Abstract
Introduction:
Institutional experience has been associated with improved outcomes for various malignancies, including testicular cancer. The present study evaluated whether institution at orchiectomy was associated with outcomes on active surveillance (AS) for clinical stage (CS) I germ cell tumours (GCT).
Methods:
815 patients with CSI GCT managed with AS at the Princess Margaret Cancer Centre were identified. Princess Margaret is a tertiary academic institution with a multidisciplinary testicular cancer clinic involving radiation oncologists, medical oncologists, and urologists, and has research experience in testicular cancer care. The association between institution of orchiectomy (Princess Margaret vs. Other) and time to progression on AS was analyzed using multivariable Cox proportional hazards models. Academic vs. non-academic institutions were compared in a sensitivity analysis.
Results:
Patients undergoing orchiectomy at Princess Margaret for non-seminoma GCT were significantly less likely to have pure embryonal carcinoma (EC) in orchiectomy pathology (odds ratio [OR] 0.33; p=0.008) and CSIB disease (OR 0.47; p=0.014). Seminoma characteristics did not differ significantly between institution groups. In non-seminoma GCT, median followup was 5.4 years, 27% progressed on AS, and institution of orchiectomy was not associated with time to progression in either univariate (hazard ratio [HR] 0.79; p=0.33) or multivariable analyses (HR 1.01; p=0.97). In seminoma, median followup was 4.7 years, 12% progressed on AS, and institution of orchiectomy was not associated with progression (univariate: HR 0.87; p=0.73; multivariable: HR 0.98; p=0.96). Sensitivity analyses demonstrated similar results.
Conclusions:
Among CSI GCT patients managed on AS at a specialized cancer centre, there appears to be no difference in oncologic outcomes based upon the institution where orchiectomy was performed.
Introduction
Following orchiectomy, progression occurs in approximately 30% of patients with clinical stage I (CSI) non-seminoma germ cell tumours (NSGCT) and approximately 15% of patients with CSI seminoma.1,2 Given the low risk of relapse in CSI disease, active surveillance (AS) has become widely accepted for CSI seminoma and CSIA NSGCT, and is adopted by most centres for CSIB NSGCT.
Several studies in various malignancies have demonstrated that institution characteristics, such as volume, specialization, and academic status, are associated with improved outcomes.3–8 However, it is unknown whether the institution at which an orchiectomy is performed is associated with outcomes on AS. Given that specialized centres tend to have longer wait times for surgery,9 and both institution experience and a physician’s specialization influence a patient’s choice of healthcare provider,10 evidence demonstrating comparable outcomes across institutions may help reassure patients to seek out a timely orchiectomy and reduce the anxiety related to a diagnosis of testicular cancer.11 On the other hand, if institution characteristics are independently associated with oncologic outcomes on AS, initiatives to improve the quality of orchiectomy at underperforming institutions may be needed.
Therefore, the objective of this study was to compare outcomes of patients being managed with AS for CSI GCT after having undergone an orchiectomy at either an institution specializing in testicular cancer care or other academic/community institutions. The primary outcome of this study was to compare progression-free survival (PFS) and the secondary outcome was to compare extent of disease at progression. The hypothesis was that outcomes would be comparable across institutions.
Methods
After obtaining Research Ethics Board approval, we obtained clinical and pathological data from our prospectively maintained surgical database. A total of 930 patients with CSI GCT managed with AS at the Princess Margaret Cancer Centre were identified. Of these, 464 patients underwent orchiectomy for NSGCT between 1980 and 2011, while 466 patients underwent orchiectomy for seminoma between 1998 and 2014. In the case of seminoma, institution of orchiectomy was not reliably recorded prior to 1998. Our AS followup protocols for NSGCT and seminoma have been previously reported.12,13 Progression was defined as imaging or physical examination evidence of metastases and/or elevated tumour markers.
Categorical variables were compared between groups using the Chi-square or Fisher’s exact test, while continuous variables were compared using the t-test.
The primary outcome assessing the association between orchiectomy institution and PFS was analyzed using Cox proportional hazards models. Covariates in these models were age at orchiectomy, institution at orchiectomy, year of orchiectomy, and depending on orchiectomy pathology, overall stage at presentation and pure embryonal carcinoma (EC) in orchiectomy pathology for NSGCT, and primary tumour size for seminoma. Pathology-specific covariates were chosen based on prior studies demonstrating their independent association with progression on AS.14–18 To avoid collinearity, lymphovascular invasion was not included in the models for NSGCT, as this information is captured in the overall stage at presentation (i.e., IA vs. IB). The proportional hazards assumption was examined by evaluating cumulative score statistics.19 Kaplan-Meier curves were constructed, stratifying by institution at orchiectomy.
The secondary outcome assessing the extent of disease at progression was analyzed using logistic regression models, adjusting for the same variables described for the Cox proportional hazard models. Extent of disease at progression was categorized as non-disseminated if visible disease was confined to the retroperitoneum and/or tumour markers were S0 or S1. All other progression was considered disseminated. Hosmer-Lemeshow goodness-of-fit tests were conducted to verify the appropriateness of each model.
Institution at orchiectomy was dichotomized between the Princess Margaret Cancer Centre and other academic or community institutions. Princess Margaret is a specialized tertiary academic institution with a multidisciplinary testicular cancer clinic involving radiation oncologists, medical oncologists, and urologists, and has extensive research experience in testicular cancer care. Outside pathology specimens were reviewed by Princess Margaret genitourinary pathologists in 90% of cases. When available, pathology review from Princess Margaret was used in the analysis.
As a sensitivity analysis, outcomes were evaluated by stratifying institutions based on academic status. An academic institution was defined as a university-affiliated hospital where urology resident physicians trained routinely.
Statistical analyses were performed using SAS (version 9.4, Cary, NC, U.S.). A two-sided p value of 0.05 was considered statistically significant.
Results
NSGCT
Patient characteristics
Of the 464 patients with CSI NSGCT being managed at the Princess Margaret for AS, information regarding the institution at orchiectomy and pathology details were available in 460 (99%) cases. Patients undergoing an orchiectomy at non-specialized institutions were significantly more likely to have pure EC in the orchiectomy pathology (odds ratio [OR] 3.06, 95% confidence interval [CI] 1.28–7.29; p=0.008) and significantly more likely to have CSIB disease (OR 2.13, 95% CI 1.15–3.94; p=0.014) compared to patients undergoing an orchiectomy at Princess Margaret (Table 1).
Table 1.
Demographic data on 460 patients with CSI NSGCT managed with AS at the Princess Margaret Cancer Centre
| Variable | Institution specializing in testicular cancer | Non-specializing institution | p value |
|---|---|---|---|
| Non-seminoma (n=460) | n=88 | n=372 | |
| Year of orchiectomy (n (%)) | |||
| 1980–1995 | 38 (43%) | 148 (40%) | 0.56 |
| 1996–2011 | 50 (57%) | 224 (60%) | |
| Age at orchiectomy (mean (SD)) | 30.7 (7.2) | 30.2 (8.9) | 0.58 |
| Left-sided orchiectomy (n (%)) | 44 (50%) | 161 (43%) | 0.25 |
| Pure EC in orchiectomy pathology (n (%)) | 6 (6.8%) | 68 (18.3%) | 0.008 |
| Overall stage at presentation | |||
| CSIA | 74 (84%) | 265 (71%) | 0.014 |
| CSIB | 14 (16%) | 107 (29%) |
AS: active surveillance; CS: clinical stage; EC: embryonal carcinoma; NSGCT: non-seminoma germ cell tumours SD: standard deviation.
Progression-free survival
Disease progression occurred in 123 (27%) patients with CS1 NSGCT. Age at orchiectomy, pure EC in orchiectomy pathology, and overall stage at presentation were significantly associated with PFS in univariate and multivariable Cox proportional hazard models, while institution at orchiectomy was not significant in either univariate or multivariable analyses (Fig. 1, Table 2). Sensitivity analyses demonstrated similar results in univariate (hazard ratio [HR] 0.76, 95% CI 0.50–1.14; p=0.18) and multivariable analyses (HR 0.90, 95% CI 0.59–1.36; p=0.62) when comparing academic to non-academic institutions.
Fig. 1.
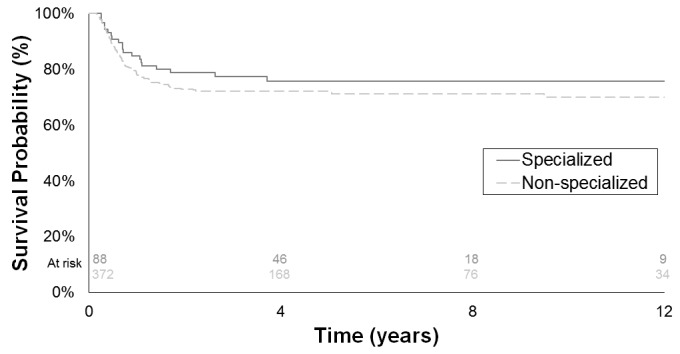
Progression-free survival by institution at orchiectomy in clinical stage I non-seminoma germ cell tumours patients managed with active surveillance.
Table 2.
Cox proportional hazard models for the association between clinical and demographic factors and progression-free survival for CSI NSGCT between specialized vs. non-specialized institutions
| Univariate | Multivariable | |||
|---|---|---|---|---|
|
| ||||
| Variable | HR (95% CI) | p value | HR (95% CI) | p value |
| Age at orchiectomy (continuous) | 0.97 (0.95–0.99) | 0.009 | 0.97 (0.95–0.99) | 0.039 |
| Year of orchiectomy (1996–2011 vs. 1980–1995) | 0.84 (0.59–1.20) | 0.35 | 1.03 (0.71–1.48) | 0.88 |
| Pure EC in orchiectomy pathology (Yes vs. No) | 2.26 (1.51–3.37) | <0.0001 | 1.73 (1.14–2.62) | 0.01 |
| Overall stage at presentation (CSIB vs. CSIA) | 2.96 (2.07–4.22) | <0.0001 | 2.60 (1.79–3.77) | <0.0001 |
| Institution at orchiectomy (Specialized vs. non-specialized institutions) | 0.79 (0.49–1.27) | 0.33 | 1.01 (0.62–1.65) | 0.97 |
CI: confidence interval; CS: clinical stage; EC: embryonal carcinoma; HR: Hazard ratio; NSGCT: non-seminoma germ cell tumour.
Extent of disease at progression
Of the 123 CSI NSGCT patients with disease progression, 30 (24%) had disseminated disease. None of the pathological or clinical variables, including institution at orchiectomy, were associated with extent of disease progression in univariate or multiple logistic regression models (Table 3). Similar results were observed in the univariate (OR 1.11, 95% CI 0.43–2.8; p=0.83) and multivariable (OR 1.00, 95% CI 0.38–2.61; p=0.99) sensitivity analyses when comparing academic to non-academic institutions.
Table 3.
Logistic regression models for the association between clinical and demographic factors and non-regional disease at progression for CSI NSGCT between specialized vs. non-specialized institutions
| Univariate | Multivariable | |||
|---|---|---|---|---|
|
| ||||
| Variable | OR (95% CI) | p value | OR (95% CI) | p value |
| Age at orchiectomy (continuous) | 1.01 (0.96–1.07) | 0.66 | 1.01 (0.96–1.07) | 0.71 |
| Year of orchiectomy (1996–2011 vs. 1980–1995) | 1.17 (0.51–2.69) | 0.70 | 1.07 (0.45–2.54) | 0.87 |
| Pure EC in orchiectomy pathology (Yes vs. No) | 0.78 (0.30–2.05) | 0.62 | 0.79 (0.29–2.12) | 0.64 |
| Overall stage at presentation (CSIB vs. CSIA) | 0.59 (0.25–1.38) | 0.22 | 0.61 (0.26–1.45) | 0.27 |
| Institution at orchiectomy (Specialized vs. non-specialized institutions) | 1.87 (0.67–5.24) | 0.23 | 1.73 (0.61–4.90) | 0.30 |
CI: confidence interval; CS: clinical stage; EC: embryonal carcinoma; NSGCT: non-seminoma germ cell tumour; OR: odds ratio.
Seminoma
Patient characteristics
Of the 466 patients with CSI seminoma being managed at Princess Margaret for AS, information regarding the institution at orchiectomy and pathology details were available in 355 (76%) cases. There was no significant difference in mean tumour size in the orchiectomy pathology between patients undergoing an orchiectomy at non-specialized institutions and at Princess Margaret (Table 4).
Table 4.
Demographic data on 355 patients with CSI seminoma managed with AS at the Princess Margaret Cancer Centre
| Variable | Institution specializing in testicular cancer | Non-specializing institution | p value |
|---|---|---|---|
| Seminoma (n=355) | n=67 | n=288 | 0.36 |
| Year of orchiectomy (n (%)) | |||
| 1998–2006 | 30 (45%) | 147 (51%) | 0.92 |
| 2007–2014 | 37 (55%) | 141 (49%) | |
| Age at orchiectomy (mean (SD)) | 37.4 (9.7) | 37.6 (9.8) | 0.88 |
| Left-sided orchiectomy (n (%)) | 33 (49%) | 139 (48%) | 0.22 |
| Tumour size (cm) (mean (SD)) | 3.32 (1.98) | 3.67 (2.18) | 0.38 |
| Overall stage at presentation | |||
| CSIA | 47 (70%) | 217 (75%) | |
| CSIB | 20 (30%) | 71 (25%) |
AS: active surveillance; CS: clinical stage; SD: standard deviation.
Progression-free survival
Disease progression occurred in 42 (12%) patients with CSI seminoma. Tumour size was significantly associated with PFS in univariate and multivariable. Age at orchiectomy, overall stage at presentation, and institution at orchiectomy were not associated with PFS in either univariate or multivariable analyses (Fig. 2, Table 5). Similar results were observed in the univariate (HR 1.21, 95% CI 0.66–2.23; p=0.53) and multivariable (HR 1.46, 95% CI 0.78–2.72; p=0.24) sensitivity analyses when comparing academic to non-academic institutions.
Fig. 2.

Progression-free survival by institution at orchiectomy in in clinical stage I seminoma patients managed with active surveillance.
Table 5.
Cox proportional hazard models for the association between clinical and demographic factors and progression-free survival for CSI seminoma between specialized vs. non-specialized institutions
| Univariate | Multivariable | |||
|---|---|---|---|---|
|
| ||||
| Variable | HR (95% CI) | p value | HR (95% CI) | p value |
| Age at orchiectomy (continuous) | 0.98 (0.95–1.01) | 0.28 | 0.98 (0.94–1.01) | 0.16 |
| Year of orchiectomy (2007–2014 vs. 1998–2006) | 1.09 (0.58–2.04) | 0.79 | 1.01 (0.54–1.90) | 0.98 |
| Tumour size (cm) | 1.17 (1.04–1.32) | 0.008 | 1.19 (1.05–1.34) | 0.006 |
| Institution at orchiectomy (Specialized vs. non-specialized institutions) | 0.87 (0.38–1.95) | 0.73 | 0.98 (0.43–2.22) | 0.96 |
CI: confidence interval; CS: clinical stage; HR: Hazard ratio.
Extent of disease at progression
Of the 42 patients experiencing progression on AS for CSI seminomas, 4 (9.5%) had non-regional metastatic disease. Given the limited number of progression events in seminoma, extent of disease progression was not compared between groups.
Discussion
The association between institutional experience and survival outcomes has been demonstrated for several malignancies, including testicular cancer.3–8 The present study found no significant difference in PFS or extent of disease at progression between an orchiectomy performed at a specialized vs. non-specialized institutions, and academic vs. non-academic institutions. This data can be used to help reassure patients and reduce the anxiety that is related to a diagnosis of testicular cancer.11
A recent systematic review found that cancers associated with high-risk surgery, such as lung or pancreatic cancer, demonstrated large trends in improved risk-adjusted mortality in higher-volume hospitals.3 Potential theories supporting the volume-outcome association are that high-volume, specialized institutions are more likely to have effective intensive care units, increased availability of fellowship-trained consultants, and improved hand-off of care for treatment in the adjuvant setting.3 This same systematic review found that cancers associated with low-risk surgery, such as surgery for colon or prostate cancer, had improved outcomes in higher-volume centres, but the difference was modest compared to that seen in cancers associated with high-risk surgery. These findings suggest that the degree of improvement in outcomes at high-volume, specialized institutions may be related to the complexity of the surgery.
While the present study demonstrates the oncologic outcomes of orchiectomy, considered a simple, low-risk procedure, are comparable across institutions, several studies in advanced stages of testicular cancer have shown that higher-volume, specialized institutions have improved survival.4–8 The majority of these studies evaluated chemotherapy as the intervention and one study was a randomized, controlled trial comparing primary retroperitoneal lymph node dissection vs. chemotherapy as adjuvant treatment for CSI NSGCT.7 This community-based trial had a relapse rate of 54% from primary retroperitoneal lymph node dissection, a relatively complicated, high-risk procedure. This rate of relapse is much higher than the experience from specialized centres, where the relapse rate is approximately 5–20%.20–23 The authors of the study suggested that lower rates of local recurrence may have been observed if surgery was performed by experienced surgeons at specialized institutions. Taken together, this supports the findings of the previously described systematic review, whereby the institution volume and specialization status are more important for high-risk, complicated procedures than for low-risk, relatively simple procedures.
Unlike procedures in other malignancies, whereby indicators of quality of surgical resection, such as margin status24,25 or node count,26,27 have been associated with outcomes, no such technical factors have been identified in patients undergoing an orchiectomy. Rather, the findings of the present study support that the outcomes on AS are driven by pathological factors.
The present study found that of all patients being managed with AS at the specialized institution, those undergoing an orchiectomy for CSI NSGCT at non-specialized institutions were more likely to have pure EC in orchiectomy pathology and have CS1B disease, both of which are risk factors for progression.14 This may represent a referral bias, whereby patients with orchiectomy features suggesting a high risk of progression are being referred to specialized centres. Despite this baseline difference in patient populations, unadjusted and adjusted analyses failed to demonstrate an association between institution at orchiectomy and PFS or extent of disease at progression.
While a referral bias may have been present for NSGCT, this did not seem to occur in seminoma, as there were no clinical or pathological differences, including tumour size, which is a known risk factor for progression,15–17 in patients undergoing orchiectomy at a specialized vs. non-specialized institutions. This may be related to the overall lower risk of progression in CSI seminoma compared to NSGCT and, therefore, reflective of the relative comfort that clinicians outside specialized institutions have in managing CSI seminoma patients. Similar to NSGCT, institution of orchiectomy was not an independent prognostic factor for outcomes on AS for seminoma patients. The overall lack of association between institution at orchiectomy and outcomes on AS are likely robust, given that they remained consistent when stratifying institutions based on academic status.
This study has limitations. First, due to its retrospective design there may have been factors that were not accounted for, which may have influenced the outcomes assessed. One potential explanation for the lack of difference between institutions is that we analyzed patients already selected as suitable for AS by Princess Margaret physicians. Thus, it could be that any orchiectomies with adverse outcomes (e.g., tumour spill, positive margin, scrotal violation) were treated with immediate adjuvant therapy or observed for a short period (not formal AS) and then received adjuvant therapy. Therefore, it may be possible that in unselected patients with CSI GCT, oncologic outcomes after orchiectomy may differ across institutions. Second, the time frame of our study is over 30 years and thus practice patterns at institutions may have evolved over time, with the increasing emphasis on ensuring adequate cancer care by central quality assurance programs.28 However, given that there was no difference in outcomes among institutions, the improvement of care across all institutions only further suggests that orchiectomy outcomes are similar among institutions in the present day. Third, there were a limited number of events and, therefore, this study may have been underpowered. Population-based studies may provide further insight on the association between institution of orchiectomy and outcomes on AS. Fourth, approximately one-quarter of seminoma patients did not have their institution of orchiectomy and pathology data available, which could represent a selection bias. However, considering that the progression rate in those without these data available was 10.4%, similar to the progression rates of both institution groups in the present study, a selection bias is unlikely. Last, all patients in this study were managed on AS at the Princess Margaret Cancer Centre. While not the focus of this study, future research should evaluate whether the centre managing AS is associated with outcomes. Despite these limitations, the present study is the first to compare outcomes on AS for CSI GCT between institutions and the findings are strengthened by extended followup in patients and consistent results in sensitivity analyses.
Conclusion
Our study of patients managed by AS for CSI GCT at the Princess Margaret Cancer Centre demonstrates that the institution at which an orchiectomy is performed is not an independent prognostic factor for oncologic outcomes. These findings may help reassure patients and reduce anxiety related to a diagnosis testicular cancer.
Footnotes
Competing interests: Dr. Jewett has been an Advisory Board member for Novartis, Pifzer, and Theralase Inc.; has received grants/honoraria from GSK, Novartis, and Pfizer; holds investments in Theralase Inc.; and has participated in clinical trials for GSK, Novartis, and Pfizer. Dr. Hamilton has been an Advisory Board member for Abbvie, Astellas, Bayer, and Janssen; and has participated in clinical trials for Janssen. The remaining authors declare no competing personal or financial interests.
This paper has been peer-reviewed.
References
- 1.Groll RJ, Warde P, Jewett MA. A comprehensive systematic review of testicular germ cell tumour surveillance. Crit Rev Oncol Hematol. 2007;64:182–97. doi: 10.1016/j.critrevonc.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Leung E, Warde P, Jewett M, et al. Treatment burden in stage I seminoma: A comparison of surveillance and adjuvant radiation therapy. BJU Int. 2013;112:1088–95. doi: 10.1111/bju.12330. [DOI] [PubMed] [Google Scholar]
- 3.Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: Importance in quality of cancer care. J Clin Oncol. 2000;18:2327–40. doi: 10.1200/JCO.2000.18.11.2327. [DOI] [PubMed] [Google Scholar]
- 4.Collette L, Sylvester RJ, Stenning SP, et al. Impact of the treating institution on survival of patients with “poor-prognosis” metastatic nonseminoma. European Organization for Research and Treatment of Cancer Genito-Urinary Tract Cancer Collaborative Group and the Medical Research Council Testicular Cancer Working Party. J Natl Cancer Inst. 1999;91:839–46. doi: 10.1093/jnci/91.10.839. [DOI] [PubMed] [Google Scholar]
- 5.Feuer EJ, Frey CM, Brawley OW, et al. After a treatment breakthrough: A comparison of trial and population-based data for advanced testicular cancer. J Clin Oncol. 1994;12:368–77. doi: 10.1200/JCO.1994.12.2.368. [DOI] [PubMed] [Google Scholar]
- 6.Aass N, Klepp O, Cavallin-Stahl E, et al. Prognostic factors in unselected patients with nonseminomatous metastatic testicular cancer: a multicenter experience. J Clin Oncol. 1991;9:818–26. doi: 10.1200/JCO.1991.9.5.818. [DOI] [PubMed] [Google Scholar]
- 7.Albers P, Siener R, Krege S, et al. Randomized phase 3 trial comparing retroperitoneal lymph node dissection with one course of bleomycin and etoposide plus cisplatin chemotherapy in the adjuvant treatment of clinical stage I nonseminomatous testicular germ cell tumours: AUO trial AH 01/94 by the German Testicular Cancer Study Group. J Clin Oncol. 2008;26:2966–72. doi: 10.1200/JCO.2007.12.0899. [DOI] [PubMed] [Google Scholar]
- 8.Harding MJ, Paul J, Gillis CR, et al. Management of malignant teratoma: Does referral to a specialist unit matter? Lancet. 1993;341:999–1002. doi: 10.1016/0140-6736(93)91082-W. [DOI] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States: Trends and predictors of delays. Ann Surg. 2011;253:779–85. doi: 10.1097/SLA.0b013e318211cc0f. [DOI] [PubMed] [Google Scholar]
- 10.Victoor A, Delnoij DM, Friele RD, et al. Determinants of patient choice of healthcare providers: A scoping review. BMC Health Serv Res. 2012;12:272. doi: 10.1186/1472-6963-12-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fosså SD, Dahl AA, Loge JH. Fatigue, anxiety, and depression in long-term survivors of testicular cancer. J Clin Oncol. 2003;21:1249–54. doi: 10.1200/JCO.2003.08.163. [DOI] [PubMed] [Google Scholar]
- 12.Sharir S, Jewett MA, Sturgeon JF, et al. Progression detection of stage I nonseminomatous testis cancer on surveillance: Implications for the followup protocol. J Urol. 1999;161:472–5. doi: 10.1016/S0022-5347(01)61926-8. discussion 475–6. [DOI] [PubMed] [Google Scholar]
- 13.Vesprini D, Chung P, Tolan S, et al. Utility of serum tumor markers during surveillance for stage I seminoma. Cancer. 2012;118:5245–50. doi: 10.1002/cncr.27539. [DOI] [PubMed] [Google Scholar]
- 14.Sturgeon JF, Moore MJ, Kakiashvili DM, et al. Non-risk-adapted surveillance in clinical stage I nonseminomatous germ cell tumours: The Princess Margaret Hospital’s experience. Eur Urol. 2011;59:556–62. doi: 10.1016/j.eururo.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Warde P, Specht L, Horwich A, et al. Prognostic factors for relapse in stage I seminoma managed by surveillance: A pooled analysis. J Clin Oncol. 2002;20:4448–52. doi: 10.1200/JCO.2002.01.038. [DOI] [PubMed] [Google Scholar]
- 16.Chung P, Daugaard G, Tyldesley S, et al. Evaluation of a prognostic model for risk of relapse in stage I seminoma surveillance. Cancer Med. 2015;4:155–60. doi: 10.1002/cam4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von der Maase H, Specht L, Jacobsen GK, et al. Surveillance following orchidectomy for stage I seminoma of the testis. Eur J Cancer. 1993;29A:1931–4. doi: 10.1016/0959-8049(93)90446-M. [DOI] [PubMed] [Google Scholar]
- 18.Kollmannsberger C, Tandstad T, Bedard PL, et al. Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol. 2015;33:51–7. doi: 10.1200/JCO.2014.56.2116. [DOI] [PubMed] [Google Scholar]
- 19.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–57. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 20.Nayan M, Jewett MA, Sweet J, et al. Lymph node yield in primary retroperitoneal lymph node dissection for nonseminoma germ cell tumours. J Urol. 2015;194(2):386–91. doi: 10.1016/j.juro.2015.03.100. [DOI] [PubMed] [Google Scholar]
- 21.Heidenreich A, Albers P, Hartmann M, et al. Complications of primary nerve-sparing retroperitoneal lymph node dissection for clinical stage I nonseminomatous germ cell tumors of the testis: Experience of the German Testicular Cancer Study Group. J Urol. 2003;169:1710–4. doi: 10.1097/01.ju.0000060960.18092.54. [DOI] [PubMed] [Google Scholar]
- 22.Sweeney CJ, Hermans BP, Heilman DK, et al. Results and outcome of retroperitoneal lymph node dissection for clinical stage I embryonal carcinoma--predominant testis cancer. J Clin Oncol. 2000;18:358–62. doi: 10.1200/JCO.2000.18.2.358. [DOI] [PubMed] [Google Scholar]
- 23.Stephenson AJ, Bosl GJ, Bajorin DF, et al. Retroperitoneal lymph node dissection in patients with low stage testicular cancer with embryonal carcinoma predominance and/or lymphovascular invasion. J Urol. 2005;174:557–60. doi: 10.1097/01.ju.0000165163.03805.37. discussion 560. [DOI] [PubMed] [Google Scholar]
- 24.Park CC, Mitsumori M, Nixon A, et al. Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: Influence of margin status and systemic therapy on local recurrence. J Clin Oncol. 2000;18:1668–75. doi: 10.1200/JCO.2000.18.8.1668. [DOI] [PubMed] [Google Scholar]
- 25.Swindle P, Eastham JA, Ohori M, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2005;174:903–7. doi: 10.1097/01.ju.0000169475.00949.78. [DOI] [PubMed] [Google Scholar]
- 26.Johnson PM, Porter GA, Ricciardi R, et al. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol. 2006;24:3570–5. doi: 10.1200/JCO.2006.06.8866. [DOI] [PubMed] [Google Scholar]
- 27.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: Data from a large U.S.population database. J Clin Oncol. 2005;23:7114–24. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg A, Angus H, Sullivan T, et al. Development of a set of strategy-based system-level cancer care performance indicators in Ontario, Canada. Int J Qual Health Care. 2005;17:107–14. doi: 10.1093/intqhc/mzi007. [DOI] [PubMed] [Google Scholar]


