Abstract
Objectives
Metalworking fluids (MWF), used to cool and lubricate metal in occupational settings, are linked to several cancers but data on kidney cancer are limited. We examine how MWF influence the rate of renal cell carcinoma (RCC) in a large prospective study.
Methods
A cohort of Michigan autoworkers consisting of 33,421 individuals was followed 1985 through 2009. The cohort was linked to the Michigan Cancer Registry to identify new cases of RCC. We analyzed RCC in relation to cumulative exposure to each specific type of MWF (straight, soluble and synthetic) and all three types pooled into a single MWF variable, with a 15-year lag. Cox Proportional Hazards Regression with splines were used to estimate Hazard Ratios (HRs) and 95% confidence intervals (95% CIs), controlling for age, gender, race, calendar year, year hired, time since hire, plant, and other MWF types.
Results
There were 135 incident cases. A linear increase in the log-HR was observed for RCC with increasing cumulative exposure to each MWF type and total MWF exposure. At the mean of total MWF exposure (18.80 mg/m3-yr), the estimated HR was 1.11 (95% CI 1.04, 1.19).
Conclusions
Our results provide evidence for a dose-dependent association between MWF exposure and RCC. The influence of components of both oil- and water-based MWF needs further examination.
Keywords: Occupational autoworkers, Metalworking fluids, Kidney cancer, Renal Cell Carcinoma
INTRODUCTION
In the US, there are approximately 61,560 newly diagnosed kidney cancers and 14,080 kidney cancer deaths annually,1 yet our knowledge of environmental and occupational risk factors for kidney cancer is limited.2 The National Institute for Occupational Safety and Health (NIOSH) estimates that millions of American workers are exposed to known or suspected carcinogens in the workplace,3 and evidence suggests that at least 3–6% of all cancers worldwide are attributable to carcinogen exposure in the workplace.4 Yet, even among occupational exposures found to be carcinogenic, the impact on kidney cancer may be unrecognized. Reflecting the kidney’s role in filtering and clearing toxicants from the blood, the kidney may be exposed to higher levels of potential carcinogens.
Among occupational exposures that may be harmful to the kidney are metalworking fluids (MWF), a complex mixture of water- and/or oil-based fluids and additives used to lubricate and cool metals during cutting and grinding operations. The International Agency for Research on Cancer (IARC) classified mineral oils as a human carcinogen in 1984,5 and NIOSH in 1998 also recognized the substantial evidence for cancer in workers based on elevated risk observed for cancers at different sites.6 NIOSH has estimated that over a million workers in the machining and metalworking operations in the US are exposed to MWF.7 MWF use initially involved dermal exposure to workers, but, as the process mechanized, MWF were increasingly aerosolized, with greater opportunity for inhalation and ingestion. Although the composition of MWF can vary over time and for different operations, they are often classified as straight (neat or mineral oils), soluble (a mixture of mineral and water-based), and synthetic (water-based, no oil) fluids. Although MWF have, in general, influenced the risk of several cancer sites, including the skin, stomach, rectum, larynx, bladder, and pancreas, those relationships have varied by fluid type.8–11 Straight MWF contain polycyclic aromatic hydrocarbons (PAHs), which can react with DNA molecules, potentially forming adducts that, if not repaired, can lead to mutations following cellular replication and, eventually, clinically apparent cancer.6,8–10 Also, in straight MWF, N-nitrosodiethanolamine12 can form. This byproduct of nitrite and amine additives is carcinogenic in animal models13 and may have similar potential in humans.14 Soluble oils are water-based oil emulsion and contain anticorrosive agents, such as amines and nitrites. Synthetic fluids do not contain oils, but instead are alkaline solutions with biocides added to ward off bacterial growth in the water-based fluid.
Previous occupational cohort studies with fewer than ten15–18 or twenty cases19–21 provide an inconsistent picture of whether MWF increase,15,17,20,22 decrease19 or have no influence16,18,21,23 on kidney cancer risk. In addition to low case numbers, these studies lacked any exposure assessment for MWF. Occupational cohort studies have not previously provided an opportunity to study a larger number of incident kidney cancer cases with quantitative exposure estimates to fully examine the relationship between MWF and kidney cancer.
We investigate the relationship between exposure to MWF and renal cell carcinoma (RCC) in the United Autoworkers-General Motors (UAW-GM) cohort. UAW-GM is unique because it is a large cohort followed for decades with quantitative exposure estimates for each of the three main types of MWF. We are interested in RCC specifically, which is the predominant type of kidney cancer and occurs in the renal parenchyma.24
MATERIALS AND METHODS
The United Autoworkers-General Motors (UAW-GM) cohort and exposure assessment have been described previously.25,26 The full cohort consists of 46,316 workers from one of three Michigan automobile manufacturing plants. All hourly autoworkers at three plants who ever worked between 1941 and 1984 were initially eligible for mortality follow-up if they worked at least 3 years and had work histories at least 50% complete. Information on date of birth, date of hire, race, plant, and gender were obtained through the employer records. With cancer incidence follow-up starting in 1985, all subjects were prevalent hires, meaning they had all been hired prior to the start of follow-up and were alive at the beginning of the observation period.27,28 About 8% of the participants in the cancer incidence follow-up were initially of unknown race but were classified as Whites based on a priori time specific and plant specific information records.26
Exposure Assessment
The estimated exposure to each type of MWF (straight, soluble, synthetic) by job, year and plant has been detailed previously.25,29,30 Briefly, airborne exposure measurements of total particulate matter (mg/m3) were taken by the company, industrial hygienists, and researchers across a combination of plant, department and job types over time. This information was linked to individual work histories, which were available through 1994 and annual exposures to each MWF was estimated. If there were gaps in the work history information for individual subjects, exposures were interpolated by averaging the exposure information from previous and subsequent jobs. Cumulative exposures to the three MWF (mg/m3-year) were then calculated as time-varying exposures for each subject. Total cumulative exposure to MWF (all three types combined) was also determined. Cumulative exposure was lagged 15 years to allow for a latency period.
Working in automotive plants potentially involved other occupational exposures that may act as confounders for the relationship of interest. In particular, more information was sought on workers’ exposure to asbestos and solvents as these have been associated with kidney cancer.31–33 For one solvent specifically, trichloroethylene (TCE), growing evidence supports a relationship with RCC.31,34
Using information gathered from industrial hygiene activities at the three plants, two members of the original study industrial hygiene (IH) team (K.H. and M.H.) together with a third industrial hygienist (S.L.) reviewed existing records gathered during study data collection periods. This included information collected when study industrial hygienists returned to two of the plants for additional data on solvents, asbestos and acids.35 Solvent use descriptions were obtained through interview of facility managers, which informed of when, where, and why solvents were used in different operations/departments and what specific solvents were used. In addition, IH air sampling records were obtained. The majority of the solvent data came from two of the three GM facilities examined in this cohort study. Data on asbestos came from all three plants. Data included plant, job, date the specific solvent, and airborne concentrations. This reflected industrial hygiene measurements taken between 1971 and 1990. We abstracted more detailed data on four chlorinated solvents, TCE, methyl chloroform, methylene chloride, and perchloroethylene, to inform on which operations these substances were used and over what time periods.
Outcome Ascertainment
We linked the cohort to the Michigan Cancer Registry, which began data collection in 1985, resulting in a follow-up period for cancer incidence from 1985 through 2009. At the start of follow-up, 33,421 subjects were alive and eligible to be included. The National Death Index was used to identify deaths and date of death.
From the cancer registry data, we classified as RCC those indicated as having International Classification of Diseases – Oncology 3rd Edition (ICD-O-3) code of C64.9 and examined morphology codes. Follow-up for each subject ended at the time of death, the time case was diagnosed, or on December 31, 2009, whichever occurred first.
Statistical Analyses
We used Cox proportional hazards regression to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) as an estimate of incidence rate ratios for the association between MWF and RCC. Risk sets were constructed based on time since hire as the time metric. We first generated lagged cumulative exposure to total MWF (all three types combined), as total exposure allows for consideration of the impact of MWF overall and provides the greatest power in terms of total number of exposed cases. Next, we lagged cumulative exposure to each of the three MWF. We used penalized splines to examine the shape of the exposure-response curves, selecting the fit of the spline by allowing the model to identify the optimal Akaike’s Information Criteria (AIC). Nearly all models chose 1 degree of freedom, indicating a linear relationship on the log-HR scale, unless otherwise indicated. We also estimated the association using a continuous term for MWF to assess a linear trend, only among those who had any exposure to MWF. Models adjusted for age (continuous), race (Black, White), gender, calendar year (time-varying), year of hire (continuous), and plant. In models with splines for a single MWF type, the other two MWF were controlled for using two linear terms. Further, we a priori stratified by race, investigating this in men only due to the low number of woman enrolled. R software (version 3.2.0, R development core team, Vienna, Austria) and SAS 9.4 (SAS institute Cary, NC) were used. The study research protocols were approved by the Institutional Review Board at University of California, Berkeley (Berkeley, CA).
RESULTS
The study population was mostly men (86.5%) and White (81.2%) (Table 1). Mean time since hire at baseline was 21.7 years and mean age was 47 years old. 51.8% of subjects were exposed to at least one type of MWF, with 29.4% exposed to straight fluids, 50.2% to soluble fluids, and 13.9% to synthetic fluids.
Table 1.
Characteristics of autoworkers at baseline in 1985
| Characteristics | |
|---|---|
| No. of subjects | 33421 |
| Age, mean (SD) | 46.98 (14.13) |
| Gender, n (%) | |
| Male | 28,908 (86.50) |
| Female | 4,513 (13.50) |
| Race, n (%) | |
| Whites | 27,148 (81.23) |
| Black | 6,271 (18.77) |
| Plant, n (%) | |
| Plant 1 | 7,515 (22.45) |
| Plant 2 | 14,281 (42.73) |
| Plant 3 | 11,639 (34.82) |
| Calendar year of hire, mean (SD) | 1964 (11.05) |
| Years since hire, mean (SD) | 21.70 (11.04) |
| Any MWF exposure, n (%) | |
| Non exposed | 16,099 (48.17) |
| Exposed | 17,322 (51.83) |
| Cumulative exposure, mean (SD) | 7.74 (16.50) |
| Straight MWF*, n (%) | |
| Non exposed | 23,585 (70.57) |
| Exposed | 9,636 (29.43) |
| Cumulative exposure, mean (SD) | 1.63 (8.56) |
| Soluble MWF*, n (%) | |
| Non exposed | 16,644 (49.80) |
| Exposed | 16,777 (50.20) |
| Cumulative exposure, mean (SD) | 5.71 (12.60) |
| Synthetic MWF*, n (%) | |
| Non exposed | 288,773 (86.09) |
| Exposed | 4,648 (13.91) |
| Cumulative exposure, mean (SD) | 0.40 (2.96) |
MWF exposure in units of mg/m3-yr with 15-yr lag
During the follow-up period, 135 RCC cases occurred, 114 among Whites (106 male and 8 female) and 21 among Blacks (19 male and 2 female). The crude incidence rate was 285/1,000,000 person-years overall subjects, 299/1,000,000 person-years for White males and 357/1,000,000 personyears for Black males. Compared to overall at-risk person-time, cases were generally older, employed for longer duration, and had higher mean exposure to the three MWF (data not shown).
The penalized spline model for incident RCC and total MWF (all three types combined) is shown in Figure 1A. There was a log-linear increase in the HR for RCC with increasing cumulative MWF exposure. The slope of the association was steeper when restricted to White males (Figure 1B). For White males, the association was statistically significant at the mean cumulative exposure for exposed cases (mean 18.8 mg/m3-yr; HR=1.11 95% CI (1.04, 1.19) (Table 2). For Black males, the low number of exposed cases provided limited power (Figure 1C).
Figure 1.
The association between Total MWF (all types combined, cumulative exposure (mg/m3-yr)) and incidence of renal cell carcinoma (RCC) (A) among all subjects, (B) White males, and (C) Black males in the United Autoworkers-General Motors cohort, 1985–2009. Cox proportional hazards regression models using penalized splines controlled for age, gender, race, calendar year, year hired, plant and used time since hire as the analytic timeline. The solid horizontal line represents the null value. The rug plot along the bottom horizontal axis indicates the person-time distribution by exposure. Each spline was truncated at the 99th percentile of total MWF (A) all subjects (91.5 mg/m3-yr), (B) White males (93 mg/m3-yr), (C) Black males (99 mg/m3-yr).
Table 2.
Estimated exposure-response for the association between metalworking fluids and renal cell carcinoma from penalized spline regression among White males in the United Autoworkers-General Motors cohort, follow-up period 1985–2009
| Renal cell carcinoma (n=106 cases, Person-years=445,056) |
||
|---|---|---|
| Cumulative MWF exposure | mg/m3-yr* | HR (95% CI) |
| Total MWF (All types combined), 15-yr lag† | ||
| Median | 9.60 | 1.06 (1.05, 1.06) |
| Mean | 18.80 | 1.11 (1.04, 1.19) |
| P75 | 21.87 | 1.13 (1.02, 1.24) |
| P90 | 43.08 | 1.27 (0.97, 1.66) |
| Straight MWF, 15-yr lagǂ | ||
| Median | 0.93 | 1.01 (0.99, 1.02) |
| Mean | 7.05 | 1.05 (0.99, 1.10) |
| P75 | 18.20 | 1.14 (0.95, 1.35) |
| P90 | 18.47 | 1.14 (0.96, 1.36) |
| Soluble MWF, 15-yr lagǂ | ||
| Median | 6.73 | 1.02 (0.99, 1.05) |
| Mean | 12.46 | 1.02 (0.92, 1.13) |
| P75 | 12.69 | 1.02 (0.92, 1.13) |
| P90 | 29.27 | 1.03 (0.74, 1.41) |
| Synthetic MWF, 15-yr lagǂ | ||
| Median | 0.48 | 1.01 (1.00, 1.01) |
| Mean | 3.54 | 1.05 (0.96, 1.14) |
| P75 | 2.80 | 1.04 (0.97, 1.10) |
| P90 | 7.60 | 1.10 (0.90, 1.34) |
Median, mean, 75th percentile (P75), and 90th percentile (P90) were based on the distribution of exposure among exposed cases.
Cox proportional hazard regression model controlled for age, calendar year, year hired, plant and with time since hire as the analytic timeline
Cox proportional hazard regression model controlled for age, calendar year, year hired, plant, and other MWF types and with time since hire as the analytic timeline
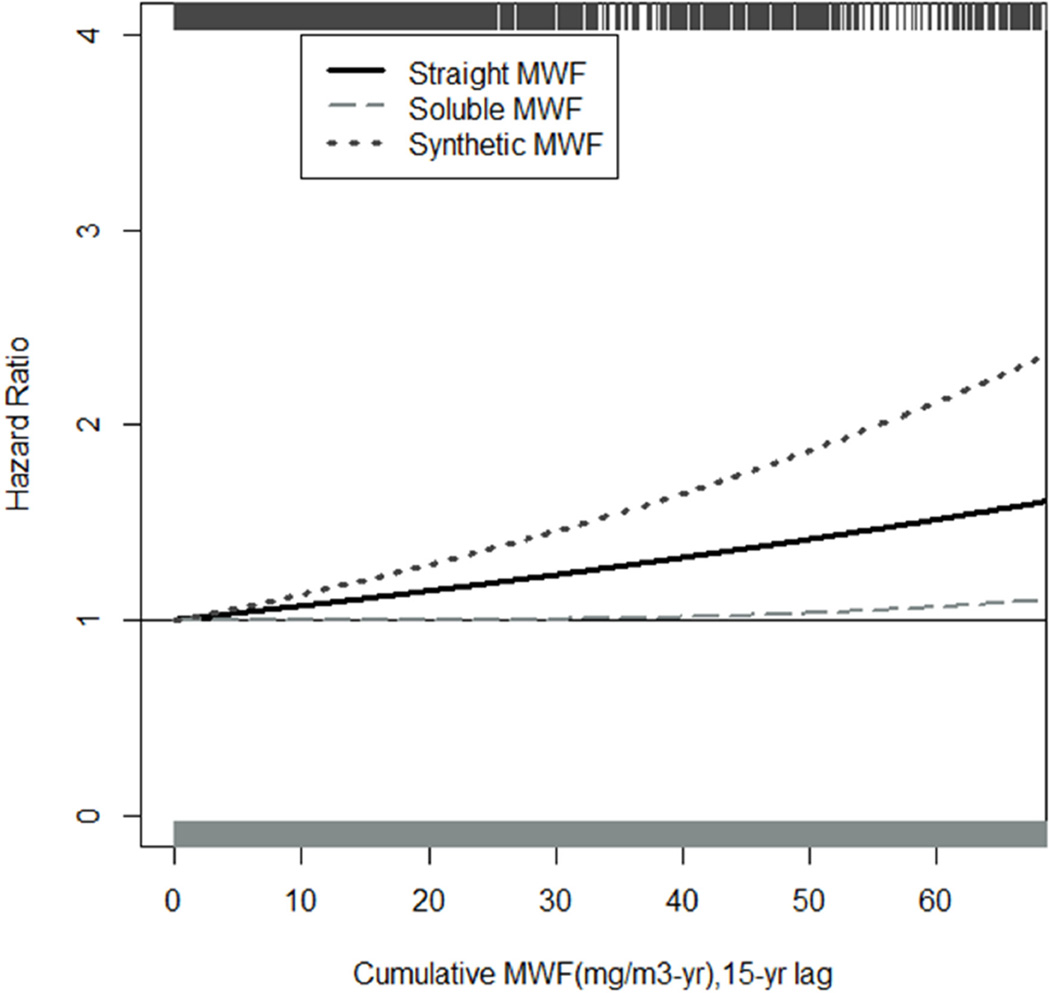
We next examined the relationship between RCC and specific MWF among White males. We observed that the risk of RCC increased regardless of fluid type (Figure 2). In Table 2, we display HRs at specific positions along the splines (e.g., 50%, 75%, and 90% of the exposure distribution) for White males. At the median, mean, and 75% for cumulative exposure to total MWF (9.60, 18.80, and 21.87 mg/m3-yr, respectively), the association with RCC was elevated and statistically significant (HR 1.06, 95% CI (1.05, 1.06), 1.11 95% CI (1.04, 1.19), 1.13 95% CI (1.02, 1.24), respectively). Examining across fluid types, the median cumulative exposure was noticeably lower for synthetic fluids (0.48 mg/m3-yr) compared with the two other fluid types, explained by its later introduction into the cohort. At this median value, synthetic fluids had an elevated HR of 1.01 (95% CI (1.00, 1.01). The HRs for straight and soluble fluids were also elevated (straight HR 1.01 95% CI (0.99, 1.02); soluble HR 1.02 95% CI (0.99, 1.05)) at their respective median cumulative exposure (straight 0.93 mg/m3-yr; soluble 6.73 mg/m3-yr).
Figure 2.
The association between specific types of MWF and incidence of RCC among White males in the United Autoworkers-General Motors cohort, 1985–2009. Cox proportional hazards regression models using penalized splines controlled for age, calendar year, year hired, plant, and used time since hire as the analytic timeline. Individual splines for each MWF were generated separately, adjusting for the other two fluids as linear terms. The solid horizontal line represents the null value. The rug plot along the bottom horizontal axis indicates the person-time distribution by cumulative soluble exposure. The rug plot along the top horizontal axis indicates the persontime distribution by cumulative synthetic exposure. Each spline was truncated at the 99th percentile of total soluble fluid for White males (66 mg/m3-yr).
With splines selecting a linear model for the log-HR, we also restricted the analysis to those who had been exposed to any MWF type and generated a Cox Proportional Hazards model with a linear term for total exposure MWF to assess trend (data not shown). Among the exposed subjects, there was a borderline statistically significant increasing linear trend for RCC (p-value of 0.05).
As for the potential for exposure to other occupational substances, i.e., solvents and asbestos, the review of IH records identified a total of 618 samples for 35 solvents and 2,913 samples for asbestos. The arithmetic mean for the 29 TCE measurements was 29.5 ppm (SD 77.2 ppm), which is higher than the current threshold limit value (TLV) of 10 ppm, but lower than the TLV of 50 ppm at the time the samples were taken. While IH sampling revealed that 8 of the TCE measurements were substantially above 10 ppm, because these measurements were mostly short-duration samples with varying sampling time (30–270 minutes), the full shift time-weighted average could be lower than the measured air concentration. TCE was used mainly for painting, solvent tank and degreaser related operations. IH measurements for the other chlorinated solvents were much lower than their current TLVs (data not shown); MWF were not used in the paint departments, and there were very few solvent tanks (<10) at these large facilities, so few of those in the MWF exposed group would have been exposed to vapors from these tanks. None of the average air concentrations for non-chlorinated solvents with more than 10 IH measurements exceeded their current TLVs. However, the maximum concentration for formaldehyde, toluene, and ethanolamine exceeded their TLVs. No asbestos parts were manufactured at any of the plants. Potential for asbestos exposure occurred in the assembly departments (Clutch Department, Transmission Assembly, Brake Assembly, and Axle Assembly) or among construction tradesmen, such as electricians and pipefitters, not the departments which used MWF, so asbestos is not a confounder for MWF.
DISCUSSION
Using time-varying quantitative exposure estimates, we observed increasing exposure-response patterns for total MWF and RCC. From penalized splines, the relationship with total MWF was strongest when examining White males only. There was a statistically significant association in the HR at the mean of exposed cases among White males mean 18.80 mg/m3-yr, HR 1.11 95% CI (1.04, 1.19).
The association was apparent for total MWF and fluid-specific analyses suggested that more than one MWF may influence this association. The exposure assessment involving quantitative estimates of specific MWF types is an advantage of the UAW-GM cohort. This detailed exposure assessment has been instrumental in identifying how specific MWF may have different relationships with a cancer site. For example, several cancer sites such as prostate,36,37 malignant melanoma,26 and bladder cancer38 were related only to fluids containing petroleum based mineral oils. On the other hand, pancreatic cancer was weakly associated39 while lung cancer was inversely associated40 with synthetic fluids. When cancer at a site is pronounced for one type of fluid, it suggests that a particular composition of the fluid is influential and provides a clue to cancer etiology. For RCC, the risk appeared to increase with more than one fluid type. Therefore, there may be additional considerations and likely more than one pathway to disease.
Mineral oil-based fluids (straight and soluble) contain PAHs, which are known carcinogens.41 These may also contain naphthenes, paraffins, sulfur and chlorine additives, and fatty oil.14 Soluble fluids contain emulsifying agents to maintain the oil-water mix and water-based fluids (synthetic and soluble) Water-based fluids may also contain organic esters, polyglycols, biocides, and corrosion inhibitors. Among anti-corrosive agents are ethanolamines and, when combined with nitrites, may form nitrosamine. N-nitrosodiethanolamine (NDELA) is one type of ethanolamine that is potentially carcinogenic.14,42 In mice, diethanolamine exposure was found to be related to renal tubule neoplasms.43 All types of MWF may also contain medium-chain chlorinated paraffin (MCCP) as a pressure additive, though there is concern that this exposure increases risk for kidney toxicity.44 Therefore, the fluids and their additives contain a number of substances that may affect the kidney.
In addition, impurities that get introduced into MWF during metalworking operations may be a factor. MWF remove from work surfaces small particles that are released from the metal during the various metalworking operations. For example, in cutting and grinding operations, the metals on which this activity occurred may release small particles and these can get mixed with the MWF and workers are exposed to the fluids and the added particles principally through inhalation and dermal routes. These contaminants may include steel, iron, and aluminum. Aluminum has previously been associated with increased risk of kidney cancer among aluminum plant workers.45 Based on both experimental and human studies, IARC considered aluminum, iron, steel as Group 1 carcinogens.46,47 In our analysis, we examined aluminum, steel, and iron based on duration of exposure in workers. These were not confounders of the relationship between MWF and kidney cancer nor were they independent risk factors. It is possible that if these metal contaminants were a factor, duration of exposure alone may not be an adequate metric.
As briefed earlier, prior studies of exposure to MWF were limited in their ability to investigate this relationship, in part, due to lacking quantitative exposure estimates. These earlier studies typically had smaller case numbers and a case definition that was broader, including additional types of kidney cancer. For example, one cohort study in the motor vehicle manufacturing company reported a large number (n=100) of deaths from kidney cancer and reported reduced standardization mortality ratios (SMR) for White men and elevated SMR for Black men, all with wide confidence intervals.48 However, there was no mention of whether the cases had ever had any direct exposure to MWF, let alone the specific types of MWF. Population-based case-control studies indicated an elevated risk of RCC, but relied on selfreporting of MWF exposure which is susceptible to recall bias.49,50 We can also compare our results with occupational studies that evaluated exposure to PAHs, a component of concern in MWF. Estimates from case-control studies ranged from 1.6 to 9.3-fold increased risk of kidney cancer associated with exposure of PAHs working with iron steel, coke, coal tar pitch and asphalt, although these studies relied on selfreported exposure.51,52
We considered other occupational exposures as potential confounders of the relationship between MWF and RCC. After examining available data on exposure to asbestos, solvents overall, chlorinated solvents, and TCE at the plants, the evidence suggests that asbestos exposure was in departments which did not use MWF while solvents were also used in a limited number of areas, only a very few of which also used MWF. Solvents were primarily used in painting, degreasers, hand cleaning of repaired parts, construction and maintenance, and laboratory analyses. MWF were not used in the paint or most of the other departments. A very small number of employees were exposed to solvents as a result of the cleaning operations, not all of whom would have been exposed to MWF, although the actual percentage of workers exposed to both MWF and solvents remains unclear. Chlorinated solvents including TCE were the major solvents used in parts cleaning operations before late 1980s when solvents were replaced by aqueous cleaners. The IH air sampling record indicates TCE had substantial air concentrations in one plant 1979–1989, but there were few tanks in the large plant so few workers would have been exposed to both MWF and TCE. However, since solvent use in general was intermittent and moderate and most of the high air concentration measurements were from short duration samples, the actual full shift time weighted average exposures was probably lower than the measured air concentration. Nearly all of the measurements for most of the other chlorinated and non-chlorinated solvent measurements were under their respective TLVs, though a few were found to have a limited number of measurements exceeding the TLV. This review of IH data indicated that worker exposure to solvents and asbestos could be above recommended levels during specific tasks but overall were uncommon and in limited operations. Moreover, asbestos exposure occurred in departments that did not use MWF and, because solvents were primarily used in painting and the few degreasing operations, very few workers experienced overlapped exposures from solvents and MWFs. Thus, asbestos and solvents are unlikely confounders for MWF associations reported here.
Analyses adjusted for age, gender, race, plant which autoworkers were employed, hired year and calendar year. Time-varying calendar year adjusted the unmeasured constituents of exposure related to time period and accounts for the industry-wide changes in MWF content, the personal protective measures and modification on plants ventilation or the changes in the diagnosis of the outcome. In addition, the present analysis involved working with a cohort that is left-truncated.28 That is, only subjects who are still alive in 1985 were eligible for cancer incidence follow-up and they had worked between 3 and more than 40 years prior to the start of follow-up in 1985 when cancer registry activities commenced. Although we examined date of hire in the analysis, the case data became too sparse to examine this issue closely. Though if left truncation was influential, the true association would be stronger than what is reported here.
We observed differences in the association of MWF and RCC among White and Black men. The trend for White males revealed a steeper exposure-response relationship. For Black males, there was a decreased risk of RCC, though there were fewer than 20 cases among Black men in this follow-up period. However, we also note that the rate of RCC was higher in the Black men of this cohort (357 per 1 million person-years) than the White men (299 per 1 million person-years). Exposure will appear to have a larger impact on a relative measure of association when the background rates are lower.53
Our study has unique advantages over the previous studies on MWF and RCC. The GM-UAW cohort has been recognized for its quantitative exposure assessment of metalworking fluids by type.6,10 Other strengths of this cohort include the diversity of the cohort and its size, as well as the length and completeness of the follow-up (less than 4% loss to follow up).
We have attempted to evaluate potential occupational exposures to asbestos and solvents based on available data collected at the facilities. Results suggest limited potential for confounding. There are several non-occupational risk factors for RCC24 that we are unable to control for, including smoking, dietary factors, genetic, and other comorbidities such as hypertension, cardiovascular, obesity, and diabetes. Though not impossible, we think these are unlikely to be correlated with the primary MWF exposures and thus unlikely to undermine our results.
CONCLUSION
We observed a clear indication of exposure-response association between MWF and RCC. In this study, we found the increased relationship with each type of MWF, particularly synthetic MWF at a lower cumulative exposure. Further research integrating other comorbidities may provide insights into etiology. Cancers attributed to occupational disease place a heavy burden on quality life of workers, in terms of financial cost as well as human suffering.2 Future research should examine specific components and contaminants of MWF as well as molecular indicators of kidney damage in order to better define pathways leading to RCC. If MWF components of concern are identified, perhaps the composition of the fluids could be modified or other engineering controls implemented to protect worker health.
What this paper adds.
Although the International Agency for Research on Cancer (IARC) has classified components of metalworking fluids (MWF) as carcinogens, research on MWF and kidney cancer generally or renal cell carcinoma (RCC) specifically has been limited. In this prospective cohort study, there was a linear increase in the log-HR for RCC incidence with increasing cumulative exposure to straight, soluble and synthetic MWF and for all three types of MWF combined.
The association between MWF and RCC was strongest for White males, though Black males had a higher background risk of RCC.
An industrial hygiene review indicated that asbestos and solvents were uncommon exposures in limited operations, and thus unlikely confounders.
Recommended exposure limits for MWF are currently based on preventing respiratory effects. However, in light of the risks MWF pose for cancer at various sites including the kidney, exposure limits deserve consideration with respect to preventing long-term health effects.
Acknowledgments
Authors appreciate the technical assistance provided by Clara Chen and Michel Winter from Boston University with dataset development. This research was supported by the Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. R01 OH010180, R01 OH010028.
Footnotes
CONFLICT OF INTEREST
The authors reported no financial conflict of interest.
AUTHOR’S CONTRIBUTIONS
KMA and EAE conceived the study. EAE provided the study data and assisted in the linkage process. DS performed statistical analyses and drafted the paper along with KMA. SL and SKH abstracted and analysed data from industrial hygiene records and summarized these findings. All authors contributed to the interpretation of the results and revising and approving the manuscript.
REFERENCES
- 1.Cancer Facts and Figures 2010. Atlanta, Georgia: American Cancer Society, Inc; 2011. [Google Scholar]
- 2.Steenland K, Burnett C, Lalich N, et al. Dying for work: The magnitude of US mortality from selected causes of death associated with occupation. Am J Ind Med. 2003;43(5):461–482. doi: 10.1002/ajim.10216. [DOI] [PubMed] [Google Scholar]
- 3.Data from the National Occupational Hazard Survey. Cincinnati, Ohio: 1974. Data from the National Occupational Hazard Survey.: NIOSH. [Google Scholar]
- 4.Driscoll T, Takala J, Steenland K, et al. Review of estimates of the global burden of injury and illness due to occupational exposures. Am J Ind Med. 2005;48(6):491–502. doi: 10.1002/ajim.20194. [DOI] [PubMed] [Google Scholar]
- 5.Polynuclear aromatic hydrocarbons, part 3: Industrial exposures in aluminum production, coal gasification, coke production, and iron and steel founding: IARC, International Agency for Research on Cancer. 1984 [Google Scholar]
- 6.Mirer F. Updated epidemiology of workers exposed to metalworking fluids provides sufficient evidence for carcinogenicity. Appl Occup Environ Hyg. 2003;18(11):902–912. doi: 10.1080/10473220390237511. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Occupational Safety and Health (NIOSH): National Occupational Exposure Survey (NOES), 1981–1983. Unpublished database.: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, NIOSH Division of Surveillance, Hazard Evaluations, and Field Studies, Surveillance Branch, Hazard Section. 1983 [Google Scholar]
- 8.Savitz DA. Epidemiologic evidence on the carcinogenicity of metalworking fluids. Appl Occup Environ Hyg. 2003;18(11):913–920. doi: 10.1080/10473220390237539. [DOI] [PubMed] [Google Scholar]
- 9.Tolbert PE. Oils and cancer. Cancer Causes Control. 1997;8(3):386–405. doi: 10.1023/a:1018409422050. [DOI] [PubMed] [Google Scholar]
- 10.Mirer FE. New evidence on the health hazards and control of metalworking fluids since completion of the OSHA advisory committee report. Am J Ind Med. 2010;53(8):792–801. doi: 10.1002/ajim.20853. [DOI] [PubMed] [Google Scholar]
- 11.Colt JS, Friesen MC, Stewart PA, et al. A case-control study of occupational exposure to metalworking fluids and bladder cancer risk among men. Occup Environ Med. 2014;71(10):667–674. doi: 10.1136/oemed-2013-102056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan TY, Morrison J, Rounbehler DP, et al. N-Nitrosodiethanolamine in synthetic cutting fluids: a part-per-hundred impurity. Science. 1977;196(4285):70–71. doi: 10.1126/science.841341. [DOI] [PubMed] [Google Scholar]
- 13.Hilfrich J, Schmeltz I, Hoffmann D. Effects of N-nitrosodiethanolamine and 1,1-diethanolhydrazine in Syrian golden hamsters. Cancer Lett. 1978;4(1):55–60. doi: 10.1016/s0304-3835(78)93412-2. [DOI] [PubMed] [Google Scholar]
- 14.Cincinnati, OH: 1998. Criteria for a recommended standard-Occupational exposure to metalworking fluids: niosh, National Institute for Occupational Safety and Health, DHHS. [Google Scholar]
- 15.Vena JE, Sultz HA, Fiedler RC, et al. Mortality of workers in an automobile engine and parts manufacturing complex. Br J Ind Med. 1985;42(2):85–93. doi: 10.1136/oem.42.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverstein M, Park R, Marmor M, et al. Mortality among bearing plant workers exposed to metalworking fluids and abrasives. J Occup Med. 1988;30(9):706–714. [PubMed] [Google Scholar]
- 17.Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41(7):556–566. doi: 10.1097/00043764-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Decoufle P. Further analysis of cancer mortality patterns among workers exposed to cutting oil mists. J Natl Cancer Inst. 1978;61(4):1025–1030. [PubMed] [Google Scholar]
- 19.Kazerouni N, Thomas TL, Petralia SA, et al. Mortality among workers exposed to cutting oil mist: update of previous reports. Am J Ind Med. 2000;38(4):410–416. doi: 10.1002/1097-0274(200010)38:4<410::aid-ajim6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Park RM, Mirer FE. A survey of mortality at two automotive engine manufacturing plants. Am J Ind Med. 1996;30(6):664–673. doi: 10.1002/(SICI)1097-0274(199612)30:6<664::AID-AJIM3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Teta MJ, Ott MG. A mortality study of a research, engineering, and metal fabrication facility in western New York State. Am J Epidemiol. 1988;127(3):540–551. doi: 10.1093/oxfordjournals.aje.a114829. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Krishnadasan A, Kennedy N, et al. Estimated effects of solvents and mineral oils on cancer incidence and mortality in a cohort of aerospace workers. Am J Ind Med. 2005;48(4):249–258. doi: 10.1002/ajim.20216. [DOI] [PubMed] [Google Scholar]
- 23.Costello S, Brown DM, Noth EM, et al. Incident ischemic heart disease and recent occupational exposure to particulate matter in an aluminum cohort. J Expo Sci Environ Epidemiol. 2014;24(1):82–88. doi: 10.1038/jes.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7(5):245–257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisen EA, Tolbert PE, Monson RR, et al. Mortality studies of machining fluid exposure in the automobile industry I: A standardized mortality ratio analysis. Am J Ind Med. 1992;22(6):809–824. doi: 10.1002/ajim.4700220604. [DOI] [PubMed] [Google Scholar]
- 26.Costello S, Friesen MC, Christiani DC, et al. Metalworking fluids and malignant melanoma in autoworkers. Epidemiology. 2011;22(1):90–97. doi: 10.1097/EDE.0b013e3181fce4b8. [DOI] [PubMed] [Google Scholar]
- 27.Applebaum KM, Malloy EJ, Eisen EA. Reducing healthy worker survivor bias by restricting date of hire in a cohort study of Vermont granite workers. Occup Environ Med. 2007;64(10):681–687. doi: 10.1136/oem.2006.031369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Applebaum KM, Malloy EJ, Eisen EA. Left truncation, susceptibility, and bias in occupational cohort studies. Epidemiology. 2011;22(4):599–606. doi: 10.1097/EDE.0b013e31821d0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woskie SR, Smith TJ, Hallock MF, et al. Size-selective pulmonary dose indices for metal-working fluid aerosols in machining and grinding operations in the automobile manufacturing industry. Am Ind Hyg Assoc J. 1994;55(1):20–29. doi: 10.1080/15428119491019221. [DOI] [PubMed] [Google Scholar]
- 30.Hallock MF, Smith TJ, Woskie SR, et al. Estimation of historical exposures to machining fluids in the automotive industry. Am J Ind Med. 1994;26(5):621–634. doi: 10.1002/ajim.4700260505. [DOI] [PubMed] [Google Scholar]
- 31.Scott CS, Jinot J. Trichloroethylene and cancer: systematic and quantitative review of epidemiologic evidence for identifying hazards. Int J Environ Res Public Health. 2011;8(11):4238–4272. doi: 10.3390/ijerph8114238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karami S, Boffetta P, Stewart PS, et al. Occupational exposure to dusts and risk of renal cell carcinoma. Br J Cancer. 2011;104(11):1797–1803. doi: 10.1038/bjc.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alanee S, Clemons J, Zahnd W, et al. Trichloroethylene Is Associated with Kidney Cancer Mortality: A Population-based Analysis. Anticancer Res. 2015;35(7):4009–4013. [PubMed] [Google Scholar]
- 34.Moore LE, Boffetta P, Karami S, et al. Occupational trichloroethylene exposure and renal carcinoma risk: evidence of genetic susceptibility by reductive metabolism gene variants. Cancer Res. 2010;70(16):6527–6536. doi: 10.1158/0008-5472.CAN-09-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisen EA, Tolbert PE, Hallock MF, et al. Mortality studies of machining fluid exposure in the automobile industry. III: A case-control study of larynx cancer. Am J Ind Med. 1994;26(2):185–202. doi: 10.1002/ajim.4700260205. [DOI] [PubMed] [Google Scholar]
- 36.Agalliu I, Eisen EA, Kriebel D, et al. A biological approach to characterizing exposure to metalworking fluids and risk of prostate cancer (United States) Cancer Causes Control. 2005;16(4):323–331. doi: 10.1007/s10552-004-4323-7. [DOI] [PubMed] [Google Scholar]
- 37.Agalliu I, Kriebel D, Quinn MM, et al. Prostate cancer incidence in relation to time windows of exposure to metalworking fluids in the auto industry. Epidemiology. 2005;16(5):664–671. doi: 10.1097/01.ede.0000173266.49104.bb. [DOI] [PubMed] [Google Scholar]
- 38.Friesen MC, Costello S, Eisen EA. Quantitative exposure to metalworking fluids and bladder cancer incidence in a cohort of autoworkers. Am J Epidemiol. 2009;169(12):1471–1478. doi: 10.1093/aje/kwp073. [DOI] [PubMed] [Google Scholar]
- 39.Mehta AJ, Malloy EJ, Applebaum KM, et al. Reduced lung cancer mortality and exposure to synthetic fluids and biocide in the auto manufacturing industry. Scand J Work Environ Health. 2010;36(6):499–508. doi: 10.5271/sjweh.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bardin JA, Eisen EA, Tolbert PE, et al. Mortality studies of machining fluid exposure in the automobile industry. V: A case-control study of pancreatic cancer. Am J Ind Med. 1997;32(3):240–247. doi: 10.1002/(sici)1097-0274(199709)32:3<240::aid-ajim9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Friesen MC, Costello S, Thurston SW, et al. Distinguishing the common components of oil- and water-based metalworking fluids for assessment of cancer incidence risk in autoworkers. Am J Ind Med. 2011;54(6):450–460. doi: 10.1002/ajim.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Man IMECRC, editor. Lyon, France: International Agency for Research (IARC); 1978. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans: some N-nitroso compounds; pp. 77–82. [Google Scholar]
- 43.NTP Toxicology and Carcinogenesis Studies of Diethanolamine (CAS No. 111-42-2) in F344/N Rats and B6C3F1 Mice (Dermal Studies): National Toxicology Program. Natl Toxicol Program Tech Rep Ser. 1999:1–212. [PubMed] [Google Scholar]
- 44.Cherrie JW, Semple S. Dermal exposure to metalworking fluids and medium-chain chlorinated paraffin (MCCP) Ann Occup Hyg. 2010;54(2):228–235. doi: 10.1093/annhyg/mep081. [DOI] [PubMed] [Google Scholar]
- 45.Gibbs GW, Labreche F. Cancer risks in aluminum reduction plant workers: a review. J Occup Environ Med. 2014;56(5 Suppl):S40–S59. doi: 10.1097/JOM.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barr DB, Wilder LC, Caudill SP, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gamble MV, Liu X. Urinary creatinine and arsenic metabolism. Environ Health Perspect. 2005;113(7):A442. doi: 10.1289/ehp.113-a442a. author reply A42-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delzell E, Macaluso M, Honda Y, et al. Mortality patterns among men in the motor vehicle manufacturing industry. Am J Ind Med. 1993;24(4):471–484. doi: 10.1002/ajim.4700240411. [DOI] [PubMed] [Google Scholar]
- 49.Hu J, Mao Y, White K. Renal cell carcinoma and occupational exposure to chemicals in Canada. Occup Med (Lond) 2002;52(3):157–164. doi: 10.1093/occmed/52.3.157. [DOI] [PubMed] [Google Scholar]
- 50.Pesch B, Haerting J, Ranft U, et al. Occupational risk factors for renal cell carcinoma: agent-specific results from a case-control study in Germany. MURC Study Group. Multicenter urothelial and renal cancer study. Int J Epidemiol. 2000;29(6):1014–1024. doi: 10.1093/ije/29.6.1014. [DOI] [PubMed] [Google Scholar]
- 51.Boffetta P, Fontana L, Stewart P, et al. Occupational exposure to arsenic, cadmium, chromium, lead and nickel, and renal cell carcinoma: a case-control study from Central and Eastern Europe. Occup Environ Med. 2011;68(10):723–728. doi: 10.1136/oem.2010.056341. [DOI] [PubMed] [Google Scholar]
- 52.Karami S, Boffetta P, Brennan P, et al. Renal cancer risk and occupational exposure to polycyclic aromatic hydrocarbons and plastics. J Occup Environ Med. 2011;53(2):218–223. doi: 10.1097/JOM.0b013e31820a40a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stayner L, Steenland K, Dosemeci M, et al. Attenuation of exposure-response curves in occupational cohort studies at high exposure levels. Scand J Work Environ Health. 2003;29(4):317–324. doi: 10.5271/sjweh.737. [DOI] [PubMed] [Google Scholar]