Abstract
Purpose:
Ultrasound neuromodulation is a promising noninvasive technique for controlling neural activity. Previous small animal studies suffered from low targeting specificity because of the low ultrasound frequencies (<690 kHz) used. In this study, the authors demonstrated the capability of focused ultrasound (FUS) neuromodulation in the megahertz-range to achieve superior targeting specificity in the murine brain as well as demonstrate modulation of both motor and sensory responses.
Methods:
FUS sonications were carried out at 1.9 MHz with 50% duty cycle, pulse repetition frequency of 1 kHz, and duration of 1 s. The robustness of the FUS neuromodulation was assessed first in sensorimotor cortex, where elicited motor activities were observed and recorded on videos and electromyography. Deeper brain regions were then targeted where pupillary dilation served as an indicative of successful modulation of subcortical brain structures.
Results:
Contralateral and ipsilateral movements of the hind limbs were repeatedly observed when the FUS was targeted at the sensorimotor cortex. Induced trunk and tail movements were also observed at different coordinates inside the sensorimotor cortex. At deeper targeted-structures, FUS induced eyeball movements (superior colliculus) and pupillary dilation (pretectal nucleus, locus coeruleus, and hippocampus). Histological analysis revealed no tissue damage associated with the FUS sonications.
Conclusions:
The motor movements and pupillary dilation observed in this study demonstrate the capability of FUS to modulate cortical and subcortical brain structures without inducing any damage. The variety of responses observed here demonstrates the capability of FUS to perform functional brain mapping.
Keywords: brain, noninvasive neuromodulation, focused ultrasound, pupillary dilation
1. INTRODUCTION
Ultrasound neuromodulation has gained attention as a promising technique to overcome limitations of current techniques such as the implantation of electrodes when using deep brain stimulation (DBS); the poor spatial resolution (≈1 cm), inadequate depth of penetration, and short-lasting effects (milliseconds) of transcranial magnetic stimulation (TMS); and the gene modification required by optogenetics.1,2 Focused ultrasound (FUS) has been shown to be capable of modulating—suppressing or stimulating—specific parts of the brain such as the motor, sensorimotor, and visual cortices.3–7 Tufail et al. 2011 (Ref. 8) presented a general protocol for the stimulation of intact mouse brain and a review with the most recent findings in ultrasonic neuromodulation is presented in Naor et al., 2016.9 The ultrasound frequencies used in most previous small animal neuromodulation studies were lower than 690 kHz. Such frequencies (in the kilohertz range) have been claimed to present not only superior transmission rate through the skull but also superior modulation efficiency.10 However, FUS with lower frequencies generally has large focal spots generating problems of target specificity, especially with small animal models (rodents). A more confined focus can be formed using higher frequencies, which allows spatial-selective modulation of the brain. Hypothetically, higher selectivity of ultrasound neuromodulation would allow stimulation of the specific groups of neurons, e.g., different brain regions or brain structures, which in turn would help understanding previous results with inconsistent lateralization of motor responses.11,12 Previous studies have demonstrated the feasibility of utilizing megahertz frequency ultrasound to elicit motor activations of limbs,11,12 tail,11 and whiskers11 of mice, but these studies failed to demonstrate consistent lateralization of muscle responses.
In addition to motor responses elicited by neuromodulation, the pupillary response can be used as an indicator of the modulation of subcortical structures of the brain associated with light reflex and cognition.13 The dilator and sphincter muscles of the iris are directly innervated by brain regions associated with cognitive and emotional processing.14 Nevertheless, the relative deep location of subcortical structures makes eliciting pupil dilation a very challenging task for most invasive neuromodulation techniques. In this study, we aimed at demonstrating the high spatial resolution and superior targeting specificity of megahertz FUS in mice. FUS-induced neuromodulation was performed at the cortex and subcortical brain structures to assess the potential of the technique to evoke specific functional brain activation.
2. MATERIAL AND METHODS
In accordance with the National Institutes of Health Guidelines for animal research, all animal procedures for these experiments were reviewed and approved by the Institutional Animal Care and Use Committee at Columbia University. The experiments were performed in wild type mice (strain: C57BL-6), which were anesthetized with intraperitoneal injection of sodium pentobarbital (65 mg/kg). After injection, the animals remained in the cage for a period of 20–30 min in order for the anesthesia to take effect. The anesthesia level was assessed by the pedal reflex and vital sign recordings. The animals were depilated on the scalp and neck and positioned in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) using ear and bite bars. Oxygen was delivered continuously at 0.8 l/min throughout the experiments. A vital sign monitor with a collar format sensor (MouseOx Plus, Starr Life Sciences Corp., Torrington, CT, USA) was placed on the throat of the animals to assess heart and breathing rates, which were recorded during sonication using an acquisition board (MP150, Biopac Systems, Inc., Santa Barbara, CA, USA).
A single-element FUS transducer (focus = 60 mm, aperture = 70 mm, inner hole diameter = 20 mm, f-number = 0.86, and focus zone = 8.7 mm long with 1.0 mm diameter; calibration data presented in Fig. S1 of the supplementary material15) was driven by a function generator (33220A, Agilent Technologies, Palo Alto, CA, USA) through a 50-dB power amplifier (ENI, Inc., Rochester, NY, USA). The transducer was attached to an acrylic cone filled with degassed water. A recipient filled with degassed water coupled acoustically the head of the animal to the transducer to allow the transducer to move freely in space using a computer controlled 3D positioning system (VXM, Velmex, Inc., NY, USA) (see Fig. S2 of the supplementary material15). The FUS sonications were carried out at 1.9 MHz at a pulse repetition frequency of 1 kHz with 50% duty cycle (950 pulses). The sonication was on for 1 s and off for 1 s and was repeated ten times at each targeted spot.
For the study of motor-evoked responses, the transducer was moved randomly within a grid of 8 by 8 mm with a resolution of 1 mm and sonication was performed one time per animal with the sequence of ten shots, unless otherwise noticed. The center of the positioning grid was placed between the bregma and lambda skull landmarks (landmarks are approximately 4.2 mm apart from each other) covering the sensorimotor cortex and part of cerebellum. An ultrasound C-scan of a reference metallic grid placed on the top of lambda16 (see Fig. S2(b) of the supplementary material15) was used to place the FUS focus at anteroposterior (AP) = −2 mm and mediolateral (ML) = 0 mm from lambda. Three mice were used during motor response exploration.
The hind limb movements evoked by FUS excitation of the sensorimotor cortex were recorded on videos by a camera (EOS Rebel T3i, Canon, Melville, NY, USA) positioned at the back of the animals. The random exploratory positioning of the transducer was performed only once per animal. However, once a responsive region was detected, the ultrasound focus was symmetrically moved to the opposite brain hemisphere, seeking for ipsilateral and contralateral activation. Thus, spots in the opposite hemisphere of first detected responsive spots were sonicated twice due to the initially defined map for the random spatial exploration of lateralization of motor response. Muscle activities of the hind limbs were recorded at different acoustic pressure levels (1.12–1.79 MPa) using an electromyography (EMG) system (BN-EMG2, Biopac Systems, Inc., Santa Barbara, CA, USA). The EMG signals were acquired from 26-gauge electrodes placed 5-mm apart at the biceps femoris in both hind limbs with the ground electrode placed on the tail (symmetrically apart from the other electrodes).
Once the robustness of modulating shallower (cortical) brain regions by FUS was confirmed, we evaluated its capability of modulating deeper structures in the brain at DV (dorsal/ventral): −3 mm. The FUS was placed over the locus coeruleus, hippocampus or superior colliculus. Locus coeruleus and hippocampus are associated with stress and panic, which modulatory responses can be indexed by pupil dilation. Superior colliculus is associated with eye movements. To evaluate responses associated with the modulation of these structures, videos of the animal’s right eye were recorded during sonications at different pressure levels (0.75–2.25 MPa, n = 3) (DMK 23U618, The Imaging Source, Bremen, Germany) (see Fig. S2 of the supplementary material15).
To evaluate the safety of the sonication in the megahertz-range, whole brain histological examinations using hematoxylin and eosin (H&E) staining were performed in five mice sonicated with 1.9 MPa at one hemisphere (AP = +2 mm, ML = +2 mm, and DV = −3 mm) and with 3.0 MPa (approximately the double of the threshold pressure observed for motor elicitation) at the opposite hemisphere (AP = +2 mm, ML = −2 mm, and DV = −3 mm). The reported pressures were derated for skull attenuation, calibrated using ex vivo skulls in a water tank. The higher pressure (3.0 MPa) was used to assess a safe range of pressure to account for variances in the skull attenuation across animals and focus position at skull. A trained observer without knowledge of the location and parameters of sonication performed the histological evaluation. The samples were evaluated for red blood cell extravasation into the brain parenchyma as well as cell and tissue loss.
3. RESULTS AND DISCUSSION
In this study, the animals remained nonresponsive to pedal pinches throughout the sonication period confirming deep anesthesia levels. The experiment was started when the heart and breathing rates were under 200 beats/min (bpm) and 70 breaths/min (brpm), respectively. During sonication, the heart rate and breathing rate were under 400 bpm and 120 brpm, respectively. The anesthesia effect lasted approximately 90 min (assessed by pedal reflex) allowing sufficient experimental time.
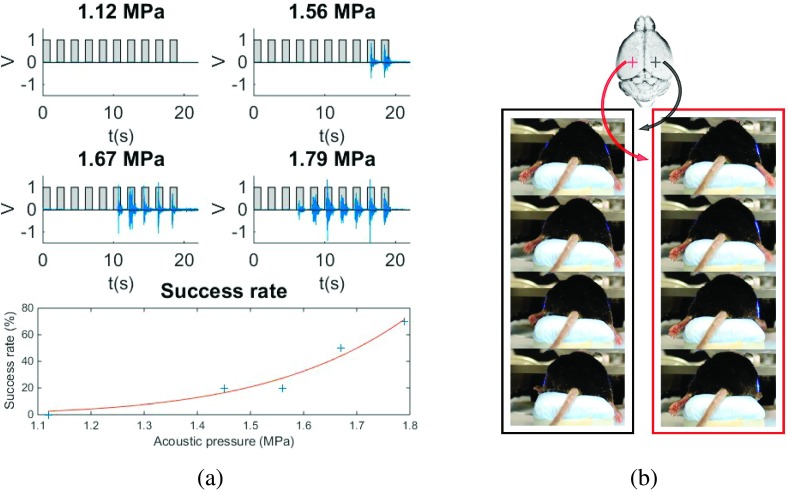
Contralateral muscle activity recorded on EMG signals [Fig. 1(a)] was observed when ultrasound neuromodulation was carried out at the sensorimotor cortex at AP (anterior/posterior): +2 mm, ML (medial/lateral): ±2 mm, and DV (dorsal/ventral): −1 mm for two out of three mice. The minimum pressure to elicit hind limb movement was determined to be 1.45 MPa between the two responsive mice. The average success rate, defined as the number of experiments during which a mouse movement was observed divided by the total number of experiments, increased with the increase of pressure for both mice together. Higher pressures increased the success rate from 20% at the threshold pressure 1.45 MPa to 70% at 1.79 MPa. The peak of the EMG signal was recorded 266 ± 37 ms after the onset of the ultrasound pulse. Figure 1(b) shows pictures of the contralateral hind limb movements recorded on a video presented in the supplementary materials (see Fig. S3 of the supplementary material15). The video shows contralateral paw movements elicited by sonications of symmetrical spots on the sensorimotor cortex (AP: +2 mm and ML: ±2 mm). The same video shows other sonications with elicitation of ipsilateral hind limb movements, the mostly elicited movements observed in all three mice (AP: +1 mm and ML: ±3 mm), and tail movements observed in one mouse (AP: 0 mm and ML: −3 mm). Although, the motor responses were not reproducible in all mice, once responsive spots were detected repeated sonications elicited same motor responses. An electric stimulation study shows that the movement representation regions in mice vary among animals,17 which for ultrasound neuromodulation may also occur due to differences in skull attenuation, precision in positioning of focus, and variances with the animal’s sensitivity to anesthesia. The last part of the video depicts the function generator used to drive the transducer, which indicates the onset of sonication and the respective tail movement observed.
FIG. 1.
FUS-induced motor responses. (a) EMG of the right hind limb during contralateral FUS stimulation at different acoustic pressure levels with success rate referred to contralateral motor response elicitation and (b) contralateral paw movement elicited by FUS neuromodulation. Video frames recorded during the left paw movement when sonicating AP = +2 mm from lambda and ML = +2 mm (left) and during the right paw movement when sonicating AP = +2 mm from lambda and ML = −2 mm (right) [see video S3 of the supplementary material (Ref. 15)].
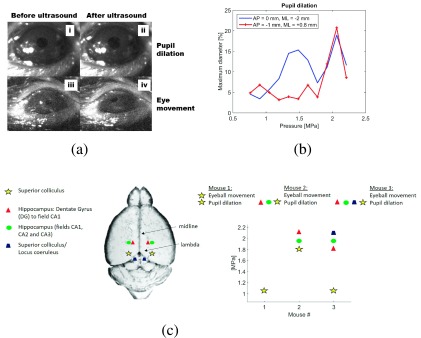
In a second set of experiments using the same transducer, we targeted subcortical brain structures where the ultrasound focus encompassed the superior colliculus (associated with motor control of the eyes), the hippocampus or the locus coeruleus (anxiety-related regions of the brain) as indicated in Fig. 2. A lower threshold in eliciting pupil dilation (1.20 MPa) was observed when targeting the region of the superior colliculus. For the same region, eyeball movements were observed when sonications were conducted at higher pressures (>1.8 MPa). Given that the 1.9 MHz beam is 1 mm in diameter, it is possible that both superior colliculus and pretectal nucleus were sonicated, since the superior colliculus reaches the pretectal nucleus near (∼0.5 mm) to the targeted region AP: 0 mm, ML: ±2 mm, and DV: −3.0 mm (Fig. 2). The pupillary dilation observed at this target may be associated with the modulation of the pretectal nucleus, which is directly involved with the pupillary light reflex. Input from retinal ganglion cells is sent to the pretectal nucleus, which projects to the Edinger–Westphal nucleus that innervates the iris sphincter muscle. Eyeball movements followed by pupillary dilation are observed in video S4 of the supplementary material15 when sonication was conducted at AP: 0 mm, ML: ±2 mm, and DV: −3.0 mm.
FIG. 2.
FUS-induced pupil dilation. (a) (i) and (ii) show pupil sizes before and after sonication, respectively, and (iii) and (iv) show the eye position before and after sonication of Mouse 1 at superior colliculus [see video S4 of the supplementary material (Ref. 15)]. (b) Representative percentage of pupil dilation for one mouse showing different thresholds at superior colliculus (AP: 0 mm and ML: −2 mm) and locus coeruleus (AP: −1 mm and ML: +0.8 mm). (c) Sonication spots where pupil dilation and eyeball movements were observed.
The hippocampus and locus coeruleus presented only pupil dilations but with higher thresholds (>1.8 MPa). Figure 2(a) shows relaxed pupil and pupil dilation of up to 20% (top, i and ii) and eyeball movements (bottom, iii and iv). In video S4 of the supplementary material,15 pupil dilation and eyeball movements are shown when sonications were carried out at superior colliculus. The breathing rate of the first part of the video showing pupillary dilation only was estimated based on the average brightness of the video frames.18 The pupillary dilation was accompanied by breathing rate variations from 38 brpm before sonication to 60 brpm during sonication. The variation of breathing rate may be an evidence of modulation of the locus coeruleus associated with stress, panic, and anxiety.
Whole brain histological examinations using hematoxylin and eosin (H&E) staining for general histology revealed no brain damage in five mice sonicated at 1.9 MPa at AP = +2 mm and ML = +2 mm and with 3.0 MPa at AP = +2 mm and ML = −2 mm (see Fig. S5 of the supplementary material15).
Sodium pentobarbital presented a working time of 60 min and was effective for keeping the animals fully anesthetized during the experiments. When targeting the cortex, the use of sodium pentobarbital seems to be an optimal choice because it does not suppress cortical evoked responses to the extent of other anesthetics (e.g., isoflurane19) and lasts longer than most other injectable agents (e.g., ketamine). Further studies are necessary to quantitatively compare the effects of different types of anesthesia on ultrasonic neuromodulation.
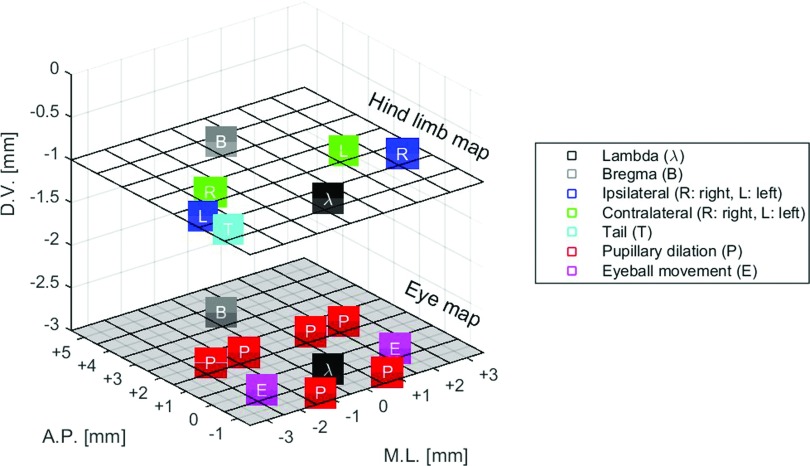
The contralateral and ipsilateral motor responses observed when targeting different locations on the cortex indicate superior spatial selectivity of our setup, which ultimately may have enabled stimulating selectively different brain regions (Fig. 3). As opposed to that, a previous study with demonstration of the efficacy of the megahertz-range to elicit motor functions reported inconsistent lateralization of muscle responses.12 The failure in obtaining lateralization of muscle responses in the previous study12 may be associated with the effect of the anesthesia on the cortex activity (isoflurane suppresses cortical activity19) or the excitation of larger regions of the brain due to the use of a transducer with higher f-number (f-number = focus/aperture; 1.33 vs 0.86 used in this study), the effect of waveguides to couple the transducer to the head of the animal, and pulse sequence (continuous wave for the same range of frequency vs pulsed in this study).
FIG. 3.
Map of most dominant responses observed during ultrasound neuromodulation of mice. Modulation of the cortex (D.V.: −1 mm) elicited tail and ipsilateral/contralateral hind limb movements. Modulation of subcortical structures of the brain (D.V.: −3 mm) elicited pupillary dilation and eyeball movements.
The eyeball movements and pupil dilation revealed the capability of the FUS to stimulate motor-related cortical structures in addition to anxiety-related and other subcortical structures of the brain (Fig. 3). Pupil dilation was observed when sonicating regions such as the limbic regions and the locus coeruleus. The superior colliculus presented a lower threshold in evoking pupil dilation (1.2 vs 1.8 MPa). The locus coeruleus, associated with responses to stress and panic, projects to superior colliculus. Pupil dilation was observed with a higher threshold when sonicating the hippocampus (part of the limbic system), which supports functions such as adrenaline flow, emotion, and behavior. At this sonication spot (AP = +2 mm and ML = ±2 mm), the ultrasound focus could reach the pretectal nucleus in the dorsal–ventral direction. Thus, there is a possibility of modulation of the pretectal nucleus associated with light reflex. We show the feasibility of using higher frequencies for modulating neuronal activity, demonstrating that the resultant smaller acoustic focus can provide superior target specificity. Based on the results obtained from the calibration of the transducer (see Fig. S1 of the supplementary material15), the transducer’s focal size can be highly improved by increasing the driving frequency (0.5 MHz: lateral resolution = 3.4 mm and axial resolution = 17.7 mm vs 1.94 MHz: lateral resolution = 1.0 mm and axial resolution = 8.5 mm). Thus, the entire ultrasound parametric space can be assessed in humans or larger animals seeking greater target specificity without being limited to submegahertz frequencies.
4. CONCLUSION
Reproducible contralateral and ipsilateral evoked motor responses demonstrated the superior target specificity of the megahertz-range for brain modulation, since previous studies failed in demonstrating such consistent responses when using lower frequencies. The sonication of deeper regions in the brain translated to pupillary dilations is used as indications of modulation of subcortical structures associated with cognition and light reflex responses. The variety of responses (motor and pupillary dilation) reported herein demonstrated the capability of FUS to perform functional brain mapping.
ACKNOWLEDGMENTS
This study was supported in part by NIH (Grant Nos. R01EB009041 and R01AG038961) and FAPESP (Grant Nos. 2011/10809-6 and 2013/08116-8). The authors thank Yang Liu, Ph.D., Edward Li, and Kathleen G. Fan for the technical support.
REFERENCES
- 1.Szobota S. and Isacoff E. Y., “Optical control of neuronal activity,” Annu. Rev. Biophys. , 329–348 (2010). 10.1146/annurev.biophys.093008.131400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Häusser M., “Optogenetics: The age of light,” Nat. Methods (10), 1012–1014 (2014). 10.1038/nmeth.3111 [DOI] [PubMed] [Google Scholar]
- 3.Tufail Y., Matyushov A., Baldwin N., Tauchmann M. L., Georges J., Yoshihiro A., Helms Tillery S. I., and Tyler W. J., “Transcranial pulsed ultrasound stimulates intact brain circuits,” Neuron (5), 681–694 (2010). 10.1016/j.neuron.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 4.Yoo S.-S., Bystritsky A., Lee J.-H., Zhang Y., Fischer K., Min B.-K., McDannold N. J., Pascual-Leone A., and Jolesz F. A., “Focused ultrasound modulates region-specific brain activity,” NeuroImage (3), 1267–1275 (2011). 10.1016/j.neuroimage.2011.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King R. L., Brown J. R., Newsome W. T., and Pauly K. B., “Effective parameters for ultrasound-induced in vivo neurostimulation,” Ultrasound Med. Biol. (2), 312–331 (2013). 10.1016/j.ultrasmedbio.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 6.Younan Y., Deffieux T., Larrat B., Fink M., Tanter M., and Aubry J.-F., “Influence of the pressure field distribution in transcranial ultrasonic neurostimulation,” Med. Phys. (8), 082902 (10pp.) (2013). 10.1118/1.4812423 [DOI] [PubMed] [Google Scholar]
- 7.King R. L., Brown J. R., and Pauly K. B., “Localization of ultrasound-induced in vivo neurostimulation in the mouse model,” Ultrasound Med. Biol. (7), 1512–1522 (2014). 10.1016/j.ultrasmedbio.2014.01.020 [DOI] [PubMed] [Google Scholar]
- 8.Tufail Y., Yoshihiro A., Pati S., Li M. M., and Tyler W. J., “Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound,” Nat. Protoc. (9), 1453–1470 (2011). 10.1038/nprot.2011.371 [DOI] [PubMed] [Google Scholar]
- 9.Naor O., Krupa S., and Shoham S., “Ultrasonic neuromodulation,” J. Neural Eng. (3), 031003 (2016). 10.1088/1741-2560/13/3/031003 [DOI] [PubMed] [Google Scholar]
- 10.Kim H., Chiu A., Lee S. D., Fischer K., and Yoo S.-S., “Focused ultrasound-mediated non-invasive brain stimulation: Examination of sonication parameters,” Brain Stimul. (5), 181–204 (2014). 10.1016/j.brs.2014.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehic E., Xu J. M., Caler C. J., Coulson N. K., Moritz C. T., and Mourad P. D., “Increased Anatomical specificity of neuromodulation via modulated focused ultrasound,” PLoS One (2), e86939 (2014). 10.1371/journal.pone.0086939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye P. P., Brown J. R., and Pauly K. B., “Frequency dependence of ultrasound neurostimulation in the mouse brain,” Ultrasound Med. Biol. (7), 1512–1530 (2016). 10.1016/j.ultrasmedbio.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilzenrat M. S., Nieuwenhuis S., Jepma M., and Cohen J. D., “Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function,” Cognit. Affective Behav. Neurosci. (2), 252–269 (2010). 10.3758/CABN.10.2.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graur S. and Siegle G., “Pupillary motility: Bringing neuroscience to the psychiatry clinic of the future,” Curr. Neurol. Neurosci. Rep. 13: 365 (2013). 10.1007/s11910-013-0365-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.See supplementary material at http://dx.doi.org/10.1118/1.4963208 E-MPHYA6-43-040610 for calibration of the transducer in water; more details about the experimental setup for motor response and pupil dilation observations; videos showing FUS-induced motor response elicitation; videos showing FUS-induced pupil dilation and eyeball movements; and whole brain histologic examination.
- 16.Choi J. J., Pernot M., Small S. A., and Konofagou E. E., “Noninvasive, transcranial and localized opening of the blood–brain barrier using focused ultrasound in mice,” Ultrasound Med. Biol. (1), 95–104 (2007). 10.1016/j.ultrasmedbio.2006.07.018 [DOI] [PubMed] [Google Scholar]
- 17.Tennant K. A., Adkins D. L., Donlan N. A., Asay A. L., Thomas N., Kleim J. A., and Jones T. A., “The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture,” Cereb. Cortex (4), 865–876 (2011). 10.1093/cercor/bhq159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao F., Li M., Qian Y., and Tsien J. Z., “Remote measurements of heart and respiration rates for telemedicine,” PLoS One (10), e71384 (2013). 10.1371/journal.pone.0071384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawaguchi M., Shimizu K., Furuya H., Sakamoto T., Ohnishi H., and Karasawa J., “Effect of isoflurane on motor-evoked potentials induced by Direct electrical stimulation of the exposed motor cortex with single, double, and triple stimuli in rats,” Anesthesiology (5), 1176–1183 (1996). 10.1097/00000542-199611000-00027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1118/1.4963208 E-MPHYA6-43-040610 for calibration of the transducer in water; more details about the experimental setup for motor response and pupil dilation observations; videos showing FUS-induced motor response elicitation; videos showing FUS-induced pupil dilation and eyeball movements; and whole brain histologic examination.