Abstract
MicroRNAs (miRNAs) regulate tumorigenesis by inhibiting gene expression. In this study, we showed that miR-320a expression is decreased in human gastric cancer tissues and correlates inversely with expression of FoxM1, a key cell cycle regulator involved in gastric carcinoma. By contrast, the expression of P27KIP1, a downstream effector of FoxM1, correlates positively with miR-320a levels. Luciferase assays indicate that miR-320a suppresses FoxM1 expression, and in vitro recovery tests using FoxM1 siRNA indicate miR-320a inhibits gastric cancer cell proliferation by suppressing activity in the FoxM1-P27KIP1 axis. In vivo, nude mice injected with BGC-823 gastric cancer cells expressing a miR-320a inhibitor exhibit faster tumor growth than mice injected with control cells. Analysis of FoxM1 and P27KIP1 expression in tumor tissues indicates that miR-320a suppression increases the tumor growth by enhancing FoxM1-P27KIP1 signaling. These results thus reveal the crucial role played by miR-320a in limiting gastric carcinoma by directly targeting FoxM1- P27KIP1 axis.
Keywords: miR-320a, FoxM1, proliferation, gastric cancer, P27KIP1
INTRODUCTION
Gastric cancer is the fourth most commonly diagnosed cancer and the second leading cause of cancer death worldwide, especially in developing countries [1, 2]. Although the incidence has gradually decreased, there are about 700,000 confirmed mortalities annually worldwide [3]. Gastric cancer is generally diagnosed at an advanced stage, which is the primary cause of its poor prognosis [4]. Infection with Helicobacter pylori is one of the most important factors contributing to the development of gastric carcinoma [5]. In order to improve the outcome of gastric cancer, identification of genetic and epigenetic events regulating the proliferation of gastric cancer cells is required. System biological analyses of gastric cancer samples have shown the importance of microRNAs (miRNAs) in this process [6, 7].
MiRNAs are small noncoding RNAs with 18-24 nucleotides in length [8]. They have emerged as important posttranscriptional regulators, which negatively regulate gene expression by directly targeting the three prime untranslated region (3′-UTR) of mRNAs, either promoting the degradation of target mRNAs or preventing their translation [9, 10]. MiRNAs are estimated to regulate up to 30% of genes in the human genome [11]. Over the past few years, studies of miRNAs have revealed that miRNAs participate in regulation of various biological processes, such as cell proliferation, apoptosis, and differentiation, as well as tumor development, metastasis, angiogenesis, and immune responses. MiRNAs play important roles in tumorigenesis [12, 13]. Some miRNAs act as tumor suppressors or oncogenes, depending on the function of their target genes [14, 15]. Recent studies also indicate that some miRNAs contribute to gastric carcinoma, including activated miRNAs (such as miR-21, miR-107, miR-222, and miR-106b) and suppressed miRNAs (such as miR-143, miR145, miR-622, and miR-148a) [16]. We have previously found that gastric carcinoma samples have activated several signal pathways, including the Forkhead box M1 (FoxM1) signaling pathway, which includes special miR-320a [17, 18]. MiR-320a has been identified to act as a tumor suppressor in some types of cancers, such as colon cancer, breast cancer, and bladder cancer [19–21]. More work needs be done to elucidate the role of miR-320a in gastric carcinoma.
FoxM1 is a member of the Fox transcription factor family, which is a key stimulator of cell proliferation and functions as an oncogene [22, 23]. We have previously reported that FoxM1 is activated in gastric cancer and that it induces gastric cancer cell proliferation by the inhibition of P27KIP1 [17]. P27KIP1 is an important negative molecular checkpoint [24]. In this study, we aimed to identify the role of miR-320a in gastric carcinoma and the down-stream FoxM1 and P27KIP1 regulatory mechanisms both in vitro and in vivo.
RESULTS
Human gastric cancer tissues exhibit low miR-320a expression and increased FoxM1 expression
We carried out microdissection to obtain gastric epithelial cells from gastric cancer and normal tissues, and extracted RNA for microarray analysis. By analyzing the microarray data, we found that some important signaling pathways, such as NF-κB, Wnt/β-catenin, Ras-MAPK, and FoxM1 were activated in gastric carcinoma. Bioinformatics databases [miRanda (http://www.microrna.org), TargetScan (http://www.targetscan.org), and miRBase (http://www.mirbase.org)] predicted that miR-320a is an up-stream regulator of FoxM1. Compared with normal human tissues, gastric cancer specimens showed significant inhibition of miR-320a expression and activation of FoxM1 (Figure 1A, 1B and 1C). By qRT-PCR and immunohistochemistry (IHC), we observed suppression of miR-320a expression and activation of FoxM1 in 10 cases (71.5%). At the same sites, we observed strong P27KIP1 expression, the down-stream target of FoxM1, in the normal tissues and weak expression in the gastric cancer tissues (Figure 1D, 1E). This clinical evidence supports the negative association of miR-320a and FoxM1 expression in gastric cancer. The gene expression was not associated with age, gender, and specimen histology (Supplementary Table S1).
Figure 1. Association of miR-320a reduced expression and increased FoxM1 expression with the inhibition of P27KIP1 in human gastric cancer.

QRT-PCR analyses of A. miR-320a and B. FoxM1 mRNA in normal and cancerous human gastric tissues. *P < 0.05. C. Correlation of miR-320a and FoxM1levels in human gastric cancer tissues after standardization with matched normal tissues. **P < 0.01. D. HE staining and IHC staining of FoxM1 and P27KIP1 in human normal (left panel) and cancerous (right panel) gastric tissues. Representative images are shown. E. Percentage of FoxM1 and P27KIP1 positive cells analyzed by IHC in human normal and cancerous gastric tissues. **P < 0.01. Data are mean ± SEM of 3 independent experiments.
FoxM1- P27KIP1 axis is a direct target of miR-320a
The effect of miR-320a on FoxM1 expression was evaluated using overexpression of miR-320a mimics and inhibitors. In AGS, BGC-823, and HGC-27 cells, the overexpression of miR-320 resulted in the inhibition of FoxM1 mRNA expression and the recovery of P27KIP1 expression (Figure 2A, 2B and 2C). Since these cells are at different differentiation stages, they have different transfection efficiency with miR-320a mimics. The protein levels of FoxM1 and P27KIP1 were also affected by miR-320a (Figure 2G-2J). On the contrary, the inhibition of miR-320a resulted in the overexpression of FoxM1 and the decreased expression of P27KIP1 both at the mRNA and protein levels (Figure 2D-2J).
Figure 2. The effect of miR-320a on FoxM1 and P27KIP1 expression in human gastric cancer cells.
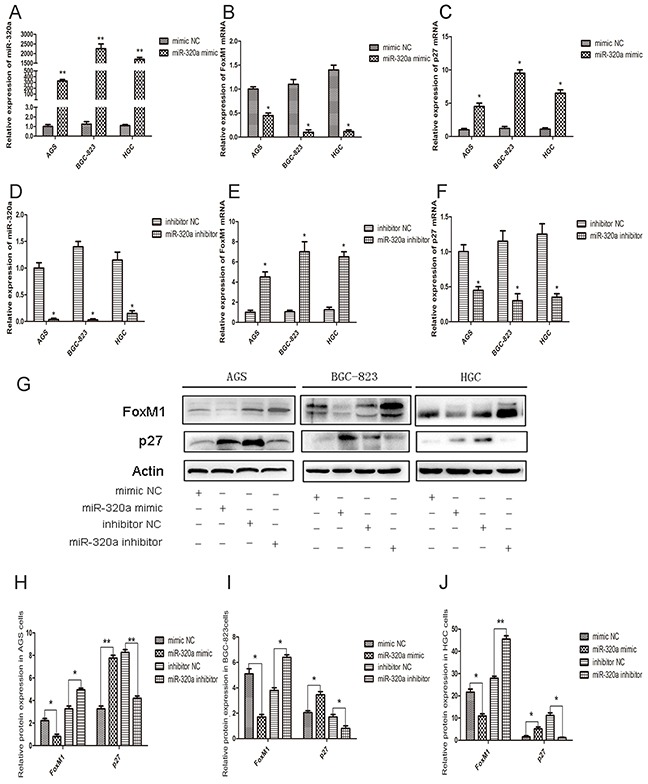
qRT-PCR analyses of A. miR-320a, B. FoxM1 and C. P27KIP1 mRNA levels in control and miR-320a mimics-transfected AGS, BGC-823 and HGC-27cell lines after 48 h. *P < 0.05 and **P < 0.01. D. miR-320a, E. FoxM1 and F. P27KIP1 mRNA levels in control and miR-320a inhibitors-transfected AGS, BGC-823 and HGC-27 cells after 48 h. *P < 0.05 and **P < 0.01. G. Western blot analyses of FoxM1 and P27KIP1 protein levels in gastric cancer cells treated with control and miR-320a mimics or inhibitors. H, I, J. Western blot analyses of FoxM1 and P27KIP1 protein levels. *P < 0.05 and **P < 0.01. Data are mean ± SEM of 3 independent experiments. The “mimic NC” means “mimic negative control”, and the “inhibitor NC” as “inhibitor negative control”.
In order to determine whether miR-320a affects the P27KIP1 expression by suppressing FoxM1, the recovery experiment was performed. With the transfection of miR-320a inhibitors, the FoxM1 expression increased with P27KIP1 inhibition in all the gastric cancer cell lines. While with the co-transfection of miR-320a inhibitors and the special FoxM1 siRNA, both the expression of FoxM1 and P27KIP1 recovered to the normal levels (Figure 3A, 3B).
Figure 3. The recovery experiment of miR-320a's regulation of P27KIP1 expression through FoxM1in human gastric cancer cells.
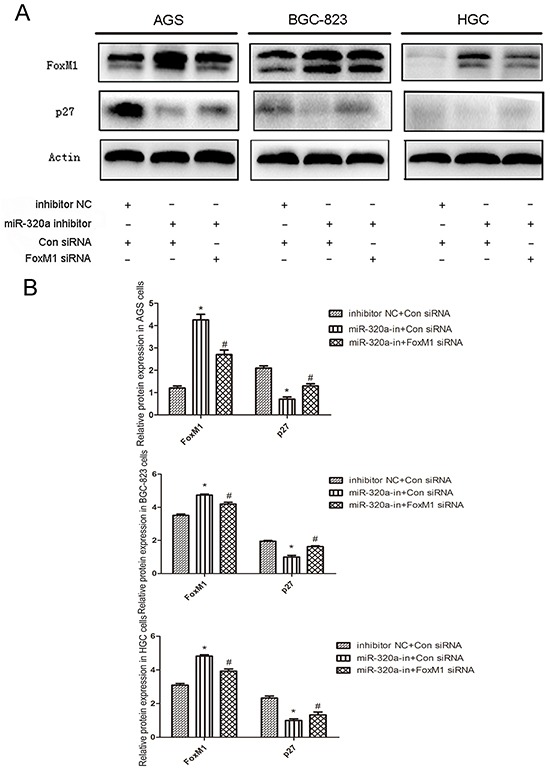
A. Western blot analyses of FoxM1 and P27KIP1 protein levels in gastric cancer cells treated with control and miR-320a inhibitors or FoxM1 siRNA for 48 h. B. Western blot analyses of FoxM1 and P27KIP1 protein levels. *P < 0.05 and **P < 0.01. Data are mean ± SEM of 3 independent experiments.
Next, we validated FoxM1 as a direct target of miR-320a by luciferase report assay. In AGS, BGC-823 and HGC-27 cells, miR-320a mimics were co-transfected with FoxM1 wild-type or mutant-type 3′-UTR plasmids. Co-transfection of miR-320a and wild-type 3′-UTR plasmid reduced the luciferase activity by approximately 62% relative to the control, whereas mutant 3′-UTR co-transfection almost restored the luciferase activity (Figure 4A, 4B and 4C). Thus, miR-320a directly targeted the binding site located at FoxM1 3′-UTR and FoxM1- P27KIP1 axis may be a direct target of miR-320a.
Figure 4. miR-320a directly bound to the 3′-UTR of FoxM1.
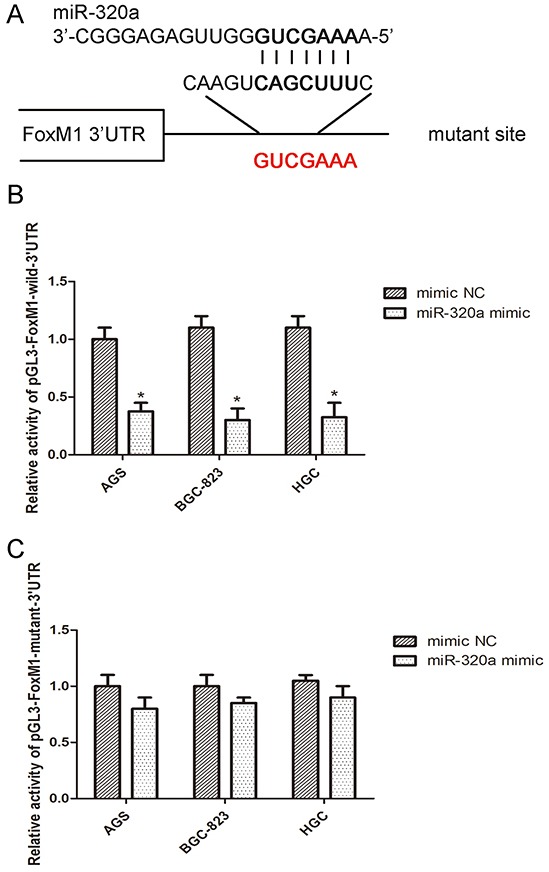
A. The wild sequence on 3′-UTR of FoxM1 that could be bound by miR-320a and the corresponding mutant sequence. B. Luciferase activity assay with pMIR-REPORT-FoxM1-3′-UTR co-transfected with miR-320a mimics or the negative control in the three gastric cancer cells for 48h. *P <0.05 vs. con. C. Luciferase activity assay with pMIR-REPORT-FoxM1-3′-UTR mutant co-transfected with miR-320a mimics or the negative control in the three gastric cancer cells for 48 h. No significant difference. Data are mean ± SEM of 3 independent experiments.
miR-320a regulates proliferation of gastric cancer cells through FoxM1- P27KIP1 axis
Colony formation assay in AGS, BGC-823, and HGC-27 cells revealed that enforced expression or knockdown of miR-320a affected cloning of cells (Figure 5A). Transfection of miR-320a mimics reduced the number of colonies, while the inhibition of miR-320a markedly increased the number of colonies (Figure 5B).
Figure 5. miR-320a was involved in gastric cells proliferation.
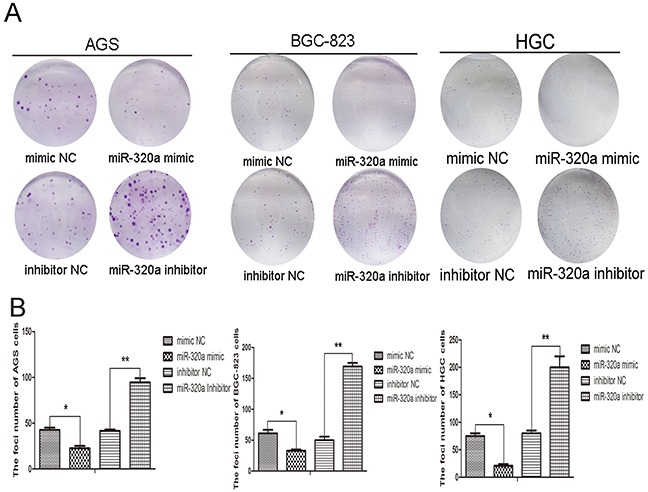
A. Colony formation ability in AGS, BGC-823 and HGC-27 cells with overexpression and knockdown of miR-320a and B. quantification. *P < 0.05 and **P < 0.01. Data are mean ± SEM of 3 independent experiments.
The recovery experiment was done also on clone genetics to determine the role of FoxM1- P27KIP1 axis in the biological activity of miR-320a. As shown in Figure 6, inhibition of miR-320a resulted in the increase of colonies in all three gastric cancer cell lines, while co-transfection of miR-320a inhibitors and FoxM1 siRNA almost recovered the number (Figure 6A, 6B). Therefore, miR-320a inhibited the proliferation of human gastric cells through the regulation on FoxM1- P27KIP1 axis in vitro.
Figure 6. The recovery experiment for miR-320a of colon genetics.
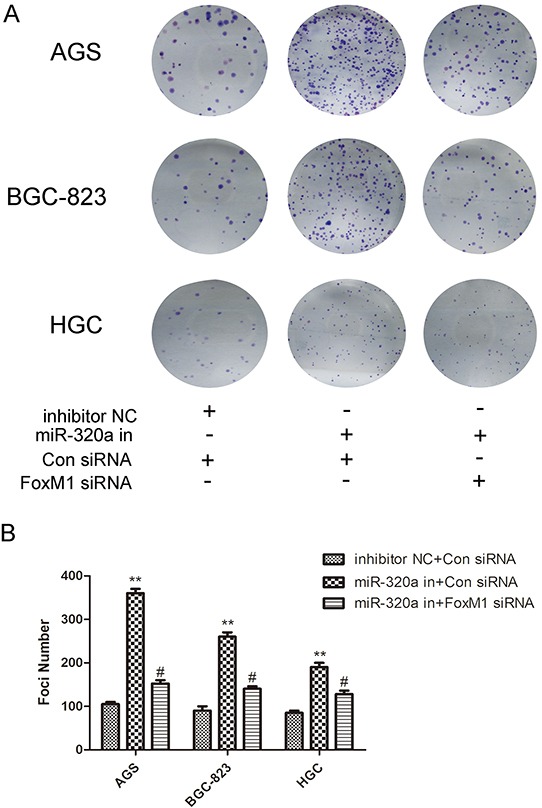
A. Colony formation ability in AGS, BGC-823 and HGC-27 cells with knockdown of miR-320a or co-knockdown of FoxM1 siRNA (5μM) and B. quantification. *P < 0.05 and **P < 0.01. Data are mean ± SEM of 3 independent experiments.
miR-320a suppression increases gastric tumor growth in nude mice through altered FoxM1- P27KIP1 signaling
To evaluate the effect of miR-320a knockdown on the gastric tumor growth in vivo, we established stable lentiviral-miR-320a inhibitor-transfected BGC-823 cells and injected them subcutaneously into nude mice. In contrast to control cells, lentiviral-miR-320a inhibitor-transfected BGC-823 cells produced much larger gastric tumors with faster growth (Figure 7A, 7B). To identify the mechanisms involved in miR-320a-associated gastric tumor growth, we examined the effect of miR-320a inhibition on FoxM1 and P27KIP1 expression in the tumors. QRT-PCR and IHC analyses demonstrated that silencing of miR-320a increased expression of FoxM1 and decreased expression of P27KIP1 (Figure 7C, 7D and 7E). These results suggest that miR-320a suppression increases the tumor growth through altered FoxM1- P27KIP1 signaling.
Figure 7. Overexpression of FoxM1 and inhibition of P27KIP1 by miR-320a knockdown in nude mice.

A. Tumorigenesis after injection of BGC-823 cells stably expressing miR-320a and controls. B. Tumor volume with stably expressing miR-320a and controls. C. Growth curve with stably expressing miR-320a and control. **P<0.01. D. QRT-PCR analysis of FoxM1 and P27KIP1 mRNA expression with stably expressing miR-320a and control. *P<0.05 and **P<0.01. E. HE and IHC staining for FoxM1 and P27KIP1 in the control group (left panel) and miR-320a inhibitors stable expression group (right panel). Representative images are shown. F. Percentage positive area for FoxM1 and P27KIP1 expression with the control and miR-320a inhibitors stable expression determined immunohistochemically. **P<0.01.
DISCUSSION
In this study, we demonstrate that miR-320a has an anti-tumor role in gastric cancer. miR-320a directly inhibits the expression of FoxM1 through the binding of FoxM1 3′-UTR, resulting in the increased expression of P27KIP1. Our in vitro and in vivo data indicate that the biological activity of miR-320a is to inhibit the gastric cancer cell proliferation.
Approximately 72% of gastric cancer occurs in developing countries, especially in Asia (e.g. Korea, Japan, and China) and parts of South America [25]. Approximately two thirds of gastric cancer patients show tumor metastasis or local invasion at diagnosis [26]. Surgery is the treatment of choice for early stages of the disease. However, the median survival time is only 6 to 9 months for later stage of disease after surgical treatment [27]. Thus, early detection and treatment of gastric cancer are the key to prolong patients’ survival. Identification and evaluation of novel molecules that are involved in the different stages of gastric cancer genesis and development are important to improve diagnosis and management of gastric cancer.
An extensive amount of research has led to the identification of miRNAs as important regulators of gene expression in cancer biology [28, 29]. Different types of miRNAs can play different roles in gastric carcinoma, such as cell survival, apoptosis, proliferation and cell death [30–33]. Microdissection and microarrays detected different mRNAs and miRNAs in human gastric cancer samples and normal tissues. Some pathways identified in gastric carcinoma, such as Ras-MAPK and Wnt/β-catenin signaling pathways, were also found in this study. We also found an important miRNA, miR-320a, in this study. There are few studies of the function of miR-320a, including its inhibition of ARDP-19/ERRr in breast cancer and targeting ITGB3 in bladder carcinoma [19, 20]. It also functions in atherogenesis and survivin/apoptosis [34, 35]. Bioinformatics analysis indicated that the target of miR-320a is FoxM1. Aberrant expression of FoxM1 is involved in several tumor types, including hepatocellular carcinoma, basal cell carcinoma, breast cancer, lung cancer, prostate cancer, glioblastomas, and gastric cancer, suggesting its oncogenic role in carcinogenesis [36]. Some miRNAs have been identified to regulate the expression of FoxM1, including miR-149, miR-134, miR-370, miR-494, miR-194, and miR-24-1 [37–43]. Thus, we analyzed the expression of miR-320a and FoxM1 in human gastric cancer species. The results of qRT-PCR and IHC showed that the expression of miR-320a was decreased in gastric cancer samples and negatively correlated with FoxM1 expression. Our previous study has shown that P27KIP1, one of the important inhibitors of cell cycle and a tumor suppressor, is the down-stream target of FoxM1 and regulated by FoxM1 activity [17]. The results in human samples also showed that miR-320a expression negatively correlated with FoxM1 expression but positively with P27KIP1. The luciferase assay and the recovery experiment further revealed that miR-320a controlled the expression of P27KIP1 through directly binding to the 3′-UTR of FoxM1, thus inhibiting FoxM1 expression. The biological activity of miR-320a was examined in vitro and in vivo. The results of clone genetics and the recovery experiment showed that the inhibition of cell proliferation with miR-320 overexpression was through the regulation on FoxM1- P27KIP1 axis. The nude mice models confirmed that the inhibition of miR-320a could improve tumorigenesis with overexpression of FoxM1 and decrease of P27KIP1. The potential mechanisms of miR-320a's down-regulation of gastric cancer may be diverse. One possibility might be the change of chromosome. Most miRNAs associated with tumors are located in the cancer-associated genomic regions (CAGRs), where they are prone to gene rearrangement, deletion, amplification, etc. [44]. In addition, genetic mutations and single nucleotide polymorphisms (SNP) may also lead to the miRNA's abnormal expression. SNP may affect the transcription of pri-pre-miRNA or produce new binding sites of mRNA-miRNA [45]. Epigenetics could also affect the level of miRNAs. We have previously shown that RBP2, an H3K4 demethylase on di- and tri- methylation, could regulate the expression of miR-21 in gastric cancer [46].
Altogether, our data have revealed a crucial role of miR-320a in limiting the gastric carcinoma by directly targeting FoxM1- P27KIP1 axis. Considering the major role of FoxM1 in many types of cancer, miR-320a might be a promising agent to treat other cancers, such as hepatic carcinoma. Based on our findings, we propose that miR-320a might be useful as an anti-gastric cancer therapeutic agent.
MATERIALS AND METHODS
Patients and tissue specimens
The study was approved by the Ethics committee of Shandong University School of Medicine (Jinan, PR China). Resected tissues from 22 gastric cancer patients and distal normal gastric tissues (>5 cm from the margin of the tumor) were harvested at surgery. The patients underwent the surgeries at Qilu Hospital, Shandong University (Jinan, PR China) during 2014 and 2015. None of the patients had received adjuvant chemotherapies before surgery. The diagnosis of all gastric cancers was histopathologically confirmed by examination of the surgical specimens. Microdissection (PALM MicroBeam, ZEISS, Germany) and microarray (Exiqon LNATM microRNA and Roche-NimbleGen, KANGCHEN, Shanghai, PR China) were used for sample analysis. Five normal and five gastric cancer samples from Qilu Hospital, Shandong University (Jinan, PR China) were analyzed. Total RNA was harvested using TRIzol (Invitrogen) and RNeasy mini kit (QIAGEN) according to manufacturer's instructions. After having passed RNA measurement on the Nanodrop instrument, the samples were labeled and detected by KANGCHENG using the miRCURY™ Hy3™/Hy5™ Power labeling kit and hybridized on the miRCURY™ LNA Array (v.14.0). The samples were hybridized on a hybridization station. The scanning was performed with the Axon GenePix 4000B microarray scanner. GenePix pro V6.0 was used to read the raw intensity of the images. The miRNA expression profiling was completed on the samples. The profiling identified that miR-320a was inhibited in human gastric cancer tissues vs. the normal controls. Detailed patient and disease characteristics are documented in Supplementary Table S1.
Animal models
Sixteen (8-10 weeks old) male nude mice were purchased from QING ZI LAN Animal Company (Nanjing, China) and divided into two groups with one as control and the other as miR-320a inhibition. The mice were subcutaneously injected with 4×105 BGC-823 cells per mouse. One group was injected with miR-320a inhibitor stable-transduction cells and the other group was injected with the matched control cells. Two weeks later, the mice were sacrificed and the tumor tissues were harvested and photographed. Tissue sections were attained with traditional method and HE staining was performed.
Cell lines and cell culture
The gastric epithelial-derived cell lines, AGS, BGC-823 and HGC-27 were used for the study. AGS cells were cultured in F12 medium supplemented with 10% fetal bovine serum (FBS, Gibco, USA). BGC-823 and HGC-27 cells were cultured in RPMI 1640 medium supplemented with 10% FBS. All cells were cultured in 5% CO2-air at 37°C. All cells were plated for 18-24 h before biological function detections were performed in 6-well plates.
RNA extraction and qRT-PCR
Total RNA of tissue samples and cultured cells was extracted using TRIzol reagent (Invitrogen, USA). Quantitative real-time PCR (qRT-PCR) assays were carried out to detect mRNA expression using the First Strand cDNA Synthesis Kit (Fermentas, Canada) and SUBR Premix Ex Taq™ (TaKaRa, Japan) according to the manufacturer's instructions. β-actin expression was used as the control. The sequences of the PCR primers were: FoxM1, 5′-TGCAGCTAGGGATGTGAATCTTC-3′ (Forward) and 5′-GGAGCCCAGTCCATCAGAACT-3′ (Reverse); p27, 5′-ATGTCAAACGTGCGAGTGTCTAA-3′ (Forward) and 5′-TTACGTTTGACGTCTTCTGAGG-3′ (Reverse). QRT-PCR analyses for miRNAs were performed by using TaqMan miRNA assays (Applied Biosystems, USA). U6 small nuclear RNA was used as endogenous control for data normalization. Real-time PCR was carried out in an ABI7500 sequence detector (Applied Biosystems, USA). All RT-PCR reactions were conducted in triplicates, and relative quantification was calculated by the 2−ΔΔCt method (95% confidence interval) with calibration to the corresponding endogenous control.
Western blotting and immunohistochemistry (IHC)
Immunoblotting was performed according to standard western blot procedures. Briefly, proteins (20 mg) were separated by 10% SDS-PAGE and transferred to PVDF membrane (Bio-Rad, USA). After blocking in 5% nonfat milk, the membranes were incubated with the primary antibodies, FoxM1 (1:1000) and P27KIP1 (1:300), followed by 1h incubation at RT with the corresponding secondary antibody (1:1000). β-actin was used as a loading control.
FoxM1 expression in tissue specimens was detected by IHC staining according to standard protocols. For incubation with primary antibodies (FoxM1 1:200 and P27KIP1 1:150), tissue slides were incubated at 4°C overnight. Negative controls were treated identically, but without the primary antibody. The primary antibodies for p27 and FoxM1 were purchased from Santa Cruz Biotechnology (USA): p27(C-19), CAT.# sc-528 and FoxM1(H-19), CAT.# sc-501.
Luciferase assay and transfection
The mimics and the inhibitor of miR-320a were purchased from Ruibo (Guangzhou, PR China). FoxM1 siRNA was purchased from Sigma (USA). The special fragment of the FoxM1 3′-UTR containing the miR-320A predicted target site was synthesized by Invitrogen (USA). Then the fragment was cloned into the multiple cloning sequence of the luciferase reporter pMIR-REPOTR (Applied Biosystems, USA), designated as pMIR-REPORT-FoxM1-3′-UTR (with the binding site CAGCUUU), which was also used in PCR to generate pMIR-REPORT-FoxM1-3′-UTRmut plasmid with mutation of the binding sites on the 3′-UTR of FoxM1(with the binding site mutation GUCGAAA). To examine the direct conjugation of miR-320a to the 3′-UTR of FoxM1, pMIR-REPORT- FoxM1-3′-UTR and pMIR-REPORT- FoxM1-3′-UTRmut were co-transfected into AGS, BGC-823 and HGC-27 cell lines with miR-320a mimics. pMIR-REPORT β-gal plasmid was used as a negative control. Luciferase activity in the cell lysates was determined by a single luciferase reporter assay (Promega, USA) 48 h after transfection, and target promoter-driven firefly luciferase activity was normalized to that of β-gal.
For transient transfection, cells were seeded in 6-well plates (3×105cells/well) for 18 to 24 h, then transfected with plasmids/miRNA mimics and inhibitors/FoxM1 siRNA by the use of Lipofectamine 3000 (Invitrogen, USA) with the standard protocol.
Clone formation assay
AGS, BGC-823, and HGC-27 cells were incubated in 6-well plates for 18-24 h, and transfected with the corresponding plasmids/ miRNA mimics and inhibitors for 48 h. Single cells were seeded on 6-well plates (300-500 cells/well). After 10-14 days of incubation, plates were stained with Giemsa for 20 min. The number of colonies with more than 50 cells was counted.
Statistical analysis
FoxM1, P27KIP1 and miR-320a expression in different tissue samples analyzed by qRT-PCR and IHC was evaluated by One-Way ANOVA. The comparison of FoxM1, P27KIP1 and miR-320a expression, and foci numbers after different treatments was made with a Student's t-test. All the tests were determined using the 2-tailed Student t test in SPSS software, version 15.0. Results are expressed as the mean ± standard error of the mean. P values of <0.05 were considered to be statistically significant.
SUPPLEMENTARY TABLE
Acknowledgments
The study was supported by the National Natural Science Foundation of China (nos. 81272654, 81172354, 81171536, and 81371781) and the National Basic Research Program of China (973 Program, 2012CB911202).
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A, Lieto E, Ciardiello F, De Vita F. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635–1649. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peek RM Jr., Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 6.Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4:143–159. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13:358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Y, Yu X, Hu S, Yu J. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics. 2009;7:147–154. doi: 10.1016/S1672-0229(08)60044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ. Biological functions of microRNAs: a review. J Physiol Biochem. 2011;67:129–139. doi: 10.1007/s13105-010-0050-6. [DOI] [PubMed] [Google Scholar]
- 11.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 12.Iorio MV, Croce CM. MicroRNA involvement in human cancer. Carcinogenesis. 2012;33:1126–1133. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, Bader AG. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70:5923–5930. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Qiu C, Zhang H, Wang J, Cui Q, Yin Y. Human microRNA oncogenes and tumor suppressors show significantly different biological patterns: from functions to targets. PLoS One. 2010;5:e13067. doi: 10.1371/journal.pone.0013067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JL, Hu Y, Kong X, Wang ZH, Chen HY, Xu J, Fang JY. Candidate microRNA biomarkers in human gastric cancer: a systematic review and validation study. PloS One. 2013;8:e73683. doi: 10.1371/journal.pone.0073683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng J, Wang L, Li Q, Li W, Björkholm M, Jia J, Xu D. FoxM1 is up-regulated in gastric cancer and its inhibition leads to cellular senescence, partially dependent on p27kip1. J Pathol. 2009;218:419–427. doi: 10.1002/path.2530. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, Wang L, Zeng J, Shen L, Liang X, Yu H, Liu S, Liu Z, Sun Y, Li W, Chen C, Jia J. FoxM1 is overexpressed in Helicobacter pylori–induced gastric carcinogenesis and is negatively regulated by miR-370. Mol Cancer Res. 2013;11:834–844. doi: 10.1158/1541-7786.MCR-13-0007. [DOI] [PubMed] [Google Scholar]
- 19.Sun JY, Huang Y, Li JP, Zhang X, Wang L, Meng YL, Yan B, Bian YQ, Zhao J, Wang WZ, Yang AG, Zhang R. MicroRNA-320a suppresses human colon cancer cell proliferation by directly targeting β-catenin. Biochem Biophys Res Commun. 2012;420:787–792. doi: 10.1016/j.bbrc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 20.Lü M, Ding K, Zhang G, Yin M, Yao G, Tian H, Lian J, Liu L, Liang M, Zhu T, Sun F. MicroRNA-320a sensitizes tamoxifen-resistant breast cancer cells to tamoxifen by targeting ARPP-19 and ERRγ. Sci Rep. 2015;5:8735. doi: 10.1038/srep08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang C, Zhang H, Guo Y, Hong Y, Liu Y, Xue Y. MiR-320a down-regulation mediates bladder carcinoma invasion by targeting ITGB3. Mol Biol Rep. 2014;41:2521–2527. doi: 10.1007/s11033-014-3110-0. [DOI] [PubMed] [Google Scholar]
- 22.Raychaudhuri P, Park HJ. FoxM1: a master regulator of tumor metastasis. Cancer Res. 2011;71:4329–4333. doi: 10.1158/0008-5472.CAN-11-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halasi M, Gartel A L. FOX (M1) news–it is cancer. Mol Cancer Ther. 2013;12:245–254. doi: 10.1158/1535-7163.MCT-12-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanchuk S M, Rutka J T. The cell cycle: accelerators, brakes, and checkpoints. Neurosurgery. 2004;54:692–699. doi: 10.1227/01.neu.0000109534.28063.5d. [DOI] [PubMed] [Google Scholar]
- 25.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 26.Bamboat ZM, DeMatteo RP. Updates on the management of gastrointestinal stromal tumors. Surg Oncol Clin N Am. 2012;21:301–316. doi: 10.1016/j.soc.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Lim do H, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, Bae JM, Ahn YC, Sohn I, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 28.Vandenboom Ii TG, Li Y, Philip PA, Sarkar FH. MicroRNA and cancer: tiny molecules with major implications. Curr Genomics. 2008;9:97–109. doi: 10.2174/138920208784139555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esquela-Kerscher A, Slack FJ. Oncomirs–microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 30.Yang H, Kong W, He L, Zhao JJ, O'Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Sun Q, Zhang Z, Ge S, Han ZG, Chen WT. Loss of microRNA-143/145 disturbs cellular growth and apoptosis of human epithelial cancers by impairing the MDM2-p53 feedback loop. Oncogene. 2013;32:61–69. doi: 10.1038/onc.2012.28. [DOI] [PubMed] [Google Scholar]
- 32.Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M, Wang S, Kiessling F, Olson EN, et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, Bao JK. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45:487–498. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C, Wang Y, Yang S, Li H, Zhao G, Wang F, Yang L, Wang DW. MiR-320a contributes to atherogenesis by augmenting multiple risk factors and down-regulating SRF. J Cell Mol Med. 2015;19:970–985. doi: 10.1111/jcmm.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farooqi AA. MicroRNA Targeted Cancer Therapy. Springer International Publishing; 2014. miRNA Regulation of DNA Damage Repair Proteins in Cancer Cells: Interplay of ATM, TRAIL and miRNA; pp. 289–307. [Google Scholar]
- 36.Halasi M, Gartel AL. Targeting FOXM1 in cancer. Biochem Pharmacol. 2013;85:644–652. doi: 10.1016/j.bcp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Ke Y, Zhao W, Xiong J, Cao R. miR-149 inhibits non-small-cell lung cancer cells EMT by targeting FOXM1. Biochem Res Int. 2013;2013:506731. doi: 10.1155/2013/506731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Wang Y, Luo J, Fu Z, Ying J, Yu Y, Yu W. miR-134 inhibits epithelial to mesenchymal transition by targeting FOXM1 in non-small cell lung cancer cells. FEBS Lett. 2012;586:3761–3765. doi: 10.1016/j.febslet.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Zeng J, Zhou M, Li B, Zhang Y, Huang T, Wang L, Jia J, Chen C. The tumor suppressive role of miRNA-370 by targeting FoxM1 in acute myeloid leukemia. Mol Cancer. 2012;11:56. doi: 10.1186/1476-4598-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Li Z, Kong X, Xie D, Jia Z, Jiang W, Cui J, Du Y, Wei D, Huang S, Xie K. Down-regulation of microRNA-494 via loss of SMAD4 increases FOXM1 and β-catenin signaling in pancreatic ductal adenocarcinoma cells. Gastroenterology. 2014;147:485–497. doi: 10.1053/j.gastro.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Ying X, Chen H, Ye P, Shen Y, Pan W, Zhang L. MicroRNA-194 inhibits the epithelial–mesenchymal transition in gastric cancer cells by targeting FoxM1. Dig Dis Sci. 2014;59:2145–2152. doi: 10.1007/s10620-014-3159-6. [DOI] [PubMed] [Google Scholar]
- 42.Inoguchi S, Seki N, Chiyomaru T, Ishihara T, Matsushita R, Mataki H, Itesako T, Tatarano S, Yoshino H, Goto Y, Nishikawa R, Nakagawa M, Enokida H. Tumour-suppressive microRNA-24-1 inhibits cancer cell proliferation through targeting FOXM1 in bladder cancer. FEBS Lett. 2014;588:3170–3179. doi: 10.1016/j.febslet.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 43.Calin GA, Croce CM. MicroRNAs and chromosomal abnormalities in cancer cells. Oncogene. 2006;25:6202–6210. doi: 10.1038/sj.onc.1209910. [DOI] [PubMed] [Google Scholar]
- 44.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer. 2010;10:389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duan R, Pak C, Jin P. Single nucleotide polymorphism associated with mature miR-125a alters the processing of pri-miRNA. Hum Mol Genet. 2007;16:1124–1131. doi: 10.1093/hmg/ddm062. [DOI] [PubMed] [Google Scholar]
- 46.Zhou M, Zeng J, Wang X, Wang X, Huang T, Fu Y, Sun T, Jia J, Chen C. Histone demethylase RBP2 decreases miR-21 in blast crisis of chronic myeloid leukemia. Oncotarget. 2015;6:1249–1261. doi: 10.18632/oncotarget.2859.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


