oxide After Acute Stress. Psychosom Med. 2014;76:716–725. doi: 10.1097/PSY.0000000000000118. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Nater UM. Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34:469–485. doi: 10.1016/j.psyneuen.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Rosenkranz MA, Busse WW, Johnstone T, Swenson CA, Crisafi GM, Jackson MM, Bosch JA, Sheridan JF, Davidson RJ. Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proc Natl Acad Sci U S A. 2005;102:13319–13324. doi: 10.1073/pnas.0504365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz MA, Busse WW, Sheridan JF, Crisafi GM, Davidson RJ. Are there neurophenotypes for asthma? Functional brain imaging of the interaction between emotion and inflammation in asthma. PLoS One. 2012;7:e40921. doi: 10.1371/journal.pone.0040921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Sacchetti L, Napolitano F, De Chiara V, Motta C, Studer V, Musella a, Barbieri F, Bari M, Bernardi G, Maccarrone M, Usiello a, Centonze D. Interleukin-1 Causes Anxiety by Interacting with the Endocannabinoid System. J Neurosci. 2012;32:13896–13905. doi: 10.1523/JNEUROSCI.1515-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Technical Report R-101: Tests of Hypotheses in Fixed-Effects Linear Models. Cary, NC: 1978. [Google Scholar]
- Schleich FN, Chevremont A, Paulus V, Henket M, Manise M, Seidel L, Louis R. Importance of concomitant local and systemic eosinophilia in uncontrolled asthma. Eur Respir J. 2014;44:97–108. doi: 10.1183/09031936.00201813. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter Ha, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silkoff PE, Carlson M, Bourke T, Katial R, Ogren E, Szefler SJ. The Aerocrine exhaled nitric oxide monitoring system NIOX is cleared by the US Food and Drug Administration for monitoring therapy in asthma. J Allergy Clin Immunol. 2004;114:1241–56. doi: 10.1016/j.jaci.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Silkoff PE, McClean PA, Slutsky AS, Furlott HG, Hoffstein E, Wakita S, Chapman KR, Szalai JP, Zamel N. Marked flow-dependence of exhaled nitric oxide using a new technique to exclude nasal nitric oxide. Am J Respir Crit Care Med. 1997;155:260–267. doi: 10.1164/ajrccm.155.1.9001322. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp. 2013;34:2944–58. doi: 10.1002/hbm.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Spielberger C. Manual for the State-Trait Anxiety Inventory: STAI. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Strine TW, Mokdad AH, Balluz LS, Berry JT, Gonzalez O. Impact of depression and anxiety on quality of life, health behaviors, and asthma control among adults in the United States with asthma, 2006. J Asthma. 2008;45:123–33. doi: 10.1080/02770900701840238. [DOI] [PubMed] [Google Scholar]
- Trueba AF, Smith NB, Auchus RJ, Ritz T. Academic exam stress and depressive mood are associated with reductions in exhaled nitric oxide in healthy individuals. Biol Psychol. 2013;93:206–12. doi: 10.1016/j.biopsycho.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Vazquez-Tello A, Halwani R, Hamid Q, Al-Muhsen S. Glucocorticoid receptor-beta up-regulation and steroid resistance induction by IL-17 and IL-23 cytokine stimulation in peripheral mononuclear cells. J Clin Immunol. 2013;33:466–78. doi: 10.1007/s10875-012-9828-3. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, Büchel C. Dyspnea and pain share emotion-related brain network. Neuroimage. 2009;48:200–206. doi: 10.1016/j.neuroimage.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Waisman A, Hauptmann J, Regen T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015:625–637. doi: 10.1007/s00401-015-1402-7. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–97. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Steinbach SF. Violence: An Unrecognized Environmental Exposure That May Contribute to Greater Asthma Morbidity in High Risk Inner-City Populations. Environ Health Perspect. 2001;109:1085–1089. doi: 10.1289/ehp.011091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Tochino Y, Chibana K, Trudeau JB, Holguin F, Wenzel SE. Nitric oxide and related enzymes in asthma: Relation to severity, enzyme function and inflammation. Clin Exp Allergy. 2012;42:760–768. doi: 10.1111/j.1365-2222.2011.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–74. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.